Abstract
Introduction:
Weight-management options include lifestyle modifications, bariatric surgery and, until recently, limited pharmacotherapy. Phentermine and topiramate extended-release (phentermine/topiramate ER) has recently been approved in the USA for chronic weight management in obese adults and overweight adults with weight-related co-morbidities in conjunction with a reduced-calorie diet and increased physical activity.
Areas covered:
This review describes the pharmacology and clinical trials data for phentermine/topiramate ER and its role in a complications-centric approach to medical care of the overweight and obese patient.
Expert opinion:
Phentermine/topiramate ER is an effective and safe weight-loss medication that can produce and sustain approximately 10% loss of body weight. This is a landmark development in the pharmacotherapy of obesity. By offering an effective medical option to complement lifestyle and surgical approaches, phentermine/topiramate ER enables a comprehensive medical model for obesity care. The overall approach to the overweight and obese patient should be to identify individuals who will benefit most from therapy based on cardiometabolic or mechanical complications, establish therapeutic targets and goals for ameliorating these complications and selecting the treatment modality and intensity for weight loss to achieve these goals. This complications-centric model emphasizes weight loss as a tool to ameliorate obesity-related complications and optimizes benefit/risk for achieving the best outcomes in overweight/obese patients.
Keywords: cardiometabolic disease, metabolic syndrome, obesity, phentermine and topiramate extended-release, prediabetes, sleep apnea, type 2 diabetes, weight loss
1. Introduction
Overweight (body mass index (BMI) ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) are global epidemics and, in the USA, affect approximately 70 and 35% of the population, respectively [1]. Because of associated co-morbidities, obesity adversely affects mortality, morbidity and quality of life [2,3]. Regarding the public health burden, foremost among obesity-related complications is the exacerbation of cardiometabolic disease, which leads to increased prevalence of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [4-6]. Obesity is also associated with mechanical complications, including osteoarthritis and obstructive sleep apnea, due to the increase in body mass [4]. In addition to patient suffering and the direct medical costs associated with treatment of these conditions, there is an indirect burden to society that includes decreased work productivity, absenteeism, disability, workplace injuries and depression [7].
This review will provide an overview of the pharmacology and clinical trials data pertaining to two recently approved medications for overweight and obesity [8,9], with a focus on phentermine and topiramate extended-release (phentermine/topiramate ER), and will offer a complications-centric model for the use of these drugs in medical management of the overweight and obese patient.
2. Overview of the market
Current treatment options for overweight and obesity include lifestyle modifications, pharmacotherapy and bariatric surgery. Lifestyle modifications include a reduced-calorie diet, physical activity and behavior changes and represent the initial approach and the cornerstone of weight-loss therapy, as recommended by National Heart, Lung, and Blood Institute [3]. Bariatric surgery can be a highly effective weight-loss option [10]. However, indications for bariatric surgery are typically limited to severely obese patients (BMI ≥ 40 kg/m2) or those with a BMI ≥ 35 kg/m2 plus weight-related co-morbidities. Patients undergoing surgery require lifelong medical monitoring, can experience weight regain over time and are at risk of a range of peri- and postoperative adverse events (AEs) and mortality related to these procedures [3,11,12]. Surgery is particularly challenging in those with common obesity-related co-morbidities, such as T2DM, which impairs wound healing [13].
Until recently, medication options were quite limited. There are four sympathomimetic medications, namely phentermine, benzphetamine, diethylpropion and phendimetrazine, which act by suppressing appetite and are approved only for short-term treatment of obesity (< 3 months) [14]. These drugs have limited applicability to the long-term treatment of obesity, which is a chronic and perhaps lifelong disease. Orlistat (Xenical, Genentech, South San Francisco, CA, USA), a gastrointestinal lipase inhibitor that reduces the absorption of dietary fat, is approved for long-term treatment of obesity; however, in a 4-year, double-blind, prospective study of 3305 obese patients, only moderate weight loss over placebo was observed (5.8 vs 3.0 kg), together with variable improvements in cardiometabolic risk factors [15]. Sibutramine (Meridia, Abbott Laboratories, Abbott Park IL), a serotonin-norepinephrine reuptake inhibitor, was withdrawn from the market in 2010 after the SCOUT Trial showed an increase in composite CVD events in patients with pre-existing vascular disease, despite the fact that the drug was contraindicated in this population [16].
In the summer of 2012, two therapies were approved by the US Food and Drug Administration (FDA) as adjuncts to a reduced-calorie diet and increased physical activity. These medications, twice-daily lorcaserin (Belviq®, Arena Pharmaceuticals, Switzerland) and once-daily phentermine/topiramate ER (Qsymia®, VIVUS, Inc., Mountain View, CA, USA), have greatly enhanced medication treatment options for clinicians and patients. Both are indicated for chronic weight management in adult patients with an initial BMI of ≥ 30 kg/m2 or BMI ≥ 27 kg/m2 in the presence of at least one weight-related co-morbid condition (e.g., hypertension, dyslipidemia, T2DM) [8,9]. This review will focus primarily on phentermine/topiramate ER.
Lorcaserin produced moderate weight loss after 1 year in two Phase III, randomized, placebo-controlled trials of overweight and obese patients [17,18]. In the BLOOM (Behavioral Modification and Lorcaserin for Overweight and Obesity Management) trial, mean weight loss in placebo- and lorcaserin-treated subgroups (10 mg twice daily) was -2.2 kg (-2.2%) and -5.8 kg (-5.8%), respectively, with 20.3 and 47.5% of these subjects achieving ≥ 5% weight loss [17]. Similarly, in the BLOSSOM (Behavioral Modification and Lorcaserin Second Study) trial, mean weight loss was -2.9 kg (-2.8%), -4.7 kg (-4.7%) and -5.8 kg (-5.8%) with placebo, lorcaserin 10 mg once daily and lorcaserin 10 mg twice daily, respectively, and 25.0, 40.2 and 47.2% of these subjects achieved ≥ 5% weight loss [18]. The most common AEs were headache, dizziness and nausea [8].
The FDA also reviewed a third obesity medication in 2012. The combination of naltrexone sustained-release (SR) and bupropion SR was studied in four Phase III clinical trials demonstrating that naltrexone SR/bupropion SR produced greater weight loss in overweight and obese patients (5 – 9.3%) when compared with placebo (1.2 – 5.1%) and led to improvements in metabolic parameters [19]. However, the FDA has requested additional preapproval trials to examine cardiovascular risk.
3. Introduction to the compound
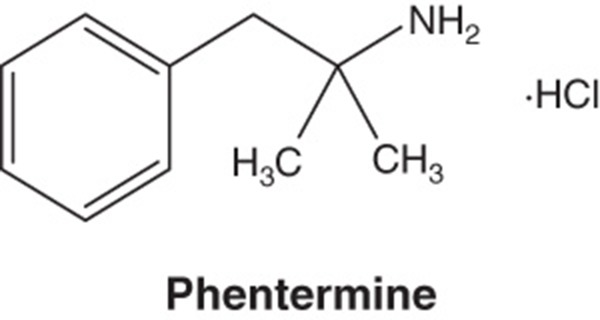
Phentermine/topiramate ER is a once-daily, oral combination of immediate-release phentermine hydrochloride and topiramate extended-release (Box 1) [9]. Phentermine hydrochloride is a sympathomimetic amine anorectic agent approved at doses up to 37.5 mg daily for short-term obesity therapy [14]. Topiramate is a sulfamate-substituted monosaccharide related to fructose approved for use as an antiepileptic agent and for the prophylaxis of migraine headaches; it is approved at doses up to 400 mg once daily for these indications [20]. The phentermine/topiramate ER combination employs the first extended-release formulation of topiramate and lower doses than the approved individual components for other indications in order to accentuate the weight loss response while diminishing adverse symptomatology.
Box 1.
