Abstract
Foxp3+ regulatory T (Treg) cells include thymic-derived natural Treg and conventional T-derived adaptive Treg cells. Both are proposed to play important roles in downregulating inflammatory immune responses. However, the mechanisms of Treg expansion in inflammatory environments remain unclear. In this study, we report that, in an autoimmune-like graft-versus-host disease model of DBA/2 (H-2d) donor to BALB/c (H-2d) recipients, donor Treg cells in the recipients predominantly originated from expansion of natural Treg cells and few originated from adaptive Treg cells. In vivo neutralization of IFN-γ resulted in a marked reduction of donor natural Treg expansion and exacerbation of graft-versus-host disease, which was associated with downregulation of host APC expression of B7H1. Furthermore, host APC expression of B7H1 was shown to augment donor Treg survival and expansion. Finally, donor Treg interactions with host APCs via B7.1/B7H1 but not PD-1/B7H1 were demonstrated to be critical in augmenting donor Treg survival and expansion. These studies have revealed a new immune regulation loop consisting of T cell-derived IFN-γ, B7H1 expression by APCs, and B7.1 expression by Treg cells.
Graft-versus-host disease (GVHD) remains a major obstacle for allogeneic hematopoietic cell transplantation (HCT) (1, 2). GVHD is considered an exaggerated and undesirable manifestation of an inflammatory response, in which donor lymphocytes encounter foreign Ags in an environment that fosters inflammation (2). Many reports have showed that the exaggerated immune response in GVHD can be prevented by infusion of donor Foxp3+ regulatory T (Treg) cells (3–9). We recently observed that, in a GVHD model of DBA/2 (H-2d) donor to MHC-matched but minor Ag-mismatched BALB/c (H-2d) recipient, the percentage of donor Treg cells was inversely associated with GVHD severity (9, 10). Although host APCs were shown to initiate the activation and expansion of pathogenic alloreactive donor T cells (11, 12), the role of host APCs in donor Treg cell activation and expansion is still largely unknown.
Foxp3+ Treg cells can be divided into natural and adaptive subsets (13). Natural Treg cells develop and mature in the thymus whereas adaptive Treg cells are converted from conventional Foxp3− T cells in the periphery during the T cell activation process under the influence of TGF-β, retinoic acid, and other factors (14–16). Conventional CD4+ T cells can be easily converted into adaptive Treg cells in vitro (17, 18), but in vivo Treg cell conversion appeared to be more difficult (19), although Treg cell conversion can take place in some in vivo circumstances (20). Additionally, the origin of donor-type Treg cells in allogeneic HCT recipients is still unclear.
IFN-γ from alloreactive T cells plays an important role in GVHD pathogenesis (21, 22); however, IFN-γ also regulates GVHD by directly inducing apoptosis of the pathogenic T cells and upregulating tissue expression of the costimulatory molecule B7H1 (also known as PD-L1) that could tolerize the activated T cells (23, 24). B7H1 is constitutively expressed by APCs (i.e., dendritic cells [DCs]) at a low level but not constitutively expressed by parenchymal cells (25). IFN-γ is identified as a major proinflammatory cytokine in upregulation of B7H1 on APCs and parenchymal cells (24, 26). B7H1 is shown to bind two receptors, PD-1 and B7.1 (27). PD-1 is expressed by activated T cells, and extensive studies demonstrate that B7H1/PD-1 interactions result in T cell suppression, anergy, and apoptosis (28). B7.1 is expressed by APCs and activated T cells. Although our recent studies indicate that T cell and APC interaction via B7H1/B7.1 contributes to induction and maintenance of conventional T cell anergy specific to orally administered Ag (29), the impact of B7.1 and B7H1 interaction on Treg cells remains unclear.
The tissue expression of B7H1 was reported to be associated with a high frequency of Treg cells in tumor and autoimmune environments (30). The expression of B7H1 by tumor cells was implicated to augment Treg cell conversion in the tumor microenvironment (31). Expression of low-level B7H1 in normal tissues was also reported to play an important role in Treg conversion in non-disease mice (20). In light of the findings that IFN-γ is the most potent cytokine found so far in the upregulation of B7H1 (25), we hypothesize that IFN-γ may augment Treg conversion via upregulation of tissue expression of B7H1. Surprisingly, we found that, in our GVHD model, 1) the Treg cells in the allogeneic HCT recipients were predominantly from the expansion of natural Treg cells, and there were few cells originating from conventional T cells; 2) IFN-γ upregulated recipient APC expression of B7H1 and subsequently augmented donor natural Treg cell expansion; and 3) host APC and donor Treg interactions via B7H1/B7.1 play an important role in augmenting the Treg cell expansion.
Materials and Methods
Mice
Wild-type (WT) C57BL/6 (H-2b), BALB/c (H-2d), and DBA/2 mice were purchased from National Cancer Institute Laboratories (Frederick, MD). Rag2−/− BALB/c and Rag2−/− C57BL/6 were purchased from Taconic. B7.1−/− C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B7H1−/− BALB/c mice were established as previously described (32), and B7H1−/−Rag2−/− BALB/c were generated through backcrossing B7H1−/− to Rag2−/− mice. Foxp3gfp/KI DBA/2 mice were generated by backcrossing Foxp3gfp.KI C57BL/6 (33) to WT DBA/2 mice for eight generations. Mice were maintained in a pathogen-free room at the City of Hope Research Animal Facilities (Duarte, CA). All animal protocols were approved by the City of Hope Research Animal Care Committee.
Induction and assessment of GVHD
Bone marrow and spleen cell harvest and donor CD4+ T purification as well as HCT procedures were described in our previous publications (9, 10, 34). In brief, recipient mice were given 800 rad total body irradiation (TBI) from a [137Cs] source and then injected with spleen cells (~50 × 106) and bone marrow (BM) cells (2.5 × 106) from DBA/2 donor mice. Thereafter, recipient mice were monitored for clinical GVHD, proteinuria, and survival. The experimental and control groups were set up at the same time and experiments were replicated two to three times. The assessment and scoring of cutaneous clinical GVHD were evaluated based on the area of alopecia as follows: 1, skin ulcers with alopecia <1 cm2 in area; 2, skin ulcer with alopecia 1–3 cm2; 3, skin ulcer with alopecia >10% body area; and 4, skin ulcer with alopecia >30% body area.
mAbs and flow cytometric analysis as well as in vivo neutralizing of cytokines
Anti–IFN-γ (R4-6A2) was obtained from the American Type Culture Collection and purified from culture supernatant using protein G columns as described in our previous publications (9). Recipients were i.v. injected with anti–IFN-γ or rat IgG (0.5 mg/ml) daily after HCT until they became moribund or developed severe ascites. For anti–PD-1 and anti-B7H1 (43H12) blockade, recipients were neutralized with anti–PD-1, anti-B7H1 (43H12), or hamster IgG (0.5 mg/mice) every day after HCT until they became moribund or developed ascites. Control hamster IgG and rat IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Measurement of autoantibodies and cytokine in serum
Anti-dsDNA IgG was measured with ELISA as previously described (9). Anti-dsDNA titers are expressed in units per milliliter, using a reference-positive standard of pooled serum from 6- to 7-mo-old NZB/W mice. A 1:100 dilution of this standard serum was arbitrarily assigned a value of 100 U/ml.
