Abstract
Objective
Compare the effects of contralaterally controlled neuromuscular electrical stimulation (CCNMES) versus cyclic neuromuscular electrical stimulation (NMES) on lower extremity impairment, functional ambulation, and gait characteristics.
Design
Twenty-six stroke survivors with chronic (≥6mo) footdrop during ambulation were randomly assigned to six weeks of CCNMES or cyclic NMES. Both groups had ten sessions per week of self-administered home application of either CCNMES or cyclic NMES plus two sessions per week of gait training with a physical therapist. Primary outcomes included lower extremity Fugl-Meyer score, modified Emory Functional Ambulation Profile, and gait velocity. Assessments were made at pretreatment, posttreatment, and at 1 and 3 months posttreatment.
Results
There were no significant differences between groups in the outcome trajectories for any of the measures. With data from both groups pooled, there were significant but modest and sustained improvements in the Fugl-Meyer score and the modified Emory Functional Ambulation Profile, but not in gait velocity.
Conclusions
The results support the hypothesis that gait training combined with either CCNMES or cyclic NMES reduces lower extremity impairment and functional ambulation, but do not support the hypothesis that CCNMES is more effective than cyclic NMES in chronic patients.
Keywords: Stroke Rehabilitation, Hemiplegia, Neuromuscular Electrical Stimulation, Footdrop
Contralaterally controlled neuromuscular electrical stimulation (CCNMES) is a new method of delivering neuromuscular electrical stimulation (NMES) to paretic muscles for the purpose of promoting motor relearning after stroke.1 With CCNMES, the stimulation intensity, and therefore, the subsequent movement of the paretic limb, is regulated by volitional movement of the patients’ contralateral unimpaired limb. A sensor on the unimpaired limb regulates the stimulation intensity. Patients are instructed to attempt to move both limbs at the same time, thereby pairing motor intention with the stimulated (and intended) motor response. Repetitive pairing of motor intention with intended motor response may promote synaptic remodeling and neural reorganization leading to improved central control of the impaired limb.2-4 Also, simultaneous bilateral movements might promote interhemispheric disinhibition, which may allow reorganization by sharing of normal movement commands from the undamaged hemisphere.5
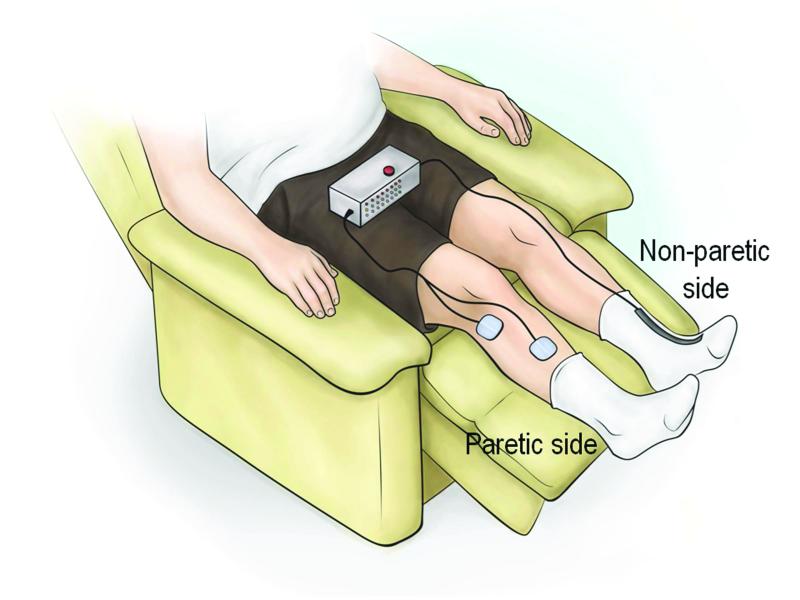
A randomized controlled trial of CCNMES applied to the upper limb of subacute stroke survivors showed that CCNMES with occupational therapy produced larger improvements in impairment and function than an equivalent dose of cyclic NMES and occupational therapy.6 Promising results of CCNMES in the upper limb1, 7 led to a case series feasibility study of its effects in the lower limb, particularly, its efficacy in promoting recovery of ankle dorsiflexion.8 When used to improve ankle dorsiflexion, CCNMES stimulates the paretic ankle dorsiflexors with an intensity that is proportional to the degree of volitional dorsiflexion of the contralateral unimpaired ankle. The unimpaired foot wears an instrumented sock that detects the degree of ankle dorsiflexion. Thus, volitional dorsiflexion of the unaffected ankle produces stimulated dorsiflexion of the affected ankle (Fig. 1).
Figure 1.
Contralaterally controlled neuromuscular electrical stimulation system for ankle dorsiflexion. Volitional dorsiflexion of the unimpaired ankle produces a proportional intensity of stimulation to the paretic ankle dorsiflexors.
CCNMES applied to the lower limb is intended to be an ankle motor retraining therapy consisting of repetitive ankle dorsiflexion exercise plus practice of a CCNMES-mediated ankle tracking task. Because CCNMES produces ankle dorsiflexion bilaterally and symmetrically, the treatment is done while seated, in contrast to peroneal nerve stimulators that assist dorsiflexion while walking.9-11 Although not used for walking, CCNMES may create a stronger temporal coupling of motor intention (central activity) and stimulated motor response (peripheral activity) than other existing NMES therapies by giving the patient direct proportional control of the stimulus intensity. Also, the active motor intention that the patient must give to dorsiflexing the ankle during CCNMES is greater than with ambulation-assisting peroneal nerve stimulators for which the patient does not consciously control the timing or intensity of stimulation. This added cognitive investment required by CCNMES may be necessary to produce neuroplastic changes leading to motor recovery.2, 12
In the case series feasibility study of ankle CCNMES in chronic (>6 mo) hemiplegia8, two of the three participants had substantial improvements in lower extremity Fugl-Meyer score, maximum active dorsiflexion, and ankle tracking performance. Adding gait training to CCNMES treatment may produce improvements in functional ambulation and spatiotemporal gait parameters in addition to reductions in ankle impairment.
