Abstract
Immune escape describes a critical event whereby tumor cells adopt an immunoresistant phenotype to escape adaptive surveillance. We show that expression of a pivotal negative regulator of T-cell function, B7-H1, correlates with PI(3) kinase activation in breast and prostate cancer patients. B7-H1-mediated immunoresistance can be attenuated by inhibitors of the PI(3) kinase pathway, and is dependent on S6K1-mediated translational regulation of B7-H1 protein. Breast and prostate carcinoma cells with activated PI(3) kinase lose the immunoresistant phenotype after treatment with B7-H1 siRNA. Conversely, breast and prostate carcinoma cells with minimal PI(3) kinase activation adopt an immunoresistant phenotype when engineered to overexpress B7-H1 protein. These observations describe a mechanism for immune escape from tumor dormancy in humans that relates to oncogenesis.
Keywords: PI(3) kinase, B7-H1, immunoresistance, breast cancer, prostate cancer, T cell
Cancer immunoediting describes the capacity of immunity to control and shape cancer development and progression (Bui and Schreiber, 2007). Immunoediting is defined by three fundamental processes: (1) elimination, whereby immunosurveillance suppresses tumor growth, (2) equilibrium, whereby growth of transformed cancer cells is controlled by adaptive immunity and (3) escape, whereby cancer cell variants develop resistance to immune responses and grow into a clinically significant tumor. There is compelling experimental support for each of these steps: (1) immunodeficient mice develop more spontaneous tumors than wild-type mice (Shankaran et al., 2001), (2) adaptive immunity maintains occult cancer in an equilibrium state in mice (Koebel et al., 2007) and (3) cancer cells from immunodeficient mice are more immunogenic than those from immunocompetent mice (Shankaran et al., 2001). In humans, examples of immunoediting steps have been inferred from clinical anecdotes such as development of cancer in immunosuppressed patients after organ transplantation (Penn, 1996). In these patients, cancer cells from the donor organ escape surveillance when adaptive immunity is attenuated in the transplant recipient. For immunocompetent patients, immune escape is likely more complex, involving a combination of oncogenic pathway activation and focal resistance of cancer cells to T-cell-mediated killing. To date, there have been no fundamental mechanisms identified that connect immunoresistance to oncogenesis in prevalent cancers known to express tumor-specific antigens, such as breast and prostate carcinoma.
Cancer cells can evade adaptive immunity by many methods including downregulation of MHC, expression of soluble factors and expression of membrane-bound proteins that prevent T-cell-mediated killing (Schartner et al., 2005; Liu et al., 2007; Parsa et al., 2007; Chang et al., 2008). A recurring observation in many late-stage cancer vaccine trials has been that some patients experience a peripheral tumor-specific immune response without apparent clinical benefit (Powell et al., 2006; Johnson et al., 2007; Meyer et al., 2007; Slingluff et al., 2007). This may be due to a specific immunoresistant phenotype that precludes T-cell-mediated killing of tumor target. We have reported earlier that transformed, pre-cancerous human astrocytes adopt an immunoresistant phenotype after the activation of the oncogenic PI(3)K pathway (Parsa et al., 2007). The mechanism for this immunoresistance involves the protein B7-H1, a negative regulator of T-cell function (Dong et al., 2002; Mazanet and Hughes, 2002). Normal tissues from mice and humans express B7-H1 transcript, but little or no B7-H1 protein (Wintterle et al., 2003). In contrast, cancerous cells can express the B7-H1 protein, which induces apoptosis of activated T cells and impedes tumor-specific T-cell killing (Dong et al., 2002). B7-H1 protein expression has been associated with a poor prognosis in multiple cancers, raising the possibility that immunoresistance confounds conventional therapies (Thompson et al., 2004, 2006, 2007; Inman et al., 2007; Krambeck et al., 2007; Geng et al., 2008; Loos et al., 2008; Routh et al., 2008). In this current study of breast and prostate cancer, we show that PI(3) kinase activation, either through PTEN loss or mutation in the gene, which encodes the catalytic subunit of PI(3)K (PIK3CA), is associated with an increase in B7-H1 protein-mediated immunoresistance.
