Abstract
Circadian phase resetting is sensitive to visual short wavelengths (450–480 nm). Selectively filtering this range of wavelengths may reduce circadian misalignment and sleep impairment during irregular light-dark schedules associated with shiftwork. We examined the effects of filtering short wavelengths (<480 nm) during night shifts on sleep and performance in nine nurses (five females and four males; mean age ± SD: 31.3 ± 4.6 yrs). Participants were randomized to receive filtered light (intervention) or standard indoor light (baseline) on night shifts. Nighttime sleep after two night shifts and daytime sleep in between two night shifts was assessed by polysomnography (PSG). In addition, salivary melatonin levels and alertness were assessed every 2 h on the first night shift of each study period and on the middle night of a run of three night shifts in each study period. Sleep and performance under baseline and intervention conditions were compared with daytime performance on the seventh day shift, and nighttime sleep following the seventh daytime shift (comparator). On the baseline night PSG, total sleep time (TST) (p < 0.01) and sleep efficiency (p = 0.01) were significantly decreased and intervening wake times (wake after sleep onset [WASO]) (p = 0.04) were significantly increased in relation to the comparator night sleep. In contrast, under intervention, TST was increased by a mean of 40 min compared with baseline, WASO was reduced and sleep efficiency was increased to levels similar to the comparator night. Daytime sleep was significantly impaired under both baseline and intervention conditions. Salivary melatonin levels were significantly higher on the first (p < 0.05) and middle (p < 0.01) night shifts under intervention compared with baseline. Subjective sleepiness increased throughout the night under both conditions (p < 0.01). However, reaction time and throughput on vigilance tests were similar to daytime performance under intervention but impaired under baseline on the first night shift. By the middle night shift, the difference in performance was no longer significant between day shift and either of the two night shift conditions, suggesting some adaptation to the night shift had occurred under baseline conditions. These results suggest that both daytime and nighttime sleep are adversely affected in rotating-shift workers and that filtering short wavelengths may be an approach to reduce sleep disruption and improve performance in rotating-shift workers. (Author correspondence: casper@lunenfeld.ca)
Keywords: Melatonin, shiftwork, short-wavelength light, sleep efficiency, total sleep time, wake after sleep onset
Introduction
Neurobehavioral processes including sleep and alertness are modulated by an interaction between circadian sleep propensity and the homeostatic need for sleep (Borbély, 1982; Dijk & Czeisler, 1994; Dijk et al., 1992). Optimal sleep occurs when both processes are highest, whereas a relative misalignment between circadian sleep propensity and homeostatic sleep pressure impairs sleep (Dijk & Czeisler, 1994). For example, night shiftwork forces individuals to work at a time when the circadian sleep drive is high and to sleep when wakefulness is high. This misalignment induces poor daytime sleep and impaired nighttime alertness (Åkerstedt, 1984; Chapdelaine et al., 2012; Czeisler et al., 1990). In addition, because of the misalignment induced by the imposed irregular light-dark schedules during shiftwork, repeated circadian phase resetting occurs in different physiologic rhythms (James et al., 2007; Stokkan et al., 2001; Yamazaki et al., 2000).
Light is the strongest circadian resetting cue and alters the phase of the pacemaker based on the timing, intensity, duration, and spectral characteristics of the photic stimulus (Brainard et al., 2001; Chang et al., 2012; Czeisler & Gooley, 2007; St Hilaire et al., 2012; Thapan et al., 2001; Zeitzer et al., 2000). Light during the early biological night induces phase delays, whereas light in the biological morning induces phase advances (Khalsa et al., 2003; St Hilaire et al., 2012). The duration and intensity of light have a nonlinear relation with phase resetting. For example, indoor light at low intensity (∼100 lux) can induce half-maximal phase resetting compared with high-intensity (10,000 lux) exposures (Zeitzer et al., 2000). Short-duration (0.2-h) exposures can induce phase shifts that are 8 times greater in magnitude per minute of exposure than long-duration (4-h) exposures (Chang et al., 2012). Therefore, irregular light-dark cycles during shiftwork, even at indoor intensities and short-duration exposures, may induce phase resetting and alter the relative alignment of the circadian sleep drive and sleep-work schedules in some individuals (Boivin & James, 2002; Crowley et al., 2003; Lee et al., 2006; Smith et al., 2009), which may adversely affect nighttime sleep.
Circadian phase resetting and the acute alterations in behavior, hormones, and gene expression induced by light exposure are preferentially sensitive to visual short wavelengths (Cajochen et al., 2006; Lockley et al., 2003, 2006; Thapan et al., 2001). We, and others, have previously shown that selectively removing wavelengths shorter than ∼500 nm from nocturnal illumination prevents melatonin suppression normally induced by nocturnal light exposure (Kayumov et al., 2005; Rahman et al., 2008, 2011; Sasseville & Hebert, 2010; Sasseville et al., 2006). Several studies have demonstrated that the combined use of bright light at night and dark goggles that reduce both light intensity and short-wavelength light transmission during the day facilitates circadian adaptation to permanent night shiftwork and improves daytime sleep and nighttime performance (Boivin et al., 2012a; Crowley et al., 2003; Eastman et al., 1994; Sasseville & Hebert, 2010; Smith et al., 2009). The use of the goggles during the day likely attenuates the phase advances induced by morning light exposure, which impair circadian adaptation to a nocturnal schedule (Crowley et al., 2003; Mitchell et al., 1997). In the present study, we hypothesized that light exposure in night-shift workers will result in a circadian phase delay leading to impairment not only of daytime sleep between consecutive night shifts but also on nighttime sleep on the first day off after consecutive night shifts. To test this hypothesis, we examined the effects of selectively filtering visual short wavelengths (<480 nm) during night shifts, on nighttime sleep following night shifts when individuals transitioned to a diurnal schedule, and daytime sleep between night shifts when individuals maintained a nocturnal schedule. In addition, we examined the effects of filtering short wavelengths on subjective and objective measures of alertness during night shifts.
Methods
Study Participants
The study protocol was approved by the Human Research Ethics Committee of the University Health Network (Toronto, Ontario, Canada) and all participants provided informed consent prior to enrolling in the study. The study conforms to international ethical standards as described (Portaluppi et al., 2010). All participants were recruited from the same hospital using recruitment flyers and meetings with the study investigator. Thirty-six full-time nurses working different rotating shifts at the same hospital were interested and further screened for the study. Fourteen nurses met the selection criteria and the results from nine participants with the same shift rotation are presented. The participants included five females and four males; mean age ± SD: 31.3 ± 4.6 yrs. Participants were screened for extreme chronotype as assessed with the composite scale of morningness (Smith et al., 1989) (exclusion range: <22 and >44; mean ± SD: 34.2 ± 4.3; range: 29–43); history of ocular/vision diseases including requirement of corrective lenses for vision and/or color blindness; depressive symptomatology based on the Center for Epidemiologic Studies Depression Scale (CES-D) score (Weissman et al., 1977) (exclusion >16; mean ± SD: 8.4 ± 3.3; range: 2–14); any medication use; and occasional or habitual nicotine use. None of the participants worked elsewhere or on other shifts within the department besides the study schedule while enrolled in the study. Participants were recruited from Emergency department, Medical/Surgical/Neurological intensive care unit, and the Musculoskeletal Health and Arthritis units.