Cytokine concentration of IL-6 serum was determined with the DuoSet ELISA development kit (R&D Systems, Minneapolis, MN).
In vivo BrdU labeling
HCT recipients were injected i.p. with BrdU (200 μg/g) twice during a 48-h period, followed by tissue collection and analysis of T cells for BrdU incorporation using BrdU flow kit protocol (BD Pharmingen).
mAbs and flow cytometric analysis
Abs to mouse CD4, CD8, TCR, CD5.1, CD11c, IL-17, IFN-γ, PD-1, B7H1, anti-BrdU, and Foxp3 were purchased from BD Biosciences (San Diego, CA) or eBioscience (San Diego, CA). For intracellular staining, cells were stimulated with plate-bound CD3/CD28 for 5 h, and brefeldin A (Sigma-Aldrich) (10 μg/ml) was added in the last 2 h. Cells were then harvested and stained for cytokines. Dead cells were excluded by a LIVE/DEAD Fixable Aqua Dead Cell Stain kit (Invitrogen, Carlsbad, CA).
Cell isolation from GVHD target organs
Mononuclear cells from liver and skin were processed and collected as previously described (24). Isolation of hepatocytes was performed as previously described (35).
Real-time PCR
Real-time PCR of Ebi3 and Il12α as well as Bcl-xL were performed as described in our previous publication (36). We used Ebi3 and Il12α primers described in a recent publication by Collison et al. (37). The Bcl-xL probe was purchased from SABiosciences (Qiagen).
Statistical analysis
Body weight and survival in different groups were compared using the log-rank test (Prism version 5.0; GraphPad Software, San Diego, CA). Comparison of two means was analyzed using an unpaired two-tail Student t test. Comparison of frequencies was analyzed by a χ2 test.
Results
Exacerbation of GVHD by neutralization of IFN-γ is associated with marked reduction of natural Treg cells in vivo
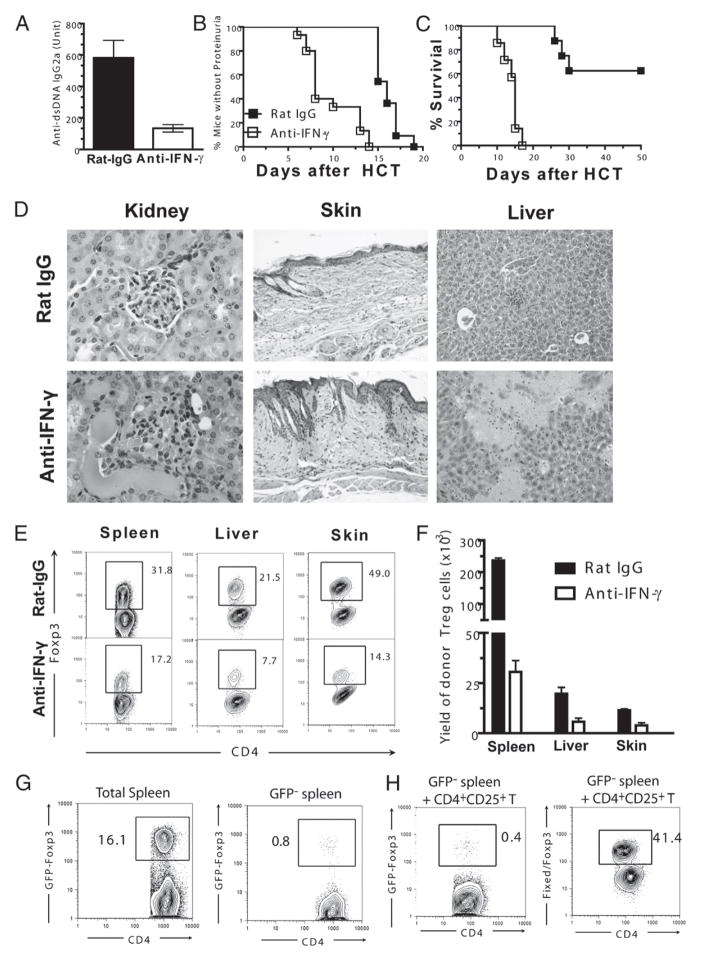
We have previously reported that, in an autoimmune-like GVHD model of DBA/2 donor into BALB/c recipient, disease development was associated with high levels of serum anti-dsDNA IgG2a auto-antibodies, glomerulonephritis, and proteinurea (9, 10). IFN-γ was shown to play a key role in augmenting B cell generation of pathogenic IgG2a autoantibodies (38). Thus, we tested whether neutralizing IFN-γ could ameliorate glomerulonephritis and proteinurea in the GVHD recipients. We observed that anti–IFN-γ neutralization reduced serum anti-dsDNA IgG2a by ~4-fold as compared with the rat IgG control (p <0.01, Fig. 1A). Interestingly, the reduction of serum autoantibodies was associated with an accelerated onset of proteinurea. Whereas all (16 of 16) recipients treated with anti–IFN-γ developed proteinurea by day 13 after HCT, none of the recipients treated with rat IgG developed proteinurea by this time point (p < 0.01, Fig. 1B). Additionally, whereas all of the former recipients died of GVHD within 20 d posttransplantation, >50% of the latter survived for >50 d (p < 0.01, Fig. 1C).
FIGURE 1.
Exacerbation of chronic GVHD by neutralization of IFN-γ is associated with a markedly reduced natural Treg cell expansion. TBI-conditioned BALB/c recipients were transplanted with DBA/2 splenocytes (50 × 106) and treated with anti–IFN-γ or rat IgG control. A, Mean (±SE) of serum levels of anti-dsDNA IgG2a 12 d after HCT (n = 8). B and C, Percentage of mice without proteinurea and percentage survival after HCT (n ≥ 12). D, One of four representative H&E staining images of kidney, skin, and liver sections of recipients 13–15 d after HCT (original magnification ×20). E and F, Tissue mononuclear cells from recipients were stained for CD5.1 (donor marker), CD4, and Foxp3 8 d after HCT. A representative flow cytometry pattern of CD4 versus Foxp3 of gated CD5.1+CD4+ T cells and mean (±SE) of the yield of Foxp3+CD4+CD5.1+ cells are shown (n ≥ 4). G, TBI-conditioned BALB/c recipients were transplanted with either whole splenocytes or GFP+ Treg cell-depleted splenocytes from Foxp3gfp.KI DBA/2 mice. Splenocytes from recipients were stained for CD4 and TCRβ 8 d after HCT. Gated CD4+TCRβ+ cells are shown as CD4 versus GFP. One representative of three replicated experiments is shown. H, TBI-conditioned BALB/c recipients were transplanted with CD25hiCD4+ natural Treg cells (0.5 × 106) and T cell-depleted splenocytes (20 × 106) from WT DBA/2 and GFP−CD4+ T cells (2 × 106) from Foxp3gfp.KI DBA/2. Eight days after HCT, splenocytes from recipients were stained for CD4, TCRβ, or CD5.1, with or without fixed Foxp3 staining. Left panel, Gated CD4+TCRβ+ cells are shown as CD4 versus GFP. Right panel, Gated CD4+CD5.1+ cells are shown as CD4 versus Foxp3. One representative of three replicated experiments is shown.