The purpose of this pilot randomized controlled trial was to estimate the effect of 6 weeks of CCNMES and gait training on lower limb impairment, functional ambulation, and gait characteristics. Cyclic NMES was chosen as the comparison treatment because it rules out the effect of muscle stimulation (e.g., muscle strengthening) as well as the effects of other factors not unique to CCNMES, such as therapist attention, expectations, practice effects, and time in the laboratory or in exercise. Any group differences in outcome could then be attributed to the characteristics of CCNMES that distinguish it from cyclic NMES, which include: 1) stimulation controlled by the participant such that motor intention produces a proportional motor response, 2) bilateral symmetric ankle movement, 3) stimulation assists a goal-oriented motor control task (tracking).
METHODS
Participants and Treatment Assignment
As a pilot study, the sample size was determined by budgetary and time constraints and the availability of eligible stroke patients. Stroke patients who were 21 years of age or older and had ankle dorsiflexion impairment from a stroke that had occurred at least 6 months before enrollment were recruited from a stroke rehabilitation outpatient clinic of an academic medical center. Qualifying impairment was defined as footdrop during ambulation and less than normal ankle dorsiflexion strength (i.e., ≤4/5 on the Medical Research Council scale) while seated. To enroll, participants also needed to exhibit NMES-elicited pain-free ankle dorsiflexion to neutral while seated with the leg supported, ambulate at least 5 m continuously with minimal assistance or less without an ankle-foot-orthosis (necessary for quantitative gait analysis), and have sufficient cognition and communication to use the study device as instructed with the assistance of a caregiver if necessary. Patients were excluded if they had a brainstem stroke, insensate lower leg, uncompensated hemi-neglect, intramuscular botulinum toxin injections in any lower limb muscle within 3 months of enrollment, acute signs or symptoms of deep vein thrombosis or thromboembolism, edema of the affected lower leg, or currently receiving physical therapy for the lower extremity. The study protocol was approved by the committee on research ethics at the university-affiliated hospital in which the research was conducted, and written informed consent was obtained from each participant.
Following baseline assessments of lower extremity impairment, functional ambulation, and gait characteristics (described below), participants were assigned in equal proportions to CCNMES or cyclic NMES. An adaptive randomization algorithm13, 14 was used that minimized group differences on the following factors: 1) level of ambulation assistance required (i.e., at least contact guard assistance vs. no physical assistance) and 2) ankle proprioception (i.e., normal vs. impaired) determined by manual testing with the participants’ eyes closed.
Electrical Stimulation Systems and Stimulation Parameters
For both groups, a single pair of 2×2-in self-adhering electrodes (PALS® Platinum Blue, Axelgaard Manufacturing Co., CA) was used to produce ankle dorsiflexion, one positioned just below the head of the fibula over the common peroneal nerve and the second positioned over the motor point of the tibialis anterior. A multipurpose custom-built stimulator that produced biphasic rectangular current pulse trains was used. Pulse frequency was set at 35 Hz for all participants. Pulse amplitude was set constant at 40, 60, 80, or 100 mA, depending on what was needed to elicit dorsiflexion to neutral without pain. The strength of stimulated muscle contraction was modulated with pulse duration, which had a range of 0 to 250 μs. For each participant, the maximum pulse duration was defined as that required to dorsiflex the ankle to neutral without pain while seated. The electrode positions were adjusted until balanced dorsiflexion was achieved without discomfort.
During stimulator use, participants in both groups were seated with both legs supported on a legrest and ankle movement unimpeded (Fig. 1). The stimulator was programmed according to group assignment. As a CCNMES stimulator, it delivered stimulation according to input received from a bend sensor (Images SI, NY) attached to a sock worn on the participants’ unimpaired foot. As the participant moved the unimpaired foot from its resting posture to full dorsiflexion, the pulse duration (stimulation intensity) delivered to the paretic dorsiflexors increased from 0 to the maximum pulse duration determined for that participant. As a cyclic NMES stimulator, it delivered stimulation according to a fixed on/off duty cycle where the stimulation automatically turned on for several seconds and then turned off for several seconds with 1-sec on and off ramps. For the cyclic NMES group, no instrumented sock was worn on the unimpaired foot.
Each participant and their caregiver, if necessary, were trained on how to position the electrodes and use the stimulator according to their group assignment. For each participant, a picture of the electrodes in their proper positions was taken and given to them to assist positioning the electrodes themselves at home. A group-specific user’s manual was also reviewed and given to each participant.
Treatments
The treatment for both groups lasted 6 weeks. It consisted of: 1) self-administered home use of the assigned stimulator, and 2) lab sessions that included gait training with a physical therapist.
