Abstract
Purpose
As the recommended radiation dose for non-small cell lung cancer (NSCLC) increases, meeting dose constraints for critical structures like the brachial plexus becomes increasingly challenging, particularly for tumors in the superior sulcus. In this retrospective analysis, we compared dose-volume histogram information with the incidence of plexopathy to establish the maximum tolerated dose to the brachial plexus.
Methods and Materials
We identified 90 patients with NSCLC treated with definitive chemoradiation from March 2007 through September 2010 who had received>55 Gy to the brachial plexus. We used a multi-atlas segmentation method combined with deformable image registration to delineate the brachial plexuson the original planning CT scans and scoredplexopathy according to the Common Terminology Criteria for Adverse Events v4.03.
Results
The median radiation dose to the brachial plexus was 70 Gy (range 56-87.5 Gy, 1.5-2.5 Gy/fraction). At a median follow-up time of 14.0 months, 14 patients had had brachial plexopathy (16%) (8 [9%] grade 1 and 6 [7%] grade ≥2); median time to symptom onset was 6.5 months (range 1.4-37.4 months). On multivariate analysis, receipt of median brachial plexus dose >69 Gy(odds ratio [OR] 10.091, 95% confidence interval [CI] 1.512-67.331, P=0.005), maximum dose >75 Gy to 2 cm3 of the brachial plexus(OR 4.909, 95% CI 0.966-24.952, P=0.038), and the presence of plexopathy before irradiation(OR 4.722, 95% CI 1.267-17.606, P=0.021) were independent predictors of brachial plexopathy.
Conclusions
For lung cancers near the apical region, brachial plexopathy is a major concern for high-dose radiation therapy. We developed a computer-assisted image segmentation method which allowed us to rapidly and consistently contour the brachial plexus and establish the dose limits to minimize the risk of brachial plexopathy. Our results could be used as a guideline in future prospective trialswithhigh dose radiation therapy for unresectable lung cancer.
Keywords: brachial plexopathy, superior sulcus tumor, dose escalation, normal tissue toxicity, deformable image registration
INTRODUCTION
Lung cancer is the leading cause of cancer-related death worldwide, with approximately 1.4 million deaths per year [1]. Around 80% of all lung cancer cases are of non-small cell histology. The current standard treatment for patients with non-small cell lung cancer (NSCLC), established by the Radiation Therapy Oncology Group (RTOG) trials 94-10 and 7310, is concurrent chemoradiation with once-[2 Gy]daily fractions to 60 Gy[2-4]. However, local failure rates remain high, up to 85% in some studies [5], prompting assessment of whether dose escalation can improve local tumor control. Initial results of the phase II trial RTOG 0117 to evaluate the feasibility of dose escalation to 74 Gy with concurrent chemotherapy for unresectable NSCLC were encouraging; the median overall survival time, 24 months, compares favorably to that produced by the lower (60-Gy) dose used in RTOG 9410 [6]. These positive findings led to RTOG 0617, a randomized phase III comparison of 74 Gy versus 60 Gy for NSCLC, expected to be completed in late 2011.
Dose escalation introduces challenges with regard to meeting dose constraints for proximal critical structures such as the brachial plexus. The maximum tolerated dose to the plexus continues to be debated; we have found this structure to be a dose-limiting factor in our phase III randomized comparison of protons versus photons for unresectable NSCLC.Radiation-induced brachial plexopathy can be quite debilitating and is difficult to treat [7]. Brachial plexopathy can present with a wide range of symptoms, often irreversibly, including numbness, pain, parasthesias, and motor impairment [8]. The underlying mechanismis thought to be due to demyelination leading to axon loss [9]. Balancing the benefit of local control with the risk of considerable toxicity is a particular challenge for tumorsof the superior sulcus or tumors with supraclavicular adenopathy.