Additionally, we compared the histopathologies of the recipients. We observed more severe tissue damage in the kidney, skin, and liver of the recipients treated with the anti–IFN-γ as compared with recipients given the rat IgG control (Fig. 1D), which was consistent with the clinical GVHD exacerbation in recipients treated with anti–IFN-γ (Fig. 1B, 1C). These results indicate that IFN-γ neutralization, while reducing autoantibody production, augments GVHD tissue damage in GVHD recipients.
We have reported that the presence of a high percentage of in vivo activated donor-type CD4+Foxp3+ Treg cells in autoimmune-like GVHD recipients has a profound effect in ameliorating GVHD tissue damage (9). Thus, we next compared the percentage and yield of the Treg cells in the GVHD recipients treated with anti–IFN-γ or rat IgG control. We found that in the recipients treated with anti–IFN-γ the percentage and yield of Foxp3+ Treg cells in spleen, liver, and skin was reduced 2- to 4-fold as compared with control recipients given rat IgG (p < 0.01, Fig. 1E, 1F). Taken together, these results indicate that neutralization of IFN-γ results in a marked reduction of donor Treg cells in autoimmune-like GVHD recipients.
Treg cells in GVHD recipients can be either expanded from natural Treg cells or converted from conventional T cells, as described in some autoimmune disease models (13). To characterize whether IFN-γ augments expansion of donor natural Treg cells or conversion of Foxp3− conventional T cells into Treg cells, we first compared the percentage of GFP+ Treg cells in recipients transplanted with either whole or GFP+ Treg cell-depleted (GFP−) splenocytes from Foxp3gfp.KI DBA/2 mice. We found that after HCT the percentage of GFP+ Treg cells among CD4+ T cells in recipient given whole splenocytes was >20-fold higher than that in recipients given GFP− splenocytes (Fig. 1G). This result indicates that few Treg cells are from conversion of conventional T cells in GVHD recipients in the absence of natural Treg cells. To exclude the possibility that natural Treg cells may exert a critical role in influencing the in vivo Treg conversion, we further used a mixed transplantation system by transferring GFP−CD4+ T cells from Foxp3gfp.KI DBA/2 donor mice combined with sorted CD4+ CD25+ T cells and T cell-depleted spleen cells from WT DBA/2 donor cells into TBI-conditioned BALB/c recipients. Similarly, there was only a small percentage (0.4–0.6%) of GFP+ Treg cells, whereas Foxp3+ Treg cells constituted >30% of total donor CD4+ T cells in the recipients (Fig. 1H). Taken together, these results indicate that in vivo Treg cells in allogeneic HCT recipients are predominantly expanded from natural Treg cells but not converted from conventional T cells, and IFN-γ plays an important role in natural Treg cell expansion in HCT recipients.
Reduction of natural Treg cell expansion in autoimmune-like GVHD recipients by neutralization of IFN-γ is associated with downregulation of recipient tissue expression of B7H1
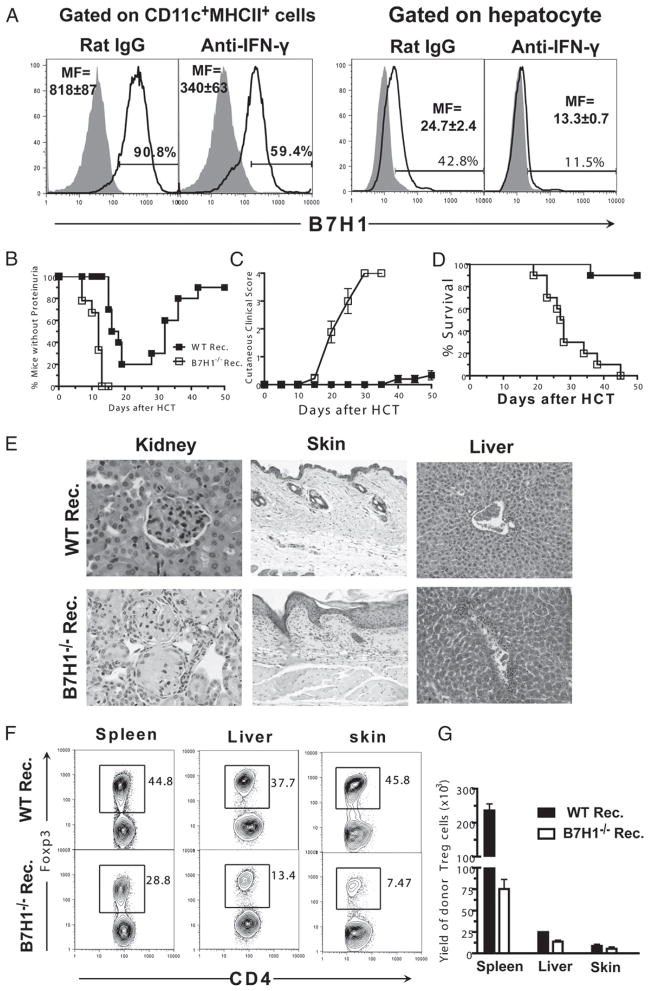
We next sought to determine how IFN-γ regulates Treg cell expansion in the autoimmune-like GVHD recipients. Because tissue expression of B7H1 was reported to be associated with a high frequency of Treg cells in tumor environments (30), we tested whether IFN-γ promoted Treg cell expansion by augmenting tissue cell expression of B7H1 in the GVHD recipients. Accordingly, 8 d after HCT, when the recipients were still at mixed chimeric stage as indicated by the coexistence of donor- and host-type T cells (Supplemental Fig. 1), B7H1 expression on hematopoietic cells (i.e., DCs) and parenchymal cells (i.e., hepatocytes) in chronic GVHD recipients treated with anti–IFN-γ or isotype control was analyzed. We observed a significant reduction of B7H1 expression level on both DCs and hepatocytes in recipients treated with anti–IFN-γ as compared with control recipients (p < 0.01, Fig. 2A).
FIGURE 2.
Reduction of natural Treg cell expansion in chronic GVHD recipients by neutralization of IFN-γ is associated with downregulation of recipient tissue expression of B7H1. A, TBI-conditioned BALB/c recipients were transplanted with DBA/2 splenocytes (50 × 106) and treated with anti–IFN-γ or rat IgG control. Eight days after HCT, enriched CD11c+ DCs or purified hepatocytes from recipients were stained for B7H1 or isotype control. The filled gray area represents isotype control, and the solid black line represents B7H1 staining. Representative flow cytometry patterns and mean (±SE) of mean fluorescence of B7H1 staining are shown (n = 4). B–G, TBI-conditioned WT or B7H1−/− BALB/c recipients were transplanted with DBA/2 splenocytes (50 × 106). B–D, Percentage of mice without proteinurea, mean (±SE) of cutaneous clinical scores, and percentage survival after HCT (n ≥ 12). E, One of four representative H&E staining images of kidney, skin, and liver sections of recipients 20–25 d after HCT (original magnification ×20). F and G, Tissue mononuclear cells from recipients were stained for CD5.1, CD4, and Foxp3 8 d after HCT. A representative flow cytometry pattern shown for gated CD5.1+CD4+ T cells as CD4 versus Foxp3 and mean (±SE) of the yield of Foxp3+CD4+CD5.1+ cells is shown (n = 4).
