Abstract
INTRODUCTION
Improving fat absorption remains a challenge in cystic fibrosis (CF). Antibiotics (AB) treatment has been shown to improve body weight in CF mice. The mechanism may include improvement in fat absorption. We aimed to determine the effect of AB on fat absorption in two CF mouse models.
RESULTS
AB did not improve total fat absorption. Interestingly, AB accelerated the absorption of isotope-labeled fats, in both Δ/Δ and WT mice. The changes observed were not related to the solubilization capacity of bile or to changes in the bacteria in the small intestine. AB reduced the fecal excretion of cholate by ∼50% (P < 0.05) in both CF mouse models, indicating improved intestinal bile salt absorption.
DISCUSSION
In conclusion, AB treatment does not improve total fat absorption in CF mice but does decrease fecal loss of bile salts and accelerate long-chain fatty acid (LCFA) absorption.
METHODS
For 3 weeks, we administered oral AB (ciprofloxacin/metronidazole) or control treatment to homozygous ΔF508 (Δ/Δ), cystic fibrosis transmembrane conductance regulator (CFTR) knockout (−/−), and wild-type (WT) mice and quantified fat absorption using a 72-h fat balance test. In Δ/Δ mice, we assessed fat absorption kinetics by administering tri-1-13C-palmitin and 1-13C-stearate intragastrically and determining the appearance of stable isotope-labeled fats in plasma. We quantified biliary and fecal bile salts (gas chromatography) and small intestinal bacteria (quantitative-PCR).
The classic phenotype of cystic fibrosis (CF) in the digestive system includes malabsorption of fats. Fat malabsorption can result in a suboptimal nutritional status, thereby contributing to recurrent pulmonary infections in patients with CF (1). Therefore, the improvement of fat absorption is an important key feature in CF care. Pancreatic insufficiency is the main cause of fat malabsorption in the majority of patients with CF. Although fat absorption is greatly improved by pancreatic enzyme replacement therapy, it still remains 10–30% lower than normal in a substantial fraction of patients (2). Other causes of fat malabsorption may be a decrease in intestinal pH, intestinal mucosal abnormalities (such as small intestinal bacterial overgrowth (SIBO), viscous mucus, and intestinal inflammation), or changes in biliary bile salt composition and/or the enterohepatic circulation of bile salts (2).
Treatment strategies other than pancreatic enzyme substitution should be developed so as to further improve fat absorption in patients with CF. Two previous studies indicated a positive effect of antibiotics (AB) treatment on nutritional status in CF conditions. Patients with CF showed improvements in body weight and BMI after 6 months of treatment with azithromycin, irrespective of changes in pulmonary function (3,4). It is unclear which of the properties of azithromycin—antimicrobial or anti-inflammatory—brought about this result. CF transmembrane conductance regulator (CFTR)-knockout mice showed increases in body weight after a 3-week treatment with broad-spectrum AB, as compared with controls (5). This may reflect effective treatment of SIBO, which is a common feature in CF conditions (2) and is known to respond well to AB treatment in CFTR-knockout mice (5). Because intestinal bacteria are able to deconjugate bile salts, they could impair the solubilization capacity of bile (6), thereby contributing to fat malabsorption in patients with CF.
CF mice are generally pancreas-sufficient, and yet they have problems with fat absorption (7). In this regard, CF mice mimic the condition in human patients with CF who are receiving treatment with pancreatic enzymes (thereby being rendered pancreas-sufficient). The CF mouse model is therefore an excellent one in which to study fat malabsorption caused exclusively by the intestinal CF phenotype. CFTR-knockout mice have a severe intestinal phenotype, and have lower fat absorption percentages in comparison with wild-type (WT) animals. CFTR homozygous ΔF508 mice, the model for the most common mutation found in CF patients, display a milder intestinal phenotype. These latter CF mice have a delayed intestinal absorption of fatty acids, as we previously demonstrated by absorption kinetics with isotope-labeled fats (7).
We aimed to determine the effect of broad-spectrum AB treatment on fat absorption in both CFTR-knockout mice and homozygous ΔF508 mice. We also analyzed small-intestinal bacterial flora and bile salt composition, because these are potentially influenced by AB treatment and could provide mechanistic information on possible changes in fat absorption.
RESULTS
AB Treatment Improved Body Weight in CFTR-Knockout Mice But Not in Homozygous ΔF508 Mice
Table 1 shows the nutritional data of homozygous ΔF508 mice (Δ/Δ), CFTR-knockout mice (−/−), and their WT littermates (+/+) after AB or control treatment. AB treatment did not improve body weight in Δ/Δ mice. As seen in a previous study (5), body weight increased in the male −/− mice as compared with untreated controls (+24%, P = 0.02). In our study, there were not enough female −/− mice for this analysis. The multiple-regression analysis showed that AB had a beneficial effect on body weight in the male mice of both genetic backgrounds irrespective of the CF phenotype (C57BL/6 mice: r2 = 0.7, P = 0.02, FVB/129 mice: r2 = 0.9, P = 0.01). Parameters for fat absorption were not dependent on gender (Tables 2 and 3).
Table 1.