The self-administered stimulation consisted of two sessions per day at home. Each session lasted 51 minutes, consisting of three 15-min sets separated by 3-min rests. During each 15-min set, CCNMES participants sat with both legs extended and supported and were prompted by light and sound cues from the stimulator to repeatedly attempt to dorsiflex both ankles for several seconds, then relax for several seconds. For cyclic NMES participants, the stimulator automatically dorsiflexed the paretic ankle repeatedly with the same duty cycle as the CCNMES group, the sound and light cues indicating when the stimulation ramped on and off. Cyclic NMES participants were specifically instructed to try to dorsiflex their paretic ankle in synchrony with the stimulation, but keep their unimpaired foot relaxed. For both groups, the ankle dorsiflex/relax cue durations were changed every 2 weeks, from 6 sec on/20 sec off, to 8 sec on/16 sec off, to 8 sec on/10 sec off. This graded duty cycle was to progressively build strength with longer duration muscle contractions and more repetitions.15 All participants were instructed to separate the two sessions by at least 2 hrs to avoid fatigue. On days they came to the lab for gait training, they were instructed to perform only one exercise session at home at least 2 hrs after the lab session. In addition, they were instructed to skip any two exercise sessions they wished during the week. Therefore, the dose prescribed was equivalent to 10 sessions per week for 6 weeks – a total of 60 sessions. Participants filled out diaries to keep track of their exercise sessions. Compliance was monitored by the usage logging capability of the stimulator.
The twice-a-week lab sessions consisted of 15 minutes of stimulation-mediated ankle exercise followed by 30 minutes of pre-gait/gait training with a physical therapist. For the CCNMES group, the 15-min exercise was a CCNMES-assisted ankle movement tracking task. The participants sat with both legs supported facing a computer screen that displayed two parallel traces scrolling right-to-left across the screen creating a “path” characterized by stretches of gradually rising and falling slopes and periods with abrupt jumps up and down. An electrogoniometer (Biometrics Ltd, UK) attached to the paretic ankle displayed a cursor on the screen, its vertical position corresponding to the degree of dorsiflexion of the paretic ankle. The participant’s task was to keep the cursor between the parallel scrolling traces by using CCNMES along with their own volition to carefully dorsiflex and plantarflex the paretic ankle. The magnitude of deviation from the path was registered as an error, which was displayed on the screen and accumulated as the task progressed. At the end of the 15-min tracking task, a final error score was recorded and used as a goal during the next session. For the cyclic NMES group, the ankle exercise was a 15-min period of cyclic NMES. Cyclic NMES does not lend itself to assisting a goal-oriented motor control task like ankle tracking because the participant does not control the stimulation. The ankle tracking task is intended to be an important aspect of CCNMES treatment 8; therefore, to ensure that both groups received equivalent doses of stimulation, the cyclic NMES group received a 15-min set of cyclic NMES at the lab sessions.
Both groups received 30 minutes of conventional post-stroke pre-gait/gait training from the same experienced physical therapist. The focus of pre-gait training was to achieve sufficient trunk and balance control and master specific gait components to ambulate safely and efficiently. Weight-shifting exercises, side and forward stepping tasks, balance training in parallel bars, walking exercises, dynamic weight shifts, and strengthening exercises were included. Gait training on flat surfaces focused on safe and energy efficient ambulation. Verbal cues and physical assistance were provided to facilitate proper sequencing of specific joint movements, coordination of paretic limb movement, and maintenance of balance. Each participant was scheduled for 2 lab sessions per week for 6 weeks for a total of 12 lab sessions.
Outcome Assessments
Assessments of lower limb motor impairment, functional ambulation, and gait characteristics were made by a blinded assessor at baseline, end-of-treatment (EOT), and at 1 and 3 months post-treatment. The primary impairment measure was the lower extremity Fugl-Meyer assessment, a valid and reliable global measure of post-stroke lower extremity impairment.16 Secondary impairment measures included maximum dorsiflexion angle while seated, ankle movement tracking error, and maximum isometric dorsiflexion moment. The modified Emory Functional Ambulation Profile was the primary functional ambulation measure,17, 18 and gait velocity was the primary measure derived from quantitative gait analysis. Secondary gait analysis outcomes included dorsiflexion angle at initial contact, peak knee flexion in swing, peak hip flexion in swing, stride length, and cadence.
The lower extremity Fugl-Meyer assessment required participants to make various isolated and simultaneous movements of the hip, knee, and ankle. Each movement was graded on a 2-point scale and summed to provide a maximum score of 34.16 Maximum dorsiflexion angle was measured with an electrogoniometer (Biometrics Ltd., UK) taped to the anterior aspect of the foot so that it spanned the ankle without impeding ankle rotation.8 While seated with the lower leg supported and the knee flexed 30°, a 4-sec audio tone prompted the participant to maximally dorsiflex the ankle; this was repeated 3 times and the maximum dorsiflexion angles from the three trials were averaged. Ankle movement tracking error was measured using a 30-sec 0.1 Hz sinusoid track and the electrogoniometer at the ankle.8 The average absolute vertical distance between the cursor and the target sine wave was calculated in units equivalent to the percentage of the participants’ rest-to-maximum dorsiflexion range of motion; the lowest error of three trials was taken as the tracking error. Maximum isometric dorsiflexion moment was measured with a torque transducer (JR3 Inc., CA) attached to the shaft of a Biodex (Biodex Medical Systems, NY) dynamometer. With the knee fixed at 10° flexion and the ankle fixed at 5° plantarflexion, a 4-sec audio tone prompted the participant to dorsiflex the ankle with maximum force; this was repeated 3 times and the maximum dorsiflexion moments from the three trials were averaged.
The modified Emory Functional Ambulation Profile17, 18 measured the time to ambulate through 5 common environmental terrains: 1) 5-m walk on a hard floor, 2) 5-m walk on a carpeted floor, 3) rise from a chair, 3-m walk, return to seated position, 4) standardized obstacle course (bricks to step over), 5) stair ascent and descent. Participants were asked to perform these ambulation tasks at a comfortable and safe rate with physical assistance as necessary to ensure safety. The 5 timed subscores were added to derive a summary score.