Overall Study Design
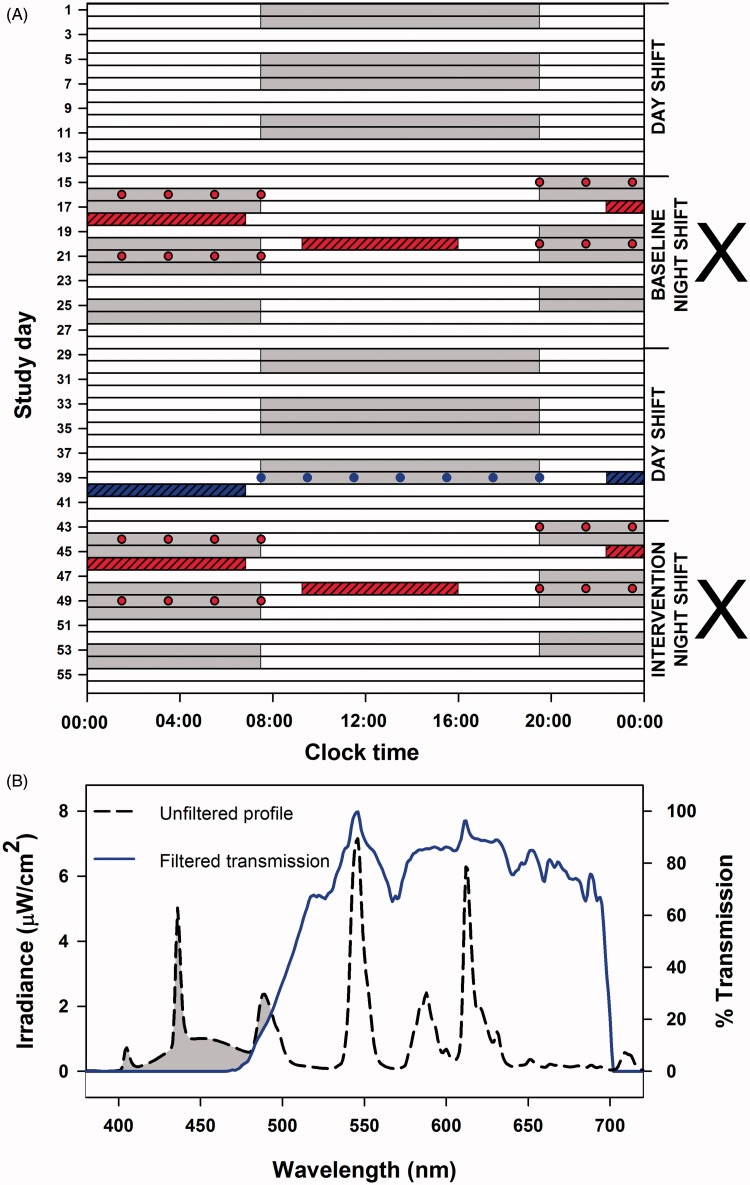
Each participant was enrolled in the study for 8 wks. Twelve-hour night (19:30–07:30 h) and day (07:30–19:30 h) shifts were worked in 2-wk stretches with no more than two or three consecutive workdays and no less than two consecutive workdays (Figure 1A). Participants did not rotate between night and day shifts during the 2-wk stretch, i.e., the subjects completed 2 wks of night shifts alternating with 2 wks of day shifts. All work shifts were separated by two to three non-working days during both night and day shift stretches. All subjects started the study with 2 wks of day shifts. Subjects were then randomized to receive glasses fitted with short-wavelength filters (0% transmission <480 nm) (Figure 1B) to be used only during night shifts during weeks 3 and 4 (days 15–28) or 7 and 8 (days 43–56). Subjects were not allowed to consume stimulants including caffeine on any of the testing nights and prior to nighttime and daytime sleep studies; however, caffeine use was allowed on other days. Participants were not allowed to nap during work.
Figure 1.
Schematic representation of study protocol and spectral transmission profile of filtered light. Nine full-time nurses participated in the 8-wk study (A). Each participant completed 8 wks consisting of 2-wk rotation periods of alternating night and day shifts (gray bars). Working days were separated by two to three non-workdays (white bars). Participants were randomized in a crossover design (marked by X) to receive glasses fitted with short-wavelength filters (0% transmission <480 nm) to be used only during night shifts (intervention) or exposed to regular unfiltered ambient artificial light (baseline). Sleep and alertness under baseline and intervention conditions (red hashed bar and red filled circles) were compared with nighttime sleep after the seventh day shift (polysomography) and alertness measures taken during the seventh day shift on the second 2-wk day shift period (comparator; blue hashed bar and blue filled circles). Baseline and intervention for nighttime and daytime sleep were assessed on four separate occasions by polysomnography (red hashed bars). Subjective and objective measures of alertness and saliva samples for melatonin assays were collected every 2 h on the first and fourth night shifts under baseline and intervention conditions (red filled circles). Transmission profile (B) of the filtered light (blue line) was generated against a standard fluorescent light source (black dashed line), demonstrating the effective visual short-wavelength range (<480 nm; gray-shaded region) removed by the interference filters.
Nighttime and daytime sleep was assessed in the sleep laboratory by polysomnography (PSG) on five separate occasions. Nighttime sleep was assessed after the first two night shifts with (intervention) and without (baseline) short-wavelength filtering glasses. The first exposure to the sleep laboraotry was randomized between baseline (five subjects) and intervention (four subjects) to control for any acclimatization and first-night effects. In addition, nighttime sleep was assessed on the last day shift of the second 2-wk stretch of day shifts (comparator) (Figure 1A). Daytime sleep was assessed between the third and fourth night shifts with (intervention) and without (baseline) short-wavelength filtering glasses. Saliva samples were collected for melatonin assays every 2 h during the first night shift and during the middle night of three night shifts under baseline and intervention conditions (Figure 1A). Subjective and objective assessment of alertness was conducted on the first and middle night shifts under baseline and intervention conditions as well as on the last day shift of the second 2-wk stretch of day shifts (Figure 1A).
Lighting Conditions
Participants were exposed to a mean light intensity of 179.4 ± 48.3 lux (mean ± SD) generated mostly from overhead fluorescent lamps. The range of lowest to highest intensities for the different hospital units where the nurses in the study worked was 41.3–480.4 lux. Exposure to light during working or non-working hours was not controlled but individuals were instructed to maintain consistent daytime and nighttime sleep-wake schedules throughout the study.
Photic Spectral Modulation
The filters used in the present study have been characterized and described in detail elsewhere (Rahman et al., 2011). Visual short wavelengths were filtered from ambient light during night shifts using identical wraparound spectacle frames fitted with polycarbonate lenses coated with thin-film Fabry-Perot interference filters that blocked all transmission (0% transmission) below 480 nm (ZircLight, Stoneham, MA, USA; Figure 1B). The direct application of the thin-film filters onto the lenses and the use of wrap-around frames ensured a single-unit filtering device minimizing stray light incident on the eyes. The spectral transmission profile generated using a common fluorescent light source (T-8, 48-inch 32 watt, F032/850 5000 K Octron Eco Fluorescent Bulb; Osram Sylvania, Mississauga, Ontario, Canada) is presented in Figure 1B. The 1931 CIE x/y chromaticity values for the filtered lenses derived from a fluorescent source was 0.487/0.500, respectively (Rahman et al., 2011). Subjectively, looking through the lenses resulted in a slight yellow cast, which did impair the ability to distinguish some colors, e.g., dark blue from dark green in the absence of backlighting. When looking at the lenses from certain angles when another person was wearing them, they occasionally appeared blue, since wavelengths in the blue range were being blocked and reflected back by the optical filter.
