Abstract
The natural history of ovarian cancer continues to be characterized by late-stage presentation, metastatic bulky disease burden and stagnant mortality statistics despite prolific drug development. Robust clinical investigation, particularly with modifications to primary treatment surgical goals and adjuvant therapy are increasing median progression-free and overall survival, although the cure rates have only modestly been affected. Maintenance therapy holds promise, but studies have yet to identify an agent and/or strategy that can affect survival. Recurrent disease is largely an incurable state; however, current intervention with selected surgery, combination and targeted therapy and investigational protocols are impacting progression-free survival. Ovarian cancer is a diverse and genomically complex disease, which commands global attention. Rational investigation must balance the high rate of discovery with lagging clinical investigation and limited patient resources. Nevertheless, armamentarium growth offers unprecedented opportunities for patients suffering with this disease. This Review presents and reviews the contemporary management of the disease spectrum termed epithelial ‘ovarian’ cancer and introduces the direction and early results of clinical investigation.
Introduction
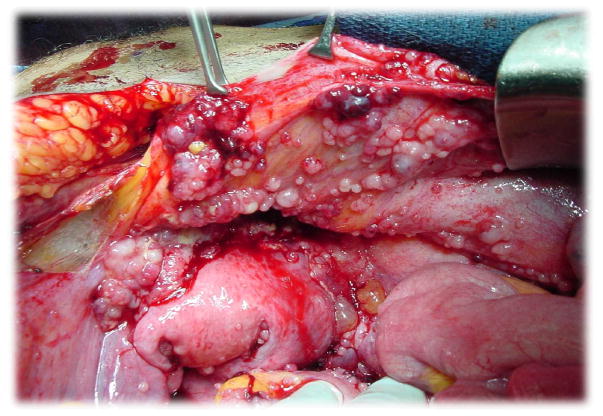
Characterized by late-stage presentation, metastatic bulky disease burden (Figure 1) and stagnant mortality statistics despite prolific drug development, ovarian cancer is facing a renaissance of discovery. These advances are fuelling renewed expectations that this disease might be more efficiently combated to impact outcomes.1 Convincing evidence already exists to indicate that the term ‘ovarian’ cancer (including primary peritoneal carcinoma) might be a misnomer, with a significant proportion of primary ‘ovarian’ tumours arising from malignant transformation of secretory cells in the fallopian tube.2 In addition, genomic characterization of different histologies, such as low-grade serous, mucinous, clear cell and endometrioid, has revealed heterogeneity in leveraged pathways for proliferation and invasion.3, 4 Even interrogation of the most common histology, high grade serous, has revealed that it could be subclassified by genomic signatures that suggest a variety of different pathways and response elements guiding their intrinsic biology.5–9 This emerging knowledge base is starting to guide and inform clinical investigation, most obviously therapeutics. Additionally, the tumour microenvironment has emerged as an important target with antiangiogenesis therapies and immunological approaches leading the way. However, it is also likely that informatics will guide the paradigm of treatment, including surgical intervention and strategic sequencing of therapy in the near future.10
Figure 1.
Typical peritoneal distribution of primary ovarian cancer
In light of the developing database of therapeutic information and emerging technology directing clinical trial development, we provide a review of the clinical state of the disease. Herein, we address the latest results from clinical trials at the key management decision points throughout the natural history of the disease. Global contribution from investigators and cooperative groups has provided a plethora of treatment options, which is expected to expand, but likely in a more-selective and individualized fashion. Although under-pacing discovery, growth in our armamentarium should enable more individualized treatment options in an attempt to increase the pace of improved survivorship.
Surgery
The role of surgery
The role of surgery in the treatment of ovarian cancer is pivotal to achieve the goal of maximally extending survival. There are essentially three goals of surgical intervention for patients with suspected ovarian malignancies: establishing the diagnosis, staging and cytoreduction or debulking.
Establishing the diagnosis
Establishing the diagnosis might sound elementary; however, in the face of widespread disease, gastrointestinal and a myriad of other solid tumours can mimic ovarian cancer at initial presentation. Often, immunohistochemical and other protein-expression biomarker tests, in combination with sophisticated imaging and endoscopy procedures, are necessary to accurately identify the site of malignant origin when widespread intraperitoneal disease exists. Furthermore, the histological confirmation of a cancer diagnosis is often made initially at surgery, as needle biopsy of large ovarian masses is generally discouraged for fear of spreading or upstaging patients.
Staging
Determining the extent, or stage (Box 1), of disease is of particular importance for those patients who have what seems to be cancer confined to the ovary. Accurate staging guides treatment and offers valuable prognostic information. Multiple reports have demonstrated that occult disease is identified nearly a third of the time when comprehensive surgical staging is conducted.11, 12, 13, 14
Box 1. Key staging components.
Laparotomy (usually midline) or laparoscopy
Inspection and palpation of tissues in the abdomen and pelvis
-
Cytologic washings
Pelvis
Bilateral paracolic gutters
Below hemidiaphragms
-
Systematic peritoneal biopsies
Pelvic cul-de-sac
Anterior bladder peritoneum
Infundibulopelvic ligament pedicles
Bilateral pelvic sidewalls
Paracolic gutters bilaterally
Hemidiaphragms bilaterally
Any other suspicious tissues (for example, adhesions)
Pelvic and paraaortic lymph node dissection
Omentectomy
Cytoreduction or debulking
The current standard of care for patients with disseminated disease is maximal surgical cytoreduction. Although such an approach is rarely practiced in other non-gynaecological tumours, cytoreduction in patients with ovarian cancer patients provides opportunities to accurately establish a diagnosis, to remove poorly perfused or under oxygenated tissue that may harbour chemoresistant disease, and to favourably alter the tumour microenvironment to enhance adjuvant therapy.13, 15 Although no prospective randomized trial has been conducted to examine the impact of residual disease on overall survival, removal of greater amounts of tumour has consistently been associated with better outcomes. The first report on the topic dichotomized post-operative tumour residuum to less than or greater than 1.5 cm.16 Patients left with disease greater than 1.5 cm had a median OS of 12 months; those found with either 1.5 cm of disease or less or in whom debulking rendered them with the same disease volume had and OS of 26 months. Since then, the definition has varied between less than 2 cm of residual disease to no gross residual disease.17 In more-contemporary accounts, optimal cytoreduction is defined as removing all disease 1 cm or greater in diameter, leaving visible, but small-volume disease at the completion of surgery.11, 13 However, recently many clinicians have proposed that the true goal of radical cytoreduction should be to reduce tumour to no grossly visible tumour.18 This suggestion stems from several systematic review articles that demonstrated a significant survival variance in tumour residuum below 1 cm.13, 18, 19 Although radical resection procedures, such as intestinal resection, diaphragm stripping or resection, splenectomy and liver resection might be necessary to achieve this result, complete resection seems to be feasible in approximately a quarter of patients.18, 19 It is still controversial whether disease that is amenable to complete resection is functionally driven by a different biology than those for which resection is infeasible.
Timing of surgery—primary versus interval
Depite the relationship between cytoreduction outcome and overall survival, investigators have not been able to identify an externally validated method to choose patients best suited for primary surgical cytoreduction, although several nomograms have been described.20 An alternative strategy leverages the innate chemosensitivity that frequently characterizes ovarian cancer at presentation, neoadjuvant chemotherapy. The concept of neoadjuvant chemotherapy, whereby chemotherapy is administered prior to comprehensive surgical cytoreduction, has been a topic of significant interest in the treatment of front-line ovarian cancer for the past decade. Patients who are thought to benefit include those with significant medical comorbidities and those with a priori non-debulkable tumors. Proponents of neoadjuvant chemotherapy have cited the improved or at least non-inferior disease-free and overall survival associated with the approach and the improved ability of the surgeon to achieve a state of optimal cytoreduction othewise known as complete resection.12, 15, 21–24 Most of the studies that have compared primary surgical cytoreduction with neoadjuvant chemotherapy followed by interval cytoreduction have demonstrated overall decreased surgical morbidity and mortality for the neoadjuvant-therapy group. The tolerability advantages that favour neoadjuvant therapy have included: decreased estimated blood loss, shorter surgical times, less intensive-care-unit admissions, fewer bowel resections, and reduced overall lengths of stay. 12, 15, 21–25 Overall operative morbidity is clearly reduced when neoadjuvant therapy is employed.
However, some authors have challenged that neoadjuvant chemotherapy may, in fact, be associated with poorer than expected overall survival.26–28 The controversy was not resolved with the publication of the only phase III randomized trial to prospectively compare the outcomes of primary debulking with neoadjuvant chemotherapy and interval cytoreduction in women with stage III–IV bulky primary ovarian cancer.29 Despite demonstrating that the neoadjuvant approach was not inferior to primary surgical cytoreduction and was associated with less morbidity, the absolute values of resection to <1 cm (41% overall) and progression-free survival (PFS) and overall survival in the neodjuvant-therapy group were substantially lower than expected (12 months and 30 months, respectively). Indeed, Chi et al.,30 have countered that patients enrolled at their institution—meeting the same eligibility criteria as the Vergote et al. study29—over the same enrollment period, fared much better with primary surgery and that the outcomes in the randomized trial seemed to be more consistent with their large-volume residual disease (suboptimal) patient population. Recently, Vergote et al. 25 have suggested that in patients with large-volume disease, neoadjuvant therapy might be preferred, yet the overall survival is not impressive even in the experimental neoadjuvant cohort (Table 1). A recent metaanalysis concluded that interval debulking surgery should not be the prefered approach to primary debulking surgery,31 yet as stated, the only phase III data currently available demonstrates similar efficacy and improved toxicity with the neoadjuvant approach. Thus, the debate continues (Box 2), and the hope is that ongoing trials of interval versus primary cytoreduction [Ed: Add the highlighted clinical trials to the reference list once the author has completed changes: NCT00075712, NCT00993655, NCT01654146] will better define the best option for patients while addressing perceived short-comings of the present trial.