Drug summary.
| Drug | Qsymia® (phentermine and topiramate extended-release) |
| Phase | Trials complete for use as a weight-loss therapy; EQUATE (NCT00563368), EQUIP (NCT00554216), CONQUER (NCT00553787), SEQUEL (NCT00796367) |
| Indication | July 2012: FDA approved as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adult patients with an initial BMI of 30 kg/m2 or greater, or 27 kg/m2 or greater in the presence of at least 1 weight-related co-morbidity such as hypertension, T2DM or dyslipidemia |
| Pharmacology description/mechanism of action | Phentermine C10H15N · HCl α,α-dimethylphenethylamine hydrochloride. Phentermine hydrochloride is a sympathomimetic amine anorectic agent. The primary mechanism of action of phentermine in treating obesity is postulated to be appetite suppression; however, other CNS actions, or metabolic effects, may also be involved. Topiramate C12H21NO8S 2,3:4,5-di-O-isopropylidene-β-d-fructopyranose sulfamate. Topiramate is a sulfamate-substituted monosaccharide related to fructose used as an antiepileptic agent and for the prophylaxis of migraine headaches. The mechanism of action of topiramate leading to weight loss is also not fully understood, but evidence suggests an effect on energy-balance regulation through a combination of pharmacologic effects, including augmenting the activity of gamma-aminobutyrate, modulation of voltage-gated ion channels, inhibition of AMPA/kainite excitatory glutamate receptors or carbonic-anhydrase inhibition |
| Route of administration | Oral |
| Chemical structure |
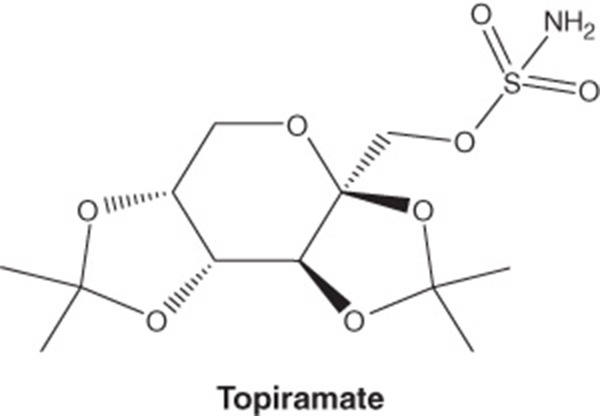
|
| Pivotal trial(s) | EQUIP (NCT00554216); CONQUER (NCT00553787) |
BMI: Body mass index; CNS: Central nervous system; T2DM: Type 2 diabetes mellitus.
4. Chemistry
Phentermine hydrochloride is a white, odorless, hygroscopic, crystalline powder that is soluble in water, methanol and ethanol (Box 1) [9]. Topiramate is a white to off-white crystalline powder with a bitter taste that is freely soluble in methanol and acetone, sparingly soluble in aqueous solutions from pH 9 to 12, and slightly soluble in aqueous solutions from pH 1 to 8 (Box 1) [9].
5. Pharmacodynamics
The exact mechanism of action of phentermine/topiramate ER combination therapy is unknown. The primary mechanism of action of phentermine in obesity treatment is postulated to be appetite suppression; however, other central nervous system (CNS) actions, or metabolic effects, may also be involved [9]. The mechanism of action of topiramate leading to weight loss is also not fully understood, but evidence suggests an effect on energy-balance regulation through a combination of pharmacologic effects, including augmenting the activity of gamma-aminobutyrate, modulation of voltage-gated ion channels, inhibition of AMPA/kainite excitatory glutamate receptors or carbonic-anhydrase inhibition [9].
Phentermine is an atypical amphetamine; as such, the more common actions of amphetamines such as CNS stimulation, elevation of blood pressure (BP), tachyphylaxis and tolerance were not observed in clinical trials with phentermine/topiramate ER at the studied doses [9]. The effect of phentermine/topiramate ER on the QTc interval was evaluated in a randomized, double-blind, placebo- and active-controlled (400 mg moxifloxacin), parallel-group/crossover study in doses up to phentermine 22.5 mg/topiramate ER 138 mg, and, in this study, phentermine/topiramate ER did not affect cardiac repolarization as measured by the change from baseline in QTc [9].
6. Pharmacokinetics and metabolism
6.1. Phentermine
On oral administration of a single dose of phentermine 15 mg/topiramate ER 92 mg, the time to maximum phentermine concentration (Cmax; Tmax) was 6 h [9]. Bioavailability was not affected by a high-fat meal prior to administration [9]. Phentermine pharmacokinetics were dose-proportional as phentermine/topiramate ER doses were increased from phentermine 3.75 mg/topiramate ER 23 mg to phentermine 15 mg/topiramate ER 92 mg [9]. When dosed alone, phentermine distribution was 17.5% plasma protein bound, with 70 – 80% of the dose unchanged in urine, and the mean terminal half-life was ∼ 20 h [9].
6.2. Topiramate
This is the first extended-release formulation of topiramate, which was designed to have a lower Cmax and delayed Tmax compared with immediate-release formulations, but with a similar AUC. On oral administration of a single dose of phentermine 15 mg/topiramate ER 92 mg, topiramate Tmax was 9 h, and, as with phentermine, a high-fat meal did not affect topiramate bioavailability [9]. Topiramate pharmacokinetics were dose-proportional as phentermine/topiramate ER doses were increased from phentermine 3.75 mg/topiramate ER 23 mg to phentermine 15 mg/topiramate ER 92 mg [9]. When dosed alone, topiramate was 15 – 41% plasma protein bound, with ∼ 70% of the dose appearing unchanged in urine, and a mean terminal half-life of ∼ 65 h [9].
7. Clinical efficacy
7.1. Phase II studies
Two Phase II studies were performed in obese subjects with specific weight-related co-morbidities in order to evaluate the effect of phentermine/topiramate ER on reducing these co-morbidities as a consequence of weight reduction.
7.1.1. Type 2 diabetes
OB-202 was a 28-week, double-blind, placebo-controlled, Phase II trial with a 28-week extension (DM-230) in 130 subjects with T2DM randomized to placebo or phentermine 15 mg/topiramate ER 92 mg for a total of 56 weeks [21]. The majority of subjects enrolled in this study had been diagnosed with T2DM for ≥ 5 years, 60% were on ≥ 2 antidiabetic medications, mean hemoglobin A1c (HbA1c) was 8.7%, mean weight was 96.3 kg and mean BMI was 35.3 kg/m2. Thus, the study population consisted of patients with moderate-to-severe T2DM with long-standing disease. During this study, patients with T2DM treated with phentermine 15 mg/topiramate ER 92 mg experienced a significant reduction in HbA1c, the primary end point, when compared with the placebo group, as well as greater decrements in body weight, fasting insulin, fasting glucose, BP and the need for antidiabetic medications (Table 1) [21]. Together, these data indicate that phentermine/topiramate ER could represent a novel approach to achieving improved glycemic control in obese patients with T2DM. The data demonstrate the utility of a weight-loss drug as a glucose-lowering agent and suggest an option for pharmacotherapy that extends beyond drugs that act primarily to enhance insulin secretion or insulin sensitivity.
Table 1.
Overview of phentermine/topiramate ER Phase II and III clinical trials*.