To test the role of B7H1 expressed by donor- and host-type DCs as well as host tissues in donor Treg cell expansion, we next compared Treg cell expansion and GVHD severity in B7H1−/− or WT BALB/c recipients. We found that, as compared with WT BALB/c recipients, B7H1−/− recipients developed markedly more severe GVHD with accelerated proteinurea, augmented cutaneous tissue damage, and increased mortality (p < 0.01, Fig. 2B–D). The augmented clinical signs of GVHD in B7H1−/− recipients were associated with elevated tissue damage in GVHD target organs, such as kidney, skin, and liver (Fig. 2E). Similar to recipients treated with anti–IFN-γ, the percentage and yield of Foxp3+ Treg cells in spleen, liver, and skin in B7H1−/− recipients were reduced by 2- to 4-fold as compared with that in WT recipients (p < 0.01, Fig. 2F, 2G). Additionally, the percentage of the converted Treg cells remained very small and was similar in WT and B7H1−/− recipients (Supplemental Fig. 2). Furthermore, neutralization of IFN-γ in B7H1−/− recipients did not result in further changes in Treg percentage (Supplemental Fig. 3).
We also tested whether host-tissue cell expression of B7H1 affected donor Treg cell suppressor function or their expression of IL-35, because it was recently reported that IL-35 specifically secreted by Treg cells is important not only for mediating Treg cell suppressor function, but also for converting conventional T cells into non–Foxp3-expressing suppressor cells (37, 39). Accordingly, sorted GFP+ Treg cells from WT or B7H1−/− recipients were added to a MLR culture for comparison of their suppressor function or measured for their expression of IL-35 components of Ebi3 and Il12α. We found that Treg cells from both recipients exerted strong suppression activity and expressed high levels of Ebi3 and Il12α, and there was no significant difference between the two groups of Treg cells (Supplemental Fig. 4). Taken together, these results indicate that IFN-γ upregulation of B7H1 expression on recipient tissue cells augments the expansion of donor Treg cells and ameliorates GVHD in the recipients.
Recipient APC expression of B7H1 is critical for donor natural Treg cell expansion
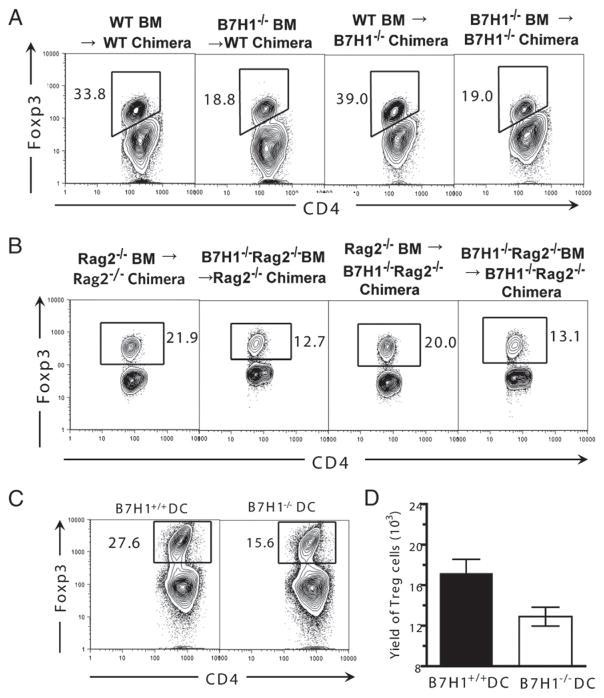
Because B7H1 can be expressed on both hematopoietic cells and parenchymal cells, we next determined which tissue cell expression of B7H1 was critical for donor Treg cell expansion in vivo. Accordingly, we set up different chimeric recipients with B7H1 expression on either hematopoietic cells or parenchyma cells by transplanting WT or B7H1−/− BM cells into TBI-conditioned WT or B7H1−/− recipients. Eight weeks later, the chimeras were again given TBI conditioning and transplanted with donor DBA/2 spleen cells. We found that the percentage of donor Treg cells in (B7H1−/− BM→WT) and (B7H1−/− BM→B7H1−/−) chimeras that had B7H1 deficiency on host-type hematopoietic cells was reduced ~2-fold, as compared with (WT BM→WT) and (WT BM→B7H1−/−) chimeras that had normal B7H1 expression on hematopoietic cells (Fig. 3A). These results indicate that lack of B7H1 expression on host-type hematopoietic cells but not parenchymal cells led to a reduced donor Treg cell expansion.
FIGURE 3.
Recipient APC expression of B7H1 is critical for donor natural Treg cell expansion. A and B, TBI-conditioned WT and B7H1−/− chimera recipients or Rag2−/− or B7H1−/−Rag2−/− chimera recipients were transplanted with DBA/2 splenocytes (50 × 106). Eight days after HCT, splenocytes from recipients were stained for CD4, CD5.1, and Foxp3. Gated CD4+CD5.1+ cells are shown as CD4 versus Foxp3. One representative of three replicated experiments is shown. C and D, CD4+ T cells (2 × 105) from GVHD recipients harvested 13 d after HCT were cocultured with irradiated CD11c+ DCs (1 × 105) from WT or B7H1−/− BALB/c spleen. Five days after culture, cells were stained for CD4 and Foxp3. Gated CD4+ T cells shown as CD4 versus Foxp3 and mean (±SE) of the yield of CD4+Foxp3+ cells in the culture are shown (n = 4).
Other than DCs, T and B cells have also been reported to express B7H1 (25). To further determine the role of recipient APC expression of B7H1 in regulating donor Treg cell expansion, we further backcrossed B7H1−/− mice to Rag2−/− mice to generate B7H1−/−Rag2−/− mice, and we then set up chimeras by transferring Rag2−/− BM or B7H1−/−Rag2−/− BM into TBI-conditioned Rag2−/− or B7H1−/−Rag2−/− recipients. Similarly, we found that the lack of host-type hematopoietic cell expression of B7H1 in T/B cell-deficient Rag2−/− recipients resulted in the reduction of the percentage of donor Treg cells ~2-fold (Fig. 3B). Furthermore, the in vivo role of recipient APC expression of B7H1 on Treg cell expansion was recapitulated in an in vitro system wherein enriched WT or B7H1−/− DCs from host-type BALB/c mice were cultured with donor-type CD4+ T cells from GVHD recipients. We found that the percentage and yield of donor Treg cells were reduced by ~2-fold when B7H1−/− DCs were used in the culture (p < 0.01, Fig. 3C, 3D). Taken together, these results demonstrate that the recipient APC expression of B7H1 augments donor Treg cell expansion in GVHD recipients.