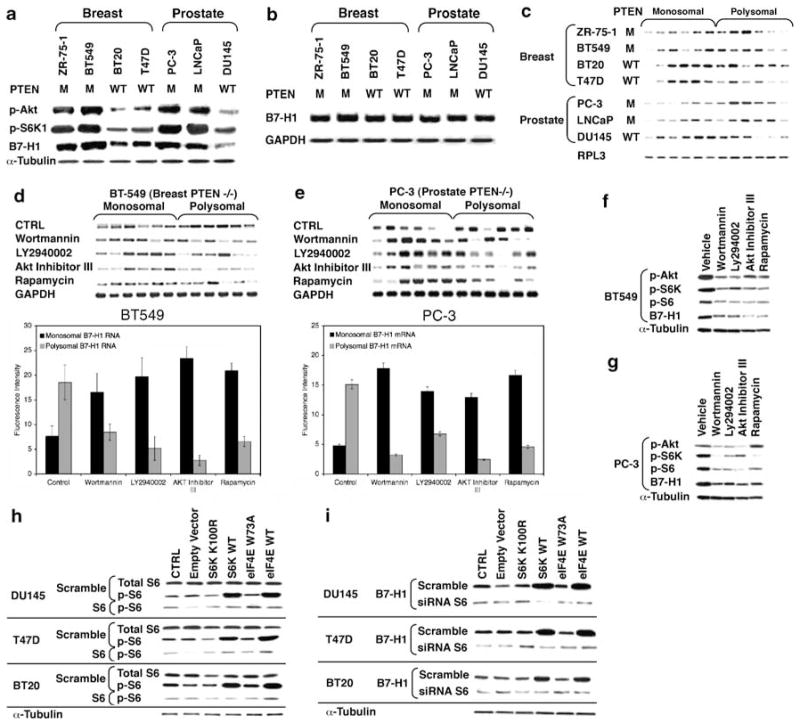
To investigate the regulation of B7-H1 expression in breast and prostate carcininoma, we measured B7-H1 RNA and protein levels in a variety of cancer cell lines with known PI(3)K activation status. As the primary phosphatase of phosphatidylinositol (3,4,5)-trisphosphate, PTEN has a central role in modulating downstream effects of PI(3)K (Tamura et al., 1998; Dey et al., 2008). Cell lines with loss of PTEN function, and activated PI(3)K, had significantly higher levels of B7-H1 protein, but similar levels of B7-H1 transcript relative to cell lines with functional PTEN (Figures 1a and b). The disparity in protein-to-transcript ratio in cells lacking PTEN function was due to polysomal recruitment of B7-H1 transcript (Figure 1c). The amount of transcript in the polysomal fractions is proportional to transcript that is actively being translated. Accordingly, any shift to polysomal fraction or fractions is indicative of an increase in translation. To assess the involvement of the PI(3)K, Akt and mammalian Target of Rapamycin (mTOR) pathway in regulating the expression of B7-H1 protein, we inhibited each of these signaling mediators and measured transcript and protein levels. Treatment of PTEN-deficient breast and prostate carcinoma cell lines with inhibitors AktIII, LY294002, Wortmannin or rapamycin resulted in significant shifts of B7-H1 transcript from the polysomal compartment to the monosomal compartment, with an associated decrease in B7-H1 protein (Figures 1d–g). Inhibition of any component of this signaling cascade had a similar effect on the quantity of polysomal mRNA and protein detected.
Figure 1.