Mice characteristics
| Genotype | Number (n) | Diet | Antibiotics (mg/kg/day) | Body weight (g) | Energy intake (kcal/day) | Fat intake (LCT, μmol/day) | Fecal excretion (g/day) | Fecal fat excretion (LCT, μmol/day) | Net fat uptake (LCT, μmol/day) | Fat absorption (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Pretreatment | Post-treatment | ||||||||||
| Δ/Δ | 4 | Chow | M: 103 ± 20 | 22.8 ± 1.6 | 22.0 ± 0.6 | 16.8 ± 1.1 | 559 ± 38 | 0.88 ± 0.15 | 40.0 ± 5.5 | 519 ± 37 | 92.1 ± 1.2 |
| C: 51 ± 10 | |||||||||||
| 4 | Chow | Control | 24.1 ± 4.1 | 24.6 ± 4.7 | 18.8 ± 1.4 | 627 ± 46 | 1.02 ± 0.03 | 55.8 ± 8.6 | 571 ± 39 | 90.2 ± 1.0 | |
| +/+ | 4 | Chow | M: 92 ± 9 | 25.1 ± 3.8 | 26.0 ± 3.6 | 18.8 ± 1.5 | 629 ± 49 | 0.92 ± 0.07 | 54.8 ± 3.2 | 574 ± 48 | 90.3 ± 0.9 |
| C: 46 ± 5 | |||||||||||
| 4 | Chow | Control | 25.2 ± 3.9 | 27.0 ± 5.0 | 19.5 ± 4.2 | 653 ± 140 | 0.96 ± 0.04 | 55.0 ± 2.3 | 598 ± 141 | 90.2 ± 2.8 | |
| −/− | 12 | Liquid | M: 93 ± 13 | 5.2 ± 1.1 | 19.2 ± 2.1* | 14.0 ± 2.0 | 313 ± 44 | 0.14 ± 0.06 | 10.6 ± 7.0 | 302 ± 43 | 96.7 ± 2.0 |
| C: 47 ± 7 | |||||||||||
| 5 | Liquid | Control | 6.5 ± 0.5 | 15.4 ± 2.5 | 14.5 ± 1.1 | 325 ± 25 | 0.15 ± 0.03 | 13.1 ± 3.1 | 312 ± 28 | 95.9 ± 1.3 | |
| +/+ | 12 | Liquid | M: 105 ± 13 | 7.6 ± 0.7 | 21.1 ± 2.8 | 15.8 ± 1.9 | 355 ± 44 | 0.14 ± 0.07 | 3.7 ± 1.7 | 351 ± 44 | 99.0 ± 0.5 |
| C: 53 ± 7 | |||||||||||
| 5 | Liquid | Control | 7.2 ± 0.2 | 20.3 ± 2.0 | 15.4 ± 3.2 | 345 ± 72 | 0.11 ± 0.02 | 3.1 ± 0.7 | 342 ± 72 | 99.0 ± 0.3 | |
Nutritional data of homozygous ΔF508 mice (Δ/Δ) (FVB/129 genetic background), CFTR-knockout mice (−/−) (C57Bl/6/129 genetic background), and wild-type littermates (+/+) after AB or control treatment. Except for body weight in the AB-treated −/− mice, no differences were observed between the AB and control-treated mice of the same genetic background (Mann–Whitney test). The ingested amount of AB was similar between all groups (Kruskal–Wallis test). Values are depicted as mean ± SD.
AB, antibiotic; C, ciprofloxacine; CFTR, cystic fibrosis transmembrane conductance regulator; LCT, long-chain triglycerides; M, metronidazole.
P < 0.05.
Table 2.
Multiple regression analysis in CFTR-knockout mice and WT littermates
| Dependent variables | Independent variables | B | P value | R2 total model |
|---|---|---|---|---|
| Fat malabsorption (total) | Genotype (WT = 0) | 2.5 | <0.001a | 0.53 |
| Treatment (control = 0) | −0.38 | 0.47 | ||
| Gender (female = 0) | 0.59 | 0.23 | ||
| Fat malabsorption (saturated) | Genotype (WT = 0) | 7 | <0.001a | 0.6 |
| Treatment (control = 0) | −1.8 | 0.16 | ||
| Gender (female = 0) | 1.5 | 0.21 | ||
| Fat malabsorption (unsaturated) | Genotype (WT = 0) | 0.77 | 0.001 | 0.33 |
| Treatment (control = 0) | 0.17 | 0.47 | ||
| Gender (female = 0) | 0.19 | 0.42 | ||
| Fecal bile salt excretion (total) | Genotype (WT = 0) | 10.8 | <0.001a | 0.54 |
| Treatment (control = 0) | −12.1 | <0.001a | ||
| Gender (female = 0) | −5.7 | 0.06 | ||
| Fecal bile salt excretion (primary) | Genotype (WT = 0) | 7.8 | <0.001a | 0.47 |
| Treatment (control = 0) | −3.2 | 0.09 | ||
| Gender (female = 0) | −0.6 | 0.7 | ||
| Fecal bile salt excretion (secondary) | Genotype (WT = 0) | 1.4 | 0.4 | 0.38 |
| Treatment (control = 0) | −6.2 | 0.003a | ||
| Gender (female = 0) | −4.9 | 0.014a | ||
| Body weight | Genotype (WT = 0) | −3.8 | <0.001a | 0.71 |
| Treatment (control = 0) | −0.9 | 0.44 | ||
| Gender (female = 0) | 0.8 | 0.51 | ||
| Gender × treatment | 3.6 | 0.02a |
B, unstandardized β coefficient; CFTR, cystic fibrosis transmembrane conductance regulator; R2, regression correlation coefficient; WT, wild-type.
Statistically significant.
Table 3.
Multiple regression analysis in homozygous ΔF508 mice and WT littermates
| Dependent variables | Independent variables | B | P value | R2 total model |
|---|---|---|---|---|
| Fat malabsorption (total) | Genotype (WT = 0) | −0.85 | 0.29 | 0.24 |
| Treatment (control = 0) | −1.0 | 0.25 | ||
| Gender (female = 0) | 0.9 | 0.33 | ||
| Fat malabsorption (saturated) | Genotype (WT = 0) | 0.08 | 0.96 | 0.25 |
| Treatment (control = 0) | −2.3 | 0.13 | ||
| Gender (female = 0) | 1.7 | 0.27 | ||
| Fat malabsorption (unsaturated) | Genotype (WT = 0) | −1.0 | 0.19 | 0.24 |
| Treatment (control = 0) | −0.64 | 0.41 | ||
| Gender (female = 0) | 0.74 | 0.34 | ||
| Fecal bile salt excretion (total) | Genotype (WT = 0) | 7.4 | <0.001a | 0.83 |
| Treatment (control = 0) | −5.1 | 0.006a | ||
| Gender (female = 0) | −7.1 | <0.001a | ||
| Fecal bile salt excretion (primary) | Genotype (WT = 0) | 9.7 | <0.001a | 0.77 |
| Treatment (control = 0) | −4.0 | 0.06 | ||
| Gender (female = 0) | −5.9 | 0.01a | ||
| Fecal bile salt excretion (secondary) | Genotype (WT = 0) | −2.5 | 0.06 | 0.34 |
| Treatment (control = 0) | −1.1 | 0.37 | ||
| Gender (female = 0) | −1.2 | 0.34 | ||
| Body weight | Genotype (WT = 0) | −3.2 | 0.004a | 0.85 |
| Treatment (control = 0) | 0.8 | 0.51 | ||
| Gender (female = 0) | 8.1 | <0.001a | ||
| Gender × treatment | −5.3 | 0.01a | ||
| Area under the curve 1-13C-palmitin | Genotype (WT = 0) | −2.2 | 0.9 | 0.46 |
| Treatment (control = 0) | 63.8 | 0.03a | ||
| Gender (female = 0) | 0.29 | 0.29 | ||
| Area under the curve 1-13C-stearate | Genotype (WT = 0) | −8.4 | 0.77 | 0.33 |
| Treatment (control = 0) | 55 | 0.09 | ||
| Gender (female = 0) | −3.5 | 0.9 |
B, unstandardized β coefficient; R2, regression correlation coefficient; WT, wild-type.