General recommendations on the dose tolerance of the plexus are based on a 20-year-old study by Emami et al [10] that found a 5% risk at 5 years if one-third of the plexus received 62 Gy, if two-thirds received 61 Gy, or if 100% received 60 Gy; a 50% risk at 5 years corresponded to 77 Gy, 76 Gy, and 75 Gy, respectively. Most studies since have recommended the maximum dose be kept under 66 Gy.However, radiation doses of that magnitude often result in local failure, which itself cancause brachial plexopathy. The purpose of this study was to identify a threshold radiation dose at which plexopathy becomes evident when that radiation is delivered using modern-day techniques to tumors in the superior sulcus, upper mediastinum, or supraclavicular regions. We also evaluated the contribution of other factors, such as having plexopathy before radiation, receipt of concurrent chemotherapy, and receipt of proton versus photon therapy, to the risk of developing brachial plexopathy. We further attempted to address the difficulties in consistently contouring this structure by using deformable image registration.
METHODS AND MATERIALS
Patient Characteristics
Patients were retrospectively identified by searching an institutional database of patients treated with radiation for lung cancer at MD Anderson Cancer Center between March 2007 and September 2010. Among 505 patients identified as having unresectable NSCLC treated with definitive chemoradiation, 90 had superior sulcus tumors or tumors involving the upper mediastinum or supraclavicular region and had received a dose of at least 55 Gy to 0.1 cm3 of the brachial plexus. Additional inclusion criteria were having at least 4 months of follow-up and having had either photon or proton therapy with 3D conformal or intensity-modulated radiation treatment planning, with or without concurrent chemotherapy. This study was approved by the appropriate institutional review board of MD Anderson.
Brachial plexopathy was documented according to the Common Terminology Criteria for Adverse Events v4.03. Minor clinical symptoms with no medical intervention required were considered grade 1; moderate symptoms requiring pain medication with good response, grade 2; and severe symptoms, treated with multiple pain medications, including neuropathic drugs or steroid injections, with some or no improvement in symptoms, grade 3. Patients with brachial plexopathy before treatment due to tumor invasion or surgical intervention were considered to have plexopathy after radiation treatment only if the plexopathy had cleared and then returned without evidence of new tumor impingement.
Brachial Plexus Image Contouring
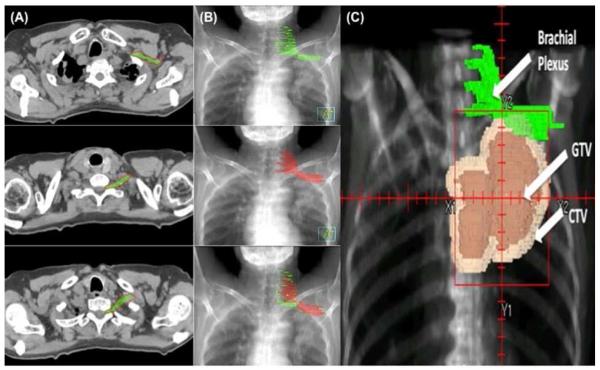
Ten of the 90 identified patients who had particularly high-quality CT images without substantial anatomic variations due to positioning or tumor invasion were selected to form the “atlas” (template) group for deformable image registration. Treatment simulation and delivery had been done with the patients’ arms above their heads. Treatment plans from those patients were de-archived from the tape backup system and restored into a research Pinnacle planning system (Philips Healthcare).The brachial plexuses of the first 10 patients were contoured with a 5-mm-diameter paint tool, with previous reports [7, 11]and Netter’s Anatomy [12] used as guides. We identified C5 through T1 roots, which served as the medial borders of the brachial plexus; the plexus was contoured from medial to lateral using the scalene muscles as landmarks[11]. The superior border of the plexus was initiated between the neural foramina at C4-C5 where the nerve was traced as it exited the foramina. Where no foramen was present, only the regions between the scalene muscles were contoured. Using the clavicle and first rib as landmarks [11], we used bony anatomy and the subclavian bundle to draw the plexus distally. The inferior and lateral borders of the plexus terminated with the subclavian vascular bundle (Fig. 1). The contours were drawn jointly by two thoracic radiation oncologists and one thoracic radiologist. These 10 images were then incorporated in the deformable registry program.
Figure 1.
Brachial plexus contouring using deformable image registration. A) Axial CT scan delineating the brachial plexus based on physician consensus (green) and computer-generated contours (red). B) Digitally-reconstructed radiographs (DRR) showing manual contours (green) and computer-generated contours (red). C) DRR showing patient with a superior sulcus tumor with contours of the brachial plexus generated by deformable image registration followed by manual modification. The planned tumor volume (PTV) received 74 Gy. This patient later developed grade 2 toxicity. GTV, gross tumor volume; CTV, clinical target volume.