Table 1.
Interval versus primary surgical cytoreduction29
| Comparator | NACT and IDS | PDS |
|---|---|---|
| n | 329 | 339 |
| PFS | 12 months | 12 months |
| OS | 30 months | 29 months |
| No residual after surgery | 53% | 21% |
| <1 cm residual after surgery | 82% | 46% |
| Mortality (0–28 days) | 0.7% | 2.7% |
| Fever grade 3 or 4 | 1.7% | 8% |
| Red blood cell transfusion | 53% | 51% |
Abbreviations: IDS, interval debulking surgery; NACT, neoadjuvant chemotherapy; PDS, primary debulking surgery; PFS, Progression-free survival; OS, overall survival.
Box 2. Pros and cons of neoadjuvant chemotherapy.
Pros
Less peri-operative morbidity and mortality
Start chemotherapy faster
Rapidly identify refractory patients
Outcomes generally seem to be comparable with primary cytoreduction in terms of survival
Phase III outcomes data are available and supportive
Less aggressive and specialized surgical procedures may be necessary
Cons
Increases theoretical odds of resistance
Chemotherapy might be less effective
Lost opportunity to cytoreduce
Less amenable to intraperitoneal therapy
Survival data worse than contemporary primary debulking surgery data
Major criticisms of surgical skill or effort and patient selection in trials
Second look surgery
A second look procedure is defined as a reassessment procedure that is conducted in patients who have had a complete clinical response to primary treatment following surgery and adjuvant therapy. Thus, physical exam, as well as tumour markers and imaging are normal. The procedure is not to be confused with interval cytoreduction or secondary cytoreduction. Second look procedures, despite a long history of use, have fallen out of favour as a standard intervention largely owing to a lack of clinical benefit or influence on overall survival.32 Improper use might result in direct harm to patients with unnecessary expense, pain, and inconvenience. However, second look operations can be valuable asset when used within the context of a clinical trial to confirm a complete pathologic response or to identify small-volume residual disease amenable to experimental therapy. The expanding role of minimally invasive surgery might increase the therapeutic index in these cases.
Chemotherapy and novel agents
First-line therapy
With the publication of the results of the Gynecologic Oncology Group (GOG) protocol 111 in 1996, intravenous paclitaxel replaced intravenous cyclophosphamide in the adjuvant treatment of advanced-stage ovarian cancer.33 This study randomly assigned 410 women with advanced-stage ovarian cancer and residual disease larger than 1 cm after initial surgery to receive cisplatin (75 mg/m2) with either cyclophosphamide (750 mg/m2) or paclitaxel (135 mg/m2 over 24 h). Among the 216 women with measurable disease, 73% on the cisplatin–paclitaxel arm responded to treatment, compared to 60% receiving the cisplatin–cyclophosphamide regimen (P =0.01). PFS was significantly longer (P <0.001) in the cisplatin–paclitaxel group than in the cisplatin–cyclophosphamide group (median PFS 18 months and 13 months, respectively). Median overall survival was also significantly longer (P <0.001) in the cisplatin–paclitaxel group (38 months versus 24 months). The paradigm of switching from cyclophosphamide to paclitaxel was later confirmed in a European and Canadian phase III clinical trial (EORTC-NCIC OV 10).34 This 680 patient study enrolled a broader patient population than GOG 111 and administered paclitaxel as a 3-h infusion instead of over 24 h. After a median follow-up of 38.5 months and despite a high rate of crossover (48%) from the cyclophosphamide arm to the paclitaxel arm at first relapse, a longer median PFS (P = 0.0005; 15.5 months versus 11.5 months) and overall survival (P = 0.0016; 35.6 months versus 25.8 months) was seen.
Two, more recent, randomized phase III trials led to the replacement of cisplatin with carboplatin when combined with paclitaxel. This regimen is widely accepted around the world as one of the current standards in treating ovarian cancer both in advanced-stage cases after debulking surgery as well as those cases with early stage disease following surgical staging. Both studies were non-inferiority phase III trials comparing paclitaxel plus cisplatin with paclitaxel plus carboplatin in patients with advanced-stage ovarian cancer; the latter regimen being better tolerated and easier to deliver as an outpatient. Since the two studies investigated different doses, the optimal doses remain unclear. The German study 35 used a carboplatin dose calculated using the method of Calvert,36 in which the required dose is obtained by the formula: carboplatin (dose in milligrams = AUC = 6 × [glomerular filtration rate + 25]) plus paclitaxel (185 mg/m2 over 3 h). The glomerular filtration rate was estimated using the Jelliffe formula.37 The GOG study used a dose of intravenous carboplatin (AUC = 7.5) plus paclitaxel (175 mg/m2 over 3 h). Either dose is acceptable. Patients with small-volume residual disease (<1 cm) treated with intravenous carboplatin plus paclitaxel in the GOG study had a median overall survival of 57.4 months.38
Most recently, the dose and schedule of paclitaxel has been questioned. A single study in Japan has suggested that intravenous paclitaxel given weekly at 80 mg/m2 combined with standard doses and schedules of carboplatin can allow dose intensification resulting in prolonged PFS and overall survival. The analysis included 631 patients. At 6.4 years of median follow-up, the median PFS after administration of weekly paclitaxel was 28.1 months compared to 17.5 months in the control (HR 0.75, 95% CI, 0.62–0.91; P=0.0037). Median survival has not yet been reached but the OS at 5 years was also higher in the dose-dense weekly group (58.6% vs. 51.0%, HR 0.79, 95% CI, 0.63–0.99; P =0.0448)..39 Such a strategy has been validated when treating patients with metastatic breast cancer.40 This idea is being tested by the GOG and the results are eagerly awaited. NCT01167712
The administration of intraperitoneal chemotherapy has also generated much controversy. By giving chemotherapy via the peritoneum, a pharmacological advantage is created but randomized trials have shown this approach to be toxic and direct comparisons of equivalent doses and schedules of agents administered intravenously to intraperitoneally have been scarce.41 The study that has generated the most disagreement is GOG 172.42 This study showed that intraperitoneal therapy increased overall survival from 49.7 months to 65.6 months compared to intravenous therapy (P = 0.03) in patients with small-volume residual disease (<1 cm) following surgery. Importantly, the intraperitoneal arm of GOG 172 also incorporated a higher dose of cisplatin and weekly treatments into the intraperitoneal arm; thus, contaminating the results.43 A well-designed study of intraperitoneal therapy has completed enrolment and should report soon. NCT00951496
The most recent advance in treating newly diagnosed ovarian cancer is adding bevacizumab, an anti-VEGF monoclonal antibody, to front-line intravenous carboplatin and paclitaxel at the doses and schedules discussed above. Angiogenesis has a fundamental role in normal ovarian physiology as well as in the pathogenesis of ovarian cancer, promoting tumour growth and progression through ascites formation and metastatic spread.44 Two phase III trials have shown that adding bevacizumab to the chemotherapy backbone prolongs PFS (Table 2).45, 46
Table 2.
Comparison and contrast of the two frontline adjuvant phase III trials in ovarian cancer patients, which studied bevacizumab.
| Study design parameter | GOG 218 | ICON 7 |
|---|---|---|
| n | 1,873 | 1,528 |
| Disease characteristics | Previously untreated EOC, PPC, or FTC | Previously untreated EOC, PPC, or FTC |
| FIGO stage | III–IV | High risk I–IIA, IIB–IV |
| Arms | Chemotherapy vs chemotherapy plus bevacizumab vs chemotherapy plus bevacizumab → bevacizumab | Chemotherapy vs chemotherapy plus bevacizumab → bevacizumab |
| Placebo controlled | Yes | No |
| Bevacizumab dosage | 15 mg/kg every 21 days | 7.5 mg/kg every 21 days |
| Duration of maintenance therapy | 16 cycles (~11 months) | 12 cycles (~8 months) |
| Primary end point | PFS* | PFS |
| Secondary end points | OS, quality of life, correlative laboratory studies | OS, RR, safety, quality of life, economics |
| Enrollment period | October 2005 to June 2009 | December 2006 to February 2009 |
| Current status | Completed; published December 201145 | Completed; published December 201146 |
Abbreviations: EOC, epithelial ovarian cancer; FIGO, International Federation of Gynecology and Obstetrics; FTC, fallopian tube cancer; GOG, Gynecologic Oncology Group; OS, overall survival; PFS, progression-free survival; PPC, primary peritoneal cancer; RR, response rate.
Originally designated as OS but modified to PFS during the trial.