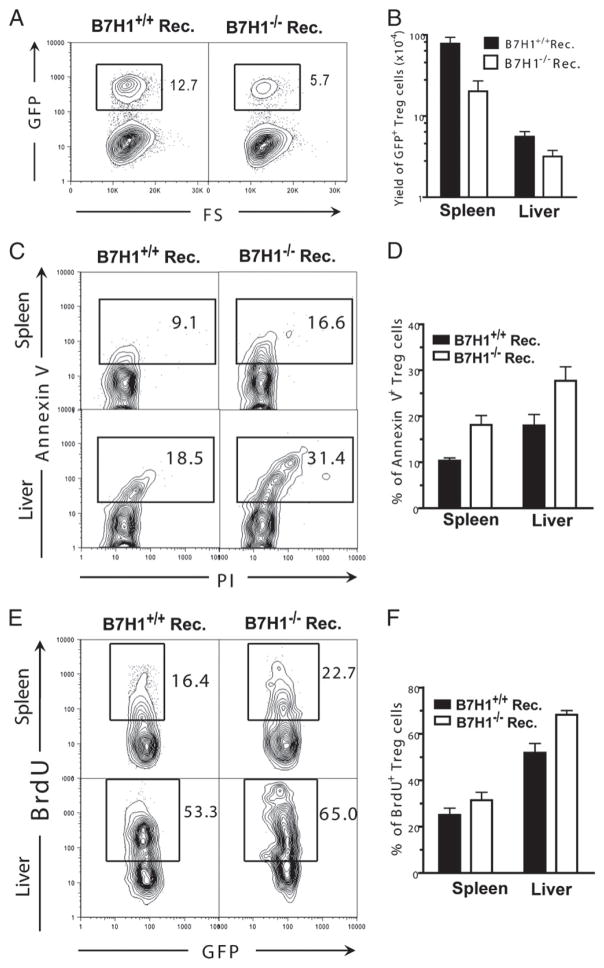
Recipient APC expression of B7H1 is critical for donor Treg survival but not proliferation
The reduced frequency and yield of Treg cells in the recipients with B7H1-deficient APCs could result from either increased death or reduced proliferation of the donor Treg cells. We next determined whether the lack of recipient B7H1 affected donor Treg cell apoptosis or proliferation. To enable isolation of the live Treg cells to measure apoptosis, we transplanted Foxp3gfp.KI DBA/2 spleen cells into TBI-conditioned B7H1−/− or WT BALB/c recipients. Consistent with results obtained from fixed Foxp3 staining (Fig. 2F, 2G), we found that the percentage and the yield of GFP+CD4+ Treg cells was reduced significantly in B7H1−/− recipients as compared with WT controls (p < 0.01, Fig. 4A, 4B). We also observed a significantly increased percentage of annexin V+ cells among freshly isolated GFP+ Treg cells in B7H1−/− recipients as compared with WT controls (p < 0.01, Fig. 4C, 4D), indicating that the lack of host tissue expression of B7H1 leads to augmented donor Treg cell apoptosis. Additionally, BrdU labeling was used to measure proliferation and turnover rate of Treg cells in vivo. We found that the percentage of BrdU+ cells among total GFP+ Treg cells in B7H1−/− recipients was slightly increased compared with WT recipients (Fig. 4E, 4F), indicating an increase in the turnover rate of GFP+ Treg cells in B7H1−/− recipients. The increased turnover rate of Treg cells in B7H1−/− recipients is most likely due to the increased apoptosis of Treg cells. Taken together, lack of host tissue expression of B7H1 augments donor Treg cell apoptosis but does not reduce Treg cell proliferation.
FIGURE 4.
Recipient APC expression of B7H1 is critical for donor Treg cell survival but not proliferation. TBI-conditioned WT or B7H1−/− recipients were transplanted with splenocytes (25 × 106) from Foxp3gfp.KI DBA/2 mice. Eight days after HCT, mononuclear cells from the spleen or liver of the recipients were stained for CD4, annexin V, propidium iodide, or BrdU. A and B, A representative flow cytometry staining pattern of gated CD4+ T cells shown as GFP versus forward scatter (FS) and mean (±SE) of the yield of GFP+CD4+ cells in the spleen and liver (n = 4). C and D, A representative flow cytometry staining pattern of gated CD4+ GFP+ T cells shown as annexin V versus propidium iodide and mean (±SE) of percentage of annexin+ GFP+CD4+ Treg cells in the spleen or liver (n = 6). E and F, A representative flow cytometry staining pattern of gated CD4+GFP+ T cells shown as BrdU versus GFP and mean (±SE) of percentage of BrdU+ CD4+GFP+ Treg cells in the spleen or liver (n = 4).
B7H1/B7.1 but not B7H1/PD-1 interactions promote Treg cell expansion in autoimmune-like GVHD recipients
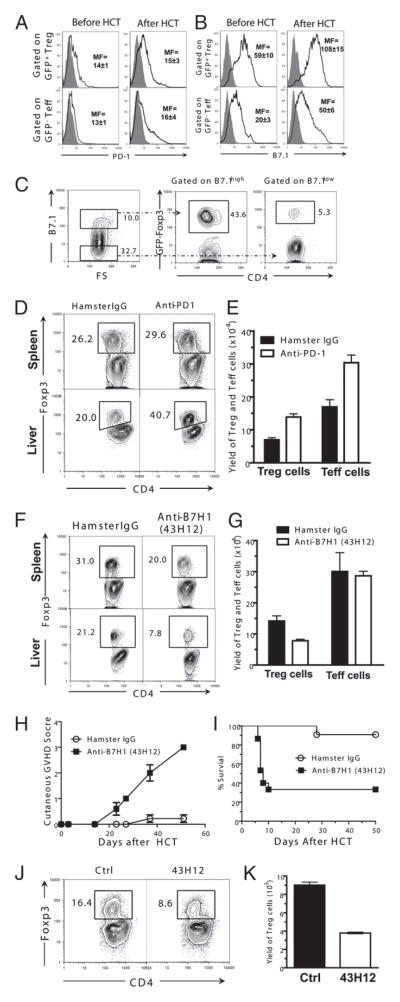
It has been reported that B7H1 can interact with both PD-1 and B7.1 (27). We next investigated which pathway was required for Treg cell expansion: B7H1/PD-1, B7H1/B7.1, or both. First, we compared the expression levels of B7.1 and PD-1 on donor CD4+ Treg and T effector (Teff) cells before and after HCT. We found that donor Treg and Teff cells expressed low levels of PD-1 before HCT, and they both upregulated the expression levels after HCT, but there was no significant difference in the expression levels between Treg and Teff cells (Fig. 5A). In contrast, Treg cells constitutively express high levels of B7.1 as compared with Teff cells (p < 0.01, Fig. 5B). After HCT, both Treg and Teff cells upregulated B7.1 expression, but the expression levels on Treg cells were still significantly higher than on Teff cells (p < 0.01, Fig. 5B). Interestingly, we found that ~50% of B7.1hi donor CD4+ T cells before HCT were Foxp3+ Treg cells, but only ~5% of the B7.1low T cells were Treg cells (Fig. 5C). These results indicate that Treg cells constitutively express higher level of B7.1 as compared with Teff cells.