Deformable Image Registration
To save time and improve the consistency of contouring, we applied a new multi-atlas segmentation method to automatically delineate brachial plexus contours as follows. The ten patients described above were selected as the atlas cases; the atlases were applied separately to register each of the remaining 80 patients (“test” cases) by using a deformable image registration algorithm based on the accelerated “demons” algorithm [13]. The resultant displacement vector fields characterizing the individual registrations were then used to deform the atlas brachial plexus contours to obtain 10 individual segmentations for each patient. Finally, the Simultaneous Truth and Performance Level Estimation (STAPLE) algorithm [14] was used to combine these 10 individual segmentations to produce a single fused contour, which was considered the best statistical estimation of the true segmentation from multiple measurements. This overall framework is illustrated in Figure 2. These auto-delineated contours for the entire cohort were then reviewed and modified individually by hand after auto-segmentation had been completed to maintain consistency in contours for all 90 patients.
Figure 2.
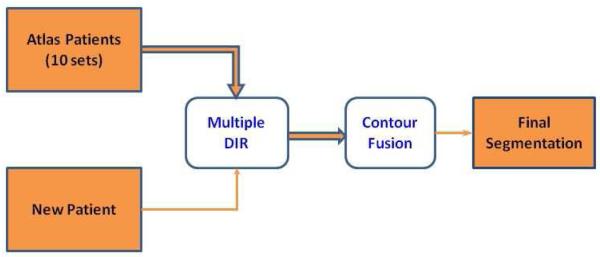
Schematic diagram for auto-contouring the brachial plexus using multiple atlases. Ten sets of atlas patients were registered to the new patient using deformable image registration (DIR) and the deformed atlas contours were fused to produce the final auto-segmented brachial plexus contours for the new patient. Please refer to the text for details.
Evaluation of Brachial Plexus Dose
The Pinnacle planning system was used to calculate the dose to the brachial plexus using the original treatment plan. When patients were treated with proton therapy using Varian Eclipse treatment planning, DICOM-RT dose plans were first exported from Eclipse planning system and then converted and imported into Pinnacle planning system for dose calculation. We calculated the maximum dose to 0.1 cm3, 0.5 cm3, 1 cm3, and 2 cm3 of the plexus and the mean and median overall dose delivered from the original treatment plans.
Statistical Analysis
Fisher’s exact test was used to assess measures of association of categorical patient and treatment factors including ethnicity, sex, smoking status, tumor histology, tumor side, disease stage, and Karnofsky Performance Status (KPS) score according to plexopathy status in frequency tables. The Mann-Whitney two-sample statistic (or Wilcoxon rank-sum test) was used to test the distribution of continuous variables according to plexopathy status. Factors assessed in this manner include patient age at treatment, body mass index, smoking pack-years, median dose to the brachial plexus and maximum dose to 0.1 cm3, 0.5 cm3, 1 cm3, and 2 cm3 of the plexus. Logistic regression analysis was used to examine the influence of patient, tumor, and other factors on the occurrence of plexopathy, including patient age, sex, body mass index group (<25 vs ≥25), tumor side, smoking history, pack-years smoked, diabetes status, tumor histology, KPS, and disease stage (I-II vs. III, IV and recurrent). The maximum doses to 0.1 cm3, 0.5 cm3, 1 cm3, and 2 cm3 of the brachial plexus were assessed using 65 Gy, 69 Gy, and 75 Gy as cut-off points. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported. P values of 0.05 or less were considered to indicate statistical significance. Statistical tests were based on a two-sided significance level.
RESULTS
Patient Characteristics
Of the 90 patients identified who had received definitive chemoradiation and at least 55 Gy to the brachial plexus, the median age was 64 years (range 33-85 years) and a slight majority (49 [54%]) were male. Most patients had stage III NSCLC (6 had stage I, 5 stage II, 69 stage III, and 7 stage IV). Three patients (3.3%) had small-cell lung cancer. Most patients had KPS scores of 80 or higher (31 [34%], 90-100; 39 [43%], 80; and 20 [22%] <80). Fifteen patients (7%) had diabetes. The median dose to the tumor was 70 Gy (range 56-87.5 Gy), and median fraction size was 2.0 Gy (range 1.8-2.5 Gy). Eighty-one patients (90%) received concurrent chemotherapy (Table 1).