Maintenance therapy
Although advances in primary surgery and adjuvant therapy have induced high rates of complete remission following therapy, maintaining disease-free status has been elusive. Indeed, many patients develop recurrent disease, even after a negative second-look operation or pathological complete response (pCR); for example, there was a high rate of recurrence (45%) in patients with a negative second-look laparotomy.47 Those patients who initially had high-stage and high-grade tumours are more likely to recur after a negative second-look operation.48 However, those who were disease free at 5 years are likely to remain disease free at subsequent follow-up. Nonetheless, this high recurrence risk has prompted several investigators to consider additional treatment at the identification of a complete response to primary treatment.49 This treatment is often termed maintenance or consolidation therapy, although the former term is favoured, given that the decision for treatment is based on the effect of primary therapy. Several randomized and nonrandomized clinical trials have been conducted in this arena, including treatment with hormones, vitamins, radiation therapy, standard and high-dose chemotherapy, radioimmunoconjugates, immunotherapy, vaccines, gene therapy, biological therapy, complementary medicines, and holistic approaches.50 These trials have been difficult to interpret because they have included patients with either partial or complete response to front-line therapy, inclusion of second-line therapy patients, inclusion of treatment in both arms of the study or are limited by sample size or clinical trial bias. A recent Cochrane Review of the subject concluded that no treatment effect on PFS or overall survival could be demonstrated among the limited sample of phase III trials, which randomized patients with complete clinical response (cCR) to treatment versus placebo or observation.51 Importantly, this review did not include the two bevacizumab trials discussed above45, 46, as these trials allowed patients with clinical partial response to proceed onto the randomized maintenance strategy. Subsequent to the Cochrane Review, another phase III trial of erlotinib (versus observation) was reported. This GCIG/EORTC-GCG trial randomly assigned 835 patients with high-risk stage I–IV disease who achieved a cCR when receiving platinum-based chemotherapy. Erlotinib (150 mg/day) was administered for 2 years; the primary end point was PFS. Unfortunately, neither PFS (median 12.7 months versus 12.4 months) nor overall survival (median 51 months versus 59 months) were impacted by therapy. No subgroup seemed to benefit, including the small sample of patients with EGFR mutation.52
An ongoing phase III trial, GOG-212, NCT 00108745 which is comparing the effect of two taxanes (paclitaxel and paclitaxel polyglutamate) to no further treatment in patients achieving cCR after front-line therapy is poised to address the impact taxane-based maintenance treatment might have on overall survival. The trial was launched in response to a phase III trial, GOG 178, which randomly assigned patients with advanced-stage ovarian cancer who were in cCR to either 3 or 12 additional months of paclitaxel.53 The trial was designed to accrue 450 patients; however, at a planned interim analysis (after 277 patients were randomized), a statistically significant benefit for the longer treatment was demonstrated and the study was closed to further accrual. The report demonstrated a 7-month improvement in median PFS (21 months versus 14 months; P = 0.006) and was not powered to show an effect on overall survival.
Several additional biological agents are being evaluated in the phase III setting in designs that mimic ICON7 and GOG218. These include nintedanibNCT01015118 and trabananib, NCT01493505 both including concomitant therapy versus placebo followed by single agent (or placebo) continuation for those patients achieving at least a partial response. In addition, pazopanib is being studied in the phase III setting as a maintenance strategy following front-line chemotherapy. NCT00866697 Although the long-term administration of chemotherapy in this setting is impractical because of mounting toxicity, the agents targeting biological processes raise the question of administration duration. Theoretically, the high percentage of recurrence, coupled with the cytostatic potential of these newer biologically targeted agents, suggests that prolonged administration could be advantageous. However, cost, toxicity, and potential adverse effects on tumour biology are relevant remaining questions, which rival those of overall survival.
Recurrent therapy
Standard of care
Modern management of recurrent ovarian, primary peritoneal, or fallopian cancers has continued to evolve and offers women the hope of extended survival with an improved quality of life; although cancer cure remains elusive once disease recurs. Unfortunately, over two-thirds of patients who originally present with advanced-stage ovarian cancer have disease that will recur.54 There is no specific hallmark of recurrent disease; many patients will report symptoms rivalling those at initial presentation, while others will acknowledge new symptoms such as lymphoedema, shortness of breath, bloating or pain.54 Some patients are diagnosed with measurable recurrent disease solely on the basis of imaging or physical exam while others might only be identified by serially rising biomarkers (such as CA-125 or HE4 levels) in the absence of imageable disease. These biological or ‘chemical ‘ recurrence patients frequently have small-volume disease on surgical investigation; however, the value of treatment in the absence of symptoms has been strongly challenged.55 A recent phase III study examined the role and impact of serial CA-125 surveillance.56 In this study, 1,442 women in complete clinical remission following front-line therapy underwent CA-125 evaluation every 3 months. Both patients and investigators were blinded to the results. Women who remained asymptomatic but whose CA-125 levels exceeded twice the upper limit of normal (n = 527) were randomly assigned to immediate unblinding and subsequent chemotherapy or to continued blinding with intervention delayed until the time of a clinical or symptomatic recurrence. As anticipated, second-line treatment was started a median of 5 months earlier in the immediate unblinding arm. However, at a median follow-up of 57 months from randomization, this early treatment did not contribute to improved survival (hazard ratio [HR] = 0.98; 95% CI 0.80–1.20) or a longer remission duration. Furthermore, immediate treatment had an adverse impact on quality of life.
Critics have argued that following patients longitudinally with biomarkers might help identify patients for surgical treatment at recurrence.57 This potential for early identification was highlighted in a small study (n = 74) of women with recurrent ovarian cancer who underwent secondary cytoreduction.57 An evaluation of factors associated with an optimal outcome (<1 cm tumour residuum) identified that an early rise in CA-125 hastened surgical intervention by over 11 weeks on the median. Despite this finding, it is still unclear whether earlier surgical intervention based on a rising biomarker translates into improved overall survival. Nonetheless, many patients are still surveyed with serial biomarkers along with physical examination in the hope that treatment at recurrence with newer active chemotherapeutic agents will improve survival. Further data, however, are needed to confirm this hopeful hypothesis.
Once recurrent disease is suspected or documented, the choice of treatment traditionally has been determined by consideration of the interval from prior therapy and specifically the time from last prior platinum-based chemotherapy. These general definitions, and the approach to treatment for patients within these categories, are used frequently in clinical practice. In actuality, the treatment-free interval irrespective of previous exposure to platinum-based therapy can be considered as well. The hope moving forward is that these crude categories that determine treatment are replaced by more-predictive, gene-expression and pathway-based assessments that facilitate individualized therapies.
Categories of recurrence
Platinum-refractory disease
Patients who have platinum-refractory disease have tumours that essentially fail to respond to front-line therapy as exhibited by ongoing growth during initial platinum-based therapy. Prognosis for these patients is very poor, characterized by low response rates (<10%) and overall survival (<12 months)58; however, alternative platinum-based doublets and some single-agent regimens have demonstrated activity in this patient population.59
Platinum-resistant disease
Platinum-resistant disease is defined as patients whose recurrence is documented within 6 months of platinum-based therapy; these patients usually receive a non-platinum single-agent regimen as second-line treatment. Most-common chemotherapeutic choices include docetaxel,60 pegylated liposomal doxorubicin (PLD),61, 62 topotecan,61, 63 and weekly paclitaxel (Table 3).64–66 As one can appreciate, the outcome data are not impressive and novel compounds and approaches are necessary as chemotherapeutics alone have not been highly successful in this cohort. One such novel approach has been the addition of bevacizumab to standard single-agent chemotherapy that has demonstrated improved responses and PFS.67 In this recently reported study, 361 women with recurrent, platinum-resistant (stratified for treatment-free interval less than 3 months, and 3–6 months and prior antiangiogenesis therapy) ovarian cancer and two or fewer prior therapies were randomly assigned to one of three chemotherapy options versus these same options plus bevacizumab. The chemotherapy cohorts (all limited to approximately 120 patients) were weekly paclitaxel (80 mg/m2, days 1, 8, 15, 22, in a 28-day cycle), PLD (40 mg/m2, every 28 days), or topotecan (either 4 mg/m2, days 1, 8, 15, in a 28-day cycle, or 1.25 mg/m2, days 1–5 of a 21-day cycle). The experimental group included patients with the same chemotherapy plus bevacizumab (10 mg/kg every 14 days or 15 mg/kg every 21 days, as appropriate for the chemotherapy schedule) and both cohorts were treated until disease progression. Prior antiangiogenesis therapy use was infrequent (approximately 7–8%). Overall, the experimental arm outperformed the control arm for PFS (median: 6.7 months versus 3.4 months; HR = 0.48; 95% CI 0.38–0.60) and response (31% versus 13%, RECIST and/or GCIG CA-125 criteria, P <0.001). Subgroup evaluation identified a robust effect across all strata. Myelosuppression was the most common grade 3 or 4 event (16–17% both cohorts) with peripheral neuropathy (5%) and hand–foot syndrome (4.5%) being more common in the experimental cohort compared to control. Overall survival has not been reported, but publication is anticipated.
Table 3.
Most-frequently used agents in platinum-resistant disease
| Agent | Response rate (%) | PFS (months) | OS (months) | Side effects | Comments |
|---|---|---|---|---|---|
| Pegylated liposomal doxorubicin61, 62 | 10–20 | 3–4 | 10–12 | Hand–foot syndrome; mucositis | Most frequently prescribed as every 4 weeks schedule |
| Topotecan61, 63 | 12–18 | 3–4 | 10–12 mos. | Myelosuppression | Daily for 5 days or weekly administration used |
| Docetaxel60 | 22 | 3.5 | 12.7 mos. | Myelosuppression | Single GOG trial with very good results |
| Gemcitabine62, 103 | 15 | 4–5 | 11.8–12.7 mos. | Myelosuppression | Also data with platinums in resistant disease104; approved for platinum-sensitive disease with carboplatinum |
| Pemetrexed105 | 15–21 | 2.9 | 11.4 mos. | Myelosuppression | Not approved in ovarian cancer |
| Etoposide106, 107 | 6–27 | 4–5 | 10–11 mos. | Myelosuppression | Activity, dose and population dependent |
| Paclitaxel64–67 | 10–30 | 4–6 | 13 mos. | Myelosuppression; Neuropathy | Usually administered weekly in this setting |
| Nab-paclitaxel108 | 23 | 4–5 | 17.4 mos. | Myelosuppression; neuropathy | Not approved in ovarian cancer |
| Bevacizumab79 | 21 | 4.7 | 17 | Hypertension; proteinuria; thrombosis | Not approved in ovarian cancer in the USA; pivotal phase II supporting phase III front-line and recurrence investigation |
| Chemotherapy* +/−bevacizumab58 | 13 vs 31 | 3.4 vs 6.7 | Pending | Adding bevacizumab increased hypertension; proteinuria | OS pending; bevacizumab not approved in ovarian cancer |
Abbreviations: GOG, Gynecologic Oncology Group; PFS, Progression-free survival; OS, overall survival.