Statistically significant.
AB Treatment Did Not Improve Fat Absorption
In accordance with the results from previous studies (7), the total absorption of dietary fat was quantitatively similar in Δ/Δ mice and +/+ mice and was not enhanced by AB treatment (Table 1). Total fat absorption was ∼4% lower in the −/− mice than in the +/+ mice, and this did not improve after AB (Figure 1a). Detailed analysis showed no changes in the absorption of saturated fatty acids in either the Δ/Δ mice or the −/− mice (Figure 1b–c). In both CF mouse models, AB did not improve the absorption of unsaturated fatty acids (control-treated Δ/Δ mice: 92% ± 1% vs. AB-treated Δ/Δ mice: 93% ± 1%, P = 0.4 and control-treated −/− mice: 99% ± 1% vs. AB-treated −/− mice: 98% ± 1%, P = 0.6).
Figure 1.
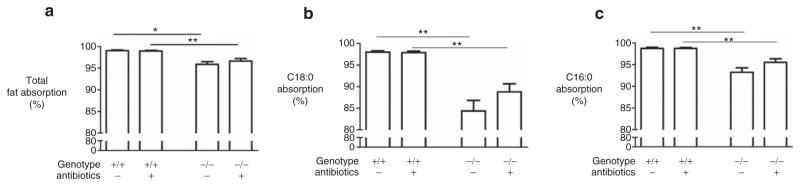
Fat absorption. (a) Total fat absorption and (b–c) absorption of the two major saturated fatty acids in cystic fibrosis transmembrane conductance regulator (CFTR) knockout mice (−/−) and their wild-type (WT) littermates (+/+) after antibiotic (AB) or control treatment. C16:0: palmitic acid. C18:0: stearic acid. Values are depicted as mean ± SEM *P value <0.05. **P value <0.01.
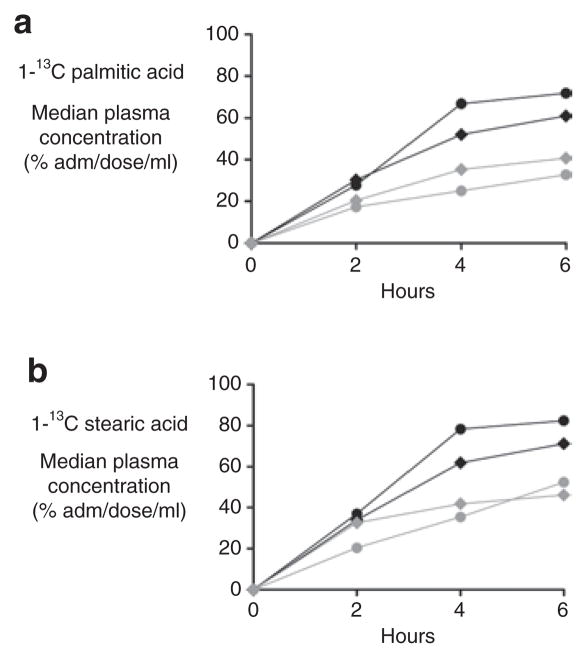
AB Treatment Accelerated the Absorption of Fatty Acids in Homozygous ΔF508 and WT Mice
Given that Δ/Δ mice have delayed fatty acid uptake (7), we also assessed the effect of AB on the kinetics of fat absorption (Figure 2). After AB treatment, a trend toward higher uptake was observed in Δ/Δ mice as compared with +/+ mice. Of interest, the total plasma appearance (area under the curve) of 1-13C-palmitic acid and 1-13C-stearic acid was higher in AB-treated Δ/Δ and +/+ mice (P = 0.04) than in control mice, compatible with an AB effect independent of CF phenotype (Tables 4 and 5, respectively). The ratio of 1-13C-palmitic acid to 1-13C-stearic acid was similar among the groups for all time points, indicating that the accelerated absorption was attributable to long-chain fatty acids (LCFAs) uptake and not to accelerated lipolysis. Total plasma triglycerides were similar among the groups (data not shown).
Figure 2.
Fatty acid kinetics. 13C-enrichment in plasma after an intragastric bolus of stable isotope–labeled (a) tri-1-13C-palmitin and (b) 1-13C-stearate in antibiotic (AB)-treated homozygous ΔF508 mice (black filled circles) and their wild-type (WT) littermates (black filled diamonds), and in control-treated homozygous ΔF508 mice (gray filled circles) and their WT littermates (gray filled diamonds). Values are depicted as medians.
Table 4.
Area under the curve 1-13C palmitic acid
| AB % | Control % | ||
|---|---|---|---|
| Δ/Δ | 167 (91–242) | 82 (47–129) | P = 0.36 |
| +/+ | 138 (128–198) | 94 (90–132) | P = 0.08 |
| P = 0.96 | P = 0.29 | P = 0.03 (AB vs. control) |
13C-enrichment in plasma after an intragastric dosage of stable isotope–labeled tri-1-13C-palmitin in homozygous ΔF508 mice (Δ/Δ) and WT littermates (+/+) after AB or control treatment. Data are expressed as median (range).