Polysomnographic Sleep Assessment
All individuals had to arrive at the sleep laboratory for nocturnal sleep studies at 20:30 h and had a mandatory lights-off no later than 24:00 h. This approach ensured a minimum 8-h sleep opportunity and wake up within 1 h of shift start time if the individuals were to return to day shift the following morning. For daytime sleep, all individuals arrived at the sleep laboratory at 08:00 h and had a mandatory lights-off no later than 10:00 h. This ensured a minimum 8-h sleep opportunity before the individuals had to return to their next night shift the same evening at 19:00 h. Individuals could choose to turn lights off and initiate sleep earlier than the mandatory lights-off time but not later than the preset time. Individuals were allowed to sleep longer than 8 h. To ensure that individuals did not forcibly truncate their sleep, participants were kept in bed for a maximum of 1 h or until the scheduled 8-h sleep was completed if they awoke prior to the 8-h sleep opportunity. In addition, participants slept in private rooms with no access to electronics including alarm clocks, cellular phones, or other devices with time-telling capability or illuminated screens. The extra hour was not added to the total sleep time or time in bed if the participants did not sleep (have stage 2 or higher or rapid eye movement [REM] sleep) during that period; however, if subjects did sleep for any portion within that 1-h period, that duration was added to total sleep time and any intervening wake was added to wake after sleep onset.
Sleep was assessed objectively using polysomnography (PSG) and data were collected and analyzed using the Sandman Elite system (Kanata, Ontario, Canada). A standard montage including electroencephalography, electrooculography, electromyography, and respiratory monitoring (oxygen saturation, nasal airflow, and breathing effort) was used. The polysomnographs were scored by a single blinded scorer according to standardized criteria (Rechtschaffen & Kales, 1968). Sleep onset latency was defined as time to the first 30-s epoch of stage 2 sleep from lights-off (Rahman et al., 2009).
In addition, all participants maintained daily sleep diaries throughout the course of the study. Participants were instructed to record their daily sleep and wake times, specifically the time at which they went to bed, how long they think it took them to fall asleep, when they woke up, and when they got out of bed for each and every sleep episode.
Melatonin Assay
Melatonin was assayed in saliva, and eating or drinking was not permitted for 30 min prior to sample collection. Saliva was collected every 2 h by passive saliva collection and stored at 4°C until all samples were collected at the end of the shift for each individual. All samples were stored at −80°C until further processing. All frozen saliva samples were defrosted on ice and centrifuged again at 1500 × g for 15 min at 4°C prior to assaying. Salivary melatonin was measured by enzyme-linked immunosorbent assay (Alpco Diagnostics, Salem, NH, USA) as per manufacturer instructions. All samples from each subject under each lighting condition were assayed in batches so that an equal number of samples from each of the two lighting conditions and subjects were processed together in each run of the assay. The intra-assay precisions were 2.1% at 1.5 pg/mL and 3.3% at 50 pg/mL, the interassay precisions were 2.9% at 1.5 pg/mL and 11.7% at 50 pg/mL, and the limit of detection was 0.5 pg/mL.
Alertness and Affect Measures
Participants completed self-report scales and objective tests programmed onto a hand-held personal digital assistant (PDA). Each participant was given a unique PDA and all tests on the PDA were commercially programmed and validated (Behavioral Neuroscience System, Springfield, MO, USA). These tests are programmed on the PDA as part of the Automated Readiness Evaluation System (ARES) (Elsmore et al., 2007), which is an extension of the Automated Neuropsychological Assessment Metric (ANAM) (Kaminski et al., 2009), the latter being a computerized version of the Walter Reed Performance Assessment Battery (PAB) (Thorne et al., 1985). The ARES battery allows selection of PAB tests to design a customized battery allowing reduction of total testing duration. Reduced testing duration can improve compliance and reduce missed tests in a field-based study (Elsmore et al., 2007). Performance tests as part of the modified ARES battery included measures of one-choice reaction and response inhibition (GO/NO-GO) tests and subjective sleepiness was assessed using a 7-item self-report scale (Elsmore et al., 2007; Thorne et al., 1985). This battery has been shown to be sensitive to circadian variation (Gillooly et al., 1990) and has been used previously to study the effects of different visual wavelengths on cognitive performance (Figueiro et al., 2009). The one-choice reaction test required the participant to tap on an assigned spot on the PDA screen when presented with a single stimulus (an asterisk *) as fast as possible. The response inhibition test presented two nonsimultaneous stimuli, where one required a screen-tapping response the other did not. The test durations were 1 and 2 min for the one-choice reaction test and the response inhibition test, respectively. At the end of each week, depressive symptomatology was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) and subjective daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS) (Johns, 1992).
Data Analysis
Data are expressed as the mean ± SEM unless otherwise stated. Data were subjected to repeated-measures one- (group) or two- (group × time) factor mixed-model analysis of variance (ANOVA) (as appropriate) with restricted maximum likelihood (REML) estimates of the variance components. If a significant main effect was observed, the analysis was followed by Dunnett’s multiple-comparison tests to assess differences between intervention and baseline groups relative to the comparator group. Comparisons between two groups were assessed using repeated-measures two-tailed Student’s t test. Change in melatonin area under the curve (AUC) and sleep variables were subjected to Pearson’s correlation analysis. A two-sided p value <0.05 was considered to indicate statistical significance. SAS version 9.2 (Cary, NC, USA) was used for all statistical analyses.
Results
Effects on Nighttime and Daytime Sleep
The main effects of group and significance levels are presented in Table 1 for nighttime sleep and Table 2 for daytime sleep. Nighttime sleep assessed under baseline and intervention conditions following two night shifts was compared with nighttime sleep after 2 wks of day shifts (comparator). Mean lights-off and lights-on times were not significantly different between the three conditions. There were significant reductions in total sleep time, sleep efficiency, and non-REM (NREM) (stage 2 + 3 + 4) sleep duration and a significant increase in wake after sleep onset (WASO) under baseline as compared with the comparator. The use of optical filters (intervention) improved all of these parameters so that they were no longer significantly different from the comparator. Total sleep time was increased by a mean of 40 min after intervention compared with baseline. WASO was reduced by a mean of 22 min and sleep onset latency was reduced by a mean of about 8 min during the night following intervention compared with baseline. There was no change in slow-wave sleep with intervention or baseline compared with the comparator night.
Table 1.
Effects of filtering visual short wavelengths (<480 nm) on nighttime sleep structure variables.
| Sleep variable | Comparator (C) Mean ± SEM | Baseline (B) Mean ± SEM | Intervention (I) Mean ± SEM | p | C vs. B | C vs. I |
|---|---|---|---|---|---|---|
| Total sleep time (min) | 476.67 ± 17.32 | 397.67 ± 18.67 | 437.78 ± 14.37 | 0.0107 | 0.0067 | 0.2375 |
| Sleep efficiency (%) | 91.42 ± 1.40 | 78.14 ± 4.01 | 85.94 ± 3.32 | 0.0221 | 0.0142 | 0.5763 |
| Stage 1 (min) | 29.00 ± 7.97 | 22.67 ± 3.92 | 20.39 ± 2.50 | 0.5416 | ||
| Stage 2 (min) | 231.08 ± 9.87 | 217.28 ± 15.17 | 238.56 ± 7.53 | 0.2371 | ||
| Stage 3 (min) | 18.58 ± 1.42 | 19.83 ± 2.53 | 22.06 ± 2.60 | 0.6210 | ||
| Stage 4 (min) | 77.83 ± 10.37 | 51.83 ± 5.11 | 63.31 ± 9.97 | 0.1667 | ||
| REM (min) | 120.17 ± 13.10 | 85.50 ± 9.17 | 92.67 ± 9.73 | 0.1168 | ||
| NREM (S2 + S3 + S4) (min) | 327.50 ± 12.64 | 288.94 ± 17.00 | 323.92 ± 12.09 | 0.0476 | 0.0771 | 0.9973 |
| SWS (S3 + S4) (min) | 96.42 ± 9.71 | 71.67 ± 5.46 | 85.37 ± 10.57 | 0.1982 | ||
| Sleep onset latency (min) | 13.27 ± 5.76 | 14.37 ± 2.33 | 10.59 ± 1.89 | 0.6643 | ||
| REM onset latency (min) | 59.25 ± 11.21 | 103.28 ± 20.78 | 72.00 ± 4.05 | 0.0907 | ||
| WASO (min) | 22.42 ± 5.80 | 73.00 ± 21.43 | 37.39 ± 17.64 | 0.0237 | 0.0338 | 0.9147 |
Data represent mean ± SEM. Data were subjected to repeated-measures one-factor (Group) mixed-model ANOVA. If a significant main effect was observed, the analysis was followed by Dunnett’s multiple-comparison tests to assess differences between intervention and baseline groups relative to the comparator group.