Allowable chemotherapy regimens in this study were paclitaxel (weekly), pegylated liposomal doxorubicin; topotecan (2 infusion styles: weekly, daily for 5 days).
Platinum-sensitive disease
Platinum-sensitive disease is defined as patients whose recurrence is documented greater than 6 months after platinum-based therapy.54, 58 These patients have a much better prognosis than the categories above in terms of longevity, as many will respond to multiple regimens. Generally these patients are treated with a platinum-based combination. Platinum-sensitive disease is a broad category that can be rather heterogeneous as those who recur just over 6 months after receiving platinum-based therapy can behave more like platinum-resistant patients than those who recur later; response rates and time to event data improve incrementally as the time interval from prior chemotherapy until recurrence increases. Thus, some authors have advocated that those who recur earlier, specifically between 6–12 months, be labelled as partially or intermediately sensitive.68 These patients can be treated like platinum-sensitive patients who recur after 12 months; however, some have advocated for the consideration of single-agent therapies used in platinum-resistant disease or non-platinum-based combinations.
A number of platinum-based combinations can be used in patients with platinum-sensitive disease (Table 4). Often, patients are treated with platinum-based and taxane-based chemotherapy again; however, concern for cumulative neuropathy can limit this choice. Furthermore, if the time from previous platinum and taxane treatment has been short, then often a regimen is selected that incorporates an alternative to taxane, such as gemcitabine or PLD. Besides altering the toxicity profile, a different mechanism of action is invoked by changing the second agent paired with platinum. More recently, with the discovery of other active cytotoxic agents, non-platinum combinations are becoming more common.69 Contemporary trials in platinum sensitive disease have incorporated targeted biological agents. (see below in Novel Targeted Agent Focus)
Table 4.
Pivotal clinical trials in the platinum-sensitive recurrent setting
| Study | Agents | RR (%) | PFS (months) | HR | OS (months) | HR |
|---|---|---|---|---|---|---|
| ICON 4 (n = 802)109 | Carboplatin* | 54 | 9 | 0.76 P <0.001 |
24 | 0.82 P = 0.02 |
| Carboplatin + paclitaxel | 66 | 12 | 29 | |||
| AGO (n = 366)110 | Carboplatin | 31 | 5.8 | 0.72 P = 0.003 |
17.3 | 0.96 P = 0.73 |
| Gemcitabin + carboplatin | 47 | 8.6 | 18 | |||
| CALYPSO (n = 976)111 | Carboplatin + paclitaxel | – | 9.4 | 0.82 P = 0.005 |
31.5 | 0.99 P = 0.87 |
| Carboplatin + PLD | – | 11.3 | ||||
| OCEANS (n = 484)112 | Gemcitabine + carboplatin + placebo | 57 | 8.4 | 0.48 P <0.0001 |
35.2 | 1.03 P = 0.84 Immature data |
| Gemcitabine + carboplatin + bevacizumab | 79 | 12.4 | 33.3 |
Other non-taxane combinations were allowed.
Abbreviations: HR, hazard ratio; OS, overall survival; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; RR, response rate.
Recurrent disease therapy
Surgery
Most patients diagnosed with recurrent disease will have a multifocal distribution of implants that mimic their presentation at initial diagnosis. In this respect, it is not difficult to understand clinicians’ and patients’ interest in ‘secondary’ surgical cytoreduction. Unfortunately, despite the significant attention this topic has received from clinical investigation, guidance as to its role in the setting of recurrent disease is not clear.17 In addition, interpretation of the available data is difficult at the minimum, and hazardous at the extreme, because of the inherent selection bias that exists in reviews of clinical experience at one or more institutions. Even reports that are based on observations of prospectively collected patients cannot be reliably compared to historical observations owing to inability to control for variations in practice patterns, patient selection and assessment models. This situation has placed a premium on phase III investigation, which fortunately is underway.
Despite the uncertainty, most practitioners strongly advocate that secondary cytoreduction has a place among carefully selected patients.70 One clear factor in the consideration for surgical intervention in medically fit patients is their underlying potential to respond to adjuvant therapy. Although this is an imperfect science, the probability of responding to secondary chemotherapy seems to be a positively sloped linear relationship with treatment and platinum-free interval.68 In this regard, patients with long treatment or platinum-free intervals (>24 months from first-line treatment completion) have anticipated outcomes, such as response rate, PFS and overall survival similar to those with treatment-naive disease. The impact of even a subtotal resection may have merit in this situation.71, 72 However, given that the median PFS from most phase III frontline trials ranges between 10 and 24 months, the majority of candidates with recurrent disease will fall into a category of intermediate and largely unknown potential chemosensitivity. In a multivariate analysis of overall survival of 46 women undergoing secondary cytoreduction for recurrent disease,73 time to recurrence of 24 months or longer, and resection to no visible residual were the only independent predictors of survival. The optimal resection rate (complete resection to no visible disease) in this study was 41%. In a prospective study of 106 patients with recurrence 6 months or longer after primary treatment,74 82% of the patients were rendered free of visible tumour. In the multivariate analysis, four variables were independent predictors of survival: disease-free interval, residual disease after secondary surgery, administration of chemotherapy before secondary surgery, and size of recurrent tumour. The authors concluded that complete resection can be obtained in most selected patients and the procedure should be considered before the administration of second-line chemotherapy. The vast majority of published series on the topic have reiterated the importance of treatment or platinum-free interval and postoperative disease residuum on treatment ‘success ‘;1 suggesting that patient and perioperative cofactors can be identified within individual series. Nevertheless, patient selection tools with sufficient external validity are problematic and limited.
Different strategies have been assessed in an effort to address this deficiency. Radiological evaluation has been frequently cited by investigators but predictive criteria identifying optimal candidates are poorly reproducible between institutions.71 Endoscopic evaluation, similar to that used by clinicians to identify appropriate candidates for primary cytoreduction, has also been used. Benedetti-Panici and colleagues reported that no visible residual disease was achieved in 79% of patients prescreened by imaging and exam who subsequently underwent preresection endoscopy.75 As in other trials, patient selection was tightly controlled with 60% of the operative sample presenting with solitary recurrence masses. The AGO identified a panel of features in their initial retrospective study on the topic (DESKTOP I trial),76 and validated their predictive model in a subsequent study.77 They found that patients who had a performance status of 0 or 1, were early stage, or had no visible tumour residuum following initial surgical cytoreduction and no ascites were likely (>67%) to have a complete surgical cytoreduction. Currently, the criteria are being used to determine eligibility for a phase III trial (DESKTOP IIINCT01166737) currently underway. Another phase III study (GOG-213NCT00565851) is being conducted to evaluate no only surgical cytoreduction but also adjuvant chemotherapy in platinum-sensitive patients. In this trial, eligibility for secondary surgery is limited to measurable disease, treatment-free interval of greater than 6 months and investigator opinion of complete cytoreduction. Both phase III studies are focused on overall survival as their primary end point.
A large, multicenter-collected data set of individual patients undergoing secondary cytoreduction was interrogated for prognostic and selection criteria characteristics.72, 78 In the first report, platinum-free interval, ascites, recurrent disease distribution, and post-operative primary residual disease were independently predictive of overall survival. From weighted scoring in a nomogram, low-risk and high-risk cohorts could be discriminated (low-risk = score 0–2 versus high-risk = score 3–8, HR = 3.65, 95% CI 3.05–4.4). In the second report aimed at candidate selection (removing secondary cytoreduction outcome) the risk model added FIGO stage, performance status, and CA-125 level. The nomogram dropped disease localization and primary cytoreduction outcome, and lowered the discriminant on platinum-free interval from 23.1 months to 16 months. The nomogram identified high-risk (score >4.8) surgical cohorts (external validity for complete resection AUC = 0.68), which was also prognostic for overall survival. When secondary cytoreduction outcome was added, the model further demonstrated prognostic value, with differential benefits seen among different risk cohorts with the same postoperative outcome (optimal versus suboptimal). Collectively, the data support interventional effects but, as mentioned, lack selection and treatment-effect bias.
Novel agent focus
Planned clinical development
Based on the initial encouraging activity of bevacizumab in newly diagnosed as well as recurrent ovarian cancer, many late-stage clinical trials have focused on other agents targeting the tumour vascular. Inhibiting VEGF has been effective in shrinking tumours as well as prolonging PFS. This class of agents even has activity when used as single agents.79 Other angiogenesis pathways involved in ovarian cancer pathogenesis include PDGF and FGF.80 Two studies have completed enrolment and the results are expected soon. The first assessed nintedanib, an oral agent that targets VEGFR-1, VEGFR-2, and VEGFR-3, PDGFR-α/β, FGFR-1, FGFR-2, and FGFR-3, members of the v-src sarcoma viral oncogene homolog (Src) family, and Flt-3. AGO-OVAR12/LUME-Ovar1 is a placebo-controlled phase III trial that is investigating the combination of nintedanib with carboplatin–paclitaxel in the first-line setting followed by nintedanib maintenance therapy until disease progression or for a maximum of 120 weeks after randomization in epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. NCT01015118 The second trial (AGO-OVAR16) assesses pazopanib, another oral agent that targets VEGFR-1, VEGFR-2, and VEGFR-3, PDGFR-α/β, FGFR-1 and FGFR-3, and c-kit. In light of its antitumour activity and acceptable tolerability, this compound is being evaluated in a phase III trial investigating pazopanib versus placebo for 24 months as maintenance therapy in patients with non-bulky stage II-IV epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer with a complete response, partial response, or stable disease after surgical debulking and at least five cycles of first-line platinum–taxane chemotherapy. NCT00866697
More recently, angiopoietin 1 and 2 have been identified as important drivers of angiogenesis in ovarian cancer via their binding to the Tie2 receptor.81–83 Both angiopoietin 1 and 2 can be inactivated by trebananib, an intravenous peptide-Fc fusion protein (or peptibody). This agent differs from VEGF inhibiting agents as it has not been shown to be associated with hypertension or bowel perforations although oedema has been seen as an attributable toxicity. Several phase III clinical trials (TRINOVA-1, NCT01204749 TRINOVA-2, NCT01281254 and TRINOVA-3NCT01493505) are evaluating trebananib in epithelial ovarian cancer, primary peritoneal cancer, or fallopian tube cancer. TRINOVA-1 is evaluating paclitaxel in combination with either trebananib or placebo in previously treated patients. The initiation date was October 2010 and the estimated primary completion date is July 2013. TRINOVA-2 is evaluating PLD in combination with either placebo or trebananib in a similar setting. To be eligible for both the TRINOVA-1 and TRINOVA-2 trials, patients also must have ECOG performance status of 0 or 1 and have received ≤ 3 prior platinum-based chemotherapy regimen(s) or had a platinum-free interval of <12 months from the first platinum-based therapy. In TRINOVA-3, trebananib is being combined with first-line carboplatin–paclitaxel. The study was initiated in December 2011, with final data collection for the primary end point of PFS expected in 2016.