Quantitative gait analysis was performed using a Vicon MX Motion Capture and Analysis System (Vicon, Oxford, UK). Participants walked 5 meters at a self-selected comfortable rate within the field of view of the motion capture system. Ten trials of data were collected per session, with a rest period between trials as needed. The 3-dimensional optical marker data were processed and analyzed using the biomechanical model contained in the Vicon software, which generated joint angles and spatiotemporal gait parameters.
Data Analysis
Baseline characteristics and scores for both groups were summarized with means (±SDs) for continuous variables, and frequencies for categorical variables. The baseline characteristics were compared between groups with Wilcoxon rank sum tests and Fisher’s exact tests, as appropriate. For each outcome measure, a linear mixed effects modeling approach was used to compare the effects of CCNMES and cyclic NMES across the post-treatment time points. The group×time interaction was of primary interest in assessing whether the two treatments had significantly different effects on the outcomes. Model estimates were made using a restricted maximum likelihood (REML) approach in SAS Version 9.2.19 Also, in case parametric assumptions were violated due to the smaller sample size, all outcomes were modeled using a more robust estimator for the standard error.20 The significance level was set at 0.05.
RESULTS
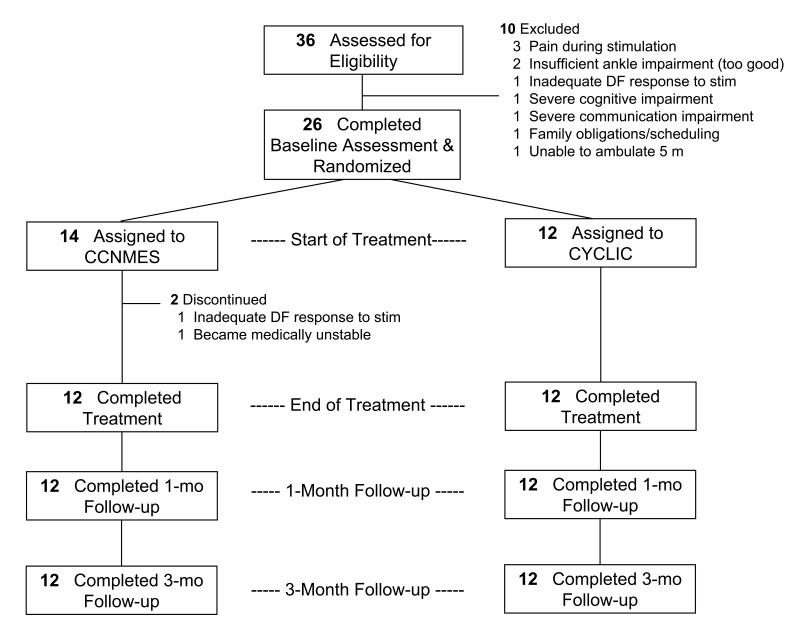
Twenty-six stroke patients were randomized to CCNMES or cyclic NMES; 2 did not complete the treatment phase (Fig. 2). The analyses are based on the remaining 24 participants, 12 in each group. There were no losses to follow-up from either group. According to the stimulators’ electronic data logger, the mean (±SD) percentage of prescribed sessions completed was 89±14 for the CCNMES group and 97±4 for the cyclic NMES group (P=0.08, two-tailed Wilcoxon rank sum test). This difference was mainly attributable to three participants in the CCNMES group who completed less than 85% of prescribed sessions. Those participants had no problems understanding the device or putting it on; rather, their lower adherence was related to personal schedules, family responsibilities, and illness. The mean (±SD) number of lab sessions competed was 10.9±0.9 for CCNMES and 11.1±1.3 for cyclic NMES (P=0.38, two-tailed Wilcoxon rank sum test). There were no treatment-related serious adverse events, but there were two cases of discomfort from stimulation, which were resolved by adjusting the position of the electrodes.
Figure 2.
Flow of participants through the study.