Table 2.
Effects of filtering visual short wavelengths (<480 nm) on daytime sleep structure variables.
| Sleep variable | Comparator (C) Mean ± SEM | Baseline (B) Mean ± SEM | Intervention (I) Mean ± SEM | p | C vs. B | C vs. I |
|---|---|---|---|---|---|---|
| Total sleep time (min) | 476.67 ± 17.32 | 307.78 ± 33.16 | 341.52 ± 25.86 | 0.0006 | 0.0004 | 0.0025 |
| Sleep efficiency (%) | 91.42 ± 1.40 | 68.32 ± 7.07 | 75.36 ± 3.99 | 0.0098 | 0.0053 | 0.0414 |
| Stage 1 (min) | 29.00 ± 7.97 | 20.22 ± 2.74 | 24.09 ± 3.13 | 0.4280 | ||
| Stage 2 (min) | 231.08 ± 9.87 | 147.22 ± 17.60 | 155.94 ± 10.59 | 0.0026 | 0.0021 | 0.0047 |
| Stage 3 (min) | 18.58 ± 1.42 | 17.00 ± 2.76 | 13.94 ± 1.74 | 0.4702 | ||
| Stage 4 (min) | 77.83 ± 10.37 | 64.33 ± 9.75 | 75.72 ± 9.32 | 0.2980 | ||
| REM (min) | 120.17 ± 13.10 | 58.50 ± 11.86 | 71.22 ± 11.67 | 0.0025 | 0.0014 | 0.0083 |
| NREM (S2 + S3 + S4) (min) | 327.50 ± 12.64 | 228.56 ± 22.05 | 245.61 ± 16.46 | 0.0026 | 0.0016 | 0.0068 |
| SWS (S3 + S4) (min) | 96.42 ± 9.71 | 81.33 ± 10.11 | 89.67 ± 10.07 | 0.4624 | ||
| Sleep onset latency (min) | 13.27 ± 5.76 | 13.24 ± 4.16 | 4.76 ± 2.06 | 0.0673 | ||
| REM onset latency (min) | 59.25 ± 11.21 | 74.72 ± 9.90 | 76.89 ± 13.71 | 0.4565 | ||
| WASO (min) | 22.42 ± 5.80 | 88.72 ± 32.24 | 66.67 ± 22.10 | 0.0460 | 0.0269 | 0.1124 |
Data represent mean ± SEM. Data were subjected to repeated-measures one-factor (Group) mixed-model ANOVA. If a significant main effect was observed, the analysis was followed by Dunnett’s multiple-comparison tests to assess differences between intervention and baseline groups relative to the comparator group.
Daytime sleep assessed under baseline and intervention conditions between two night shifts was also compared with the comparator, i.e., the same nighttime sleep episode after 2 wks of day shifts was used for both day sleep and night sleep comparisons. There was a significant increase in WASO during day sleep under baseline compared with the comparator. Under intervention, WASO was not different from the comparator night sleep. There were significant reductions in total sleep time, sleep efficiency, stage 2 duration, REM and NREM (stage 2 + 3 + 4) sleep duration under baseline during day sleep compared with the comparator (Table 2). Under intervention, all day sleep parameters were improved toward the comparator values but these changes did not reach statistical significance. Furthermore, individual self-reported habitual sleep-wake characteristics including sleep duration, sleep onset latency, and sleep efficiency averaged over 2 wks of day shifts (period of the comparator condition) showed no significant correlation with the magnitude of changes in sleep structure parameters observed under laboratory conditions.
Effects on Melatonin Levels During Night Shifts
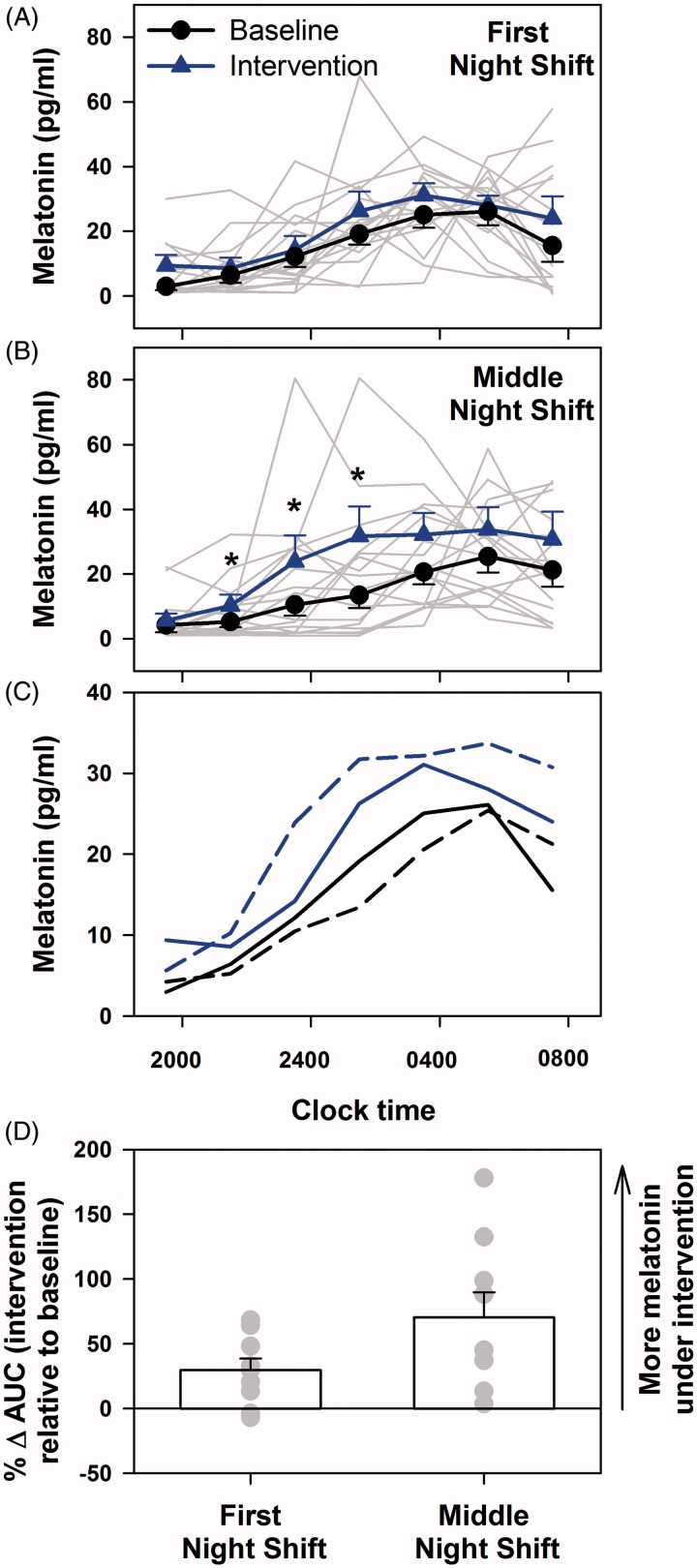
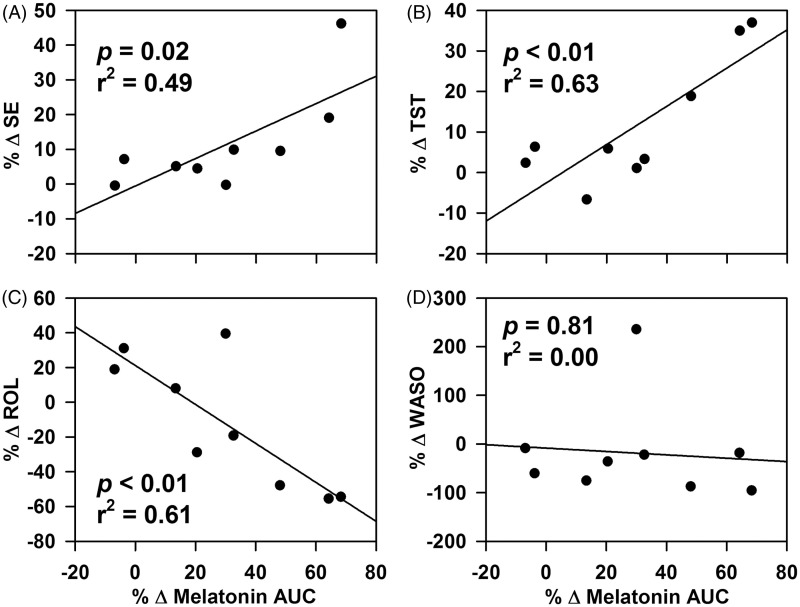