Table 1.
Patient/Treatment Characteristics
| Characteristics | Value or No. of Patients (%) |
|
|---|---|---|
| Age | median (range) | 64 (33-85) |
| Sex | Female | 41 (45.6%) |
| Male | 49 (54.4%) | |
| Disease stage | I | 6 (6.7%) |
| II | 5 (5.6%) | |
| III | 69 (76.7%) | |
| IV | 7 (7.8%) | |
| Limited | 3 (3.3%) | |
| Tumor histology | Squamous cell | 33 (36.7%) |
| Adenocarcinoma | 34 (37.8%) | |
| NSCLC NOS | 18 (20.0%) | |
| Other | 5 (5.6%) | |
| Karnofsky Performance Status score |
90-100 | 31 (34.4%) |
| 80 | 39 (43.3%) | |
| < 80 | 20 (22.2%) | |
| Smoking status | Current | 32 (35.6%) |
| Former | 50 (55.6%) | |
| Never | 8 (8.9%) | |
| BMI | median (range) | 25.7 (16.1-56.9) |
| Diabetes | Yes | 15 (16.7%) |
| No | 75 (83.3%) | |
| Radiation dose, Gy | median (range) | 70 (56-87.5) |
| Concurrent Chemotherapy | Yes | 81 (90.0%) |
| No | 9 (10.0%) | |
| Plexopathy before radiation | Yes | 13 (14.4%) |
| No | 77 (85.6%) | |
| Max. Brachial Plexus Dose, Gy |
median (range) | |
| 0.1 cm3 | 69.7 (56.2-81.8) | |
| 0.5 cm3 | 67.8 (51.1-81.3) | |
| 1.0 cm3 | 66.7 (47.9-81.0) | |
| 2.0 cm3 | 65.8 (43.8-80.5) | |
| Median dose to plexus, Gy | median (range) | 41.9 (10.0-96.0) |
| Plexopathy after radiation | Grade 0 | 76 (84.4%) |
| Grade 1 | 8 (8.9%) | |
| Grade 2 | 4 (4.4%) | |
| Grade 3 | 2 (2.2%) | |
| Time to plexopathy, mo | median (range) | 6.5 (1.4-37.4) |
Abbreviation: NSCLC NOS, non-small cell cancer not otherwise specified.
Validation of Deformable Image Registration
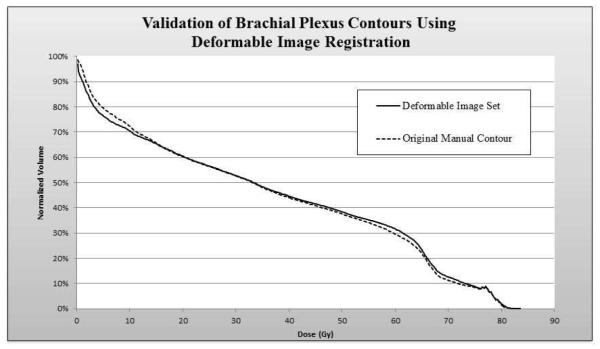
Auto-segmentation using deformable image registration followed by modification was found to be accurate for the majority of the cases, with only slight modification needed, mostly based on aberrant arm position. Dose-volume histograms (DVHs) of the brachial plexuses for the first 10 patients (original training set) contoured manually werecompared to those created by deformable image registration (deformable image set) (Fig. 3). The two curves were nearly superimposable. The V10 values (volume of brachial plexus volume receiving 10 Gy) were 71.8 Gy for the manually contoured scans and 73.2 Gy for the automatically contoured scans; the corresponding V20 values were 65.6 Gy and 66.0; the V50, 33.2 Gy and 33.2 Gy; and the V60, 20.4 Gy and 20.4 Gy.
Figure 3.
Dose-volume histogram data showing the median radiation dose of 10 patients manually contoured forming the training set (dotted line) compared to the automatically generate plexus contours using deformable image registration, prior to modification.