| Placebo (n = 55) |
PHEN/TPM ER 15/92
(n = 75) |
p-value vs placebo | |||
|---|---|---|---|---|---|
| Diabetes: 56 weeks [21] | |||||
| HbA1c, % | -1.2 | -1.6 | 0.0381 | ||
| Weight loss, % | -2.7 | -9.4 | < 0.0001 | ||
| SBP, mm Hg | -2.4 | -7.2 | < 0.05 | ||
| DBP, mm Hg | -1.7 | -2.6 | NS | ||
| Fasting glucose, mg/dl | -27.4 | -42.1 | < 0.05 | ||
| Fasting insulin, μIU/ml | 5.9 | 2.1 | < 0.05 | ||
| Placebo (n = 23) |
PHEN/TPM ER 15/92
(n = 22) |
p-value vs placebo | |||
| Obstructive sleep apnea: 28 weeks [22] | |||||
| AHI, events/h | -16.6 | -31.5 | 0.0084 | ||
| Weight loss, % | -4.2 | -10.3 | 0.0006 | ||
| Weight loss, kg | -4.7 | -10.8 | 0.0006 | ||
| SBP, mm Hg | -7.3 | -15.0 | 0.0431 | ||
| DBP, mm Hg | -5.6 | -6.3 | 0.7991 | ||
|
Placebo
(n = 103) |
PHEN/TPM ER 7.5/46 (n = 103) | p-value vs placebo |
PHEN/TPM ER 15/92
(n = 103) |
p-value vs placebo | |
| EQUATE: 28 weeks [25] | |||||
| Weight loss, % | -1.7 | -8.5 | < 0.0001 | -9.2 | < 0.0001 |
| SBP, mm Hg | -1.8 | -7.0 | < 0.05 | -5.2 | < 0.05 |
| DBP, mm Hg | -0.7 | -2.2 | NS | -2.0 | NS |
|
Placebo
(n = 498) |
PHEN/TPM ER 3.25/23 (n = 234) | p-value vs placebo |
PHEN/TPM ER 15/92
(n = 498) |
p-value vs placebo | |
| EQUIP: 56 weeks [26,52] | |||||
| Weight loss, % | -1.6 | -5.1 | < 0.0001 | -10.9 | < 0.0001 |
| Weight loss, kg | -1.9 | -5.9 | < 0.0001 | -12.6 | < 0.0001 |
| SBP, mm Hg | 0.9 | -1.8 | 0.0019 | -2.9 | < 0.0001 |
| DBP, mm Hg | 0.4 | -0.1 | NS | -1.5 | 0.0002 |
| Fasting glucose, mg/dl | 1.9 | 0.8 | NS | -0.6 | < 0.0001 |
| Triglycerides, % | 9.1 | 5.2 | NS | -5.2 | < 0.0001 |
| LDL-C, % | -5.5 | -7.7 | NS | -8.4 | 0.0157 |
| HDL-C, % | 0 | 0.5 | NS | 3.5 | 0.0005 |
|
Placebo
(n = 979) |
PHEN/TPM ER 7.5/46
(n = 488) |
p-value vs placebo |
PHEN/TPM ER 15/92
(n = 981) |
p-value vs placebo | |
| CONQUER: 56 weeks [27] | |||||
| Weight loss, % | -1.2 | -7.8 | < 0.0001 | -9.8 | < 0.0001 |
| Weight loss, kg | -1.4 | -8.1 | < 0.0001 | -10.2 | < 0.0001 |
| SBP, mm Hg | -2.4 | -4.7 | 0.0008 | -5.6 | < 0.0001 |
| DBP, mm Hg | -2.7 | -3.4 | NS | -3.8 | 0.0031 |
| Fasting glucose, mmol/l | 0.13 | -0.01 | 0.0047 | -0.07 | < 0.0001 |
| HbA1c, % | 0.1 | 0 | < 0.0001 | -0.1 | < 0.0001 |
| Fasting insulin, ρmol/l | 5.1 | -24.0 | 0.0004 | -27.6 | < 0.0001 |
| Triglycerides, % | 4.7 | -8.6 | < 0.0001 | -10.6 | < 0.0001 |
| LDL-C, % | -4.1 | -3.7 | NS | -6.9 | 0.0069 |
| HDL-C, % | 1.2 | 5.2 | < 0.0001 | 6.8 | < 0.0001 |
|
Placebo
(n = 227) |
PHEN/TPM ER 7.5/46 (n = 153) | p-value vs placebo |
PHEN/TPM ER 15/92
(n = 295) |
p-value vs placebo | |
| SEQUEL: 108 weeks [29] | |||||
| Weight loss, % | -1.8 | -9.3 | < 0.0001 | -10.5 | < 0.0001 |
| Weight loss, kg | -2.1 | -9.6 | < 0.0001 | -10.9 | < 0.0001 |
| SBP, mm Hg | -3.2 | -4.7 | NS | -4.3 | NS |
| DBP, mm Hg | -3.9 | -3.7 | NS | -3.5 | NS |
| Fasting glucose, mg/dl | 3.7 | 0.1 | NS | -1.2 | 0.0048 |
| HbA1c, % | 0.2 | 0.01 | 0.0042 | 0.00 | 0.0003 |
| Fasting insulin, μIU/ml | -2.6 | -5.3 | 0.0051 | -5.2 | 0.0012 |
| Triglycerides, % | 0.4 | -12.5 | < 0.01 | -13.7 | < 0.0001 |
| LDL-C, % | -10.7 | -4.6 | < 0.01 | -5.6 | < 0.01 |
| HDL-C, % | 4.7 | 7.3 | NS | 11.9 | < 0.0001 |
*All subjects in all treatment arms received standardized lifestyle and management to standard of care.
AHI: Apnea–hypopnea index; DBP: Diastolic blood pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; PHEN/TPM ER: Phentermine/topiramate extended-release; SBP: Systolic blood pressure.
7.1.2. Obstructive sleep apnea
A second double-blind, placebo-controlled, single-center, Phase II trial randomized 45 obese patients (BMI 30 – 40 kg/m2) with moderate to severe obstructive sleep apnea not receiving positive airway pressure treatment to placebo or phentermine 15 mg/topiramate ER 92 mg for 28 weeks [22]. At study end, those receiving phentermine/topiramate ER experienced significant improvements versus placebo in parameters related to both sleep apnea and body weight (Table 1) [22]. Further, a significant positive correlation was found between the percent change in weight and marked improvements in the apnea–hypopnea index score between baseline and week 28 (n = 43; r = 0.5218; p = 0.0003) [22]. Currently, there are no medications approved for the treatment of obstructive sleep apnea, and there is a high rate of non-compliance with the standard therapeutic approach of employing continuous positive airway pressure devices [23]. Therefore, phentermine/topiramate ER could offer a new treatment option for patients affected by obstructive sleep apnea.
7.2. Phase III studies
The efficacy and safety of phentermine/topiramate ER in the treatment of overweight and obese patients have been evaluated in conjunction with lifestyle modification in three Phase III trials and one extension study. In all four studies, patients randomized to both placebo and drug-treatment arms received lifestyle modification counseling based on the LEARN® program for weight management [24].
7.2.1. EQUATE
EQUATE was a randomized, double-blind, placebo-controlled Phase III trial that was conducted in 756 obese subjects (BMI ≥ 30 and ≤ 45 kg/m2) assigned to once-daily treatment with placebo, phentermine 7.5 mg monotherapy, phentermine 15 mg monotherapy, topiramate ER 46 mg monotherapy, topiramate ER 92 mg monotherapy, phentermine 7.5 mg/topiramate ER 46 mg or phentermine 15 mg/topiramate ER 92 mg for 28 weeks. At study end, those patients receiving phentermine/topiramate ER experienced significantly greater weight loss when compared with placebo (p < 0.0001, all comparisons; Table 1) [25]. The combination of phentermine/topiramate ER also produced greater weight loss than either phentermine or topiramate ER in equivalent doses given as monotherapy. Importantly, phentermine 7.5 mg/topiramate ER 46 mg demonstrated greater weight loss efficacy than either phentermine 15 mg or topiramate ER 92 when used individually [25]. These data support the rationale of the combination therapy to produce greater weight loss at lower doses than the individual components when used alone. EQUATE also evaluated the cardiovascular effects of phentermine/topiramate ER versus placebo and demonstrated a significant reduction versus placebo in systolic BP (SBP; p < 0.05; Table 1) without a significant change in resting heart rate [25].
7.2.2. EQUIP
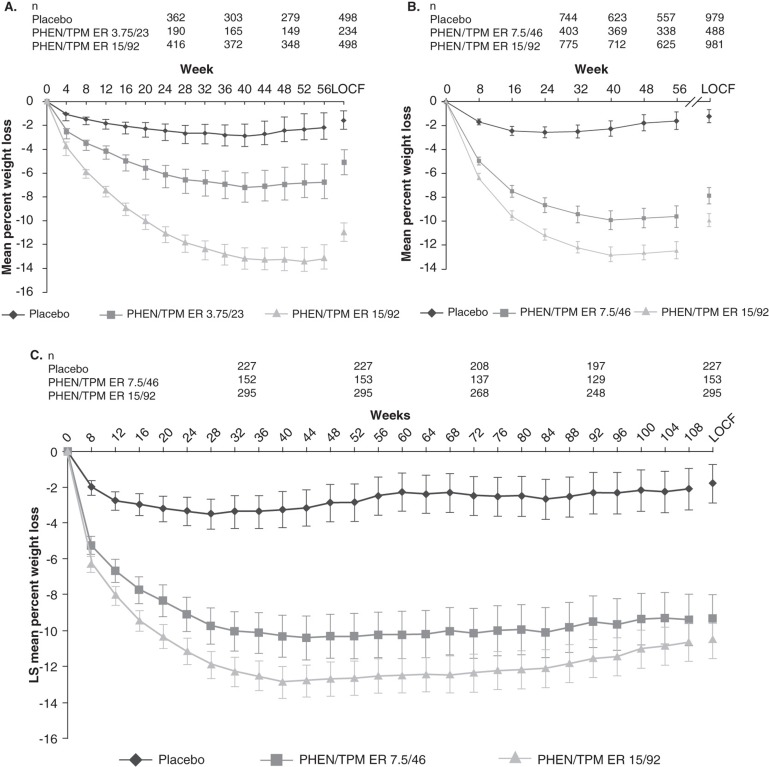
EQUIP was a 56-week, randomized, double-blind, placebo-controlled Phase III trial that evaluated 1267 obese subjects (BMI ≥ 35 kg/m2) assigned to once-daily treatment with placebo, phentermine 3.75 mg/topiramate ER 23 mg or phentermine 15 mg/topiramate ER 92 mg [26]. Overall, mean age was 42.7 years, mean BMI was 42.0 kg/m2 and 83% were female [26]. Subjects with a fasting serum glucose > 110 mg/dl were excluded from this analysis and, overall, the study population was otherwise healthy and largely free of cardiometabolic disease (i.e., T2DM, hypertension, dyslipidemia) [26]. Even so, the majority of subjects met eligibility criteria for bariatric surgery [3]. After 56 weeks, subjects receiving phentermine/topiramate ER experienced significantly greater mean percent weight loss when compared with placebo: 1.6% for placebo, 5.1% for phentermine 3.75 mg/topiramate ER 23 mg and 10.9% for phentermine 15 mg/topiramate ER 92 mg (intent-to-treat, last observation carried forward (ITT-LOCF); p < 0.0001; Table 1), which was maintained over the course of the study (completers; Figure 1A) [26]. Likewise, significantly more subjects receiving phentermine/topiramate ER achieved ≥ 5% weight loss after 56 weeks of treatment versus placebo (p < 0.0001): 17.3% for placebo, 44.9% for phentermine 3.75 mg/topiramate ER 23 mg and 66.7% for phentermine 15 mg/topiramate ER 92 mg (ITT-LOCF) [26]; a significant percentage of subjects also achieved ≥ 10% weight loss versus placebo (p < 0.0001): 7.4, 18.8 and 47.2%, respectively [26]. Cardiometabolic parameters were assessed, and subjects receiving phentermine 15 mg/topiramate ER 92 mg experienced greater improvements in these risk factors when compared with placebo (p < 0.05; Table 1) [26]. This study demonstrated that phentermine/topiramate ER produced substantial weight loss in patients with severe obesity who met criteria for bariatric surgery [3].