As compared with baseline, there was a modest but significant increase in melatonin levels with intervention (group effect p = 0.04) (Figure 2A) on the first night shift and a more robust difference on the middle night shift (p < 0.01) (Figure 2B). There was a significant effect of time on melatonin profiles on both nights (p < 0.01) but no interaction effects of the two factors (first night: p = 0.95; fourth night: p = 0.69). There was considerable interindividual variation in absolute levels and peak time on the first and middle night shifts and under both baseline and intervention conditions. The average melatonin profiles suggest a greater difference in phase on the middle night shift between baseline and intervention than on the first night shift (Figure 2C). In addition, the 12-h area under the curve (AUC) for each individual compared between baseline and intervention conditions revealed significantly greater change in melatonin AUC on the middle night as compared with first night shift (p = 0.02) (Figure 2D). Moreover, the change in melatonin AUC was more variable on the middle night shift (range: 3.7–178.1; SD: 58.2) as compared with the first night shift (range: −6.8–68.3; SD: 27.0) (Figure 2D). There were significant correlations on the first night shift between changes in melatonin AUC and the changes in sleep efficiency (p = 0.02; Figure 3A) and total sleep time (p < 0.01; Figure 3B) for nighttime sleep but not daytime sleep (Figure 3C and D).
Figure 2.
Changes in melatonin levels during night shifts with exposure to visual short-wavelength-filtered light and standard ambient artificial light. Melatonin levels were significantly higher under intervention (exposure to short-wavelength [<480 nm]-filtered light during night shifts) as compared with baseline (exposure to standard unfiltered ambient light during night shifts) on the first night shift (A) and middle night shift of a series of three consecutive night shifts (B). Individual profiles are presented as gray lines and group mean ± SEM profiles are presented as blue lines. Mean melatonin profiles are compared between the two conditions and two nights of testing (C). Data were subjected to two-factor (group × time) mixed-model ANOVA for the first and fourth night shifts individually. Statistically significant values at specific times as revealed by post hoc analysis are represented by *. Percentage change in melatonin AUC between baseline and intervention conditions on the first and second nights were calculated to assess changes in melatonin levels on an individual basis (D). Group mean ± SEM for each night is expressed as the bars and individual levels are expressed as gray filled circles. The change in AUC was significantly greater on the fourth night as compared with the first night.
Figure 3.
Correlation between percentage change in melatonin AUC between baseline and intervention conditions on the first night shift and the change in nighttime sleep structure variables. There was a significant correlation between the change in melatonin AUC between baseline and intervention conditions on the first night shift and change in nighttime sleep efficiency (A) and total sleep time (B) between baseline and intervention conditions but there was no correlation between the same variables for daytime sleep (C and D).
Effects on Alertness, Subjective Mood, and Daytime Sleepiness
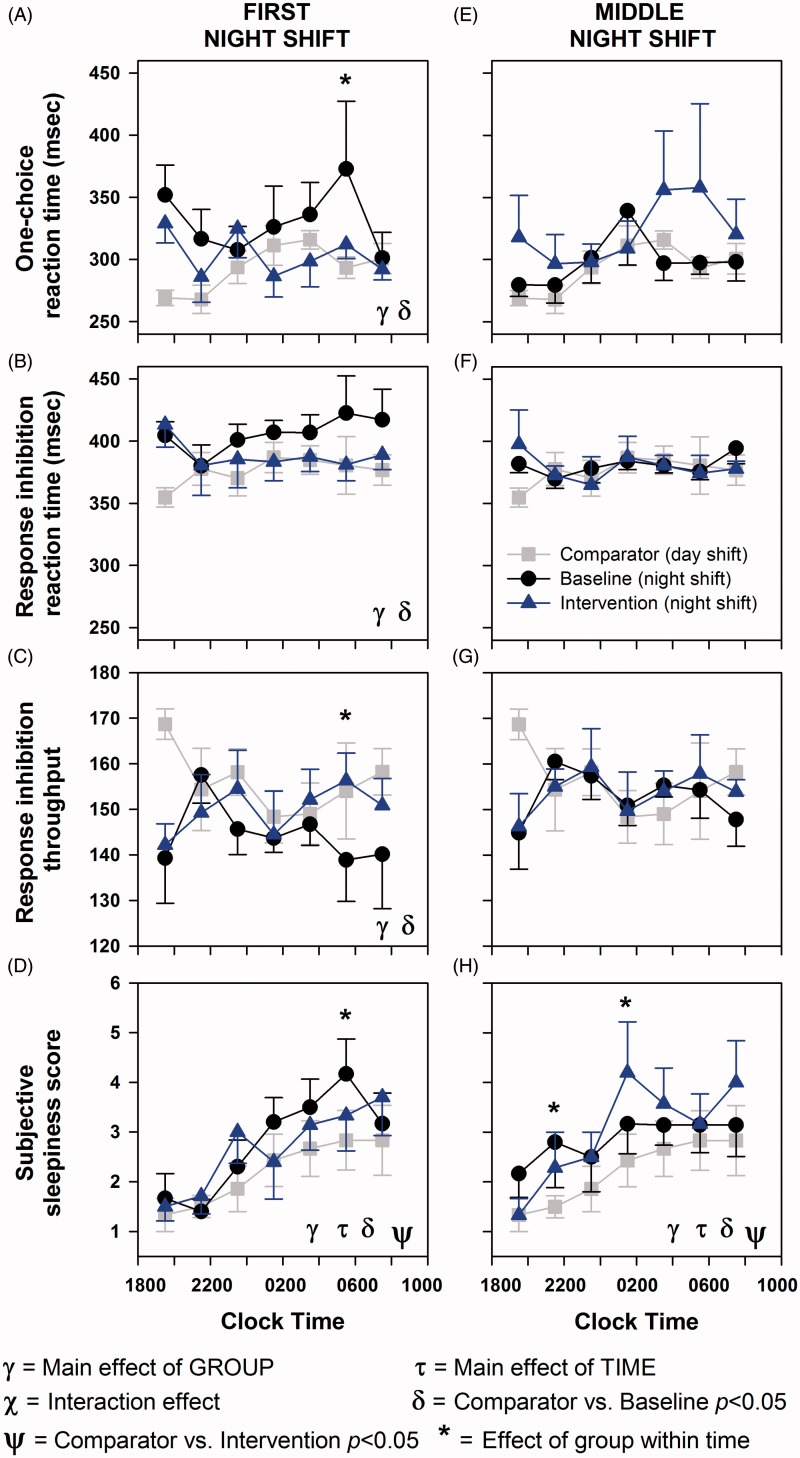
On the first night shift, there was a significant effect of group on one-choice reaction time (p < 0.01), response inhibition task reaction time (p = 0.03), response inhibition throughput (p < 0.01), and subjective sleepiness (p < 0.01) (Figure 4A–D). There was a significant main effect of time only on subjective sleepiness (p < 0.01). There was no significant effect of the interaction of the two factors on any of the cognitive parameters.