AB, antibiotic-treated; WT, wild-type.
Table 5.
Area under the curve 1-13C stearic acid
| AB % | Control % | ||
|---|---|---|---|
| Δ/Δ | 198 (145–250) | 111 (43–158) | P = 0.12 |
| +/+ | 172 (134–200) | 122 (105–198) | P = 0.29 |
| P = 0.64 | P = 0.63 | P = 0.03 (AB vs. control) |
13C-enrichment in plasma after an intragastric dosage of stable isotope–labeled 1-13C-stearate in homozygous ΔF508 mice (Δ/Δ) and WT littermates (+/+) after AB or control treatment. Data are expressed as median (range).
AB, antibiotic-treated; WT, wild-type.
Homozygous ΔF508 Mice Do Not Exhibit SIBO
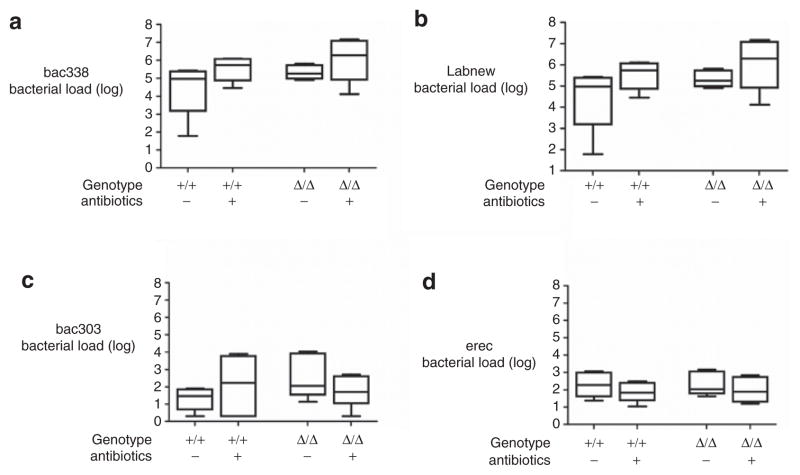
As previously reported, the −/− mice displayed SIBO, and these bacteria were markedly reduced after AB (5) (data not shown). We analyzed the data to determine whether the Δ/Δ mice also had SIBO. We found that the total bacterial load was similar in the small intestines of the Δ/Δ and +/+ mice, and did not change after AB treatment (Figure 3). Given that certain bacterial species can modulate bile acid metabolism and influence fat absorption (8), and allowing for the possibility that the bacterial composition might change after 3 weeks of oral AB, we additionally analyzed different subgroups of bacteria (Table 6). We did not find a change in bacterial composition in either the Δ/Δ or the +/+ mice after AB treatment. As previously reported in the case of AB-treated mice (9), AB significantly increased the weight of the cecum in both Δ/Δ and +/+ mice relative to controls (controls: 296 ± 99 mg, AB: 489 ± 190 mg, P = 0.03) and also the length of the cecum (controls: 2.8 ± 0.3 cm, AB: 3.6 ± 0.7 cm, P = 0.02) (Figure 4).
Figure 3.
Bacterial load in small intestine. Number of copies of the bacterial load in the small intestine in homozygous ΔF508 mice (Δ/Δ) and their wild-type (WT) littermates (+/+) after antibiotic (AB) or control treatment. Categories of bacterial groups: (a) Bac338, (b) Labnew, (c) Bac303, (d) Erec (Table 1). Box plot represents median and 25th and 50th centiles. Whiskers represent the lowest and highest values of the bacterial load.
Table 6.
Bacterial-specific primers
| Group | Reference strain | Primers | Product length (bp) |
|---|---|---|---|
| Bac 338 | Escherichia coli | UniF340-ACTCCTACGGGAGGCAGCAGT | 175 |
| Eubacteria | UniR514-ATTACCGCGGCTGCTGGC | ||
| Bac303 | Bacteroides fragilis | BactF285-GGTTCTGAGAGGAGGTCCC | 75 |
| Bacteroides | DSM 2151 | UniversalR338-GCTGCCTCCCGTAGGAGT | |
| Erec | Ruminococcus productus | UniversalF338-ACTCCTACGGGAGGCAGC | 139 |
| Eubacterium rectale/Clostridium coccoides | DSM 2950 | CcocR491-GCTTCTTAGTCAGGTACCGTCAT | |
| Labnew | Lactobaccillus acidophilus | LABF362-AGCAGTAGGGAATCTTCCA | 315 |
| Lactobacillus/Enterococcus | NIZO B228 | LABR677-CACCGCTACACATGGAG |
Figure 4.
Colon. The colons of (a) antibiotic (AB)-treated homozygous ΔF508 mice and (b) their wild-type (WT) littermates, and (c) control-treated homozygous ΔF508 mice and (d) their WT littermates.
AB Treatment Tended to Normalize Bile Salt Composition in Gallbladders of Homozygous ΔF508 Mice
We analyzed the bile salts in gallbladder bile of the Δ/Δ and +/+ mice to determine whether the accelerated absorption of fatty acids after AB treatment could be attributable to an improvement in the solubilization capacity of bile. In accord with the results of previous studies (10), the contribution of the primary bile salt cholic acid (CA) in gallbladder bile was found to be higher in the Δ/Δ mice (Δ/Δ: 68% ± 3%, +/+: 44% ± 1%, P = 0.04) (Figure 5). AB reduced the percentage contribution of CA and increased the percentage contribution of β-muricholic acid, resulting in a more hydrophilic bile salt composition in the AB-treated Δ/Δ mice (Heuman index: −0.28 ± 0.1 for AB-treated vs. −0.59 ± 0.09 for control-treated Δ/Δ mice, P = 0.03). Because hydrophobic bile salts are better able to solubilize lipids than hydrophilic bile salts are, the accelerated fatty acid uptake cannot be explained on the basis of changes in bile salt composition.
Figure 5.