Figure 1.
Weight loss with phentermine/topiramate ER in (A) EQUIP, (B) CONQUER and (C) SEQUEL studies from baseline to study end [26,27,29]. Least-squares mean change (95% CI). Weight-change curves are plotted for completers by visit. (A) EQUIP Study from baseline to week 56. Shown to the right of the graph are data derived from the analyses of the ITT-LOCF. p < 0.0001 vs placebo for all time points assessed (adapted from Allison et al. [26]); (B) CONQUER Study from baseline to week 56. Shown to the right of the graph are data derived from the analyses of the ITT-LOCF. p < 0.0001 vs placebo for all time points assessed (adapted from Gadde et al. [27]); (C) SEQUEL Study from baseline to week 108. p < 0.0001 vs placebo for all time points assessed.
Adapted from Garvey et al. [29].
CI: Confidence interval; ITT-LOCF: Intent-to-treat, last observation carried forward; MI: Multiple imputation; PHEN/TPM ER: Phentermine/topiramate extended-release.
7.2.3. CONQUER
The largest of the Phase III studies was the CONQUER trial. This randomized, double-blind trial enrolled 2487 overweight and obese subjects (BMI ≥ 27 to ≤ 45 kg/m2) with ≥ 2 weight-related co-morbidities, including hypertension, dyslipidemia, T2DM, prediabetes or abdominal obesity [27]. Subjects were assigned to once-daily treatment with placebo, phentermine 7.5 mg/topiramate ER 46 mg or phentermine 15 mg/topiramate ER 92 mg for 56 weeks [27]. Overall, the majority of subjects were female (70%) and Caucasian (86%), with a mean age of 51.1 years, baseline mean weight of 103.1 kg and mean BMI of 36.6 kg/m2 [27]. The patients in CONQUER experienced a high burden of cardiometabolic disease as evidenced by the fact that 52% of enrollees had hypertension, 36% were dyslipidemic, 45% had prediabetes and 16% had T2DM [27,28].
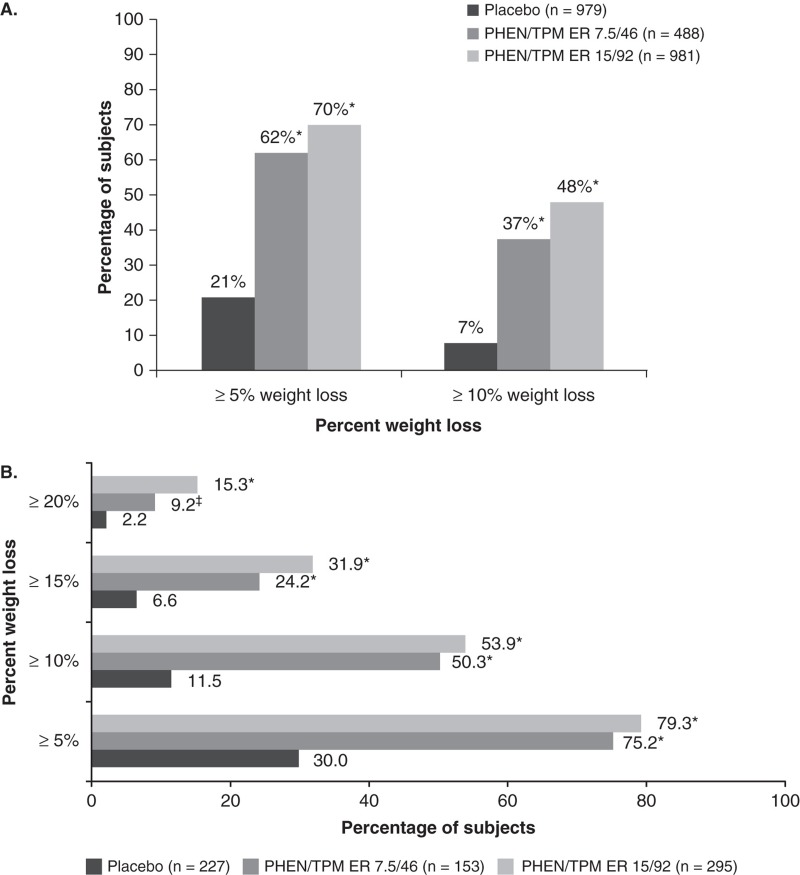
After 56 weeks of treatment, subjects receiving phentermine/topiramate ER achieved significantly greater weight loss than placebo: -1.4 kg (-1.2%) for placebo, -8.1 kg (-7.8%) for phentermine 7.5 mg/topiramate ER 46 mg and -10.2 kg (-9.8%) for phentermine 15 mg/topiramate ER 92 mg (ITT-LOCF; p < 0.0001 vs placebo; Table 1), with weight loss maintained throughout the study (Figure 1B) [27]. In addition, significantly more subjects receiving phentermine/topiramate ER achieved ≥ 5% weight loss (21, 62 and 70% for placebo, phentermine 7.5 mg/topiramate ER 46 mg and phentermine 15 mg/topiramate ER 92 mg, respectively) or ≥ 10% weight loss (7, 37 and 48% for placebo, phentermine 7.5 mg/topiramate ER 46 mg and phentermine 15 mg/topiramate ER 92 mg, respectively) (ITT-LOCF; p < 0.0001 vs placebo; Figure 2A) [27]. Participants in CONQUER also experienced significant improvements in cardiometabolic risk factors (Table 1), and more phentermine/topiramate ER-treated subjects required fewer medications than those receiving placebo to manage hypertension and T2DM [27]. Importantly, among subjects without T2DM at baseline, phentermine/topiramate ER was able to prevent progression to T2DM when compared with placebo: the annualized incidence rate for development of T2DM was 9.0% for placebo, 6.7% for phentermine 7.5 mg/topiramate ER 46 mg and 4.4% for phentermine 15 mg/topiramate ER 92 mg [28].
Figure 2.
Achievement of categorical weight loss goals in (A) CONQUER and (B) SEQUEL [27,29]. (A) CONQUER Study, subjects with ≥ 5% or ≥ 10% weight loss at week 56; (B) SEQUEL Study, percentage (95% CI) of subjects achieving ≥ 5, ≥ 10, ≥ 15 or ≥ 20% weight loss from baseline to week 108 (ITT-LOCF).
A. Adapted from Gadde et al. [27]. B. Adapted from Garvey et al.
[29].
*p < 0.0001 compared with placebo.
‡p = 0.0072 compared with placebo.
CI: Confidence interval; ITT-LOCF: Intent-to-treat, last observation carried forward; PHEN/TPM ER: Phentermine/topiramate extended-release.
7.2.4. SEQUEL
Participants who completed CONQUER on treatment were eligible to enroll in the SEQUEL extension study and maintain their original randomized treatment assignment and lifestyle-modification program for an additional 52 weeks, for a total of 108 weeks of treatment [29]. Of 866 eligible subjects, 676 (78%) elected to continue in the extension. The weight loss experienced in CONQUER was sustained through 108 weeks: -1.8% for placebo, -9.3% for phentermine 7.5 mg/topiramate ER 46 mg and -10.5% for phentermine 15 mg/topiramate ER 92 mg (ITT-LOCF; p < 0.0001 vs placebo; Table 1, Figure 1C) [29]. Similarly, phentermine/topiramate ER-treated subjects achieved greater categorical weight loss than placebo-treated subjects (Figure 2B) and maintained improvements in cardiometabolic disease parameters (Table 1) [29]. Importantly, patients experienced marked reductions in rates of progression to T2DM, up to 75%, when compared with the placebo group [29].