Figure 4.
Effects of filtering short wavelengths (<480 nm) from ambient light during night shifts on subjective and objective measures of alertness. Alertness measures were conducted every 2 h from start to the end of the shift on the first night shift and the middle night shift in a series of three night shifts under baseline (black filled circles and black line) and intervention (blue filled triangles and blue line) conditions and the seventh day shift used as the comparator condition (gray filled squares and gray line). Tests included one-choice reaction task, response inhibition (GO/NO-GO) task, and subjective sleepiness. Data represent mean ± SEM. Data were subjected to two-factor (group × time) mixed-model ANOVA followed by Dunnett’s post hoc analysis using the comparator as the control group.
Reaction times under baseline night shifts were significantly slower as compared with daytime performance (p < 0.01), whereas during intervention reaction times were the same as during the daytime comparator (p = 0.55) (Figure 4A). Similarly, reaction time on the response inhibition task was significantly slower under baseline (p = 0.02) but under intervention was similar (p = 0.44) to daytime performance (Figure 4B). In addition, throughput on the response inhibition test was significantly reduced under baseline (p < 0.01) but maintained under intervention (p = 0.24) compared with daytime performance (Figure 4C). In contrast to objective performance, subjective sleepiness significantly increased over the course of the night shift under both baseline (p < 0.01) and intervention (p = 0.01) as compared with daytime sleepiness levels (Figure 4D).
On the middle night shift of the run of three night shifts, there was a significant effect of group on subjective sleepiness (p < 0.01) but not on any of the objective alertness measures (Figure 4E–F). There was a significant main effect of time only on subjective sleepiness (p < 0.01). There was no significant effect of the interaction of the two factors on any of the cognitive parameters. Similar to the first night shift, subjective sleepiness during the middle night shift was also significantly greater under baseline (p < 0.01) and intervention (p < 0.01) as compared with daytime sleepiness levels (Figure 4D).
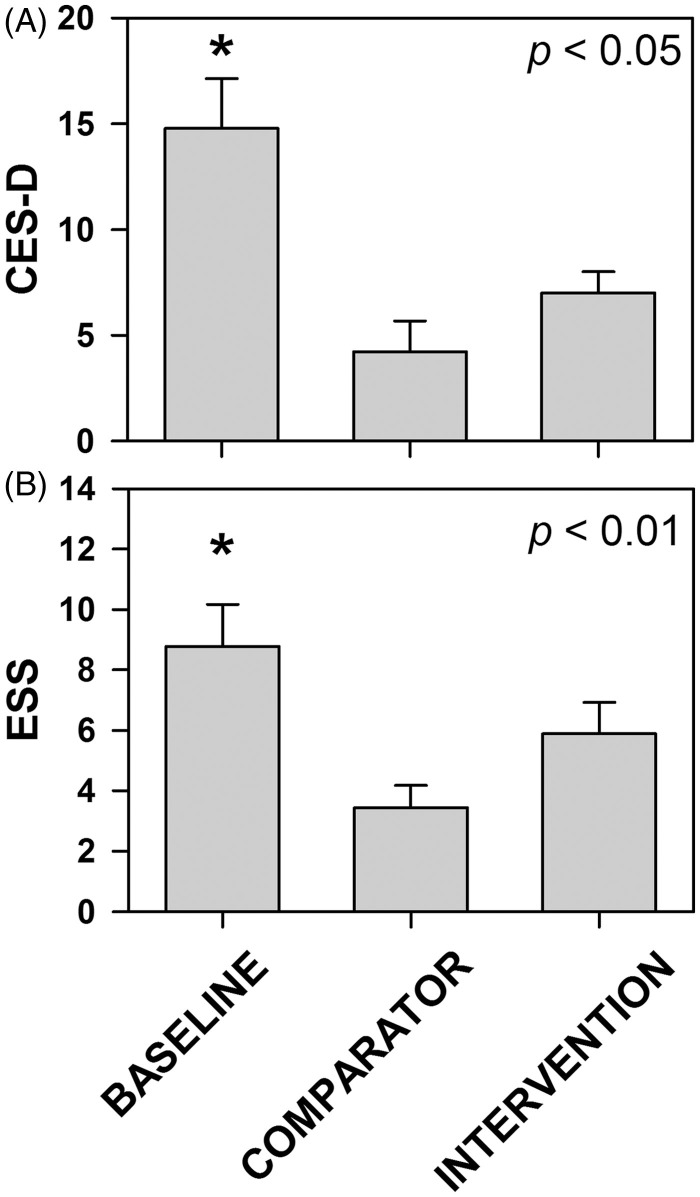
After the first week of night shifts without intervention (baseline), subjective mood was significantly worse (Figure 5A) and daytime sleepiness (Figure 5B) was significantly higher as compared with the comparator (day shift), whereas under intervention condition mood and daytime sleepiness were not different.
Figure 5.
Effects of filtering short wavelengths (<480 nm) from ambient light during night shifts on subjective mood and daytime sleepiness. Subjective mood and daytime sleepiness were assessed at the end of each study week using the CES-D (A) and ESS (B) scales, respectively. Data represent mean ± SEM. Data were subjected to two-factor (group × time) mixed-model ANOVA followed by Dunnett’s post hoc analysis using the comparator as the control group. *Statistically different as compared with the comparator condition.
Discussion
Previous studies have documented impaired daytime sleep and nighttime performance in shiftworkers (Åkerstedt, 1984). There is evidence that rotating shiftwork may be associated with greater sleep complaints and performance decrements than permanent night work (Gold et al., 1992), although most permanent night workers also maintain daytime schedules on non-workdays and therefore also rotate between nocturnal and diurnal schedules. The present study is in agreement with previous reports of significant daytime sleep impairment in rotating-shift workers (Åkerstedt, 1984), and further suggests that rotating-shift workers may also have impaired nighttime sleep on non-workdays following night shifts. Although, the reductions in total sleep time and sleep efficiency during post-night-shift nighttime sleep were smaller in comparison with the reductions in daytime sleep parameters, the adverse effects on cognitive performance and well-being may be compounded by poor daytime sleep in between night shifts and poor nighttime recovery sleep on non-workdays.
We found that total sleep time after intervention was increased by a mean of 40 min compared with baseline after night shifts without using optical filters. To put these results into perspective, total sleep time was shown to be increased by about 40 min in healthy elderly subjects taking the potent hypnotic, zopiclone 7.5 mg (Hemmeter et al., 2000), and by 40 to 70 min after zopiclone 10 mg in young healthy subjects on delayed or advanced sleep schedules, respectively (Kanno et al., 1993). Subjects in the present study also fell asleep faster and woke up less frequently after sleep onset, resulting in an improvement in sleep efficiency.