Breast and prostate cancer cell lines lacking functional PTEN upregulate B7-H1 protein expression by increasing mTOR/S6 pathway-dependent translation. Breast and prostate cancer cell lines of either mutant (M) or wild-type (WT) PTEN expression were analyzed for expression levels of phospho-Akt, phospho-S6K and B7-H1 protein or B7-H1 mRNA by northern blot using target-specific oligonucleotides (b). Total mRNA was fractionated using sucrose density fractionation as described earlier (Parsa et al., 2007). Monosomal and polysomal mRNA fractions were probed for B7-H1 mRNA by northern blot (c). PTEN-deficient BT549 cells (d) or PC-3 cells (e) were treated with inhibitors to individual components of the mTOR signaling pathway (Wortmannin 100 μM, LY249002 100 μM, AKT inhibitor III 50 μM, rapamycin 100 ng) for 24 h prior to monosomal and polysomal mRNA fractionations. Fractions were subsequently analyzed for B7-H1 mRNA as in panel c. BT549 (f) or PC-3 (g) cells were treated with mTOR pathway inhibitors as in panel d, and levels of phospho-AKT, phospho-S6K, phospho-S6 (Thr 389) and B7-H1 proteins were examined by western blot (antibodies from Abcam, Cambridge, MA, USA). (h–i) PTEN WT cell lines overexpressing WT or kinase inactive S6K (kinase inactive: K100R) or eIF4E (kinase inactive: W73A) were assayed by western for total S6 and phospho-S6 (h) or B7-H1 (i) after transfection with either a nonspecific (scramble) siRNA or siRNA targeting S6 (Ambion, Cambridge, MA, USA). Results are from a representative experiment performed in triplicate. Error bars represent ± s.d.
Activation of the PI(3)K, Akt and mTOR pathway increases translation through S6K1 activation and phosphorylation of eIF4E-binding proteins (Gingras et al., 1998). Phosphorylation of ribosomal protein S6 by S6K1 facilitates translation of transcripts with 5′-TOP elements, and is most likely involved in regulating the translation of other transcripts. The eIF4E protein binds the 5′ mRNA cap to initiate the assembly of the eIF4F complex, and in some cells can cross-activate S6K1 (Martin and Blenis, 2002). Transfection of cells with constructs encoding S6K1 or eIF4E can simulate the effects of constitutive PI(3)K, Akt and mTOR pathway activity. To demonstrate this, we transfected wild-type or catalytically inactive mutant versions of eIF4E and S6K1 into breast and prostate carcinoma cell lines with wild-type PTEN. Transfection of both eIF4E and S6K1 resulted in an increase in B7-H1 protein expression, which was dependent on wild-type S6K activity, and this effect could be suppressed through S6K1 siRNA transfection (Figures 1h and i). Collectively, these data suggest that loss of PTEN function promotes increased B7-H1 protein expression through the activation of the PI(3)K, Akt and mTOR pathway, which is secondary to S6K1-dependent polysomal recruitment of the B7-H1 transcript.