7.2.5. Post-marketing surveillance
VIVUS, Inc., will be conducting a large cardiovascular outcomes study focused on demonstrating superiority of phentermine/topiramate ER plus lifestyle modification compared with lifestyle intervention alone with respect to incidence of and time to major cardiovascular events. This trial not only is designed to rule out any cardiovascular risks of treatment with phentermine/topiramate ER, but will also attempt to demonstrate benefit of treatment through a superiority design.
8. Safety and tolerability
Overall, phentermine/topiramate ER was generally well tolerated, with the most common treatment-emergent AEs being paresthesia, dry mouth and constipation [26,27,29]. In the 1-year cohort of the combined EQUIP and CONQUER trials, heart rate was increased by 1.6 beats/min on average over the baseline rate in the phentermine 15 mg/topiramate ER 92 mg dose [30], but these increases were seen in conjunction with a substantial decrease in BP, and there were no associated adverse clinical sequelae (Tables 2 and 3) [26,27,29]. Serious AE rates were the same across treatment groups and across studies (Tables 2 and 3), and the rates of discontinuation due to AEs were low [26,27,29].
Table 2.
Overview of safety and tolerability in the EQUIP, CONQUER and SEQUEL trials.
|
Placebo
(n = 513) |
PHEN/TPM ER 3.25/23
(n = 240) |
PHEN/TPM ER 15/92
(n = 511) |
|
|---|---|---|---|
| EQUIP: 56 weeks [26] | |||
| Change in HR, bpm | -0.2 | -0.3 | 1.2 |
| SAEs, % | 2.5 | 2.5 | 2.5 |
| Discontinuation due to AEs, % | 8.4 | 11.3 | 16.0 |
| Change in bicarbonate, mEq/l | -0.3 | -1.6 | -1.7 |
|
Placebo
(n = 979) |
PHEN/TPM ER 7.5/46
(n = 488) |
PHEN/TPM ER 15/92
(n = 981) |
|
| CONQUER: 56 weeks [27] | |||
| Change in HR, bpm | -0.1 | 0.1 | 1.7 |
| SAEs, % | 4.0 | 3.0 | 5.0 |
| Discontinuation due to AEs, % | 9.0 | 11.6 | 19.3 |
| Change in bicarbonate, mEq/l | 0.5 | -0.3 | -1.0 |
|
Placebo
(n = 227) |
PHEN/TPM ER 7.5/46
(n = 153) |
PHEN/TPM ER 15/92
(n = 295) |
|
| SEQUEL: 108 weeks [29] | |||
| Change in HR, bpm | 0.4 | 1.3 | 1.7 |
| SAEs, % | 6.2 | 5.9 | 8.1 |
| Discontinuation due to AEs, % | 3.1 | 4.5 | 4.4 |
| Change in bicarbonate, mEq/l | 2.2 | 0.7 | 0.2 |
AE: Adverse event; HR: Heart rate; PHEN/TPM ER: Phentermine/topiramate extended-release; SAE: Serious adverse event.
Table 3.
AEs with a frequency of ≥ 5% in any treatment group.
| Preferred term, n (%) |
Placebo
(n = 513) |
PHEN/TPM ER 3.25/23
(n = 240) |
PHEN/TPM ER 15/92
(n = 511) |
|||
|---|---|---|---|---|---|---|
| EQUIP: 56 weeks [26] | ||||||
| Paresthesia | 10 (1.9) | 10 (4.2) | 96 (18.8) | |||
| Dry mouth | 19 (3.7) | 16 (6.7) | 87 (17.0) | |||
| Constipation | 35 (6.8) | 19 (7.9) | 72 (14.1) | |||
| Upper respiratory tract infection | 56 (10.9) | 38 (15.8) | 63 (12.3) | |||
| Headache | 52 (10.1) | 25 (10.4) | 61 (11.9) | |||
| Nasopharyngitis | 37 (7.2) | 30 (12.5) | 46 (9.0) | |||
| Dysgeusia | 5 (1.0) | 3 (1.3) | 43 (8.4) | |||
| Insomnia | 25 (4.9) | 12 (5.0) | 40 (7.8) | |||
| Nausea | 24 (4.7) | 14 (5.8) | 37 (7.2) | |||
| Sinusitis | 28 (5.5) | 18 (7.5) | 37 (7.2) | |||
| Dizziness | 21 (4.1) | 7 (2.9) | 29 (5.7) | |||
| Back pain | 26 (5.1) | 13 (5.4) | 28 (5.5) | |||
| Bronchitis | 22 (4.3) | 16 (6.7) | 28 (5.5) | |||
| Cough | 18 (3.5) | 8 (3.3) | 26 (5.1) | |||
| Influenza | 24 (4.7) | 18 (7.5) | 26 (5.1) | |||
| Diarrhea | 23 (4.5) | 12 (5.0) | 24 (4.7) | |||
| Fatigue | 17 (3.3) | 12 (5.0) | 23 (4.5) | |||
| Vision blurred | 16 (3.1) | 15 (6.3) | 23 (4.5) | |||
| Preferred term, n (%) |
Placebo
(n = 993) |
PHEN/TPM ER 7.5/46
(n = 498) |
PHEN/TPM ER 15/92
(n = 994) |
|||
| CONQUER: 56 weeks [27] | ||||||
| Dry mouth | 24 (2.4) | 67 (13.5) | 207 (20.8) | |||
| Paresthesia | 20 (2.0) | 68 (13.7) | 204 (20.5) | |||
| Constipation | 59 (5.9) | 75 (15.1) | 173 (17.4) | |||
| Upper respiratory tract infection |
128 (12.9) | 61 (12.2) | 133 (13.4) | |||
| Nasopharyngitis | 86 (8.7) | 53 (10.6) | 98 (9.9) | |||
| Dysgeusia | 11 (1.1) | 37 (7.4) | 103 (10.4) | |||
| Insomnia | 47 (4.7) | 29 (5.8) | 102 (10.3) | |||
| Headache | 90 (9.1) | 35 (7.0) | 101 (10.2) | |||
| Dizziness | 31 (3.1) | 36 (7.2) | 99 (10.0) | |||
| Sinusitis | 67 (6.7) | 34 (6.8) | 85 (8.6) | |||
| Back pain | 49 (4.9) | 28 (5.6) | 72 (7.2) | |||
| Nausea | 42 (4.2) | 18 (3.6) | 68 (6.8) | |||
| Fatigue | 50 (5.0) | 22 (4.4) | 67 (6.7) | |||
| Diarrhea | 48 (4.8) | 32 (6.4) | 58 (5.8) | |||
| Blurred vision | 36 (3.6) | 20 (4.0) | 60 (6.0) | |||
| Urinary tract infection | 37 (3.7) | 26 (5.2) | 54 (5.4) | |||
| Arthralgia | 54 (5.4) | 23 (4.6) | 44 (4.4) | |||
| Bronchitis | 43 (4.3) | 22 (4.4) | 52 (5.2) | |||
| Preferred term, n (%) | 0 – 56 Weeks | 56 – 108 Weeks | ||||
|
Placebo
(n = 227) |
PHEN/TPM ER 7.5/46
(n = 153) |
PHEN/TPM ER 15/92
(n = 295) |
Placebo
(n = 227) |
PHEN/TPM ER 7.5/46
(n = 153) |
PHEN/TPM ER 15/92
(n = 295) |
|
| SEQUEL: 108 weeks [29] | ||||||
| Constipation | 16 (7.1) | 25 (16.3) | 62 (21.0) | 7 (3.1) | 11 (7.2) | 12 (4.1) |
| Paresthesia | 6 (2.6) | 21 (13.7) | 62 (21.0) | 0 (0.0) | 1 (0.7) | 10 (3.4) |
| Dry mouth | 5 (2.2) | 21 (13.7) | 59 (20.0) | 1 (0.4) | 1 (0.7) | 4 (1.4) |
| Upper respiratory tract infection |
47 (20.7) | 23 (15.0) | 55 (18.6) | 42 (18.5) | 26 (17.0) | 45 (15.3) |
| Nasopharyngitis | 35 (15.4) | 20 (13.1) | 39 (13.2) | 26 (11.5) | 13 (8.5) | 26 (8.8) |
| Dysgeusia | 4 (1.8) | 18 (11.8) | 39 (13.2) | 0 (0.0) | 1 (0.7) | 3 (1.0) |
| Sinusitis | 19 (8.4) | 17 (11.1) | 39 (13.2) | 18 (7.9) | 12 (7.8) | 28 (9.5) |
| Headache | 21 (9.3) | 8 (5.2) | 28 (9.5) | 6 (2.6) | 4 (2.6) | 12 (4.1) |
| Insomnia | 15 (6.6) | 12 (7.8) | 24 (8.1) | 8 (3.5) | 9 (5.9) | 11 (3.7) |
| Diarrhea | 12 (5.3) | 14 (9.2) | 21 (7.1) | 3 (1.3) | 3 (2.0) | 11 (3.7) |
| Back pain | 19 (8.4) | 11 (7.2) | 21 (7.1) | 7 (3.1) | 9 (5.9) | 15 (5.1) |
| Dizziness | 6 (2.6) | 9 (5.9) | 20 (6.8) | 2 (0.9) | 2 (1.3) | 1 (0.3) |
| Nausea | 13 (5.7) | 5 (3.3) | 19 (6.4) | 4 (1.8) | 10 (6.5) | 4 (1.4) |
| Bronchitis | 8 (3.5) | 9 (5.9) | 17 (5.8) | 7 (3.1) | 8 (5.2) | 10 (3.4) |
| Fatigue | 11 (4.9) | 7 (4.6) | 17 (5.8) | 2 (0.9) | 2 (1.3) | 4 (1.4) |
| Procedural pain | 6 (2.6) | 7 (4.6) | 17 (5.8) | 4 (1.8) | 8 (5.2) | 14 (4.7) |
| Arthralgia | 20 (8.8) | 13 (8.5) | 13 (4.4) | 14 (6.2) | 7 (4.6) | 16 (5.4) |
| Influenza | 11 (4.9) | 11 (7.2) | 13 (4.4) | 8 (3.5) | 10 (6.5) | 19 (6.4) |
| Urinary tract infection | 11 (4.9) | 8 (5.2) | 13 (4.4) | 13 (5.7) | 14 (9.2) | 18 (6.1) |
| Gastroenteritis | 12 (5.3) | 3 (2.0) | 12 (4.1) | 6 (2.6) | 2 (1.3) | 9 (3.1) |
AE: Adverse event; HR: Heart rate; PHEN/TPM ER: Phentermine/topiramate extended-release; SAE: Serious adverse event.