Filtering visual short wavelengths can attenuate melatonin suppression under nocturnal light exposure (Kayumov et al., 2005; Rahman et al., 2008, 2011; Sasseville & Hebert, 2010; Sasseville et al., 2006). Recent work has shown that reducing intensity of daytime light exposure coupled with filtering short wavelengths in the visual range (<550 nm), which induce maximal circadian phase resetting, accelerates circadian adaptation to a nocturnal schedule in simulated shiftwork and field-based studies, improving daytime sleep and nocturnal performance (Boivin et al., 2012a, 2012b; Crowley et al., 2003; Sasseville & Hebert, 2010; Smith & Eastman, 2008). In the present study, the use of short-wavelength filters during the night improved nighttime sleep on the first non-working day. Whether nighttime sleep is also adversely affected beyond the first non-workday was not assessed in the present study, although some reports suggest that it may take two or three non-workdays to recover from consecutive night shifts (Chung et al., 2012).
Moreover, in the present study, individual habitual sleep-wake characteristics including sleep duration, sleep onset latency, and sleep efficiency assessed from self-report sleep diaries did not correlate with the magnitude of change observed in sleep structure parameters under laboratory conditions at an individual level. However, there was considerable loss of data (∼37%) across all participants due to noncompliance with daily reporting and there was no objective verification of the self-reports using actigraphy. Taken together, these factors greatly limit the reliability of these results. Therefore, further studies are needed to assess how spectral modulation affects sleep parameters for each individual based on their unique sleep structure profiles.
Without any bright light exposure or sleep-wake scheduling intervention, there can be large interindividual variation in phase resetting induced by shiftwork as confirmed in simulated shiftwork studies (Crowley et al., 2003; Lee et al., 2006; Smith & Eastman, 2008; Smith et al., 2009) and field-based trials (Boivin et al., 2012a, 2012b). Although the phase shifts are not spontaneously large enough to improve daytime sleep and nighttime performance, they may be large enough to affect nighttime sleep. Although we did not assess circadian phase in the present study, the change in melatonin levels with and without intervention reflects individual sensitivity to light and the change in melatonin AUC with and without intervention suggests phase alteration between the first night shift and the middle night shift of a run of three night shifts. As compared with baseline, the melatonin AUC on the first night shift was ∼30% greater under intervention, whereas on the middle night shift the change in melatonin AUC was ∼60% greater than baseline under intervention. Since the individuals were exposed to similar lighting conditions on both nights, it is unlikely that the change in melatonin levels can be attributed to direct suppressive effects of light exposure alone. Instead, the results suggest an additive effect of suppression and phase shifts that may account for the increased difference in melatonin AUC on the middle night. Moreover, there was considerable interindividual difference in the change in melatonin AUC and there was a modest but significant correlation with changes in sleep efficiency and total sleep time. Taken together, the results suggest that the nighttime sleep impairment following two night shifts was likely mediated by phase alterations and is in agreement with previous reports that support phase alterations instead of acute suppression of melatonin by light at night in nurses working in dim ambient lighting (Dumont et al., 2012). In contrast, changes in daytime sleep efficiency and duration between baseline and intervention conditions was not correlated with changes in melatonin AUC, suggesting that melatonin changes even after a total of four night shifts are not sufficient to facilitate adaptation to a night schedule and improve daytime sleep (Boivin et al., 2012a, 2012b; Crowley et al., 2003; Czeisler et al., 1990; Lee et al., 2006; Smith et al., 2009).
Circadian phase resetting has a nonlinear relationship with photic intensity and duration (Chang et al., 2012; Zeitzer et al., 2000). Previous studies have demonstrated significant phase resetting and melatonin suppression induced by photic stimuli at similar levels to those to which participants were exposed in the present study (Boivin et al., 1995; Zeitzer et al., 2000). One of the limitations of the study was the lack of ambulatory light measurements at the individual level. However, a recent report in full-time rotating-shift-work nurses documented that light levels between 29 and 223 lux was not sufficient to induce measurable melatonin suppression during night work but was associated with significantly less 24-h melatonin levels, suggesting phase resetting and misalignment (Dumont et al., 2012). Moreover, the current study was conducted in the winter season between September and March and the shorter photoperiod and reduced exposure to phase-advancing morning light may have increased the sensitivity to phase-delaying nighttime indoor lighting (Mitchell et al., 1997).
In the present study, filtering short wavelengths was not associated with reduced cognitive performance during the night shift as compared with the day shift. In contrast, under baseline conditions during the night shift, there was a significant increase in reaction time and reduction in throughput as compared with daytime performance. Similar to our previous report (Rahman et al., 2011), an improvement in cognitive performance with intervention compared with baseline was observed toward the end of the night shift. Our results are consistent with previous studies that reduced the spectral power in the short-wavelength range from nocturnal ambient lighting and did not observe a reduction in cognitive performance (Hoffmann et al., 2008; Kayumov et al., 2005). Although we cannot rule out a placebo effect in the present study, we believe this to be unlikely, since subjective sleepiness was significantly increased under both intervention and baseline conditions contrary to cognitive performance. One possible explanation may be that filtering short wavelengths prevented the phase delay induced by nocturnal light exposure and, therefore, under intervention conditions, individuals were tested at a relatively earlier circadian phase as compared with baseline conditions. As a result, the early morning tests under intervention benefited from the increasing circadian wake drive past the circadian nadir, whereas under baseline condition the phase-delayed individuals continued to show cognitive decrements associated with the circadian nadir. Importantly, both groups showed significantly higher subjective sleepiness at the end of the night shift compared with the day shift, suggesting that the modest improvement in cognitive performance observed with the optical filters may reduce the incidence of accidents and errors associated with increased fatigue at the end of shift.
The middle night shift of a run of three night shifts revealed no differences in cognitive performance between the baseline condition and day shift performance, although subjective sleepiness was significantly higher. This suggests that there may have been some level of adaptation after several night shifts. This suggestion is in agreement with previous reports showing that cognitive performance impairment is greatest on the first night shift and gradually improves in successive shifts (Lamond et al., 2003; Santhi et al., 2007), although some reports have suggested otherwise (Folkard & Lombardi, 2006). Recent work demonstrates that short wavelengths are most efficacious in improving alertness when homeostatic sleep pressure is high (Chellappa et al., 2012). It may be that the impaired daytime sleep and successive night shifts increased sleep pressure on the middle night shift, greater than on the first night shift. As a result, filtering short wavelengths on the middle night shift may have negated the alerting effects otherwise imparted by short wavelengths in polychromatic light.
In summary, the present study demonstrates that filtering visual short wavelengths thought to induce maximal circadian phase resetting during nocturnal work improves sleep duration and efficiency on the first non-working day after consecutive night shifts. This approach may be associated with acute improvements in cognitive performance at a critical time when the incidence of errors and accidents are high. However, additional studies are required to investigate the interaction effects of homeostatic sleep pressure, shift rotation, and individual tolerance to shiftwork. Future research on countermeasures instituted to improve night shift performance need to consider the effects of nocturnal light on nighttime sleep during subsequent recovery days.
Declaration of Interest
The study was funded by the Canadian Institutes of Health Research Operating Grant MOP 89896. S.A.R. was funded by Government of Ontario/Pharmacia Canada Inc./Genesis Research Foundation/OBGYN Graduate Scholarship in Science and Technology at the University of Toronto, Faculty of Medicine, and the Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award from the Canadian Institutes of Health Research.
F.W. H.A. S.K. and T.J.B. report no conflicts of interest. S.A.R., C.M.S. and R.F.C. report the following conflicts of interest, S.A.R. and R.F.C. have Intellectual Property filed for prevention of circadian rhythm disruption by using optical filters. S.A.R., C.M.S, and R.F.C. own shares in ZircLight Inc.