Abbreviations: CCNMES, contralaterally controlled neuromuscular electrical stimulation; CYCLIC, cyclic neuromuscular electrical stimulation; DF, dorsiflexion
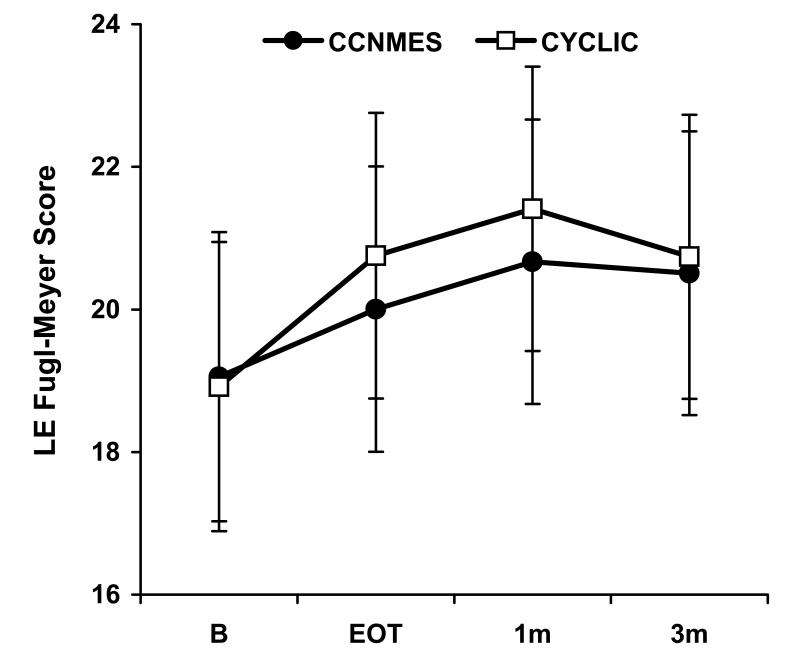
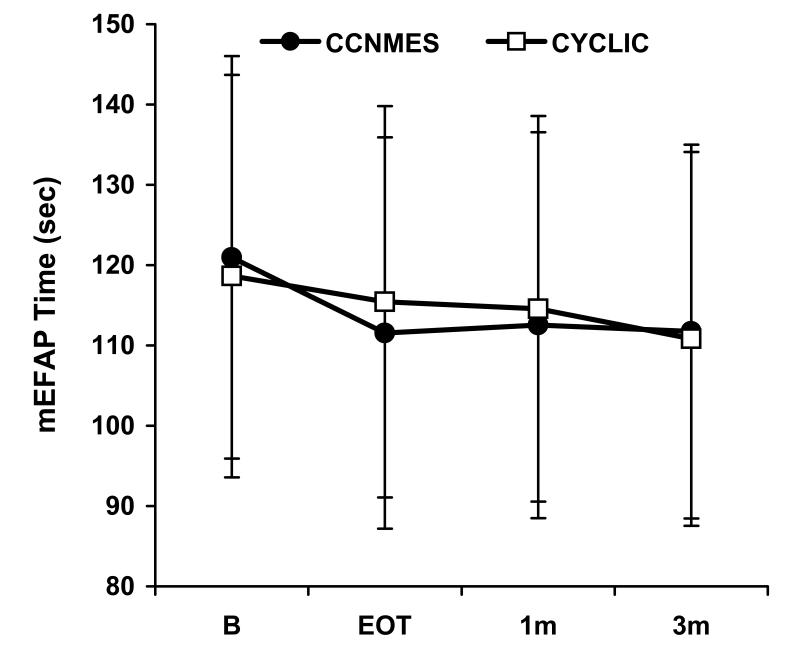
There were no significant differences between the two groups with respect to the baseline variables (Table 1). The mixed effects models estimated no significant group×time effects for any of the outcome measures (P>0.05). The two groups were not following significantly different trajectories from each other, as can be seen in plots of least squares mean over time for the primary outcome measures (Fig. 3A-C). Covariates for baseline values and for the percentage of prescribed home sessions completed were also included in the models, and neither was significant.
Table 1.
Baseline Characteristics and Scores*
| Characteristic | CCNMES (N=12) |
CYCLIC (N=12) |
P Value |
|---|---|---|---|
| Age, years | 56.7 ± 13.7 | 59.3±9.1 | 0.54 |
| Gender, n (%) male | 8 (67) | 6 (50) | 0.68 |
| Race, n (%) white | 6 (50) | 7 (58) | >0.99 |
| Years since stroke | 2.7 ± 1.8 | 3.6 ± 3.9 | >0.99 |
| Paresis of prestroke dominant foot, n (%) | 9 (75) | 9 (75) | >0.99 |
| Right side hemiparesis, n (%) | 9 (75) | 9 (75) | >0.99 |
| Ischemic stroke, n (%) | 11 (92) | 8 (67) | 0.32 |
| Ankle proprioception intact, n (%) | 11 (92) | 12 (100) | >0.99 |
| Taking anti-spasticity medication, n (%) | 2 (17) | 3 (25) | >0.99 |
| Need physical assistance to walk, n (%) | 3 (25) | 2 (17) | >0.99 |
| LE Fugl-Meyer Score, range 0 - 34 | 19.1 ± 6.8 | 18.9 ± 7.0 | 0.59 |
| Maximum dorsiflexion angle (°)** | −25 ± 18 | −13 ± 20 | 0.16 |
| Ankle movement tracking error | 13.8 ± 7.0 | 12.4 ± 7.0 | 0.63 |
| Maximum dorsiflexion isometric moment (Nm) | 5.4 ± 6.9 | 8.7 ± 11.2 | 0.44 |
| Time to complete mEFAP (sec) | 120.7 ± 86.3 | 118.6 ± 86.5 | 0.93 |
| Dorsiflexion angle at initial contact (°) | −10.0 ± 8.0 | −5.8 ± 6.7 | 0.18 |
| Peak knee flexion during swing (°) | 25.8 ± 13.2 | 24.4 ± 9.6 | 0.89 |
| Peak hip flexion during swing (°) | 34.4 ± 6.3 | 35.3 ± 7.4 | >0.99 |
| Gait velocity (cm/sec) | 39.9 ± 22.3 | 38.3 ± 19.1 | 0.93 |
| Stride length (cm) | 68.6 ± 23.3 | 61.7 ± 21.4 | 0.41 |
| Cadence (steps/min) | 64.2 ± 22.2 | 71.5 ± 19.3 | 0.32 |
Abbreviations: CCNMES, contralaterally controlled neuromuscular electrical stimulation; CYCLIC, cyclic neuromuscular electrical stimulation; LE, lower extremity; mEFAP, modified Emory Functional Ambulation Profile
Plus-minus values are means±SD. P values were calculated from Wilcoxon rank sum test and Fisher’s exact test where appropriate.