Antiepileptic drugs, including topiramate, carry a labeled precaution that they may increase the risk of suicidal thoughts or behavior [9]. In EQUIP, CONQUER and SEQUEL, based on reported AEs or the clinician-administered Columbia Suicide Severity Rating Scale questionnaire, the incidence of suicidality was not increased versus placebo and there were no suicidal behaviors [26,27,29]. It is important to consider that all Phase III trials were open to patients with controlled depression at baseline and included patients on stable doses of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors and bupropion with no loss in weight-loss efficacy or increase in AEs [9].
As expected, given the inhibition of carbonic anhydrase by topiramate, a decrease in the concentration of serum bicarbonate was observed in all studies of phentermine/topiramate ER; however, the decrements were small and bicarbonate levels tended to return toward baseline over time without need for clinical intervention [26,27,29].
9. Regulatory affairs
Teratogenicity data derived from epidemiologic studies and claims data in women using immediate-release topiramate for approved migraine and epilepsy indications showed an increased risk of cleft lip with or without cleft palate in infants exposed to topiramate during the first trimester of pregnancy [31]. However, a subsequent retrospective analysis that quantified the association between topiramate exposure during pregnancy and the risk of oral cleft or major congenital malformations found little or no increase in risk when compared with exposure to other anti-epileptic drugs [31]. The FDA approval of phentermine/topiramate ER in July 2012 included a risk evaluation and mitigation strategy (REMS) to inform patients and providers about teratogenic risk and the need to avoid fetal exposure [9]. The labeling includes recommendations for adequate contraception and monthly pregnancy testing in women of childbearing potential [9]. To comply with the REMS program requirements, phentermine/topiramate ER is available from certified pharmacies initially via mail order only, with access through retail pharmacies under regulatory review for mid-2013.
10. Conclusion
The availability of a weight-loss drug with the therapeutic profile of phentermine/topiramate ER represents a landmark development in obesity pharmacotherapy, as it enables an evidence-based medical model that incorporates a more effective and comprehensive application of lifestyle, medication and surgical treatment options. As demonstrated by several Phase II and III clinical studies, phentermine/topiramate ER may produce substantial sustained weight loss, which is unmatched by available pharmacotherapy, when compared with placebo. These studies also have demonstrated that phentermine/topiramate ER-associated weight loss can effectively prevent progression to T2DM, improve glycemia and BP and ameliorate functional indicators in patients with obstructive sleep apnea. Thus, phentermine/topiramate ER represents a well-tolerated, effective option for the treatment of obesity and its related co-morbidities, when used in conjunction with lifestyle changes. By offering an effective medical option to complement lifestyle and surgical approaches, phentermine/topiramate ER offers a medical model for comprehensive obesity care that can be used to promote the health of individuals by ameliorating obesity complications.
The clinical trial data raise three salient considerations. First, all enrollees in clinical trials were engaged in a lifestyle intervention regardless of whether randomized to placebo or drug-treatment arms [26,27,29]. Therefore, the efficacy of phentermine/topiramate ER reflects the use of the drug as an adjunct to lifestyle therapy. All patients being considered for phentermine/topiramate ER, or any other weight loss drug [32], should also participate in a lifestyle intervention in order to achieve optimal outcomes. The second point is that there is variability in the weight loss response to any given dose of phentermine/topiramate ER, as is the case for any drug [26,27,29]. Patients are not assured that the mean weight loss demonstrated in the clinical trials will be achieved, and some patients will represent primary drug failures. Therefore, it is important to follow the prescribing information that outlines scenarios for drug discontinuation when weight loss is not sufficient (e.g., < 5% on phentermine 15 mg/topiramate ER 92 mg) [9]. The third point is that while efficacy of phentermine/topiramate ER was demonstrated for 2 years [29], longer-term results are unknown. Obesity is a lifelong chronic disease and will require prolonged therapy. Combining clinical experience with protracted clinical trials will be needed to assess longer-term efficacy and strategies that employ all treatment options to sustain weight loss over decades.
11. Expert opinion
Obesity is a disease with genetic, environmental and behavioral determinants that confers increased morbidity and mortality [33]. Prior to 2012, healthcare professionals employed lifestyle modification and a limited number of modestly effective medications in efforts to combat this disease, with bariatric surgery reserved for more intractable cases [3,14]. In the summer of 2012, the FDA approved two medications to be used as adjuncts to lifestyle modification for the treatment of overweight patients (BMI ≥ 27 and < 30 kg/m2) with co-morbidities such as T2DM, hypertension and dyslipidemia, and of obese patients (BMI ≥ 30 kg/m2) regardless of whether co-morbidities are present [8,9]. Although head-to-head studies have not been conducted, clinical trial data support the contention that phentermine/topiramate ER is more efficacious than lorcaserin. For example, lorcaserin resulted in 3.6% placebo-subtracted weight loss after 1 year in the BLOOM Study (5.8% weight loss with lorcaserin vs 2.2% for placebo), with some weight regain in lorcaserin-treated patients over the second year of the study [17]. On the other hand, phentermine/topiramate ER produced up to 9.4% placebo-subtracted weight loss after 1 year in the EQUIP Study (11% with phentermine 15 mg/topiramate ER 92 mg vs 1.6% on placebo) [26]. The weight loss produced by phentermine/topiramate ER is intermediate between that commonly achieved by lifestyle modifications or other less effective medications and the average weight loss following gastric bypass bariatric surgical procedures (∼ 30%) [14,15,34-36]. In many patients, the amount of weight loss that can be achieved using phentermine/topiramate ER will predictably obviate the need for invasive surgical procedures.
What is the appropriate medical model for obesity management? Any intervention entails risk, and treatment must be targeted at those patients who will derive the greatest benefits from the intervention according to considerations that optimally balance benefit and risk. With almost 70% of the US adults being overweight or obese [1], and owing to considerations of safety and cost, it is not desirable or feasible to treat all overweight and obese patients with medical or surgical therapy. Furthermore, the treatment goal should not be a cosmetic outcome. The average weight loss achieved by lifestyle and medical therapy, and in many bariatric surgery patients, is not sufficient to achieve optimal cosmetic results, or even to bring many obese patients below the BMI threshold for obesity (i.e., 30 kg/m2) [3-5].