References
- Åkerstedt T. Work schedules and sleep. Experientia. 1984;40:417–22. doi: 10.1007/BF01952374. [DOI] [PubMed] [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. J Biol Rhythms. 2002;17:556–67. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Zeitzer JM, Czeisler CA. Resetting of the endogenous circadian rhythm of plasma melatonin and body temperature by ordinary room light in humans. J Sleep Res. 1995;5:S20. [Google Scholar]
- Boivin DB, Boudreau P, James FO, Kin NM. Photic resetting in night-shift work: impact on nurses' sleep. Chronobiol Int. 2012a;29:619–28. doi: 10.3109/07420528.2012.675257. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Boudreau P, Tremblay GM. Phototherapy and orange-tinted goggles for night-shift adaptation of police officers on patrol. Chronobiol Int. 2012b;29:629–40. doi: 10.3109/07420528.2012.675252. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Jud C, Munch M, et al. Evening exposure to blue light stimulates the expression of the clock gene PER2 in humans. Eur J Neurosci. 2006;23:1082–6. doi: 10.1111/j.1460-9568.2006.04613.x. [DOI] [PubMed] [Google Scholar]
- Chang AM, Santhi N, St Hilaire M, et al. Human responses to bright light of different durations. J Physiol. 2012;590:3103–12. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapdelaine S, Paquet J, Dumont M. Effects of partial circadian adjustments on sleep and vigilance quality during simulated night work. J Sleep Res. (2012);21:380–9. doi: 10.1111/j.1365-2869.2012.00998.x. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Viola AU, Schmidt C, et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–7. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- Chung MH, Kuo TB, Hsu N, et al. Recovery after three-shift work: relation to sleep-related cardiac neuronal regulation in nurses. Ind Health. 2012;50:24–30. doi: 10.2486/indhealth.ms1305. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, et al. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. J Biol Rhythms. 2003;18:513–23. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–97. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, et al. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–9. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–8. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–17. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Dumont M, Lanctot V, Cadieux-Viau R, Paquet J. Melatonin production and light exposure of rotating night workers. Chronobiol Int. 2012;29:203–10. doi: 10.3109/07420528.2011.647177. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Stewart KT, Mahoney MP, et al. Shiftwork: dark goggles and bright light improve circadian rhythm adaptation to night-shift work. Sleep. 1994;17:535–43. doi: 10.1093/sleep/17.6.535. [DOI] [PubMed] [Google Scholar]
- Elsmore TF, Reeves DL, Reeves AN. The ARES test system for palm OS handheld computers. Arch Clin Neuropsychol. 2007;22:S135–44. doi: 10.1016/j.acn.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Figueiro MG, Bierman A, Plitnick B, Rea MS. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci. 2009;10:1–11. doi: 10.1186/1471-2202-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkard S, Lombardi DA. Modeling the impact of the components of long work hours on injuries and “accidents”. Am J Ind Med. (2006);49:953–63. doi: 10.1002/ajim.20307. [DOI] [PubMed] [Google Scholar]
- Gillooly PB, Smolensky MH, Albright DL, et al. Circadian variation in human performance evaluated by the Walter Reed performance assessment battery. Chronobiol Int. 1990;7:143–53. doi: 10.3109/07420529009056966. [DOI] [PubMed] [Google Scholar]
- Gold DR, Rogacz S, Bock N, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–14. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmeter U, Muller M, Bischof R, et al. Effect of zopiclone and temazepam on sleep EEG parameters, psychomotor and memory functions in healthy elderly volunteers. Psychopharmacology (Berl.) 2000;147:384–96. doi: 10.1007/s002130050007. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Leichtfried V, Griesmacher A, et al. Effects of light with reduced short wavelength components on parameters of circadian rhythm and performance in an experimental night shift model. Open Physiol J. 2008;1:34–43. [Google Scholar]
- James FO, Cermakian N, Boivin DB. Circadian rhythms of melatonin, cortisol, and clock gene expression during simulated night shift work. Sleep. 2007;30:1427–36. doi: 10.1093/sleep/30.11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- Kaminski TW, Groff RM, Glutting JJ. Examining the stability of Automated Neuropsychological Assessment Metric (ANAM) baseline test scores. J Clin Exp Neuropsychol. 2009;31:689–97. doi: 10.1080/13803390802484771. [DOI] [PubMed] [Google Scholar]
- Kanno O, Watanabe H, Kazamatsuri H. Effects of zopiclone, flunitrazepam, triazolam and levomepromazine on the transient change in sleep-wake schedule: polygraphic study, and the evaluation of sleep and daytime condition. Prog. Neuropsychopharmacol. Biol Psychiatry. 1993;17:229–39. doi: 10.1016/0278-5846(93)90044-s. [DOI] [PubMed] [Google Scholar]
- Kayumov L, Casper RF, Hawa RJ, et al. Blocking low-wavelength light prevents nocturnal melatonin suppression with no adverse effect on performance during simulated shift work. J Clin Endocrinol Metab. 2005;90:2755–61. doi: 10.1210/jc.2004-2062. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. (Lond.) 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond N, Dorrian J, Roach GD, et al. The impact of a week of simulated night work on sleep, circadian phase, and performance. Occup Environ Med. 2003;60:e13. doi: 10.1136/oem.60.11.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Smith MR, Eastman CI. A compromise phase position for permanent night shift workers: circadian phase after two night shifts with scheduled sleep and light/dark exposure. Chronobiol Int. 2006;23:859–75. doi: 10.1080/07420520600827160. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- Mitchell PJ, Hoese EK, Liu L, et al. Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms. 1997;12:5–15. doi: 10.1177/074873049701200103. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rahman SA, Kollara A, Brown TJ, Casper RF. Selectively filtering short wavelengths attenuates the disruptive effects of nocturnal light on endocrine and molecular circadian phase markers in rats. Endocrinology. 2008;149:6125–35. doi: 10.1210/en.2007-1742. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Kayumov L, Tchmoutina EA, Shapiro CM. Clinical efficacy of dim light melatonin onset testing in diagnosing delayed sleep phase syndrome. Sleep Med. 2009;10:549–55. doi: 10.1016/j.sleep.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Marcu S, Shapiro CM, et al. Spectral modulation attenuates molecular, endocrine, and neurobehavioral disruption induced by nocturnal light exposure. Am J Physiol Endocrinol Metab. 2011;300:E518–27. doi: 10.1152/ajpendo.00597.2010. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human Subjects. Washington, DC: US Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- Santhi N, Horowitz TS, Duffy JF, Czeisler CA. Acute sleep deprivation and circadian misalignment associated with transition onto the first night of work impairs visual selective attention. PLoS ONE. 2007;2:e1233. doi: 10.1371/journal.pone.0001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville A, Hebert M. Using blue-green light at night and blue-blockers during the day to improves adaptation to night work: a pilot study. Prog. Neuropsychopharmacol. Biol Psychiatry. 2010;34:1236–42. doi: 10.1016/j.pnpbp.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Sasseville A, Paquet N, Sevigny J, Hebert M. Blue blocker glasses impede the capacity of bright light to suppress melatonin production. J Pineal Res. 2006;41:73–8. doi: 10.1111/j.1600-079X.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off. Sleep. 2008;31:1639–45. doi: 10.1093/sleep/31.12.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. J Biol Rhythms. 2009;24:161–72. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- St Hilaire MA, Gooley JJ, Khalsa SB et al. Human phase response curve (PRC) to a 1-hour pulse of bright white light. J Physiol. (2012);590:3035–45. doi: 10.1113/jphysiol.2012.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan K-A, Yamazaki S, Tei H, et al. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehav. Toxicol. Teratol. 1985;7:415–18. [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol (Lond.) 2000;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]