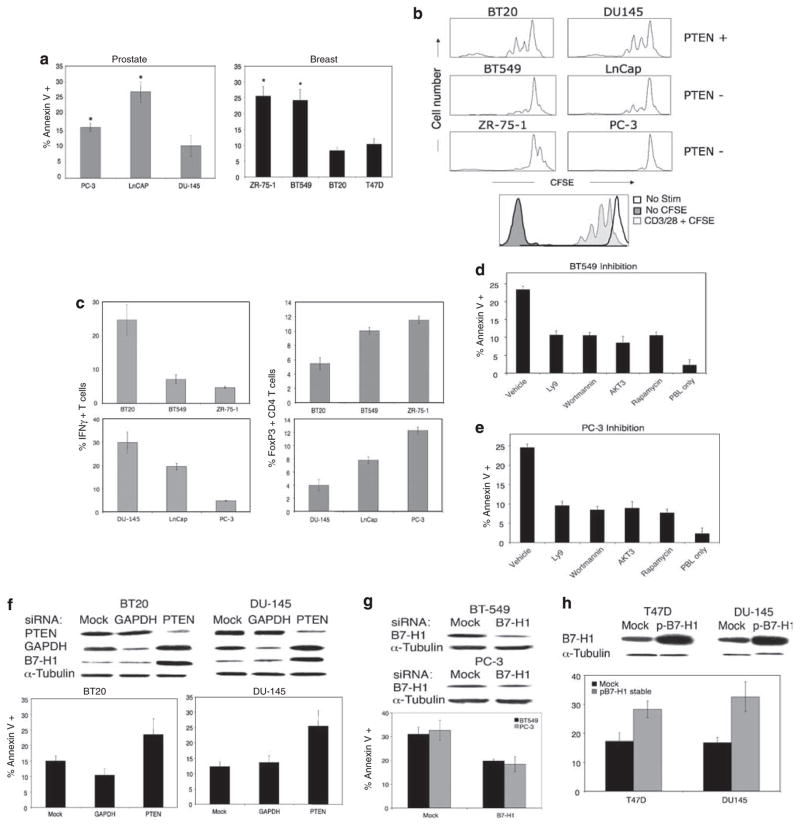
These results demonstrate that in breast and prostate cancer cell lines, loss of PTEN function is associated with enhanced B7-H1 protein levels. To address the functional consequences of these findings, we measured T-cell apoptosis, T-cell proliferation and T-cell cytokine production after exposing activated T cells to breast and prostate carcinoma. A significant increase in T-cell apoptosis (Figure 2a), decrease in T-cell proliferation (Figure 2b) and decrease in Th1 cytokine production (Figure 2c) were noted when co-culturing T cells with breast and prostate carcinoma cells deficient in PTEN. Taken together, these data indicate that PTEN loss can mitigate pro-inflammatory T-cell responses while inducing apoptosis of activated lymphocytes. PTEN-deficient tumor cell lines significantly also increased the percentage of FoxP3-expressing T cells (Figure 2c). These FoxP3+ cells are CD4+ and CD25+ (data not shown), a phenotype consistent with regulatory T cells, a cell type that can effectively suppress effector T-cell function. Our observation is consistent with recent study demonstrating that B7-H1 interaction with its ligand can induce FoxP3+ regulatory T cells (Wang et al., 2008). Increased frequency of regulatory T cells may also contribute to the observed inhibition of pro-inflammatory cytokine production and proliferation. In contrast, breast and prostate carcinoma cells with functional PTEN had minimal impact on T-cell function, consistent with the relatively low expression of B7-H1 protein. The induction of T-cell apoptosis by PTEN-deficient, B7-H1-positive breast and prostate carcinoma cells was reversible by treatment with inhibitors of the PI(3)K, Akt and mTOR pathway (Figures 2d and e), whereas siRNA knockdown of PTEN in wild-type breast and prostate carcinoma cells increased apoptosis of activated T cells (Figure 2f).
Figure 2.
Breast and prostate cancer cell lines lacking functional PTEN and expressing B7-H1 mitigate T-cell pro-inflammatory responses and cytotoxic function. (a) Breast and prostate cancer cell lines were co-cultured with CD3/CD28-stimulated T cells and assayed by flow cytometry for T-cell apoptosis using Annexin V/7-AAD/CD45 staining. (b) CFSE-labeled CD3/CD28-stimulated T cells were co-cultured with tumor cell lines for 72 h and analysed for CFSE dilution by flow cytometry. (c) Stimulated T cells were stained for CD45, fixed and permeabilized using saponin-containing buffer (BD Pharmingen, San Diego, CA, USA). T cells were then permeabilized for intracellular IFN-γ or FoxP3 expression after a 72 h co-culture with tumor cell lines. Brefeldin A (10 μg/ml; Sigma-Aldrich, St Louis, MO, USA) was added to the final four hours of co-culture. (d and e) PTEN-deficient breast (d) or prostate (e) cancer cell lines were treated with inhibitors of mTOR pathway components as described in Figure 1 and co-cultured with CD3/CD28-stimulated T cells. T cells were then analysed for apoptosis as in panel a. (f) PTEN WT cell lines were transfected with 100 pg/ml siRNA specific for PTEN. Levels of PTEN and B7-H1 were assayed by western blot to confirm knockdown 48 h later. Stimulated T cells were then co-cultured with PTEN-silenced tumor cells and assayed for apoptosis as in panel a. (g and h) B7-H1 siRNA-treated PTEN-deficient cell lines (g) or PTEN WT cell lines transfected to overexpress B7-H1 (h) were assayed for B7-H1 protein expression as in panel f and co-cultured with stimulated T cells. Results are from a representative experiment performed in triplicate. Error bars represent ± s.d.