The folate (vitamin B9) receptor is overexpressed on the surface of almost all epithelial ovarian cancers making it an excellent ‘tumor-associated antigen’. 59 Two strategies have been used to preferentially target carcinomas overexpressing the folate receptor. First, farletuzamab is a humanized monocolonal antibody to folate receptor alpha and is being evaluated in a phase III placebo controlled trial with second-line carboplatin–paclitaxel in patients with platinum-sensitive relapsed ovarian cancer. NCT00849667 Second, folate can be linked to traditional cytoxins. Vintafolide is an intravenous conjugate consisting of folate linked to a potent vinca alkaloid chemotherapy agent, desacetylvinblastine monohydrazide (DAVLBH). It is currently being evaluated in a phase III clinical trial for platinum-resistant ovarian cancer, (PROCEED trialNCT01170650) and uses an investigational companion diagnostic agent, etarfolatide (EC20).
Finally, cytotoxins alone continue to have a major role in treating ovarian cancer and will continue to be studied in clinical trials. Trabectedin is a novel marine antineoplastic alkaloid with a unique mechanism of action originally isolated from the Caribbean sea squirt, Ecteinascidia turbinate. Trabectedin binds covalently to the minor groove of DNA, bending DNA towards the major groove, and disrupts transcription leading to G2–M cell-cycle arrest and ultimately apoptosis.84 It is being studied in a randomized phase III trial in combination with PLD compared to carboplatin–PLD in patients with partially-platinum-sensitive recurrent ovarian cancer (progression-free interval 6–12 months). This study will assess if prolonging the platinum-free interval can improve survival and is known as the INternational OVArian cancer patients Trial with YONdelis. NCT01379989
Biology and new directions in drug development
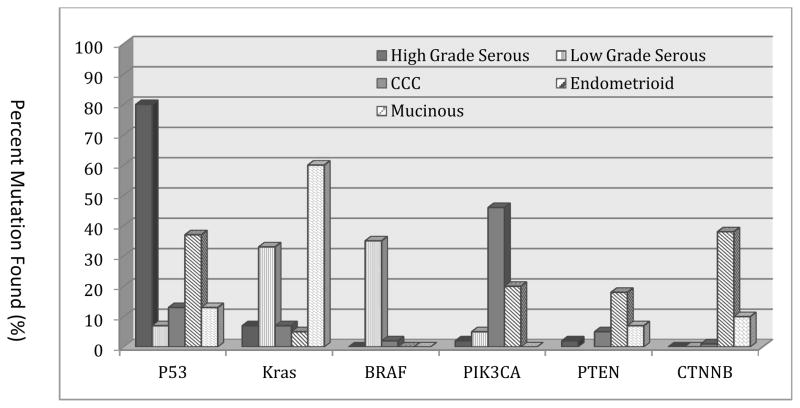
New discovery knowledge and technological advances are creating unprecedented opportunities for accelerating identification of new targets and compounds capable of engaging the biological drivers of epithelial ovarian cancer. Paramount to the translation of this knowledge is the realization of the fact that ovarian cancer is actually many diseases rather than a single entity. The diverse histological subtypes (that is, serous, mucinous, clear cell, and endometrioid) are highly distinct diseases with unique molecular features (Figure 2).85, 86,87 Even within the same histological subtype (for example, serous cancers), low-grade and high-grade differentiation is related to very distinct molecular features and clinical behaviour.85 In recognition of these findings, a Rare Tumor Committee has been formed at the NRG (formerly, GOG) to develop histology-specific trials. One completed trial in patients with low-grade serous ovarian cancer evaluated the MEK inhibitor, AZD6244, in a single-arm, two-stage design.88 An objective response rate was observed in 16% of patients, with a median PFS of 11 months. This response compares favourably with the experience with chemotherapy where response rates to chemotherapy have been less than 5%.89 Other trials in mucinous (Src, HER2, and MEK inhibitors), clear cell (PI3K pathway, HIF-1α, and MET inhibitors) and endometrioid (PI3K pathway inhibitors, and aromatase inhibitors) ovarian cancer are being designed to exploit pathway aberrations identified. Remarkably, some ovarian and breast cancers have more in common with each other than with other cancers from those organs. For example, high-grade serous ovarian cancer and basal breast cancer have more molecular similarities with each other than basal breast cancers do with luminal breast cancer.90 The term ovarian cancer might in itself require re-thinking. Although it has been used as a broad term to capture many of the malignancies noted above, it has become clear that a considerable proportion of tumours do not actually arise from the ovarian tissues (for example, distal fallopian tube as a source for many high-grade serous cancers; endometriosis as a source for endometrioid and clear cell cancers).85 However, given the current use of this term in the community, additional scientific debate will be required prior to making any changes in the terminology.
Figure 2.
Mutation profile of several genes represented against different ovarian cancer histologies. TP53 mutations are frequent in high-grade, but not low-grade serous tumours, where BRAF and KRAS mutations are more common. Mutations in the PI3K pathway (PTEN, PIK3CA) are more common in clear cell and endometrioid tumours. RAS mutations are frequently identified in mucinous ovarian cancers. (Modified from Kuo, et al, ref. 74)
Given the high prevalence and mortality associated with high-grade serous ovarian cancer, much of the large-scale discovery work has focused on this disease. Although there were few surprises from The Cancer Genome Atlas (TCGA) work, it functioned to confirm many points including: TP53 mutations are present in almost all tumours (96%); there is a high prevalence of somatic mutations, but there is a low prevalence of recurrent mutations (some genes with recurrent mutations include NF1, BRCA1, BRCA2, RB1, and CDK12); and there are large-scale copy number aberrations.5 Initial analyses also suggest that homologous recombination is defective in about half of the tumours analyzed, and that NOTCH and FOXM1 signalling are involved in ovarian cancer pathogenesis.5 Expanded and integrated analyses of the TCGA data have revealed that among women with high-grade serous ovarian cancer, BRCA2 mutation is associated with significantly better survival, improved chemotherapy response, and genome instability compared to those with BRCA1 mutation or BRCA wild type.91 Such molecular knowledge has already had an impact on developing personalized therapies. For example, poly (ADP)ribose polymerase (PARP) inhibitors have been developed to leverage BRCA (and other homologous recombination genes) dysfunction by promoting mitotic catastrophe in targeting the compensatory DNA-repair mechanism.92 Several clinical trials have documented proof of the mechanism, including a large phase I study of the PARP inhibitor olaparib in women with BRCA1 or BRCA2 germline mutations.93 In the expansion cohort of patients with ovarian cancer, objective responses were noted prompting phase II studies. In one randomized, three-arm phase II trial, two doses of olaparib (200 mg twice daily and 400 mg twice daily) were compared to PLD (50 mg/m2) in women carrying a germline BRCA1 or BRCA2 mutation, with a platinum-free interval of 12 months or less.94 The median PFS was similar between the three arms (6.5 months in the 200-mg olaparib group, 8.8 months in the 400-mg group, and 7.1 months in the PLD arm), as was response. The impressive performance of the PLD arm highlights the impact DNA-damaging agents have in this cohort of women. A second provocative randomized phase II trial addressed the impact of maintenance olaparib in women with platinum-sensitive recurrent ovarian cancer who achieved a response to platinum-based chemotherapy.95 In this trial, 265 women with both known BRCA mutation status (positive: 22%, wild-type: 15%) and unknown status were treated with olaparib (400 mg twice daily) or placebo until progression. Median PFS was significantly longer in the olaparib arm (median: 8.4 months versus 4.8 months; HR = 0.35, 95% CI 0.25–0.49). Similarly, a randomized phase II of paclitaxel–carboplatin with or without olaparib followed by olaparib or placebo maintenance has been reported.96 In this trial 162 women with high-grade serous ovarian cancer were treated with combination therapy for up to six cycles and then proceeded to maintenance in the absence of disease progression. To accommodate the combination with chemotherapy, olaparib was administered at 200 mg twice daily during chemotherapy and at 400 mg twice daily during maintenance; carboplatin was given at AUC 4 in this arm as well. Overall, the median PFS was extended significantly in the experimental arm (median: 12.2 months versus 9.6 months; HR = 0.51, 95% CI 0.34–0.77). Of interest, it seemed that separation of the curves only occurred after the completion of chemotherapy. Extensive investigation with other PARP inhibitors is underway. (e.g. NCT01618136, NCT00664781, NCT01623349, NCT0098965) Targeting tumours with homologous recombination defects with additional drugs that are complementary to PARP inhibitors, such as PI3K/Akt/mTOR and antiangiogenesis inhibitors, offers near-term opportunities. Moreover, emerging technologies, such as use of biocompatible nanoparticles for systemic therapeutic gene silencing using RNA interference, offer opportunities for rapidly functionalizing the genome and targeting even the ‘undruggable ‘. 97
Given the high rate of genomic instability in high-grade serous ovarian cancer, targeting the stroma has been considered an attractive option. As discussed previously, arguably the most clinically mature is the platform for targeting the VEGF–VEGFR axis. Drugs targeting this pathway, such as bevacizumab, have demonstrated clinical activity, although only to a modest extent in the upfront adjuvant setting in combination with chemotherapy.45 Understanding the primary resistance and adaptive responses to anti-VEGF therapy constitutes an important area of current research. The mechanisms for such evasive responses include hypoxia, adaptation of tumour endothelial cells, leveraged alternative growth factors (FGFR and so on) and alterative signalling (Dll4–Notch), and roles of other cell types in the tumour microenvironment including pericytes, fibroblasts, leukocytes, and platelets,80 and many of these will likely offer therapeutic opportunities in combination with anti-VEGF drugs and other vascular effecting agents.