FIGURE 5.
B7H1/B7.1 but not B7H1/PD-1 interactions promote Treg cell expansion in chronic GVHD recipients. A–C, Spleen cells from Foxp3gfp.KI DBA/2 mice or GVHD recipients 3 d after HCT were stained for CD4, PD-1, B7.1, or isotype control. Gated CD4+GFP+ cells or CD4+GFP− cells are shown as isotype control, PD-1, or B7.1 staining. The filled gray area represents isotype control, and the solid black line represents B7H1 or PD-1 staining. Mean (±SE) of mean fluorescence of B7H1or PD-1 staining is shown (n = 4). Gated CD4+B7.1hi or CD4+B7.1low cells are shown as Foxp3 versus CD4. D and E, TBI-conditioned BALB/c recipients were transplanted with DBA/2 splenocytes (50 × 106) and treated with anti–PD-1 or hamster IgG control. Mononuclear cells from spleen or liver of recipients were stained for CD4, CD5.1, and Foxp3 8 d after HCT. Gated CD4+CD5.1+ are shown as Foxp3 versus CD4. Mean (±SE) of CD4+CD5.1+Foxp3+ Treg cells and CD4+CD5.1+Foxp3− conventional T cells in the spleen are shown (n = 4). F–I, TBI-conditioned BALB/c recipients were transplanted with DBA/2 splenocytes (50 × 106) and treated with anti-B7H1 (43H12) or hamster IgG control. Mononuclear cells from the spleen or liver of recipients were stained for CD4, CD5.1, and Foxp3 8 d after HCT. Gated CD4+CD5.1+ are shown as Foxp3 versus CD4. Mean (±SE) of CD4+CD5.1+Foxp3+ Treg cells and CD4+CD5.1+Foxp3− Teff cells in the spleen (n = 4) is shown. H and I, Mean (±SE) of cutaneous clinical scores and percentage survival of recipients after HCT (n = 12). J and K, CD4+ T cells (2 × 105) from GVHD recipients 13 d after HCT were cocultured with irradiated CD11c+ DCs (1 × 105) purified from BALB/c spleen with or without anti-B7H1 (43H12). Five days after HCT, cultured cells were stained with CD4 and Foxp3. J, Gated CD4+ T cells are shown as CD4 versus Foxp3; K, mean (±SE) of yield of CD4+Foxp3+ cells (n = 5).
Next, we measured the effect of blocking B7H1/PD-1 interactions on donor Treg expansion in the GVHD recipients. Accordingly, BALB/c recipients were treated with control rat IgG or anti–PD-1 blocking Ab, which was previously reported to block the B7H1/PD-1 interactions (40). We found that although anti–PD-1 treatment did not significantly change the percentage of Treg cells in spleen, it increased the percentage of Treg cells in the liver (Fig. 5D). This is consistent with a previous report showing that blockade of PD-1/PDL1 interaction results in Foxp3+ Treg expansion as judged by the increased percentage of Treg cells among intrahepatic T cells in hepatitis C virus patients (41). However, we also found that anti–PD-1 treatment not only significantly increased the yield of Treg cells but also increased the yield of Teff cells (p < 0.01, Fig. 5E). These results indicate that, consistent with the conventional view, B7H1/PD-1 interactions result in the deletion of both Treg and Teff cells. Therefore, expansion of donor Treg cells mediated by host APC expression of B7H1 is unlikely a result from B7H1/PD-1 interaction.
Finally, we explored the role of B7H1/B7.1 pathway in donor Treg cell expansion. Because B7.1 is not only expressed on T cells but also on DCs, direct blockade of B7.1 receptor in vivo cannot specifically test the role of B7H1 interactions with B7.1 on T cells alone. Therefore, we used an anti-murine B7H1 (43H12) that specifically blocks B7H1 interactions with B7.1 without interfering with B7H1 interactions with PD-1 or B7.1 interactions with CTLA4, as described in our recent publication (29). We found that in recipients treated with anti-B7H1 (43H12), the percentage of Treg cells in spleen and liver tissues was reduced 2- to 3-fold as compared with control hamster IgG-treated recipients (Fig. 5F). Additionally, the yield of Treg cells in the spleen was also reduced ~2-fold in recipients with the blockade of B7H1/B7.1 interactions (p < 0.01), although there was no significant change in the yield of Teff cells (Fig. 5G). Additionally, administration of anti-B7H1 (43H12) augmented clinical GVHD and increased mortality in recipients (p < 0.01, Fig. 5H, 5I). Furthermore, blockade of B7H1/B7.1 interactions also reduced donor Treg cell expansion in in vitro culture (p < 0.01, Fig. 5J, 5K). Taken together, these results indicate that B7H1/B7.1 but not B7H1/PD-1 interactions augment donor Treg cell expansion in autoimmune-like GVHD recipients.
B7.1 is intrinsically critical for Treg cell expansion driven by the inflammatory alloimmune response
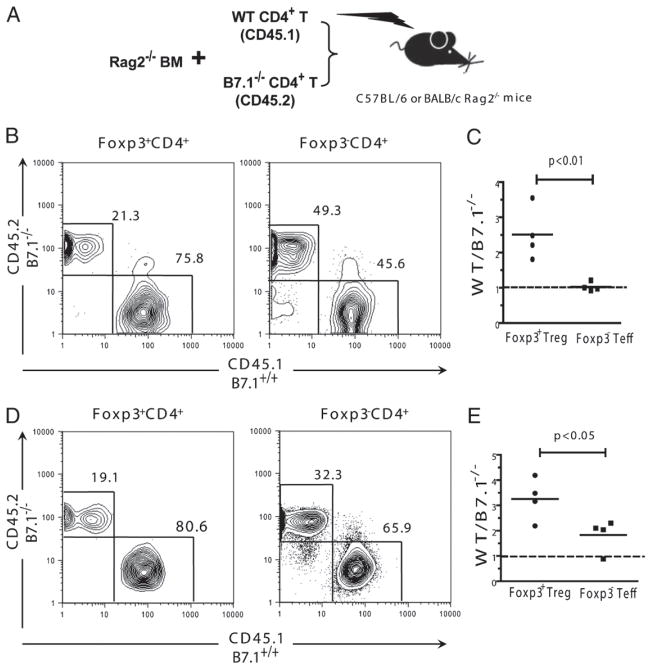
To further test the intrinsic role of B7.1 signaling in Treg cell expansion, we conducted a mixed transplantation system by transferring equal amounts of CD4+ T cells from B7.1−/− (CD45.2) or WT (CD45.1) mice with Rag2−/− BM into TBI-conditioned syngeneic Rag2−/− C57BL/6 or allogeneic Rag2−/− BALB/c recipients, as shown in the diagram in Fig. 6A. We found that the expansion of WT Treg cells (CD45.1+) was 2- to 3-fold higher than B7.1−/− Treg cells (CD45.2+) in syngeneic recipients, although the expansion of WT or B7.1−/− Foxp3− conventional T cells was similar (p < 0.01, Fig. 6B, 6C). These results suggest that B7.1 is intrinsically critical for Treg but not Teff cells in homeostatic expansion.