Brachial Plexopathy as Related to Treatment Characteristics
Fourteen patients experienced plexopathy (16%): eight individuals had grade 1, four had grade 2, and two had grade 3 toxicity. The median time to symptom onset was 6.5 months (range 1.4-37.4 months). Plexopathy in most cases presented as shoulder/arm pain (12 patients [86%]) or arm numbness/weakness (6 patients [43%]. The median maximum doses to 0.1 cm3, 0.5 cm3, 1.0 cm3 and 2.0 cm3 of the brachial plexus was 69.7 Gy (range 56.2-81.8 Gy), 67.8 Gy (range 51.1-81.3 Gy), 66.7 Gy (range 47.9-81.0 Gy), and 65.8 Gy (range 43.8-80.5 Gy) respectively. The median overall dose to the brachial plexus was 41.9 Gy (range 10.0-96.0 Gy). Thirteen patients (14%) had brachial plexopathy before treatment. Of these, 5 patients (38.5%) developed plexopathy after treatment.
Overall, patients with a median brachial plexus dose >69 Gy developed plexopathy 60% (3/5) of the time compared to 13% (11/85) in those receiving ≤69 Gy to the plexus (P=0.005). Patients with >75 Gy to 2.0 cm3had plexopathy 43% (3/7) of the time versus 13% (11/83) in those receiving ≤75 Gy to 2.0 cm3 (P=0.038). On multivariate analysis, a median dose to the entire brachial plexus of >69 Gy was associated with plexopathy(OR 10.091, 95% CI 1.512-67.331, P=0.005) (Fig. 4A), as was receiving >75 Gy to 2.0 cm3 of the plexus(OR 4.909, 95% CI 0.966-24.952, P=0.038) (Fig 4B). Other significant risk factors were having plexopathy before treatment (OR 4.722, 95% CI 1.267-17.606, P=0.021) and perhaps having a body mass index ≤25 (OR 0.291, 95% CI 0.084-1.011, P=0.052) and a maximum dose to 0.1 cm3>75 Gy (OR 3.283; 95% CI 0.925-11.646, P=0.056). Gender, concurrent chemoradiation, and the presence of diabetes were not associated with risk of brachial plexopathy (Table 2).
Figure 4.
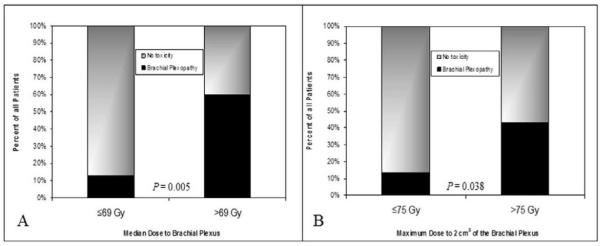
Bar graphs representing the percent risk for brachial plexopathy according to a cutoff median dose of 69 Gy to the entire brachial plexus (panel A) and a 75 Gy dose cutoff to 2 cm3 of the brachial plexus (panel B). At median brachial plexus dose > 69 Gy and 2 cm3dose >75 Gy, 60% and 43% experienced toxicity respectively.
Table 2.
Association Between Variables and Brachial Plexopathy
| Characteristics | With Plexopathy |
Total | OR | 95% CI | P Value | |
|---|---|---|---|---|---|---|
| Brachial Plexus Dose | ||||||
| Max dose to 0.1 cm3 | >75 Gy | 5 | 16 | 3.283 | 0.925-11.646 | 0.056 |
| ≤75 Gy | 9 | 74 | ||||
| Max dose to 0.5 cm3 | >75 Gy | 3 | 13 | 1.800 | 0.427-7.593 | 0.419 |
| ≤75 Gy | 11 | 77 | ||||
| Max. dose to 1.0 cm3 | >75 Gy | 3 | 12 | 2.030 | 0.474-8.689 | 0.332 |
| ≤75 Gy | 11 | 78 | ||||
| Max. dose to 2.0 cm3 | >75 Gy | 3 | 7 | 4.909 | 0.966-24.952 | 0.038 |
| ≤75 Gy | 11 | 83 | ||||
| Median dose | >69 Gy | 3 | 5 | 10.091 | 1.512-67.331 | 0.005 |
| ≤69 Gy | 11 | 85 | ||||
| Plexopathy before radiation | Yes | 5 | 13 | 4.722 | 1.267-17.606 | 0.021 |
| No | 9 | 77 | ||||
| Sex | Male | 8 | 49 | 1.138 | 0.360-3.597 | 0.825 |
| Female | 6 | 41 | ||||
| Body mass index | >25 | 4 | 47 | 0.291 | 0.084-1.011 | 0.052 |
| ≤25 | 10 | 43 | ||||
| Diabetes | Yes | 2 | 15 | 0.808 | 0.161-4.047 | 0.795 |
| No | 12 | 75 | ||||
| Concurrent chemotherapy | Yes | 13 | 81 | 1.529 | 0.176-13.286 | 0.700 |
| No | 1 | 9 |
Abbreviations: OR, odds ratio; CI, confidence interval.