Zero degrees is neutral position; positive angles correspond to dorsiflexion; negative angles correspond to plantarflexion
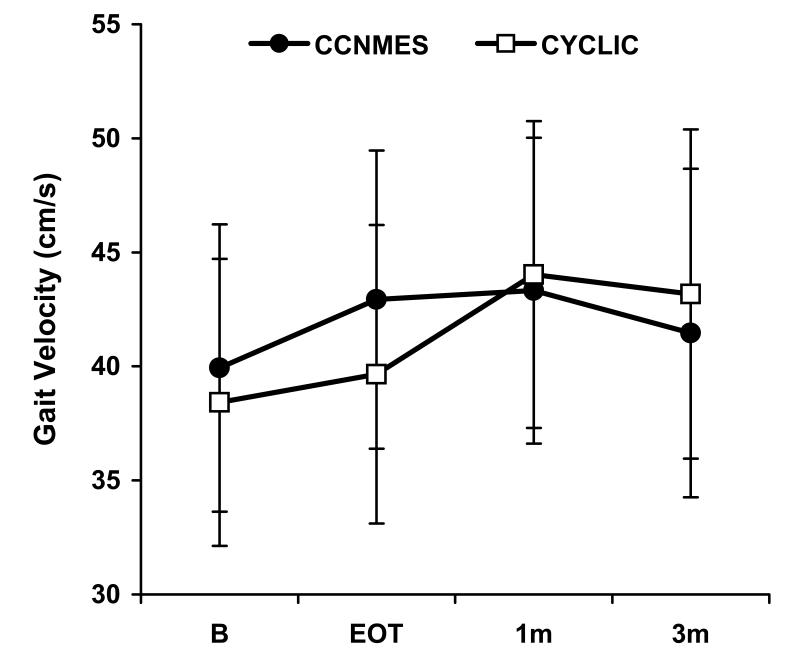
Figure 3.
Least square means ± standard error bars for the primary outcome measures. A) Fugl-Meyer Score, B) Modified Emory Functional Ambulation Profile, C) Gait Velocity
Since there were no significant group×time interactions, the data from both groups were pooled and, within the linear mixed effects modeling framework, the overall time effect for each outcome measure was examined. With data from both groups pooled, a significant overall time effect was found for Fugl-Meyer score, maximum dorsiflexion angle, tracking error, maximum dorsiflexion moment, and modified Emory Functional Ambulation Profile (P<0.05). These outcomes showed improvement over time as exhibited by significant model estimates of the time effect between baseline and EOT in the direction of improvement (Table 2). For the measures with significant EOT improvements, there were no significant differences between EOT and 1-month or between 1-month and 3-month follow-up. The same conclusions were found when the more robust estimates of standard errors were used in the models.
Table 2.
Changes in Outcome at End-Of-Treatment, Data Pooled from Both Groups*
| Outcome | Change at EOT* [95% CI] |
P Value |
|---|---|---|
| Change in score on LE Fugl-Meyer Assessment | 1.4 [0.4, 2.4] | <0.01 |
| Change in maximum dorsiflexion angle (°) | 6.5 [2.1, 10.9] | <0.01 |
| Change in ankle movement tracking error ** | −1.9 [−3.3, −0.5] | 0.01 |
| Change in maximum dorsiflexion moment (Nm) | 1.4 [0.2, 2.6] | 0.02 |
| Change in time to complete mEFAP (sec) ** | −6.3 [−11.1, −1.5] | 0.01 |
| Change in dorsiflexion angle at initial contact (°) | −0.7 [−2.3, 0.9] | 0.41 |
| Change in peak knee flexion during swing (°) | −0.1 [−4.0, 3.8] | 0.96 |
| Change in peak hip flexion during swing (°) | 0.0 [−2.5, 2.5] | 0.97 |
| Change in gait velocity (cm/sec) | 2.1 [−1.2, 5.4] | 0.21 |
| Change in stride length (cm) | 2.9 [−0.7, 6.5] | 0.12 |
| Change in cadence (steps/min) | −0.2 [−2.2, 1.8] | 0.81 |
Abbreviations: CCNMES, contralaterally controlled neuromuscular electrical stimulation; CYCLIC, cyclic neuromuscular electrical stimulation; EOT, end of treatment; LE, lower extremity; mEFAP, modified Emory Functional Ambulation Profile
Change estimates at end-of-treatment derived from linear mixed model.
On this scale, a reduction in the score indicates improvement in the condition being evaluated.
DISCUSSION
In this pilot randomized controlled trial of CCNMES for patients with chronic (> 6 mo) post-stroke ankle dorsiflexor hemiparesis, no significant benefit of CCNMES over cyclic NMES was found. With data from both groups combined, there were significant changes on all of the impairment measures tested and on the functional ambulation measure. Significant time effects at EOT persisted throughout the 3-month follow-up phase. The treatments and doses appeared to be acceptable to stroke patients, with 93% of the home sessions completed and 92% of the lab sessions kept, on average.
The study failed to show a benefit of CCNMES over cyclic NMES. This suggests that the features that distinguished CCNMES from cyclic NMES – stimulation directly linked to motor intention, bilateral symmetric ankle dorsiflexion, stimulation-assisted ankle tracking – were inadequate to produce greater improvement on lower limb impairment and function than NMES plus gait training within the experimental conditions of the present trial. Several possible explanations are offered. First, the default inter-limb coordination for the legs is anti-phase, 21-23 which is in contrast to the in-phase manner in which CCNMES was applied in this study (i.e., both ankles dorsiflexed together).21, 23, 24 The in-phase approach was used here because it was associated with positive findings in the case series feasibility study.8 Future studies might investigate CCNMES applied at the ankle in an anti-phase manner so that dorsiflexor stimulation of the paretic ankle occurs in response to plantarflexion of the non-paretic ankle. Second, the distinctions between CCNMES and cyclic NMES might have been diminished by having participants in the cyclic NMES group exert dorsiflexion effort in synchrony with the electrical stimulation. This simultaneous effort may have the same effect as linking stimulation directly to motor intention through a proportional control strategy even though the stimulation intensity in the cyclic NMES group was not controlled by the participant. Third, an acute or subacute stroke population would have greater potential for neuroplasticity than chronic stroke patients, and therefore may respond more to one treatment than the other.