The mechanisms of cytotoxic drug resistance in epithelial ovarian cancer remain elusive and indeed are probably very pleiotropic. In other words, the mechanisms of resistance to platinates and taxanes probably translate to cross-resistance to other cytotoxic agents. This is both a biological as well as a clinical phenomenon and has led clinicians to use the term ‘chemotherapy -resistant’ recurrent ovarian cancer rather than the more restrictive term ‘platinum -resistant’ disease. Accordingly, no studies have shown any improvement in survival when treating this subset of patients. In order to circumvent resistance to traditional approved agents in ovarian cancer, clinical trials are ongoing that investigate a host of targeted agents including mitotic checkpoint inhibitors, PI3K/AKT inhibitors and agents engaging HER3.98, 99 In this context, collecting tumour tissue at relapse for genomic analysis and comparison with pre-treatment tumour data, is of critical importance
To accelerate clinical drug development and to practically address the massive number of new biologically targeted drugs, improvements in clinical trial design are needed. It is simply impractical to carry out the number of phase III trials needed to study the new agents and a greater emphasis should be placed on earlier phase trial designs that incorporate molecular studies. Phase 0 trials looking at target engagement and mechanisms of action may offer earlier and faster opportunities for readouts on pathway inhibition.100, 101 Moreover, contemporary clinical trials should avoid aggregating the various ovarian cancer histological subtypes unless there is compelling scientific reason to do so. The molecular correlates or biomarkers could be considered in three broad contexts: resistance (which drugs are likely not to work); response (which drugs are likely to work in a given patient); and risk (which drugs pose a high risk of adverse events for a given patient).102 Although this strategy further divides the therapeutic population, it provides the setting and context to demonstrate the impact of targeted, actionable aberrations that may best translate into significant response metrics.
Conclusions
Ovarian cancer remains a formidable management challenge as most patients present with disease burdens that are difficult to completely eradicate despite excellent response to state-of-the-art therapies and treatment approaches. It is clear from existing survival data that early detection and prevention have the greatest potential to permanently and substantially effect long-term outcomes. Current investigation of ‘targeted ‘ therapeutics is identifying individuals with disease characteristics that are uniquely vulnerable to certain agents. However, in the absence of early detection, these are likely to have limited impact on overall survival. General population screening, such as that being conducted in the UK (UKCTOC Study) seems to be more promising to achieve the kind of stage migration that could impact overall survival. NCT00058032 However, low prevalence of disease and imperfect screening algorithms are placing high bars on reaching this goal.
Measurable impact on this disease’s clinical course requires careful consideration of all aspects of care including how it is delivered and how much it costs. Clinical drug development is grossly outpacing clinical investigation, and untethered oversight is bringing multitudes of agents to patients with limited promise of effecting measureable alterations in the natural history. Careful use of patient resources combined with robust translational science is necessary to maximize the risk:benefit considerations as we interrogate new mechanisms of action and propose the next generation of iterative clinical investigation.
Key points.
Ovarian cancer continues to be characterized by late stage presentation and bulky intraperitoneal disease burden at presentation
Surgery and chemotherapy are the mainstays of primary therapy; “optimal” surgical cytoreduction is being re-defined as resection of all macroscopic disease.
Recent advances in adjuvant chemotherapy have leveraged intraperitoneal administration, dose-dense paclitaxel and addition of biological agents predominately targeting angiogenesis
Maintenance therapy is a promising strategy as a primary or subsequent adjuvant, but as yet is unproven to increase overall survival
Recurrence therapy has improved post-progression outcomes although cures are elusive.
Closely tied to an wider understanding of the underlying biology of ovarian cancer, drug development is increasingly focused on specific new targets in the hopes of optimizing the therapeutic index
Review criteria.
We reviewed clinical data from online databases such as MedLine, PubMed, and Google Scholar, as well as abstracts from International Oncology meetings regarding topics of ovarian cancer from 1950 until 2012. Search terms used were “Ovarian Cancer”, “Primary Peritoneal Cancer”, “Fallopian Tube Cancer”, “Epithelial Cancer”, “Surgical Debulking”, “Secondary Cytoreduction”, “Chemotherapy”, “Targeted Therapeutics”, “Maintenance Therapy”, and “Clinical Trials” to identify publications and abstracts underlying treatment standards and emerging technologies for management of this disease. Clinical trials were identified by their ClinicalTrials.gov identifier.
Acknowledgments
Portions of this work were supported by the Cancer Prevention & Research Institute of Texas (CPRIT, RP120214 ), NIH (P50 CA083639, P50 CA098258), Ovarian Cancer Research Fund, Inc, The Marcus Foundation, and The Ann Rife Cox Chair in Gynecology (R.L.C); NIH (CA109298, P50 CA083639, P50 CA098258, CA128797, RC2GM092599, U54 CA151668), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC073399, W81XWH-10-1-0158, BC085265), the RGK Foundation, the Gilder Foundation, the estate of C. G. Johnson, Jr., the Marcus Foundation, the Blanton-Davis Ovarian Cancer Research Program, the Betty Anne Asche Murray Distinguished Professorship (A.K.S.);
Would like to thank Robert Bristow, MD for image used in Figure 1.
Biographies
Robert L. Coleman, MD is professor of Gynecologic Oncology and Vice-Chair, Clinical Research at The University of Texas MD Anderson Cancer Center in Houston, Texas. Dr. Coleman’s research interests include the development and translation of novel therapeutics for ovarian, uterine, and cervical cancers, clinical trial development, execution and statistical design, surgical innovations, and graduate education. He is He currently sits on the American Board of Obstetrics and Gynecology, Division of Gynecologic Oncology and serves on the Councils of the Society of Gynecologic Oncology and the International Gynecologic Cancer Society. He holds Ann Rife Cox Chair in Gynecology.
Bradley Monk, MD is Professor and Director of the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Creighton University School of Medicine at St. Joseph’s Hospital and Medical Center in Phoenix, AZ. He is also a Professor on the Clinical Scholar Track at the University of Arizona College of Medicine – Phoenix. Dr. Monk’s research interests include chemotherapy to treat ovarian and cervical carcinoma; etiology and biomarkers of gynecologic cancers; and quality of life among women with advanced malignancies. He is on the Board of Directors for the Gynecologic Oncology Group (GOG) and is the Group’s Cervical and Vulvar Committee Chair and also a member of the Publications committee, Protocol Development committee and Immunotherapy subcommittee.
Anil K. Sood, MD is Professor and Vice Chairman for Translational Research in the Department of Gynecologic Oncology and has a joint appointment in the Department of Cancer Biology at The University of Texas MD Anderson Cancer Center in Houston. He is also Co-Director of the Center for RNA Interference and Non-Coding RNA and Director of the Blanton-Davis Ovarian Cancer Research Program. His main research interests include RNAi therapeutics, identification of mechanisms underlying the effects of neuroendocrine hormones on cancer metastasis, and development of new strategies for targeting angiogenesis. He is an elected fellow of the ASCI and AAAS.
Thomas J. Herzog, MD is Director of Gynecologic Oncology and the Physicians & Surgeons Endowed Professor of Clinical Gynecology and Obstetrics at Columbia University in New York City, New York. Dr. Herzog also serves as Fellowship Director in Gynecologic Oncology for the Columbia/Cornell program. His research interests include innovative cancer surgery, minimally invasive surgery, tumor suppressor genes, molecular genetics, cytokines, pre-invasive cervical cancer, and novel cancer treatments. He is an active participant in the GOG, and has served as a Principal Investigator in a number of GOG trials with a special emphasis in ovarian and endometrial cancers. He has served on the leadership council of the Society of Gynecologic Oncology and the Foundation for Women’s Cancer as well as on the Board of Governors for the American College of Surgeons.
Footnotes
Author contributions
All the authors researched data for the article, made a substantial contribution to discussion of the content, wrote the article and edited it prior to submission.
Competing interests
R. L. Coleman declares associations with the following companies: Research Funding:, Amgen, AstraZeneca, Esperance Pharmaceuticals, Genentech/Roche, Merck, Millennium, Novartis, Scientific Advisory Board: Abbott, BioMarin Pharmaceutical, Boehringer-Ingelheim, Bristol-Myers Squibb, Clovis Pharmaceuticals, GlaxoSmithKline, Johnson & Johnson, Morphotek/Easai, Nektar. B. J. Monk declares associations with the following companies: Research Funding: Novartis, Amgen, Genentech, Lilly, Speaker’s Bureau: Roche/Genentech, Johnson & Johnson; Scientific Advisory Board: Astellas, Array, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Qiagen, Roche/Genentech. T. J. Herzog declares associations with the following companies: Research funding: Bayer, Scientific Advisory Board: Genentech/Roche, GlaxoSmithKline, Johnson & Johnson. See the article online for full details of the relationships. A. K. Sood declares no competing interests.