DISCUSSION
At present, the maximum tolerated radiation dose for the brachial plexus remains a matter of debate. The suggested maximum of 66 GyfromEmami et al[10] caused few problems when the definitive dose for lung cancer was 60 Gy. However, with current trials evaluating 74 Gy, the dose constraints for the brachial plexus need to be revisited, particularly because most of the literature on brachial plexus toxicity comes from studies of head and neck or breast cancer. Our findings here, focusing specifically on patients treated for lung cancer, indicate that the median dose to the brachial plexus should be kept below 69 Gy, and the maximum dose to 2 cm3 below 75 Gy,for patients with NSCLC.
Interestingly, we found that doses to 0.1 cm3, 0.5 cm3, and 1.0 cm3 of the brachial plexus did not predict plexopathy; rather, the larger maximum dose to 2 cm3 and the median dose to the entire plexus allowed us to define dose cut-off points. Several explanations are possible, including the difficulty of accurately predicting the dose to a very small portion of a structure that is itself quite small in relation to other surrounding organs; tumor motion, change in tumor size, and variations in patient anatomy and positioning during treatment would all be further sources of inaccuracy. Also, the borders of the brachial plexus, unlike those of other organs can be difficult to define. For these reasons, estimates of smaller point doses may not have been accurate enough to predict the development of plexopathy.
In this study we found that plexopathy before treatment was also associated with greater risk of toxicity after treatment. It is well known that peripheral nerves are sensitive to recurrent episodes of trauma, whether from tumor invasion or from surgical intervention [9, 15]; multiple traumas might be expected to reduce the threshold for development of symptoms. Unfortunately, these are the very patient likely to justify dose escalation as they often have gross tumor pushing on the nerve, and perhaps the risk is justified because recurrent tumors will also result in further morbidity. Other studies have also noted correlations between receipt of concurrent chemoradiotherapy or use of large radiation doses per fraction [16, 17]; these other findings suggest that use of twice-daily fractionation may reduce toxicity and may provide particular benefit in patients with plexopathy prior to treatment.
Contouring the brachial plexus on CT scans continues to be challenging. Deformable image registration is a valuable tool, especially for contouring difficult structures like the brachial plexus. The contours created by the image registration provided a good approximate location of the brachial plexus. Only minor modifications were made (mostly as a result of arm position) for these structures. There were minimal differences in DVHs between the auto-segmented contours and the modified contours. The multi-atlas segmentation technique we used has the potential to reduce inter-subject, inter-observer, or even intra-observer variability in contouring the brachial plexus. Even with the differences in anatomy and positioning among patients, we noticed excellent correlation between the STAPLE fused contours and the manually generated contours, suggesting that STAPLE fusion of multiple individual segmentations can reduce variability and produce accurate contours.
Our study had several limitations. The original radiation treatment plans were based on non-contrast CT images rather than on diagnostic scans with contrast, which are often better for visualizing the brachial plexus. To reduce variability in our contouring of the brachial plexus, we followed guidelines based on easily delineated structures such as the sternocleidomastoid and scalene muscles and bony landmarks. Also, changes in arm position can affect the visibility of the brachial plexus and can contribute to inaccuracies in deformable image registration. This was corrected withminor modifications for each individual to ensure consistency. Another potential shortcoming of our study was the need to base judgments of brachial plexus toxicity grade on clinicians’ notes, which may not have been consistent among different clinicians. Finally, because brachial plexopathy is relatively rare, the number of events in our study was low, which complicates our ability to generalize our defined dose limits to a larger population of patients with lung cancer.