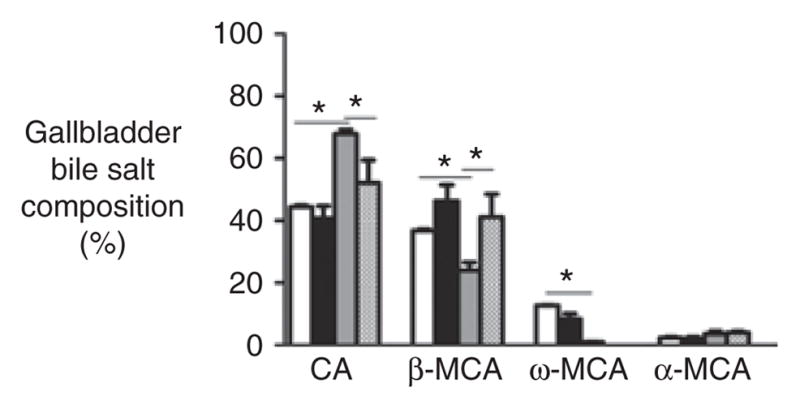
Biliary bile salt composition. Biliary bile salt composition in the gallbladders of antibiotic (AB)-treated homozygous ΔF508 mice (hatched pattern) and their wild-type (WT) littermates (black), and in the gallbladders of control-treated homozygous ΔF508 mice (gray) and their WT littermates (white). Bile salts representing <2% of total bile salts are not depicted. CA, cholic acid; MCA, muricholic acid. *P value <0.05.
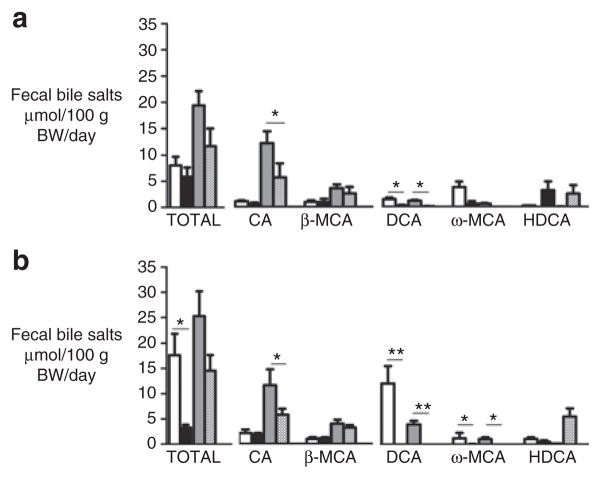
AB Treatment Reduced Fecal CA Excretion in Homozygous ΔF508 and CFTR-Knockout Mice
CF mice as well as human patients with CF have increased excretion of fecal bile salts (2,11). AB treatment did not significantly change the fecal excretion of total bile salts in Δ/Δ or −/− mice (−40% in both CF mouse models, P = 0.06). However, in comparison with controls, AB reduced the excretion of CA by ∼50% in Δ/Δ and −/− mice (P = 0.04, Figure 6). In all AB-treated mice, the fecal excretion of the “classic” secondary bile salts deoxycholic acid and ω-muricholic acid was reduced, whereas the fecal excretion of hyodeoxycholic acid was increased.
Figure 6.
Fecal bile salt excretion. Fecal bile salt excretion in (a) homozygous ΔF508 mice and (b) cystic fibrosis transmembrane conductance regulator (CFTR)-knockout mice and their respective wild-type (WT) littermates. Antibiotic (AB)-treated cystic fibrosis (CF) mice (hatched pattern) and their WT littermates (black); control-treated CF mice (gray) and their wild-type (WT) littermates (white). CA, cholic acid; DCA, deoxycholic acid; HDCA, hyoxydeoxycholic acid; MCA, muricholic acid. Values are depicted as mean ± SEM *P value <0.05, **P value <0.01.
AB Treatment Reduced mRNA Expression of Muc2 in the Small Intestines of Homozygous ΔF508 Mice
A reduction in mRNA expression of mucin 2 was observed after AB treatment in Δ/Δ mice (AB-treated: 0.51 ± 0.06 vs. 0.96 ± 0.30 control-treated, P = 0.02).
DISCUSSION
It has been previously shown that AB treatment eradicates SIBO and increases body weight in CFTR-knockout mice (5). Our study adds the important finding that the positive effect of AB on body weight is not mediated by increasing the absorption of long-chain triglycerides. AB treatment of homozygous ΔF508 mice without SIBO neither augmented body weight nor increased fat absorption. Collectively, the data indicate that the positive effect of AB on body weight in CFTR-knockout mice may be attributable to the treatment of SIBO and not to enhanced absorption of long-chain triglycerides.
The improved nutritional status that was observed in the CFTR-knockout mice could possibly be explained in terms of other AB-related effects. It has previously been reported that AB effects on the small intestine in CFTR-knockout mice (reduction in bacterial load, inflammation, and mucus) can decrease energy demand and thereby improve nutritional status (12–14). Given that these intestinal mucosal abnormalities may also (theoretically) impair the uptake of proteins and carbohydrates, we cannot exclude the possibility that the absorption of these macronutrients also improved after AB treatment. However, we do not have indications that their absorption is suboptimal in CF mice. Malabsorption of medium-chain triglycerides is almost completely corrected after pancreatic enzyme replacement therapy in human patients with CF (15). Therefore, medium-chain triglyceride malabsorption is not likely to contribute to fat malabsorption in the pancreatic-sufficient CF mice in our study. A decrease in energy requirement is the most likely explanation for the improved body weight. To what extent the previous reported effects of AB on the intestine decreased the energy demand, and whether these changes are inversely related to body weight, remain to be evaluated. In veterinary medicine, several AB are used as growth promoters, and these are probably mediated through suppression of Gram-positive intestinal bacteria (16). Of interest, our multiple-regression analysis revealed that the effect of AB treatment on bacterial growth was gender-related, whereas none of the other parameters (e.g., bacterial classes in the small intestine (data not shown), fat absorption) were gender-related. There is no mechanism-based explanation for the observed effect.