The measures for which there was a significant time effect at EOT included all the impairment measures tested and the functional ambulation measure. There were no significant EOT time effects on any of the gait kinematic or spatiotemporal parameters. The improvement in functional ambulation may have been due to improvements in motor impairment or improved conditioning and practice effects of gait training. It is unclear what other aspects of gait training might have led to improving functional ambulation without also improving some gait kinematics or spatiotemporal parameters. The significant time effects on motor impairment at EOT may be attributed to: 1) the gait training that both groups received, 2) NMES of either kind tested in this study, or 3) the combination of gait training and NMES of either kind. This study was not designed to discern which of those three possibilities is responsible for the time effect at EOT, but the finding that none of the gait parameters showed a significant time effect suggests that NMES rather than gait training was important in achieving the significant time effects on the ankle impairment measures, especially since ankle movement is what the NMES specifically targeted. This is consistent with the case series CCNMES feasibility study 8 and other studies of cyclic NMES applied to ankle dorsiflexors,25, 26 which showed reductions in ankle impairment.
Improvements in gait kinematics did not accompany the reductions in non-ambulatory impairment measures, perhaps because walking imposed additional factors (e.g., neuromuscular, biomechanical, compensatory, etc.) that negatively affected gait but were not significantly corrected by the dose and/or application of NMES or gait training used in this study. Yet, it is possible for an ankle-specific intervention that is applied while seated, to not only reduce ankle impairment, but also improve spatiotemporal gait parameters.27 The NMES component might have contributed to the improvement in the functional ambulation measure by improving ankle stability or power.
While the magnitude of improvements at EOT reached statistical significance for the impairment and functional ambulation measures, it is questionable whether those changes were large enough to have clinical relevance since they were not associated with improvements in gait velocity. Nevertheless, the significant time effects suggest that gait training combined with NMES of either kind tested in this study has positive effects on ankle impairment and functional ambulation. Optimizing the dose or enhancing other features of the treatment (e.g., anti-phase rather than in-phase CCNMES) might lead to larger and more meaningful improvements.
The outcomes of previous studies of lower limb post-stroke interventions bear similarities and differences to this one. Like this study, reductions in chronic lower limb and/or ankle impairment have been demonstrated in response to functional electrical stimulation.26, 28, 29 Also like this study, significant group effects are not always found in randomized controlled trials comparing two or more post-stroke lower limb therapies.26, 30 Unlike this study, others have demonstrated significant improvements in gait velocity and/or other spatiotemporal gait parameters after various lower limb therapies.27, 29, 31-33 This study might not have shown improvement in gait velocity because the study population was more severely impaired, as demonstrated by slower baseline gait velocities, as compared to other studies that showed improvements in gait velocity. Patients with slower baseline walking speeds are known to improve less than those who walk faster at baseline.30
A limitation of this study is the lack of a group receiving gait training with no electrical stimulation for comparison with the two groups that received gait training plus electrical stimulation. Without such a comparison, the relative contribution of gait training and electrical stimulation to the significant pooled data time effects could not be discerned. Also, the lack of gait kinetic measures limited the ability to interpret the significant improvement in functional ambulation. Finally, the small sample size limited the power to detect small but significant effects.
CONCLUSIONS
In stroke survivors with chronic (> 6 months) ankle dorsiflexion hemiparesis, sixty 51-min sessions of CCNMES ankle therapy plus twelve 30-min gait training sessions over 6 weeks did not significantly improve lower extremity motor function more than an equivalent dose of cyclic NMES applied at the ankle plus gait training. However, both treatments resulted in significant but modest improvements in lower extremity impairment and functional ambulation. Future studies will target the acute/subacute patient population and evaluate the effect of a revised CCNMES paradigm in which stimulation to the paretic dorsiflexors more closely replicates the reciprocal pattern of normal walking. Applying anti-phase CCNMES ankle training in the earlier stages of stroke recovery in combination with physical therapy may lead to more clinically relevant improvements in gait.
ACKNOWLEDGMENTS
We gratefully acknowledge Peggy Maloney, RN, for her contributions to this study.
This work was supported by the National Institutes of Health (NIH) National Institute of Child Health and Human Development (NICHD) grant number R21HD061593 and the NIH National Center for Research Resources (NCRR) Clinical and Translational Science Collaborative of Cleveland grant number UL1RR024989. The stimulators used in this study were developed and provided by the Technical Development Laboratory of the Cleveland FES Center and are limited by federal law to investigational use. Declaration of Possible Conflicting Interests: Jayme Knutson, PhD and John Chae, MD are co-inventors on the CCNMES patent assigned to Case Western Reserve University, Patent 8,165,685: System and Method for Therapeutic Neuromuscular Electrical Stimulation.