References
- 1.Chen CY, Chang HP, Ng KK, Wang CC, Lai CH, Chao A. Long-term disease-free survival in three ovarian cancer patients with a single relapse. European journal of gynaecological oncology. 2012;33(3):321–3. [PubMed] [Google Scholar]
- 2.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 3.Singer G, Shih Ie M, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor) International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 2003;22(1):37–41. doi: 10.1097/00004347-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Wu R, Hu TC, Rehemtulla A, Fearon ER, Cho KR. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(23):7359–72. doi: 10.1158/1078-0432.CCR-11-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berns EM, Bowtell DD. The changing view of high-grade serous ovarian cancer. Cancer research. 2012;72(11):2701–4. doi: 10.1158/0008-5472.CAN-11-3911. [DOI] [PubMed] [Google Scholar]
- 7.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nature reviews Cancer. 2010;10(11):803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 8.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(16):5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZC, Birkbak NJ, Culhane A, Drapkin RI, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu S, Lu X. Integrating genome and functional genomics data to reveal perturbed signaling pathways in ovarian cancers. AMIA Summits Transl Sci Proc. 2012;2012:72–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American Journal of Obstetrics & Gynecology. 1994;170(4):974–9. doi: 10.1016/s0002-9378(94)70090-7. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 12.Inciura A, Simavicius A, Juozaityte E, Kurtinaitis J, Nadisauskiene R, Svedas E, et al. Comparison of adjuvant and neoadjuvant chemotherapy in the management of advanced ovarian cancer: a retrospective study of 574 patients. BMC Cancer. 2006;6:153. doi: 10.1186/1471-2407-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 14.Young RC, Decker DG, Wharton JT, Piver MS, Sindelar WF, Edwards BK, et al. Staging laparotomy in early ovarian cancer. Jama. 1983;250(22):3072–6. [PubMed] [Google Scholar]
- 15.Le T, Faught W, Hopkins L, Fung Kee Fung M. Primary chemotherapy and adjuvant tumor debulking in the management of advanced-stage epithelial ovarian cancer. Int J Gynecol Cancer. 2005;15(5):770–5. doi: 10.1111/j.1525-1438.2005.00134.x. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. National Cancer Institute monograph. 1975;42:101–4. [PubMed] [Google Scholar]
- 17.Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25(20):2873–83. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- 18.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115(6):1234–44. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Aghajanian C, Barakat RR, Chi DS. The effect of maximal surgical cytoreduction on sensitivity to platinum-taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecol Oncol. 2008;108(2):276–81. doi: 10.1016/j.ygyno.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 20.de Jong D, Eijkemans MJ, Lie Fong S, Gerestein CG, Kooi GS, Baalbergen A, et al. Preoperative predictors for residual tumor after surgery in patients with ovarian carcinoma. Oncology. 2007;72(5–6):293–301. doi: 10.1159/000113051. [DOI] [PubMed] [Google Scholar]
- 21.Ivanov S, Khadzhiolov N. Prognostic factors and better survival rate after the treatment of advanced ovarian cancer with neoadjuvant chemotherapy. Akush Ginekol (Sofiia) 2004;43(6):17–9. [PubMed] [Google Scholar]
- 22.Lee SJ, Kim BG, Lee JW, Park CS, Lee JH, Bae DS. Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epithelial ovarian cancer who are inadequate for optimum primary surgery. The journal of obstetrics and gynaecology research. 2006;32(1):99–106. doi: 10.1111/j.1447-0756.2006.00359.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz PE. Neoadjuvant chemotherapy for the management of ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2002;16(4):585–96. doi: 10.1053/beog.2002.0304. [DOI] [PubMed] [Google Scholar]
- 24.Vergote IB, De Wever I, Decloedt J, Tjalma W, Van Gramberen M, van Dam P. Neoadjuvant chemotherapy versus primary debulking surgery in advanced ovarian cancer. Semin Oncol. 2000;27(3 Suppl 7):31–6. [PubMed] [Google Scholar]
- 25.Vergote I, Trope CG, Amant F, Ehlen T, Reed NS, Casado A. Neoadjuvant chemotherapy is the better treatment option in some patients with stage IIIc to IV ovarian cancer. J Clin Oncol. 2011;29(31):4076–8. doi: 10.1200/JCO.2011.36.9785. [DOI] [PubMed] [Google Scholar]
- 26.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103(3):1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 27.du Bois A, Marth C, Pfisterer J, Harter P, Hilpert F, Zeimet AG, et al. Neoadjuvant chemotherapy cannot be regarded as adequate routine therapy strategy of advanced ovarian cancer. Int J Gynecol Cancer. 2012;22(2):182–5. doi: 10.1097/IGC.0b013e31821d419a. [DOI] [PubMed] [Google Scholar]
- 28.Steed H, Oza AM, Murphy J, Laframboise S, Lockwood G, DDEP, et al. A retrospective analysis of neoadjuvant platinum-based chemotherapy versus up-front surgery in advanced ovarian cancer. Int J Gynecol Cancer. 2006;16 (Suppl 1):47–53. doi: 10.1111/j.1525-1438.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- 29.Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 30.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124(1):10–4. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2012;8:CD005343. doi: 10.1002/14651858.CD005343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer BE, Bundy BN, Ozols RF, Fowler JM, Clarke-Pearson D, Burger RA, et al. Implications of second-look laparotomy in the context of optimally resected stage III ovarian cancer: a non-randomized comparison using an explanatory analysis: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;99(1):71–9. doi: 10.1016/j.ygyno.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 33.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 34.Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92(9):699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 35.du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95(17):1320–9. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 36.Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7(11):1748–56. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 37.Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol. 2002;22(4):320–4. doi: 10.1159/000065221. [DOI] [PubMed] [Google Scholar]
- 38.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 39.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 40.Norton L. Use of dose-dense chemotherapy in the management of breast cancer. Clinical advances in hematology & oncology : H&O. 2006;4(1):36–7. [PubMed] [Google Scholar]
- 41.Echarri Gonzalez MJ, Green R, Muggia FM. Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncology (Williston Park) 2011;25(2):156–65. 70. [PubMed] [Google Scholar]
- 42.Gore M, du Bois A, Vergote I. Intraperitoneal chemotherapy in ovarian cancer remains experimental. J Clin Oncol. 2006;24(28):4528–30. doi: 10.1200/JCO.2006.06.0376. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt J, Matei D. Targeting angiogenesis in ovarian cancer. Cancer treatment reviews. 2012;38(4):272–83. doi: 10.1016/j.ctrv.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 46.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine. 2011;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 47.Rubin SC, Hoskins WJ, Saigo PE, Chapman D, Hakes TB, Markman M, et al. Prognostic factors for recurrence following negative second-look laparotomy in ovarian cancer patients treated with platinum-based chemotherapy. Gynecologic Oncology. 1991;42(2):137–41. doi: 10.1016/0090-8258(91)90333-z. [DOI] [PubMed] [Google Scholar]
- 48.Chu CS, Rubin SC. Second-look laparotomy for epithelial ovarian cancer: a reappraisal. Current oncology reports. 2001;3(1):11–8. doi: 10.1007/s11912-001-0037-0. [DOI] [PubMed] [Google Scholar]
- 49.Markman M. Maintenance chemotherapy: an evolving and increasingly acceptable strategy in cancer management. Current oncology reports. 2010;12(6):349–51. doi: 10.1007/s11912-010-0121-4. [DOI] [PubMed] [Google Scholar]
- 50.Herzog TJ, Coleman RL, Markman M, Cella D, Thigpen JT. The role of maintenance therapy and novel taxanes in ovarian cancer. Gynecologic oncology. 2006;102(2):218–25. doi: 10.1016/j.ygyno.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Mei L, Chen H, Wei DM, Fang F, Liu GJ, Xie HY, et al. Maintenance chemotherapy for ovarian cancer. Cochrane database of systematic reviews. 2010;(9):CD007414. doi: 10.1002/14651858.CD007414.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Vergote I, Joly F, Katsaros D, Coens C, Reinthaller A, Hall M, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: A GCIG and EORTC-GCG study. J Clin Oncol. 2012;30(Suppl) doi: 10.1200/JCO.2013.50.5669. [DOI] [PubMed] [Google Scholar]
- 53.Markman M, Liu PY, Wilczynski S, Monk B, Copeland LJ, Alvarez RD, et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(13):2460–5. doi: 10.1200/JCO.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Herzog TJ, Pothuri B. Ovarian cancer: a focus on management of recurrent disease. Nature clinical practice Oncology. 2006;3(11):604–11. doi: 10.1038/ncponc0637. [DOI] [PubMed] [Google Scholar]
- 55.Kew F, Galaal K, Bryant A, Naik R. Evaluation of follow-up strategies for patients with epithelial ovarian cancer following completion of primary treatment. Cochrane Database Syst Rev. 2011;(6):CD006119. doi: 10.1002/14651858.CD006119.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376(9747):1155–63. doi: 10.1016/S0140-6736(10)61268-8. [DOI] [PubMed] [Google Scholar]
- 57.Fleming ND, Cass I, Walsh CS, Karlan BY, Li AJ. CA125 surveillance increases optimal resectability at secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecologic oncology. 2011;121(2):249–52. doi: 10.1016/j.ygyno.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, et al. Secondline platinum therapy in patients with ovarian cancer previously treated with cisplatin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9(3):389–93. doi: 10.1200/JCO.1991.9.3.389. [DOI] [PubMed] [Google Scholar]