AB accelerated LCFA uptake in homozygous ΔF508 mice as well as in WT mice. The anticipated coefficient of variation of 10% was higher than expected, which may explain why there was significantly accelerated uptake of LCFA in the AB-treated animals (WT plus ΔF508), whereas the rate of increase of uptake was not significant in the ΔF508 mice group alone. The mechanism through which AB increase the plasma levels of orally administered fat is unclear. Given that AB did not alter the gallbladder bile salt composition in WT mice, and also did not change the major subclasses of bacteria in the small intestine, these factors probably do not contribute to the changes in fat absorption kinetics. Also, the fact that accelerated fatty acid uptake was observed in both CF and WT mice suggests an aspecific treatment effect on intestinal physiology, for example, on intestinal transit time, mucus composition, or the unstirred water layer. It has been previously shown that AB prolong the transit time in the small intestine in WT mice (13). The authors of the study hypothesized that normal microflora might play an important role in the regulation of intestinal transit time (13). Given that homozygous ΔF508 mice have the same intestinal flora as WT, it is possible that intestinal transit is prolonged in ΔF508 mice also, thereby enhancing intestinal fatty acid uptake. In addition, AB-induced bile flow is described in relation to some AB, and this should be considered as a possible underlying mechanism (17). The biological significance of the changes in fatty acid kinetics remains to be determined. One could speculate that a reduction in the rate of fatty acid absorption indicates a more rate-limiting milieu in the small intestinal lumen, and a more gradual appearance of the dietary fat into the circulation. Whereas for carbohydrates the rate of appearance into the circulation is clearly associated with metabolic effects (“slow and rapid carbohydrates”), the corresponding effects of fat absorption rates remain largely unexplored. In the homozygous ΔF508 mice, the decreased rate of uptake is adequately compensated for by an increase in the absorption capacity of the small intestine, resulting in a normal overall fat absorption. This compensatory capacity may be insufficient in patients with CF, in whom other phenomena also contribute to fat malabsorption (e.g., impaired lipolysis because of pancreatic insufficiency) or where more overt fat malabsorption is present. This concept is especially interesting, because AB treatment has been shown to increase fat absorption by ∼20% in premature (non-CF) infants with overt fat malabsorption (18). Future studies in this area would lead to a better understanding of the exact role of AB in the absorption kinetics and/or absorption of fat.
AB treatment decreased fecal CA excretion in both CF mouse models. Our observation corresponds to the findings of an earlier report in patients with CF. In that study, a 7-day treatment with metronidazole reduced fecal bile salt excretion by ∼30% in three of four CF patients (11). Some studies have suggested that AB may have an inhibitory effect on bile salt synthesis in the liver (19,20); this raises the possibility that the decrease in fecal CA is merely because of a reduced synthesis. Although we found a decrease in the percentage contribution of CA in the bile of AB-treated homozygous ΔF508 mice, this effect was not observed in WT animals. Therefore, we consider it more likely that the decrease in biliary CA is explained by a reduction in the synthesis of CA secondary to increased intestinal absorption. Fecal excretion of orally administered 24-[14C] CA decreased after AB treatment in human patients with CF (21). Our data therefore suggest an improved enterohepatic circulation of CA. Although we did not perform histology on the small intestine, we found a reduced mRNA expression of Muc2, a structural component of the intestinal mucus layer, in agreement with findings of previous studies in CFTR-knockout mice (12). We consider it unlikely that intestinal transit time could explain the observed changes in fecal bile salt excretion. The reduction in fecal excretion of CA in CFTR-knockout mice was similar to the reduction in the homozygous ΔF508 mice, whereas (as previously indicated) AB did not change intestinal transit time in the CFTR-knockout mice (13). We found that the levels of the “classic” secondary bile salts such as deoxycholic acid and ω-muricholic acid were reduced in the AB-treated animals. Of interest, however, the level of hyodeoxycholic acid (formed by 7β-hydroxylation) increased. Given that only certain strains of bacteria are capable of 7β-hydroxylation, it seems reasonable to assume that this shift in secondary bile salt formation is caused by AB-induced changes in bacterial colonization in the colon (8).
In conclusion, we showed that the growth-enhancing effect of AB treatment in CFTR-knockout mice is not attributable to increased fat absorption. AB treatment accelerates fatty acid absorption in both homozygous ΔF508 and WT mice and partially corrects the increased fecal excretion of bile salts in both CF mouse models. The potential therapeutic effect of AB on these parameters needs to be further explored, both in CF and in non-CF conditions.
METHODS
Mice and Diet
CFTRtm1Unc-knockout mice with a C57BL/6 genetic background (n = 17) and WT littermates (n = 17) were reared in an environmentally controlled facility at the University of Kansas Medical Center. From the age of 10 days, the mice were maintained on a complete elemental liquid diet to prevent lethal intestinal obstruction (Peptamen; Nestlé, Deerfield, IL). The liquid diet contained 33% energy derived from fat (23% of medium-chain triglycerides and 10% of long-chain triglycerides). The LCFAs in the diet were composed of: 16.4 mol% palmitic acid, 6.7 mol% stearic acid, 22.2 mol% oleic acid, 43.6 mol% linoleic acid, 4.6 mol% α-linolenic acid, 0.1 mol% arachidonic acid, and 0.06 mol% docosahexaenoic acid. The experiment started when the animals were 3 weeks of age, and was performed as previously described (5). CFTRtm1Eur homozygous ΔF508 mice with an FVB/129 genetic background (n = 8) and WT littermates (n = 8) were reared in an environmentally controlled facility at the Erasmus Medical Center (Rotterdam, the Netherlands). The mice had free access to water and a standard semisynthetic chow diet (Hope Farms, Woerden, the Netherlands). The diet contained 13% energy derived from fat. The LCFA composition was similar to that in the liquid diet. The experiment was performed in mice between 8 and 11 weeks of age. Except for the CFTR-knockout mice in the control-treated group (male, n = 4), both genders were equally represented in the groups after randomization. Except for body weight, no gender-related differences between parameters were observed.