To further investigate the role of B7-H1 in the immunoresistant phenotype of PTEN-deficient cells, we treated PTEN-deficient breast and prostate carcinoma cells with B7-H1 siRNA (Figure 2g), and with B7-H1-blocking antibody (Supplementary Figure 2d), which increased T-cell survival. Transfection of breast and prostate carcinoma cells with a B7-H1 expression vector promoted an increase in T-cell apoptosis despite the presence of functional PTEN (Figure 2g). Collectively, these results support the premise that activation of the PI(3)K, Akt and mTOR pathway in breast and prostate carcinoma cells results in an immunoresistant phenotype mediated in part by an increase in B7-H1 protein expression.
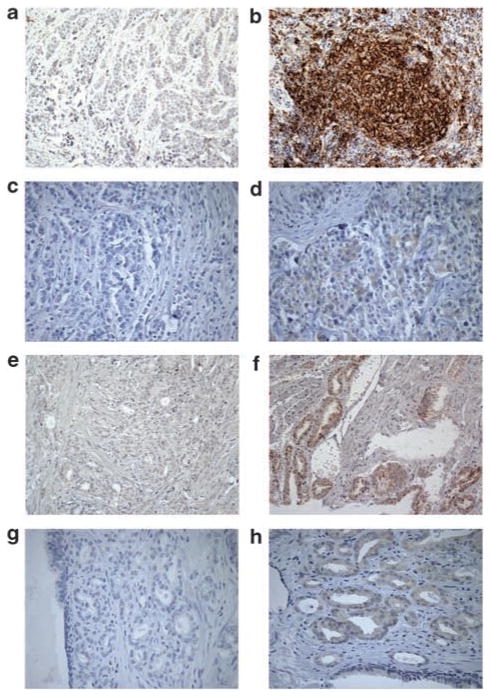
To underscore the clinical relevance of our in vitro findings, we examined B7-H1 protein expression in breast and prostate cancer tissue, which was stratified on the basis of PI(3)K activation status (Table 1). In breast and prostate cancer patients, activation of this pathway occurs through the loss of tumor suppressor PTEN function, as well as mutation of the gene encoding the PI(3)K catalytic subunit p110alpha (the PIK3CA gene) (Miled et al., 2007). The prevalence of these and other mutations that ultimately lead to S6 kinase activation, and B7-H1 protein translation, suggest that many breast and prostate cancer patients have tumors with an immunoresistant phenotype. In the tumor specimens from breast and prostate cancer patients examined in this study, we found a significant correlation between B7-H1 protein expression and PI(3)K activation (Figures 3b and f). Activation of the PI(3)K pathway, either through PTEN loss or PIK3CA mutation, resulted in detectable levels of B7-H1 protein by immunohistochemistry, with an associated increase in phospho-AKT staining (Figures 3d and h). In contrast, specimens without PI(3)K pathway activation had minimal or undetectable levels of B7-H1 protein (Figures 3a and e) and minimal phospho-AKT staining (Figures 3c and g).
Table 1.