FIGURE 6.
B7.1 is intrinsically critical for Treg cell expansion derived by inflammatory alloimmune response. Enriched CD4+ T cells from CD45.1+ C57BL/6 (1.0 × 106) and CD45.2+B7.1−/− C57BL/6 (1.0 × 106) mice were mixed with bone marrow cells (5 × 106) from Rag2−/− C57BL/6 mice and injected into TBI-conditioned C57BL/6 or BALB/c Rag2−/− mice. A, Diagram of the transfer procedure. B and C, Splenocytes from C57BL/6 Rag2−/− recipients are stained for CD45.1, CD45.2, CD4, and Foxp3 five days after HCT. Gated CD4+Foxp3+ or CD4+Foxp3− cells are shown as CD45.1 versus CD45.2. Mean (±SE) of the ratios of WT (CD45.1+) versus B7.1 (CD45.2+) cells are shown (n = 4). D and E, Splenocytes from C57BL/6 Rag2−/− recipients were stained for CD45.1, CD45.2, CD4, and Foxp3 5 d after HCT. Gated CD4+Foxp3+ or CD4+Foxp3− cells are shown as CD45.1 versus CD45.2. Mean (±SE) of the ratios of WT (CD45.1+) versus B7.1 (CD45.2+) cells are shown (n = 4).
We also found that the expansion of WT Treg cells (CD45.1+) was 3- to 4-fold higher than B7.1−/− Treg (CD45.2+) cells in allogeneic recipients (p < 0.01, Fig. 6D). Additionally, the expansion of WT Foxp3− Teff cells (CD45.1+) was only 2-fold higher than B7.1−/− Teff cells (CD45.2+) (p < 0.05, Fig. 6D). However, Treg cell expansion was still more dependent on B7.1 signaling as compared with Teff cells, because the ratio of WT/B7.1−/− of Treg cells was significantly higher than that of Teff cells (p < 0.05, Fig. 6E). These findings indicate that B7.1 signaling is critical for Treg cell expansion in alloimmune responses.
Discussion
We have demonstrated that, in an MHC-matched but minor Ag-mismatched HCT model of DBA/2 donor to BALB/c recipient, IFN-γ augments donor Treg cell expansion via upregulation of host APC expression of B7H1 that interacts with B7.1 expressed on donor Foxp3+ Treg cells. Subsequently, the expansion of Treg cells ameliorates GVHD. Our findings thus reveal a new mechanism by which Treg cells expand and function in inflammatory tissues.
First, we observed that neutralization of IFN-γ early after HCT exacerbated clinical GVHD and tissue damage and increased mortality of the recipients, which was associated with a marked reduction of Foxp3+ Treg cells in host lymphoid tissues (i.e., spleen) and GVHD target tissues (i.e., liver and skin). Second, we observed that neutralizing IFN-γ in recipients downregulated recipient DC and parenchymal cell expression of B7H1. Exacerbation of GVHD in B7H1−/− recipients was also associated with a marked reduction of Treg cells in lymphoid and GVHD target tissues. Third, through the use of bone marrow chimeras, we found that B7H1 expression by recipient hematopoietic cells such as DCs but not parenchymal cells played an important role in donor Treg cell expansion. Fourth, we found that B7H1-mediated signaling augmented donor Treg cell survival rather than proliferation, as the percentage of annexin V+ apoptotic cells was increased by 2-fold in B7H1−/− recipients, but there was no reduction in the percentage of BrdU+-proliferating Treg cells in B7H1−/− recipients. Additionally, the role of IFN-γ on Treg cell expansion was not observed in the B7H1−/− recipients. Finally, Treg cells from WT or B7H1−/− recipients both had strong suppressor function and expressed high levels of IL-35, and no significant difference was observed. All together, our results show that IFN-γ plays a pivotal role in the regulation of Treg expansion through B7H1 expression.
Although ample evidence supports the idea that interactions of B7H1 with PD-1 on T cells is a major mode in regulating T cell response (25, 42), we observed that augmentation of donor Treg cell survival and expansion occurred via B7H1/B7.1 but not B7H1/PD-1 interactions. We found that blockade of B7H1/PD-1 interactions resulted in an increase rather than decrease of Treg cells in the recipients. In contrast, blockade of B7H1/B7.1 interactions resulted in decrease rather than increase of Treg cells in the recipients. This in vivo effect of B7H1/B7.1 on expansion of Treg cells was confirmed by in vitro culture assays. Consistent with previous observations that B7.1-mediated signaling on T cells plays an important role on T cell survival (43–45), we found that, although lack of B7.1 signaling led to decreased expansion of both Treg and Teff cells in the alloimmune response, the decrease was more profound with Treg cells. Our results indicate that Treg cells are more dependent on B7.1 signaling for survival, which is probably due to the fact that Treg cells constitutively express higher levels of B7.1 than do Teff cells.
The molecular mechanism by which B7.1 controls the survival and expansion of Treg cells is not yet clear. It has been recently reported that cross-linking B7.1 on CD4+ T cells can activate a calcium-dependent signaling pathway, which leads to increased transcription of T-bet and elevated expression levels of the anti-apoptotic molecule, Bcl-xL (44, 45). We also found that mRNA levels of Bcl-xL in Treg cells isolated from B7H1−/− recipients were lower than those in WT controls (Supplemental Fig. 5). This may explain why Treg cells have increased apoptosis in B7H1−/− recipients. The calcium signaling pathway has been also reported to be a key pathway for Treg cell development and function (46).
This is the first evidence that the proinflammatory cytokine IFN-γ can elicit downregulation of inflammation through B7H1-mediated Treg cell expansion. Cytokine IFN-γ produced by Th1 cells plays an important role in Th1-mediated tissue damage such as in acute and chronic GVHD (2, 21, 22). However, IFN-γ can also downregulate the Th1 inflammatory response by inducing Th1 cell apoptosis and by upregulating tissue expression of co-stimulatory molecules such as B7H1 that mediates anergy and apoptosis of activated infiltrating T cells (23, 24, 47, 48). In contrast, IL-2, another Th1 proinflammatory cytokine, has been shown to downregulate inflammation by augmenting Treg cell survival and expansion (49), as Treg cells express high levels of high-affinity IL-2Rα (CD25). There have been several reports that IFN-γ deficiency is associated with a marked reduction of Treg cells in the inflammatory environment (50–52). Our study has linked IFN-γ, tissue expression of B7H1, and Treg cell expansion together in a concerted pathway. This regulatory loop may be important for modulating the effect of IFN-γ on activation of tissue DCs and macrophages, while the IL-2–Treg cell regulatory loop is important for modulating the effect of IL-2 on activation of conventional T cells.