The experimental protocols were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee and by the Ethical Committee for Animal Experiments of the Erasmus Medical Center in Rotterdam. The design was selected to allow comparison of the results with previously published data relating to the two genotypes (5,7).
Experimental Procedures
AB treatment
The CF mice and their respective WT littermates were individually housed. The experimental group received a 3-week (broad-spectrum) AB treatment (ciprofloxacin, 50 mg/kg/day and metronidazole, 100 mg/kg/day), as previously described (5). AB were administered to the liquid diet of the CFTR-knockout mice and their WT littermates. For the homozygous ΔF508 mice and their WT littermates, AB were administered in the drinking water, which was refreshed on a daily basis. Because of the light sensitivity of ciprofloxacin, the drinking bottles were covered with aluminium foil. Prior to the administration of AB, the daily intakes of liquid diet and water per mouse were measured. Thereafter, the dose of AB required per mouse was calculated and added to ensure an equal AB dose, on average, for each of the mice. Addition of AB did not affect the drinking behavior of the mice.
Fat balance study
After 3 weeks of AB treatment, a 72-h fat balance test was performed. During the last 3 days of treatment, the amount of dietary intake was noted and feces collected. Fecal pellets were freeze-dried and mechanically homogenized. Lipids were extracted from the samples, hydrolyzed, and methylated in accordance with the procedure described by Muskiet et al. (22). The resulting fatty acid methyl esters of LCFA were analyzed and quantified by gas chromatography, using heptadecanoic acid as an internal standard. The fat absorption coefficient (%) was calculated by subtracting the fecal fat output (g/day) from the fat intake (g/day), divided by the fat intake (g/day) multiplied by 100%.
Bile salt analysis
The total bile salt concentration was measured in an aliquot of feces (collected for the 3-day fat balance). In the homozygous ΔF508 mice and their WT littermates, the bile salt concentration was measured in gallbladder bile. After deconjugation, bile salt profiles were determined by extracting the bile salts with commercially available Sep-Pak-C18 (Mallinckrodt Baker, Deventer, The Netherlands) cartridges and converting them to their methylester/trimethylsilyl derivatives (23). Bile salt profiles were analyzed using capillary gas chromatography. The hydrophobicity of bile salts in gall-bladder bile was calculated using the Heuman index, corresponding to the ability of bile salts to solubilize lipids (24).
Kinetics of fat absorption
The rate of intestinal uptake of triglycerides and fatty acids labeled with stable isotopes was determined in homozygous ΔF508 mice and their WT littermates. After 3 weeks of AB or control treatment (after finishing the 3-day fecal collection for estimation of fat balance), the mice were intraperitoneally injected with 1 mg/kg poloxamer of 407 to block lipoprotein lipase-dependent lipolysis (25). Subsequently, a 100 μl bolus composed of olive oil (25%) and medium-chain triglyceride oil (75%) was intragastrically administered. This bolus contained 2 mg 13C-labeled tripalmitin and 2 mg 13C-labeled stearate per 30 g body weight. After bolus administration, the mice were deprived of food. Blood samples of ∼75 μl were taken from the lateral tail vein before bolus administration and at 2, 4, and 6 h after bolus administration, using heparin-coated microhematocrit tubes. The mice were then killed by puncturing the heart. Plasma and erythrocytes were separated by centrifugation (2,000 r.p.m., 10 min at 4 °C). The plasma samples were derivatized to their α-bromopentafluorobenzyl esters, and 13C-enrichment of 1-13C-palmitin and 1-13C-stearate was measured by gas chromatography combustion isotope ratio mass spectrometry (Delta S/GC Finnigan MAT, Bremen, Germany). 13C-enrichment in plasma was calculated from the fatty acid concentration and expressed as the percentage of the administered dose/ml of plasma (% dose/ml). Total plasma triglyceride concentrations were determined using commercially available kits (Roche Diagnostics, Mannheim, Germany).
Bacterial load in the small intestine
After the mice were killed, the small intestine was flushed with phosphate-buffered saline containing 10 mmol/l DTT as a mucolytic agent. The eluent was centrifuged at 5,000 r.p.m. for 45 min to pellet the bacteria. Bacterial genomic DNA was isolated using the Qiagen DNA stool kit (Qiagen, Venlo, The Netherlands). Quantitative-PCR with an SYBR Green detection system (Bio-Rad MyIQ; Qiagen) was performed, using both group-specific and kingdom-specific primers (26,27). Kingdom-specific primers for 16S rDNA were used for the amplification of the total bacterial load, whereas group-specific primers were used for the quantification of each bacterial group. Each primer set was evaluated against reference bacterial strains for primer efficiency and specificity (Table 6).
mRNA expression in the small intestine
Total RNA was isolated from the whole small intestine with TriReagent (Sigma, St Louis, MO), washed with an RNA cleanup kit (RNeasy MinElute Cleanup kit; Qiagen) and quantified using a NanoDrop ND100 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Relative mRNA expression for mucin 2 was determined using 5 μl cDNA. Primers for mucin 2 were kindly provided by I. Renes, and PCR was carried out as previously described (28). PCR products were loaded on a 1% agarose gel and electrophoresed in a 1× tris-acetate-EDTA (TAE) buffer in the presence of ethidium bromide. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS, Chicago, IL). We calculated, with an anticipated coefficient of variation of 10%, a required sample size of four per group to demonstrate a 30% decrease in fat malabsorption as compared to WT animals. Differences between experimental and control groups were calculated using the Mann–Whitney test. Data are reported as mean values ± SD (SEM in figures) or as median (range) where the data were not normally distributed. P values <0.05 were considered statistically significant. To exclude possible gender-related differences in treatment effect, we performed an additional multiple-regression analysis. Parameters for fat absorption, fecal bile salt excretion, and weight were included as dependent variables, whereas genotype, treatment, gender, and the interaction variable gender × treatment were included as independent variables.
Acknowledgments
We thank H. Jorna, T. Boer, I. Martini, R. Boverhof, and S.C. Beijer-Liefers for their technical assistance.