The patients who will benefit most from obesity treatment have obesity-related complications that can be ameliorated by weight loss. These complications can be classified into two general categories, namely those related to insulin resistance and cardiometabolic disease and those related to the mechanical consequences of excess body weight [14]. The degree of weight loss produced by phentermine/topiramate ER is sufficient to exert powerful benefits regarding obesity complications [3-5,9]. In addition to weight loss, phentermine/topiramate ER treatment was sufficient to improve cardiometabolic disease manifestations such as triglycerides, BP, high-density lipoprotein cholesterol (HDL-C) and biomarkers of cardiovascular risk, such as C-reactive protein and fibrinogen; improve glucose intolerance and enhance insulin sensitivity; prevent progression to T2DM and markedly improve the apnea–hypopnea index in patients with obstructive sleep apnea [22,26,27,29,37].
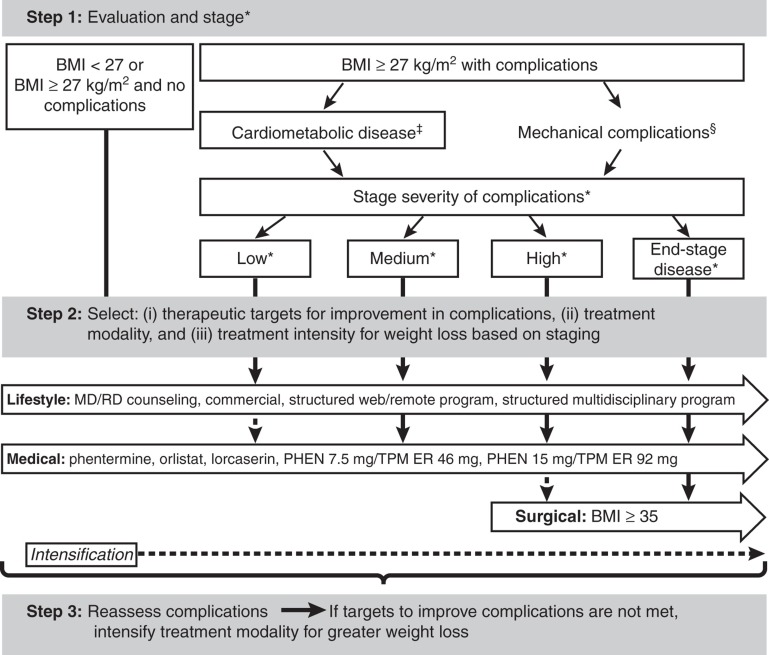
Therefore, a rational medical model for the treatment of the overweight or obese patient should emphasize complications remediable by weight loss (Figure 3). Step 1 of the complications-centric approach is to evaluate and stage the patient for the presence and severity of obesity-related complications and the impact of these complications on the patient's well-being. The clinician should assess BMI, waist circumference, fasting and 2-h oral glucose tolerance test glucose, the lipid panel, BP and presence of CVD. It will also be important to recognize patients who meet criteria for metabolic syndrome [38] and prediabetes [39] since this effectively identifies individuals at high risk of future T2DM and CVD, albeit with high specificity and low sensitivity [40]. The initial evaluation should also screen for other disease entities that may be ameliorated by weight loss, including liver disease, and mechanical complications, such as obstructive sleep apnea, problematic degenerative joint disease or chronic pulmonary disease [4]. It is important to note that not all patients with overweight or obesity have cardiometabolic disease or mechanical complications; up to 30% have been observed to be insulin sensitive without cardiometabolic disease and may not progress to T2DM or CVD, giving rise to the term ‘healthy obese' to characterize these patients [41,42].
Figure 3.
Complications-centric medical model for treatment of the overweight or obese patient. Step 1 is to evaluate patients for the presence and severity of obesity-related complications. Step 2 is to select therapeutic targets for improvement in complications and to determine the appropriate treatment modality and intensity. Step 3 is to reassess complications, and if targets for improvements in complications are not met, intensify lifestyle and/or medical treatment modality for greater weight loss.
*Definitions of stage severity: low: 1 or 2 metabolic syndrome traits; mild mechanical; medium: IFG or IGT or metabolic syndrome; moderate mechanical; high: any 2 of 3: IFG, IGT, metabolic syndrome; and/or severe mechanical; end-stage disease: T2DM, CVD, sleep apnea, NASH.
‡Cardiometabolic disease manifestations include high waist circumference and elevated fasting and 2-h glucose, blood pressure and lipids; NAFLD; T2DM; CVD.
§Mechanical complications include osteoarthritis, sleep apnea, GERD, asthma, pseudotumor cerebri, venous stasis, urinary incontinence.
BMI: Body mass index; CVD: Cardiovascular disease; IFG: Impaired fasting glucose; IGT: Impaired glucose tolerance; GERD: Gastroesophageal reflux disease; MD/RD: Medical doctor/registered dietician; NASH: Non-alcoholic steatohepatitis; NAFLD: Non-alcoholic fatty liver disease; PHEN: Phentermine; TPM ER: Topiramate extended-release; T2DM: Type 2 diabetes mellitus.
Step 2 for medical treatment of obesity is for the clinician and patient to set therapeutic targets and goals for improvements in complications, to identify the modality and intensity of therapy needed to achieve these goals and to set therapeutic targets for the improvement in complications based on the initial evaluation and staging. This approach will optimize the benefit/risk ratio for the intervention and achieve the best outcomes by aligning specific therapy with those patients who will derive the greatest benefit. Many cardiometabolic and mechanical complications exist to a large degree independent of baseline BMI. From this perspective, baseline BMI is less important than the existence and severity of complications at baseline, and by the same consideration, the degree of improvement in obesity-related complications is more important that the absolute amount of weight that is lost [43,44].
Following the initiation of the selected therapeutic option, Step 3 involves reassessment of the patient for the impact of weight loss on complications after equilibrium weight loss is achieved. If the target for improvement in complications is not reached, then the weight-loss therapy should be intensified in order to achieve the desired goal. It is important to consider that all three treatment approaches for obesity (lifestyle modification, pharmacotherapy and bariatric surgery options) are characterized by a wide range of intensities that can be employed to achieve a greater or lesser degree of weight loss. For example, the clinician could proceed to a more highly structured, intensive lifestyle therapy program or increase the daily treatment dose of phentermine 7.5 mg/topiramate ER 46 mg to full-dose phentermine 15 mg/topiramate ER 92 mg [9]. Thus, the medical model is a complications-centric approach to weight loss rather than a BMI-driven approach, in which weight loss is used as a tool to ameliorate the cardiometabolic and mechanical complications of overweight/obesity.
11.1. Potential new applications
A highly important potential benefit of phentermine/topiramate ER-associated weight loss, in terms of public health and containment of heathcare costs, is the prevention of T2DM in high-risk individuals [45]. Given the high relative costs of T2DM care and the morbidity and mortality that accompany this disease, the targeted treatment of overweight and obese patients with metabolic syndrome and prediabetes would have a pronounced impact on reducing the burden of T2DM [5,6,46].
It could be argued that, given its therapeutic profile, phentermine/topiramate ER should be considered for any overweight or obese patient with overt T2DM. Based on data from clinical trials and the known favorable effects of weight loss on T2DM pathophysiology, it is likely that phentermine/topiramate ER could be an effective second-line drug in patients who fail metformin, used in conjunction with metformin as initial dual therapy, or used as first-line therapy in newly diagnosed patients. Although additional clinical trials are necessary to comprehensively establish the role for phentermine/topiramate ER in T2DM, this drug has the potential to become integral in the T2DM drug armamentarium and could change the landscape of how we therapeutically approach T2DM, expanding the notion of ‘antidiabetes drugs' beyond those that act primarily to increase insulin secretion and action.
Beyond these topics, there are many additional unmet needs where phentermine/topiramate ER may be able to play an important role. Obesity and T2DM are ever-increasing problems in children and adolescents, for whom no obesity medications have been approved. Clinical trials to assess the efficacy and safety of phentermine/topiramate ER in children and adolescents should be initiated. In addition, phentermine/topiramate ER has several potential applications in bariatric surgery; the drug could be used to reduce operative risk via induction of preoperative weight loss, to achieve greater weight-loss results when used in conjunction with bariatric surgical procedures, or to counteract weight regain following surgery [45,47]. Treatment with phentermine/topiramate ER may be appropriate for patients with moderate to severe obstructive sleep apnea, steatohepatitis, severe osteoarthritis or stress incontinence, and in women with polycystic ovary syndrome [48-51]. Clinical trials investigating weight loss as a therapeutic option for these conditions may be beneficial.
Acknowledgement
The author would like to thank The Lockwood Group for editorial assistance (funding was provided by VIVUS, Inc.), and VIVUS, Inc., internal contributors.