- 59.Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71(11):1397–412. doi: 10.2165/11591720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Rose PG, Blessing JA, Ball HG, Hoffman J, Warshal D, DeGeest K, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88(2):130–5. doi: 10.1016/s0090-8258(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 61.Gordon AN, Tonda M, Sun S, Rackoff W. Long-term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95(1):1–8. doi: 10.1016/j.ygyno.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26(6):890–6. doi: 10.1200/JCO.2007.13.6606. [DOI] [PubMed] [Google Scholar]
- 63.ten Bokkel Huinink W, Gore M, Carmichael J, Gordon A, Malfetano J, Hudson I, et al. Topotecan versus paclitaxel for the treatment of recurrent epithelial ovarian cancer. J Clin Oncol. 1997;15(6):2183–93. doi: 10.1200/JCO.1997.15.6.2183. [DOI] [PubMed] [Google Scholar]
- 64.Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;101(3):436–40. doi: 10.1016/j.ygyno.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 65.Rosenberg P, Andersson H, Boman K, Ridderheim M, Sorbe B, Puistola U, et al. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol. 2002;41(5):418–24. doi: 10.1080/028418602320404998. [DOI] [PubMed] [Google Scholar]
- 66.Piccart MJ, Green JA, Lacave AJ, Reed N, Vergote I, Benedetti-Panici P, et al. Oxaliplatin or paclitaxel in patients with platinum-pretreated advanced ovarian cancer: A randomized phase II study of the European Organization for Research and Treatment of Cancer Gynecology Group. J Clin Oncol. 2000;18(6):1193–202. doi: 10.1200/JCO.2000.18.6.1193. [DOI] [PubMed] [Google Scholar]
- 67.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. AURELIA: A randomized phase III trial evaluating bevacizumab plus chemotherapy for platinum-resistant recurrent ovarian cancer. J Clin Oncol. 2012;30(Suppl) doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 68.Monk BJ, Coleman RL. Changing the paradigm in the treatment of platinumsensitive recurrent ovarian cancer: from platinum doublets to nonplatinum doublets and adding antiangiogenesis compounds. Int J Gynecol Cancer. 2009;19 (Suppl 2):S63–7. doi: 10.1111/IGC.0b013e3181c104fa. [DOI] [PubMed] [Google Scholar]
- 69.Monk BJ, Dalton H, Benjamin I, Tanovic A. Trabectedin as a new chemotherapy option in the treatment of relapsed platinum sensitive ovarian cancer. Current pharmaceutical design. 2012;18(25):3754–69. doi: 10.2174/138161212802002814. [DOI] [PubMed] [Google Scholar]
- 70.Lorusso D, Mancini M, Di Rocco R, Fontanelli R, Raspagliesi F. The role of secondary surgery in recurrent ovarian cancer. Int J Surg Oncol. 2012;2012:613980. doi: 10.1155/2012/613980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–9. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 72.Tian WJ, Chi DS, Sehouli J, Trope CG, Jiang R, Ayhan A, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Annals of surgical oncology. 2012;19(2):597–604. doi: 10.1245/s10434-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 73.Tay EH, Grant PT, Gebski V, Hacker NF. Secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Obstet Gynecol. 2002;99(6):1008–13. doi: 10.1016/s0029-7844(02)01977-4. [DOI] [PubMed] [Google Scholar]
- 74.Eisenkop SM, Friedman RL, Spirtos NM. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer. 2000;88(1):144–53. doi: 10.1002/(sici)1097-0142(20000101)88:1<144::aid-cncr20>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 75.Benedetti Panici P, De Vivo A, Bellati F, Manci N, Perniola G, Basile S, et al. Secondary cytoreductive surgery in patients with platinum-sensitive recurrent ovarian cancer. Annals of surgical oncology. 2007;14(3):1136–42. doi: 10.1245/s10434-006-9273-8. [DOI] [PubMed] [Google Scholar]
- 76.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702–10. doi: 10.1245/s10434-006-9058-0. [DOI] [PubMed] [Google Scholar]
- 77.Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective Validation Study of a Predictive Score or Operability of Recurrent Ovarian Cancer. The Multicenter Intergroup Study DESKTOP II. A Project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21(2):289–95. doi: 10.1097/IGC.0b013e31820aaafd. [DOI] [PubMed] [Google Scholar]
- 78.Zang RY, Harter P, Chi DS, Sehouli J, Jiang R, Trope CG, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. British journal of cancer. 2011;105(7):890–6. doi: 10.1038/bjc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 80.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and Escape From Antiangiogenesis Therapy: Clinical Implications and Future Strategies. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.41.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hata K, Nakayama K, Fujiwaki R, Katabuchi H, Okamura H, Miyazaki K. Expression of the angopoietin-1, angopoietin-2, Tie2, and vascular endothelial growth factor gene in epithelial ovarian cancer. Gynecologic oncology. 2004;93(1):215–22. doi: 10.1016/j.ygyno.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 82.Sallinen H, Anttila M, Grohn O, Koponen J, Hamalainen K, Kholova I, et al. Cotargeting of VEGFR-1 and -3 and angiopoietin receptor Tie2 reduces the growth of solid human ovarian cancer in mice. Cancer gene therapy. 2011;18(2):100–9. doi: 10.1038/cgt.2010.56. [DOI] [PubMed] [Google Scholar]
- 83.Sood AK, Coleman RL, Ellis LM. Moving beyond anti-vascular endothelial growth factor therapy in ovarian cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(4):345–7. doi: 10.1200/JCO.2011.38.8413. [DOI] [PubMed] [Google Scholar]
- 84.Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28(19):3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 85.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–25. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuo KT, Guan B, Feng Y, Mao TL, Chen X, Jinawath N, et al. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer research. 2009;69(9):4036–42. doi: 10.1158/0008-5472.CAN-08-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. The American journal of pathology. 2009;174(5):1597–601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farley J, Brady WE, Birrer M, Lankes HA, Coleman RL, Morgan M, et al. A PHASE II TRIAL OF SELUMETINIB (AZD6244) IN WOMEN WITH RECURRENT LOW-GRADE SEROUS CARCINOMA OF THE OVARY OR PERITONEUM: A Gynecologic Oncology Group. Trial European Society of Gynecologic Oncology Annual Meeting. 2012 (Abstract) [Google Scholar]
- 89.Gershenson DM, Sun CC, Bodurka D, Coleman RL, Lu KH, Sood AK, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecologic oncology. 2009;114(1):48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 90.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–65. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 93.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 94.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 95.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 96.Oza A, Cibula D, Benzaquen A, Poole C, Mathijssen RH, Sonke G, et al. Olaparib plus paclitaxel and carboplatin followed by olaparib maintenance treatment in patients with platinum-sensitive recurrent serous ovarian cancer: A randomized, open-label Phase II study. J Clin Oncol. 2012;30(Suppl; Abstr 5001) [Google Scholar]
- 97.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11(1):59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strait KA, Warnick CT, Ford CD, Dabbas B, Hammond EH, Ilstrup SJ. Histone deacetylase inhibitors induce G2-checkpoint arrest and apoptosis in cisplatinum-resistant ovarian cancer cells associated with overexpression of the Bcl-2-related protein Bad. Molecular cancer therapeutics. 2005;4(4):603–11. doi: 10.1158/1535-7163.MCT-04-0107. [DOI] [PubMed] [Google Scholar]
- 99.Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, et al. PIK3CA cooperates with other phosphatidylinositol 3’-kinase pathway mutations to effect oncogenic transformation. Cancer research. 2008;68(19):8127–36. doi: 10.1158/0008-5472.CAN-08-0755. [DOI] [PubMed] [Google Scholar]
- 100.Hill TP. Phase 0 clinical trials: towards a more complete ethics critique. Ecancermedicalscience. 2012;6:248. doi: 10.3332/ecancer.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kapiriri L, Lavery JV, Singer PA, Mshinda H, Babiuk L, Daar AS. The case for conducting first-in-human (phase 0 and phase 1) clinical trials in low and middle income countries. BMC Public Health. 2011;11:811. doi: 10.1186/1471-2458-11-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jackson DB, Sood AK. Personalized cancer medicine--advances and socio-economic challenges. Nat Rev Clin Oncol. 2011;8(12):735–41. doi: 10.1038/nrclinonc.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2007;25(19):2811–8. doi: 10.1200/JCO.2006.09.6735. [DOI] [PubMed] [Google Scholar]
- 104.Brewer CA, Blessing JA, Nagourney RA, Morgan M, Hanjani P. Cisplatin plus gemcitabine in platinum-refractory ovarian or primary peritoneal cancer: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006;103(2):446–50. doi: 10.1016/j.ygyno.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 105.Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, et al. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: a study of the Gynecologic Oncology Group. J Clin Oncol. 2009;27(16):2686–91. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rose PG, Blessing JA, Mayer AR, Homesley HD. Prolonged oral etoposide as second-line therapy for platinum-resistant and platinum-sensitive ovarian carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 1998;16(2):405–10. doi: 10.1200/JCO.1998.16.2.405. [DOI] [PubMed] [Google Scholar]
- 107.Markman M, Hakes T, Reichman B, Curtin J, Barakat R, Rubin S, et al. Phase 2 trial of chronic low-dose oral etoposide as salvage therapy of platinum-refractory ovarian cancer. Journal of Cancer Research & Clinical Oncology. 1992;119(1):55–7. doi: 10.1007/BF01209489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Coleman RL, Brady WE, McMeekin DS, Rose PG, Soper JT, Lentz SS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecologic oncology. 2011;122(1):111–5. doi: 10.1016/j.ygyno.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361(9375):2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 110.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 111.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal Doxorubicin and Carboplatin compared with Paclitaxel and Carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol. 2010;28(20):3323–9. doi: 10.1200/JCO.2009.25.7519. [DOI] [PubMed] [Google Scholar]
- 112.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Chemotherapy With or Without Bevacizumab in Patients With Platinum-Sensitive Recurrent Epithelial Ovarian, Primary Peritoneal, or Fallopian Tube Cancer. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]