STATEMENT OF FINANCIAL SUPPORT This work was supported by National Institutes of Health grant R21 AI083479 and a pilot project as part of NIH grant P20 RR024214 (to R.C.D.).
References
- 1.Sinaasappel M, Stern M, Littlewood J, et al. Nutrition in patients with cystic fibrosis: a European Consensus. J Cyst Fibros. 2002;1:51–75. doi: 10.1016/s1569-1993(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 2.Borowitz D, Durie PR, Clarke LL, et al. Gastrointestinal outcomes and confounders in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2005;41:273–85. doi: 10.1097/01.mpg.0000178439.64675.8d. [DOI] [PubMed] [Google Scholar]
- 3.Saiman L, Anstead M, Mayer-Hamblett N, et al. AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2010;303:1707–15. doi: 10.1001/jama.2010.563. [DOI] [PubMed] [Google Scholar]
- 4.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Macrolide Study Group. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 5.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004;72:6040–9. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rana SV, Bhardwaj SB. Small intestinal bacterial overgrowth. Scand J Gastroenterol. 2008;43:1030–7. doi: 10.1080/00365520801947074. [DOI] [PubMed] [Google Scholar]
- 7.Bijvelds MJ, Bronsveld I, Havinga R, Sinaasappel M, de Jonge HR, Verkade HJ. Fat absorption in cystic fibrosis mice is impeded by defective lipolysis and post-lipolytic events. Am J Physiol Gastrointest Liver Physiol. 2005;288:G646–53. doi: 10.1152/ajpgi.00295.2004. [DOI] [PubMed] [Google Scholar]
- 8.Martin FP, Dumas ME, Wang Y, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage DC, Dubos R. Alterations in the mouse cecum and its flora produced by antibacterial drugs. J Exp Med. 1968;128:97–110. doi: 10.1084/jem.128.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strandvik B, Einarsson K, Lindblad A, Angelin B. Bile acid kinetics and biliary lipid composition in cystic fibrosis. J Hepatol. 1996;25:43–8. doi: 10.1016/s0168-8278(96)80326-6. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien S, Mulcahy H, Fenlon H, et al. Intestinal bile acid malabsorption in cystic fibrosis. Gut. 1993;34:1137–41. doi: 10.1136/gut.34.8.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lisle RC, Roach EA, Norkina O. Eradication of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J Pediatr Gastroenterol Nutr. 2006;42:46–52. doi: 10.1097/01.mpg.0000189322.34582.3e. [DOI] [PubMed] [Google Scholar]
- 13.De Lisle RC. Altered transit and bacterial overgrowth in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G104–11. doi: 10.1152/ajpgi.00548.2006. [DOI] [PubMed] [Google Scholar]
- 14.Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1032–41. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- 15.Durie PR, Newth CJ, Forstner GG, Gall DG. Malabsorption of medium-chain triglycerides in infants with cystic fibrosis: correction with pancreatic enzyme supplement. J Pediatr. 1980;96:862–4. doi: 10.1016/s0022-3476(80)80559-2. [DOI] [PubMed] [Google Scholar]
- 16.de Somer P, Eyssen H, Evrard E. The influence of antibiotics on fecal fat in chicks. In: Frazer AC, editor. Biochemical Problems of Lipids. Amsterdam: Elsevier; 1963. pp. 84–90. [Google Scholar]
- 17.Gonzalez J, Fernandez C, Marino E, Morales A, Jimenez R. Biliary excretion and choleretic effect of cefmetazole in rats. Antimicrob Agents Chemother. 1989;33:1970–4. doi: 10.1128/aac.33.11.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkade HJ, van Asselt WA, Vonk RJ, et al. Fat absorption in premature infants: the effect of lard and antibiotics. Eur J Pediatr. 1989;149:126–9. doi: 10.1007/BF01995863. [DOI] [PubMed] [Google Scholar]
- 19.Le Dafniet M, Pessayre D, Le Quernec L, Erlinger S. Effects of troleandomycin administration on cholesterol 7 alpha-hydroxylase activity and bile secretion in rats. J Pharmacol Exp Ther. 1981;219:558–62. [PubMed] [Google Scholar]
- 20.Toda T, Ohi K, Kudo T, et al. Ciprofloxacin suppresses Cyp3a in mouse liver by reducing lithocholic acid-producing intestinal flora. Drug Metab Pharmacokinet. 2009;24:201–8. doi: 10.2133/dmpk.24.201. [DOI] [PubMed] [Google Scholar]
- 21.Eklund A, Norman A, Strandvik B. Excretion of bile acids in healthy children and children with cystic fibrosis. Scand J Clin Lab Invest. 1980;40:595–608. doi: 10.3109/00365518009091970. [DOI] [PubMed] [Google Scholar]
- 22.Muskiet FA, van Doormaal JJ, Martini IA, Wolthers BG, van der Slik W. Capillary gas chromatographic profiling of total long-chain fatty acids and cholesterol in biological materials. J Chromatogr. 1983;278:231–44. doi: 10.1016/s0378-4347(00)84782-9. [DOI] [PubMed] [Google Scholar]
- 23.Setchell KD, Worthington J. A rapid method for the quantitative extraction of bile acids and their conjugates from serum using commercially available reverse-phase octadecylsilane bonded silica cartridges. Clin Chim Acta. 1982;125:135–44. doi: 10.1016/0009-8981(82)90190-5. [DOI] [PubMed] [Google Scholar]
- 24.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–30. [PubMed] [Google Scholar]
- 25.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005;46:2023–8. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Barman M, Unold D, Shifley K, et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–15. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salzman NH, de Jong H, Paterson Y, Harmsen HJ, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology. 2002;148(Pt 11):3651–60. doi: 10.1099/00221287-148-11-3651. [DOI] [PubMed] [Google Scholar]
- 28.van der Sluis M, Melis MH, Jonckheere N, et al. The murine Muc2 mucin gene is transcriptionally regulated by the zinc-finger GATA-4 transcription factor in intestinal cells. Biochem Biophys Res Commun. 2004;325:952–60. doi: 10.1016/j.bbrc.2004.10.108. [DOI] [PubMed] [Google Scholar]