Abbreviations
- CCNMES
contralaterally controlled neuromuscular electrical stimulation
- CYCLIC
cyclic neuromuscular electrical stimulation
- B
baseline
- EOT
end of treatment
- 1m
1-month follow-up
- 3m
3-month follow-up
- mEFAP
modified Emory Functional Ambulation Profile
- LE
lower extremity
Footnotes
Disclosures: Financial disclosure statements have been obtained, and no other conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Knutson JS, Harley MY, Hisel TZ, et al. Improving hand function in stroke survivors: a pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch Phys Med Rehabil. 2007;88:513–20. doi: 10.1016/j.apmr.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 3.Rushton DN. Functional electrical stimulation and rehabilitation--an hypothesis. Med Eng Phys. 2003;25:75–8. doi: 10.1016/s1350-4533(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 4.Kimberley TJ, Lewis SM, Auerbach EJ, et al. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154:450–60. doi: 10.1007/s00221-003-1695-y. [DOI] [PubMed] [Google Scholar]
- 5.Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- 6.Knutson JS, Harley MY, Hisel TZ, et al. Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early-phase randomized clinical trial in subacute stroke patients. Neurorehabil Neural Repair. 2012;26:239–46. doi: 10.1177/1545968311419301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knutson JS, Hisel TZ, Harley MY, et al. A novel functional electrical stimulation treatment for recovery of hand function in hemiplegia: 12-week pilot study. Neurorehabil Neural Repair. 2009;23:17–25. doi: 10.1177/1545968308317577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knutson JS, Chae J. A novel neuromuscular electrical stimulation treatment for recovery of ankle dorsiflexion in chronic hemiplegia: a case series pilot study. Am J Phys Med Rehabil. 2010;89:672–82. doi: 10.1097/PHM.0b013e3181e29bd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–67. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 10.Sheffler LR, Hennessey MT, Naples GG, et al. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair. 2006;20:355–60. doi: 10.1177/1545968306287925. [DOI] [PubMed] [Google Scholar]
- 11.Kottink AI, Oostendorp LJ, Buurke JH, et al. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28:577–86. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 12.Khaslavskaia S, Sinkjaer T. Motor cortex excitability following repetitive electrical stimulation of the common peroneal nerve depends on the voluntary drive. Exp Brain Res. 2005;162:497–502. doi: 10.1007/s00221-004-2153-1. [DOI] [PubMed] [Google Scholar]
- 13.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–53. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 14.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–52. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 15.Powell J, Pandyan AD, Granat M, et al. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30:1384–9. doi: 10.1161/01.str.30.7.1384. [DOI] [PubMed] [Google Scholar]
- 16.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–10. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 17.Baer HR, Wolf SL. Modified emory functional ambulation profile: an outcome measure for the rehabilitation of poststroke gait dysfunction. Stroke. 2001;32:973–9. doi: 10.1161/01.str.32.4.973. [DOI] [PubMed] [Google Scholar]
- 18.Liaw LJ, Hsieh CL, Lo SK, et al. Psychometric properties of the modified Emory Functional Ambulation Profile in stroke patients. Clin Rehabil. 2006;20:429–37. doi: 10.1191/0269215506cr950oa. [DOI] [PubMed] [Google Scholar]
- 19.Enhancements in SAS/STAT 9.2 Software. SAS Institute Inc.; Cary, North Carolina: 2008. [Google Scholar]
- 20.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford Science; Oxford: 1994. [Google Scholar]
- 21.Swinnen SP. Intermanual coordination: from behavioural principles to neural-network interactions. Nat Rev Neurosci. 2002;3:348–59. doi: 10.1038/nrn807. [DOI] [PubMed] [Google Scholar]
- 22.Kawashima N, Nozaki D, Abe MO, et al. Alternate leg movement amplifies locomotor-like muscle activity in spinal cord injured persons. J Neurophysiol. 2005;93:777–85. doi: 10.1152/jn.00817.2004. [DOI] [PubMed] [Google Scholar]
- 23.Vasudevan EV, Zehr EP. Multi-frequency arm cycling reveals bilateral locomotor coupling to increase movement symmetry. Exp Brain Res. 2011;211:299–312. doi: 10.1007/s00221-011-2687-y. [DOI] [PubMed] [Google Scholar]
- 24.Tuller B, Kelso JA. Environmentally-specified patterns of movement coordination in normal and split-brain subjects. Exp Brain Res. 1989;75:306–16. doi: 10.1007/BF00247936. [DOI] [PubMed] [Google Scholar]
- 25.Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–5. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- 26.Mesci N, Ozdemir F, Kabayel DD, et al. The effects of neuromuscular electrical stimulation on clinical improvement in hemiplegic lower extremity rehabilitation in chronic stroke: a single-blind, randomised, controlled trial. Disabil Rehabil. 2009;31:2047–54. doi: 10.3109/09638280902893626. [DOI] [PubMed] [Google Scholar]
- 27.Forrester LW, Roy A, Krebs HI, et al. Ankle training with a robotic device improves hemiparetic gait after a stroke. Neurorehabil Neural Repair. 2011;25:369–77. doi: 10.1177/1545968310388291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabut SK, Sikdar C, Mondal R, et al. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32:1594–603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 29.Daly JJ, Zimbelman J, Roenigk KL, et al. Recovery of coordinated gait: randomized controlled stroke trial of functional electrical stimulation (FES) versus no FES, with weight-supported treadmill and over-ground training. Neurorehabil Neural Repair. 2011;25:588–96. doi: 10.1177/1545968311400092. [DOI] [PubMed] [Google Scholar]
- 30.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–36. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornby TG, Campbell DD, Kahn JH, et al. Enhanced gait-related improvements after therapist-versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39:1786–92. doi: 10.1161/STROKEAHA.107.504779. [DOI] [PubMed] [Google Scholar]
- 32.Stein RB, Chong S, Everaert DG, et al. A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair. 2006;20:371–9. doi: 10.1177/1545968306289292. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil. 2002;83:683–91. doi: 10.1053/apmr.2002.32488. [DOI] [PubMed] [Google Scholar]