It is of interest that host tissue APC expression of B7H1 predominantly augments Treg cell expansion but not conversion in GVHD recipients. B7H1 expression has been reported to be associated with high-level Treg cell infiltration in autoimmune patient tissues (30). Tumor tissue expression of B7H1 augments Teff cell conversion to Treg cells (31), and the tissue expression of B7H1 plays an important role in the development, maintenance, and function of the induced Treg cells in non-disease mice (20). In contrast, our studies demonstrated that Treg expansion mediated by tissue B7H1 in the GVHD inflammatory environment is predominantly due to natural Treg cell expansion but not conversion. This is consistent with previous observations that there was little Foxp3+ Treg cell conversion in inflammatory autoimmune mice, such as type I diabetes (19). These reports and our studies provide strong evidence that Treg expansion is dominant in inflammatory environments rather than the Foxp3+ Treg cell conversion. This may be due to the fact that inflammatory cytokines such as IL-6 can block Foxp3+ Treg cell conversion. In support of this, we observed that there were high serum levels of IL-6 in recipients given DBA/2 donor spleen cells (Supplemental Fig. 6). As IL-35 secreted by natural Treg cells was recently reported to mediate conversion of conventional T cells into non-Foxp3 expression Treg cells in autoimmune and tumor environment (39), it would be interesting to test whether this is the case in GVHD recipients in future studies.
Note that it is not yet clear how B7H1/B7.1 interaction affects conventional T cell activation in inflammatory environment, although our recent publication indicates that B7H1/B7.1 interaction is required for induction and maintenance of conventional T cell anergy specific to orally administered Ag in a noninflammatory environment (29). This subject is under investigation and is beyond the scope of current report.
In summary, our study has identified a new immune regulatory loop in which T cell-derived cytokine IFN-γ can upregulate APC expression of B7H1, and APC interactions with Treg cells via B7H1/B7.1 result in expansion of Treg cells that subsequently downregulate T cell immune response. Additionally, we and others have reported that B7H1/PD-1 interaction mediates conventional T cell anergy and apoptosis and downregulates auto-immunity and GVHD (24, 28, 42). Therefore, therapies that augment tissue expression of B7H1 could be vital for the prevention of inflammatory tissue damage.
Supplementary Material
Acknowledgments
We thank Dr. Vijay Kuchroo at Harvard Medical School for providing the Foxp3gfp.KI C57BL/6 mice. We also thank Lucy Brown and her staff at City of Hope Flow Cytometry Facility as well as Sofia Loera and her staff at City of Hope Anatomic Pathology Laboratory for excellent technical assistance.
This work was supported by National Institutes of Health Grant R01AI066008 (to D.Z.).
Abbreviations used in this article
- BM
bone marrow
- DC
dendritic cell
- GVDH
graft-versus-host disease
- HCT
hematopoietic cell transplantation
- TBI
total body irradiation
- Teff
T effector
- Treg
regulatory T
- WT
wild-type
Footnotes
The online version of this article contains supplemental material
Disclosures
The authors have no financial conflicts of interest.
T.Y. conceptualized and initiated research project, designed and performed research, and wrote the manuscript; X.L. designed and performed experiments; L.W., Y.C., D.Z., H.F.J., J.S.Y., H.L., and I.T. assisted in some experiments; S.Y. generated and characterized anti-B7H1 (43H12) Ab; S.J.F. reviewed the manuscript; L.C. provided critical reagents and contributed to the research design and manuscript preparation; and D.Z. designed research, wrote the manuscript, and supervised the research project.
References
- 1.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Antin JH. The pathophysiology of graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas’ Hematopoietic Cell Transplantation. Blackwell Publishing; Malden, MA: 2004. pp. 353–368. [Google Scholar]
- 3.Albert MH, Liu Y, Anasetti C, Yu XZ. Antigen-dependent suppression of alloresponses by Foxp3-induced regulatory T cells in transplantation. Eur J Immunol. 2005;35:2598–2607. doi: 10.1002/eji.200526077. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PA, Noelle RJ, Blazar BR. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao D, Zhang C, Yi T, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang C, Todorov I, Zhang Z, Liu Y, Kandeel F, Forman S, Strober S, Zeng D. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 11.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, Ferrara JL. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 12.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 13.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 14.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 16.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-β, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 19.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francisco LM, V, Salinas H, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor PA. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-γ-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 22.Yi T, Zhao D, Lin CL, Zhang C, Chen Y, Todorov I, LeBon T, Kandeel F, Forman S, Zeng D. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asavaroengchai W, Wang H, Wang S, Wang L, Bronson R, Sykes M, Yang YG. An essential role for IFN-γ in regulation of alloreactive CD8 T cells following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:46–55. doi: 10.1016/j.bbmt.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi T, Chen Y, Wang L, Du G, Huang D, Zhao D, Johnston H, Young J, Todorov I, Umetsu DT, Chen L, Iwakura Y, Kandeel F, Forman S, Zeng D. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 27.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox RA, Feldman AL, Wada DA, Yang ZZ, Comfere NI, Dong H, Kwon ED, Novak AJ, Markovic SN, Pittelkow MR, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 34.Zhao D, Young JS, Chen YH, Shen E, Yi T, Todorov I, Chu PG, Forman SJ, Zeng D. Alloimmune response results in expansion of autoreactive donor CD4+ T cells in transplants that can mediate chronic graft-versus-host disease. J Immunol. 2011;186:856–868. doi: 10.4049/jimmunol.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N, Chen Y, He W, Yi T, Zhao D, Zhang C, Lin CL, Todorov I, Kandeel F, Forman S, Zeng D. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood. 2009;113:953–962. doi: 10.1182/blood-2008-06-165522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 38.Snapper CM, Peschel C, Paul WE. IFN-γ stimulates IgG2a secretion by murine B cells stimulated with bacterial lipopolysaccharide. J Immunol. 1988;140:2121–2127. [PubMed] [Google Scholar]
- 39.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 41.Franceschini D, Paroli M, Francavilla V, Videtta M, Morrone S, Labbadia G, Cerino A, Mondelli MU, Barnaba V. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J Clin Invest. 2009;119:551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blazar BR, Sharpe AH, Taylor PA, Panoskaltsis-Mortari A, Gray GS, Korngold R, Vallera DA. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J Immunol. 1996;157:3250–3259. [PubMed] [Google Scholar]
- 44.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:2948–2958. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 45.Podojil JR, Miller SD. Cross-linking of CD80 on CD4+ T cells activates a calcium-dependent signaling pathway. J Immunol. 2009;182:766–773. doi: 10.4049/jimmunol.182.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oh-hora M, Rao A. The calcium/NFAT pathway: role in development and function of regulatory T cells. Microbes Infect. 2009;11:612–619. doi: 10.1016/j.micinf.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burman AC, Banovic T, Kuns RD, Clouston AD, Stanley AC, Morris ES, Rowe V, Bofinger H, Skoczylas R, Raffelt N, et al. IFNγ differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110:1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 48.Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-γ protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- 49.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-γ. Arthritis Res Ther. 2005;7:R402–R415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishibori T, Tanabe Y, Su L, David M. Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-γ production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.