Declaration of interest
WT Garvey is an advisor for Alkermes, Plc., Daiichi-Sankyo, Inc., LipoScience, VIVUS, Inc., Janssen Pharmaceuticals, Boehringer-Ingelheim and Tethys, is a speaker for Merck and is a stockholder for Bristol-Myers Squibb Co., Isis/Genzyme, Merck, Pfizer, Inc., Eli Lilly and Co. and VIVUS, Inc.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010 . JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity . Obesity Rev. 2001;2(4):219–29. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 3.National Heart, Lung, and Blood Institute The clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Institutes of Health; Bethesda: 1998. NIH Publication No. 98-4083. [PubMed] [Google Scholar]
- 4.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation . Circulation. 2004;110(18):2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes–2013 . Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaven GM. Importance of identifying the overweight patient who will benefit the most by losing weight . Ann Intern Med. 2003;138(5):420–3. doi: 10.7326/0003-4819-138-5-200303040-00012. [DOI] [PubMed] [Google Scholar]
- 7.Schmier JK, Jones ML, Halpern MT. Cost of obesity in the workplace . Scand J Work Environ Health. 2006;32(1):5–11. doi: 10.5271/sjweh.970. [DOI] [PubMed] [Google Scholar]
- 8.Belviq® [package insert] Arena Pharmaceuticals; Zofingen, Switzerland: [Google Scholar]
- 9.Qsymia® [package insert] VIVUS, Inc; Mountain View, CA: [Google Scholar]
- 10.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events . JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 11.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery . Nat Rev Endocrinol. 2012;8(9):544–56. doi: 10.1038/nrendo.2012.48. [DOI] [PubMed] [Google Scholar]
- 12.Sjöström L, Gummesson A, Sjöström CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial . Lancet Oncol. 2009;10(7):653–62. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 13.Keidar A. Bariatric surgery for type 2 diabetes reversal: the risks . Diabetes Care. 2011;34(Suppl 2):S361–6. doi: 10.2337/dc11-s254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray GA, Ryan DH. Medical therapy for the patient with obesity . Circulation. 2012;125(13):1695–703. doi: 10.1161/CIRCULATIONAHA.111.026567. [DOI] [PubMed] [Google Scholar]
- 15.Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients . Diabetes Care. 2004;27(1):155–61. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 16.James WP, Caterson ID, Coutinho W, et al. SCOUT Investigators Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects . N Engl J Med. 2010;363(10):905–17. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 17.Smith SR, Weissman NJ, Anderson CM, et al. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group Multicenter, placebo-controlled trial of lorcaserin for weight management . N Engl J Med. 2010;363(3):245–56. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 18.Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial . J Clin Endocrinol Metab. 2011;96(10):3067–77. doi: 10.1210/jc.2011-1256. [DOI] [PubMed] [Google Scholar]
- 19.Makowski CT, Gwinn KM, Hurren KM. Naltrexone/bupropion: an investigational combination for weight loss and maintenance . Obesity Facts. 2011;4(6):489–94. doi: 10.1159/000335352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Topamax® [package insert] Ortho-McNeil Neurologics, Inc; Titusville, NJ: [Google Scholar]
- 21.Rueger R, Garvey WT, Morelos S, Troupin B. Glycemic improvement and weight loss with low-dose, controlled-release phentermine/topiramate. Abstract 181580 . American Association of Diabetes Educators 37th Annual Meeting (AADE); 4 – 7 August 2010; San Antonio, Texas, USA. [Google Scholar]
- 22.Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults . Sleep. 2012;35(11):1529–39. doi: 10.5665/sleep.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Effects of phentermine/topiramate ER on obstructive sleep apnea, apnea/hypopnea index, O2 saturation and BP in obese adults.
- 23.Chowdhuri S. Continuous positive airway pressure for the treatment of sleep apnea . Otolaryngol Clin North Am. 2007;40(4):807–27. doi: 10.1016/j.otc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Brownell K. The LEARN program for weight management. The Life Style Co; Dallas: 2000. [Google Scholar]
- 25.Jordan J, Astrup A, Day WW. Effects of phentermine and extended-release topiramate alone and in combination on cardiovascular risk factors. Poster 697 . 48th Annual Meeting of the European Association for the Study of Diabetes; 1 – 5 October 2012; Berlin, Germany. [Google Scholar]
- 26.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) . Obesity. 2012;20(2):330–42. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phentermine/topiramate ER EQUIP Study of weight loss in morbidly obese patients, including improvements in cardiovascular and metabolic variables.
- 27.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial . Lancet. 2011;377(9774):1341–52. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]; •• Phentermine/topiramate ER CONQUER Study of percent change in bodyweight and improvements in cardiometabolic parameters in overweight and obese subjects with ≥ 2 weight-related co-morbidities.
- 28.Garvey WT, Peterson CA, Troupin B. Weight loss with low-dose, controlled-release phentermine/topiramate therapy improves glycaemic status and prevents progression to type 2 diabetes mellitus [abstract T5:P102] . 18th European Congress of Obesity; 25 – 28 May 2011; Istanbul, Turkey. [Google Scholar]
- 29.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study . Am J Clin Nutr. 2012;95(2):297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Phentermine/topiramate ER 2-year SEQUEL Study of percent change in bodyweight and improvements in cardiometabolic parameters in overweight and obese subjects with ≥ 2 weight-related co-morbidities (an extension of the CONQUER Study).
- 30.United States FDA Slides for the February 22, 2012 Meeting of the Endocrinologic and Metabolic Drugs Advisory Committee . http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm293903.htm. [Last accessed 8 January 2013]
- 31.Green MW, Seeger JD, Peterson C, Bhattacharyya A. Utilization of topiramate during pregnancy and risk of birth defects . Headache. 2012;52(7):1070–84. doi: 10.1111/j.1526-4610.2012.02190.x. [DOI] [PubMed] [Google Scholar]
- 33.Mechanick JI, Garber AJ, Handelsman Y, Garvey WT. American Association of Clinical Endocrinologists' position statement on obesity and obesity medicine . Endocrine Pract. 2012;18(5):642–8. doi: 10.4158/EP12160.PS. [DOI] [PubMed] [Google Scholar]
- 34.Hofsø D, Jenssen T, Bollerslev J, et al. Beta cell function after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention . Eur J Endocrinol. 2011;164(2):231–8. doi: 10.1530/EJE-10-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Look AHEAD Research Group Pi-Sunyer X, Blackburn G, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial . Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Look AHEAD Research Group Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial . Arch Intern Med. 2010;170(17):1566–75. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wadden TA, Berkowitz RI, Womble LG, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity . N Engl J Med. 2005;353(20):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 37.Davidson MH, Tonstad S, Oparil S, et al. Changes in cardiovascular risk associated with phentermine and topiramate extended release in participants with comorbidities and a body mass index ≥ 27 kg/m2 . Am J Cardiol. 2013 doi: 10.1016/j.amjcard.2012.12.038. published on-line January 29, 2013. doi: 10.1016/j.amjcard.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 38.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity . Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association Diagnosis and classification of diabetes mellitus . Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao Y, Kwon S, Shaughnessy S, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia . Diabetes Care. 2004;27(4):978–83. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 41.Blüher M. Are there still healthy obese patients? . Curr Opin Endocrinol Diabetes Obes. 2012;19(5):341–6. doi: 10.1097/MED.0b013e328357f0a3. [DOI] [PubMed] [Google Scholar]; • Discussion on healthy obese patient paradox.
- 42.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) . Arch Intern Med. 2008;168(15):1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 43.Kip KE, Marroquin OC, Kelley DE, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study . Circulation. 2004;109(6):706–13. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 44.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study . Lancet. 2005;366(9497):1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 46.American Diabetes Association Economic costs of diabetes in the U.S. in 2007 . Diabetes Care. 2008;31(3):596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 45.Benotti PN, Still CD, Wood GC, et al. Preoperative weight loss before bariatric surgery . Arch Surg. 2009;144(12):1150–5. doi: 10.1001/archsurg.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Benefits of weight loss before surgery.
- 47.Heber D, Greenway FL, Kaplan LM, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline . J Clin Endocrinol Metab. 2010;95(11):4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 48.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis . Ann Rheum Dis. 2007;66(4):433–9. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escobar-Morreale HF, Botella-Carretero JI, Alvarez-Blasco F, et al. The polycystic ovary syndrome associated with morbid obesity may resolve after weight loss induced by bariatric surgery . J Clin Endocrinol Metab. 2005;90(12):6364–9. doi: 10.1210/jc.2005-1490. [DOI] [PubMed] [Google Scholar]
- 50.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis . Hepatology. 2010;51(1):121–9. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuomilehto HP, Seppä JM, Partinen MM, et al. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea . Am J Respir Crit Care Med. 2009;179(4):320–7. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 52.Church T, Troupin B. Improvements in quality of life by magnitude of weight loss in obese and overweight subjects. Abstract 1623811 . 36th Annual Society of General Internal Medicine; 24 – 27 April 2013; Denver, Colorado. [Google Scholar]