DNA from fresh-frozen or formalin-fixed paraffin-embedded specimens were sequenced by PCR amplification, scanning by mismatch cleavage Surveyor analysis and mutations validated by double-strand sequencing
| Mutation | Genewindow NT | Wild-type codon | Mutant codon | Sequence change | % Mutant allele | Amino acid no. | Wild-type amino acid | Mutant amino acid | B7-H1 staining | |
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer samples | ||||||||||
| UCSFB1 | PI3KCA | Exon 9 NT85431240 |
GAA | AAA | G/A | 30 | 579 | GLU | LYS | ++++ |
| UCSFB2 | PI3KCA | Exon 9 NT85431240 |
GAA | AAA | G/A | 30 | 563 | GLU | LYS | ++++ |
| UCSFB3 | PI3KCA | Exon 9 NT85431249 |
GAG | AAG | G/A | 20 | 579 | GLU | LYS | ++++ |
| UCSFB4 | PI3KCA | Exon 19 85447243 |
CAT | CGT | A/G | 40 | 1047 | HIS | ARG | ++++ |
| UCSFB5 | PI3KCA | Exon 9 NT85431240 |
GAA | AAA | G/A | 60 | 563 | GLU | LYS | ++++ |
| UCSFB6 | Wild type | Wild type | + | |||||||
| UCSFB7 | Wild type | Wild type | + | |||||||
| UCSFB8 | Wild type | Wild type | + | |||||||
| UCSFB9 | Wild type | Wild type | + | |||||||
| UCSFB10 | Wild type | Wild type | + | |||||||
| Prostate cancer samples | ||||||||||
| UCSFP1 | PI3KCA | Exon 20 85447243 |
CAT | CGT | A/G | 30 | 1047 | HIS | ARG | +++ |
| UCSFP2 | PTEN | Exon 5 INVS 4–29 |
1 bp ins | 50 | INVS 4–29 | Frameshift | +++ | |||
| UCSFP3 | PTEN | Exon 1 8372813 |
GAC | GGC | A/G | 10 | 24 | ASP | GLY | +++ |
| UCSFP4 | PI3KCA | Exon 20 85447293 |
CAG | TAG | C/T | 10 | 1064 | GLN | STOP | +++ |
| UCSFP5 | Wild type | Wild type | + | |||||||
| UCSFP6 | Wild type | Wild type | + | |||||||
| UCSFP7 | Wild type | Wild type | + | |||||||
| UCSFP8 | Wild type | Wild type | + | |||||||
PTEN exons 1–6 and PIK3CA exons 9 and 20 were analyzed (including splice junctions).
+ indicates <25% positive staining; ++, 25–50%; +++, 50–75% and ++++, >75%.
Figure 3.
B7-H1 and phospho-AKT are expressed in breast and prostate carcinoma samples with activated PI(3)K. Patient samples were characterized histologically and classified on the basis of PI(3)K activation, either through PTEN loss or PIK3CA mutation. Representative histology slides are shown. (a) PI(3)K low-activity breast carcinoma shows minimal to no staining with B7-H1-specific antibody, (b) PI(3)K high-activity breast carcinoma shows positive staining, (c) PI(3)K low-activity breast carcinoma shows minimal to no staining with phopsho-AKT-specific antibody (d) B7-H1-positive breast carcinoma sample shows positive staining for phospho-AKT. (e) PI(3)K low-activity prostate carcinoma shows minimal to no staining with B7-H1-specific antibody, (f) PI(3)K high-activity prostate carcinoma shows positive staining, (g) PI(3)K low-activity prostate carcinoma shows minimal to no staining with phopsho-AKT-specific antibody, (h) B7-H1-positive prostate carcinoma sample shows positive staining for phospho-AKT.