In conclusion, the encouraging findings from a recently completed phase II study RTOG 0117, and soon to be complete phase III trial RTOG 0617, may lead clinicians to push the radiation dose limits for unresectable apical tumors. This may prove to be problematic for complying with dose constraints to structures like the brachial plexus. The potential benefit of tumor control must be balanced against the risk of treatment-related side effects on a case-by-case basis. As improvements in surgical and radiation techniques and chemotherapy regimens lead to longer survival for patients with lung cancer, the need to monitor toxicity and adapt our practice accordingly becomes ever more imperative. Next we plan to validate these dose constraints in an ongoing randomized phase III trial looking at dose escalation for lung cancer.
Acknowledgments
Research Support: This work was made possible through the generosity of the family of M. Adnan Hamed to the MD Anderson Cancer Center Thoracic Radiation Oncology program and was also supported in part by the Cancer Center Support grant CA016672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentations: This work was submitted for presentation at the 53rd (2011) ASTRO meeting.
Conflicts of Interest: The authors declare no conflicts of interest regarding the work presented here.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45(11):2744–53. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Curran WJ, Scott C, Langer C, et al. Phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer (NSCLC): Initial report of Radiation Therapy Oncology Group (RTOG) 9410. Proc Am Soc Clin Oncol. 2000;19(1891a) (abstract) [Google Scholar]
- 4.Curran W, Scott C, Langer C, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresectable NSCLC: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22(621a) (abstract 2499) [Google Scholar]
- 5.Le Chevalier T, Arriagada R, Tarayre M, et al. Significant effect of adjuvant chemotherapy on survival in locally advanced non-small-cell lung carcinoma. J Natl Cancer Inst. 1992;84(1):58. doi: 10.1093/jnci/84.1.58. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JD, Kyounghwa B, Graham MV, et al. Primary Analysis of the Phase II Component of a Phase I/II Dose Intensification Study Using Three-Dimensional Conformal Radiation Therapy and Concurrent Chemotherapy for Patients With Inoperable Non–Small-Cell Lung Cancer: RTOG 0117. Journal of Clinical Oncology. 28(14):2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall WH, Guiou M, Lee NY, et al. Development and validation of a standardized method for contouring the brachial plexus: preliminary dosimetric analysis among patients treated with IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2008;72(5):1362–7. doi: 10.1016/j.ijrobp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Schierle C, Winograd JM. Radiation-induced brachial plexopathy: review. Complication without a cure. J Reconstr Microsurg. 2004;20(2):149–52. doi: 10.1055/s-2004-820771. [DOI] [PubMed] [Google Scholar]
- 9.Ferrante MA. Brachial plexopathies: classification, causes, and consequences. Muscle Nerve. 2004;30(5):547–68. doi: 10.1002/mus.20131. [DOI] [PubMed] [Google Scholar]
- 10.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–22. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 11.Kong FM, Ritter T, Quint DJ, et al. Consideration of Dose Limits for Organs at Risk of Thoracic Radiotherapy: Atlas for Lung, Proximal Bronchial Tree, Esophagus, Spinal Cord, Ribs, and Brachial Plexus. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netter FH. Atlas of human anatomy. 4th ed Saunders/Elsevier; Philadelphia, PA: 2006. p. 548.p. 47. [Google Scholar]
- 13.Wang H, Dong L, Lii MF, et al. Implementation and validation of a three-dimensional deformable registration algorithm for targeted prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61(3):725–35. doi: 10.1016/j.ijrobp.2004.07.677. [DOI] [PubMed] [Google Scholar]
- 14.Warfield SK, Zou KH, Wells WM. Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE Trans Med Imaging. 2004;23(7):903–21. doi: 10.1109/TMI.2004.828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kori SH, Foley KM, Posner JB. Brachial plexus lesions in patients with cancer: 100 cases. Neurology. 1981;31(1):45–50. doi: 10.1212/wnl.31.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Galecki J, Hicer-Grzenhowicz J, Grudzien-Kowalska M, et al. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer--a review. Acta Oncol. 2006;45(3):280–4. doi: 10.1080/02841860500371907. [DOI] [PubMed] [Google Scholar]
- 17.Olsen NK, Pfeiffer P, Mondrup K, et al. Radiation-induced brachial plexus neuropathy in breast cancer patients. Acta Oncol. 1990;29(7):885–90. doi: 10.3109/02841869009096384. [DOI] [PubMed] [Google Scholar]