Collectively, our data reveal an association between activation of an oncogenic pathway and immune escape in human breast and prostate carcinoma, supporting the concept of immunoediting in humans. After an oncogenic event resulting in the loss of PTEN function, it is possible that these cells persist, avoiding immune detection in part through B7-H1-mediated apoptosis of tumor-infiltrating T cells. Along with the other known mechanisms of immune modulation by tumor cells, this may contribute to the increased tumor aggression observed in patients with a loss of PTEN function.
Breast and prostate cancer patients are possibly ideal candidates for immunotherapy: each type of tumor has methods for early detection, clinical markers for monitoring recurrence and well-documented tumor-specific antigens are known for each. The safety profile of most cancer vaccines suggests that combination with standard adjuvant therapy is well tolerated and offers the best chance of clinical success within the context of minimal residual disease.
Our data are consistent with recent study in this field highlighting the association of B7 family members with the progression of prostate cancer (Roth et al., 2007; Zang et al., 2007; Baleeiro and Barbuto, 2008; Siva et al., 2008), suggesting that these tumors escape immune recognition in part by impairing T-cell effector function. These patients may therefore be resistant to currently used T-cell-mediated immunotherapies, including peptide (Meyer et al., 2007; Slingluff et al., 2007)- or antibody (Small et al., 2007)-based vaccines. Currently, the selection of patients for immunotherapy protocols does not take into account the immunoresistant phenotype of a tumor, with emphasis placed instead on criteria such as antigen expression and histology. Immunoresistance of tumors has been well documented in breast and prostate cancer systems (reviewed in (Mittendorf et al., 2007; Slovin, 2008)), indicating a need for consideration of proteins that can modulate anti-tumor immunity. We propose that the observed association of PTEN loss and/or activation of PI(3)K, with the increase in B7-H1 expression and the resulting effects on T-cell function can further contribute to the failure of adaptive immunotherapies in some breast and prostate cancer patients. Accordingly, the data that we describe here, correlating PI(3)K activation with B7-H1 expression and immunoresistance, may be particularly important to investigators evaluating the results of completed immunotherapy trials.
Supplementary Material
Acknowledgments
We thank Pramod Srivastava and C David James for critical review of the manuscript, as well as the UCSF cancer center for providing tissue with known PI3K activation status. ATP was supported in part by a K08 grant from the National Institute of Neurological Disorder and Stroke, a career development award from Brain Specialized Programs of Research Excellence (SPORE) of the National Cancer Institute, the Seibrandt Vaccine Fund and the Khatib Foundation.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Baleeiro RB, Barbuto JA. Local secretion/shedding of tumor-derived CD83 molecules as a novel tumor escape mechanism. Mol Immunol. 2008;45:3502–3504. doi: 10.1016/j.molimm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chang YC, Chen TC, Lee CT, Yang CY, Wang HW, Wang CC, et al. Epigenetic control of MHC-II expression in tumor-associated macrophages by decoy receptor 3. Blood. 2008;111:5054–5063. doi: 10.1182/blood-2007-12-130609. [DOI] [PubMed] [Google Scholar]
- Dey N, Crosswell HE, De P, Parsons R, Peng Q, Su JD, et al. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, et al. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134:1021–1027. doi: 10.1007/s00432-008-0364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, O’Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- Johnson LE, Frye TP, Chinnasamy N, Chinnasamy D, McNeel DG. Plasmid DNA vaccine encoding prostatic acid phosphatase is effective in eliciting autologous antigen-specific CD8+ T cells. Cancer Immunol Immunother. 2007;56:885–895. doi: 10.1007/s00262-006-0241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase Akt. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- Krambeck AE, Dong H, Thompson RH, Kuntz SM, Lohse CM, Leibovich BC, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–1756. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+CD25− T cells into CD4+CD25+ T regulatory cells: role of tumor-derived TGF-beta. J Immunol. 2007;178:2883–2892. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- Martin KA, Blenis J. Coordinate regulation of translation by the PI 3-kinase and mTOR pathways. Adv Cancer Res. 2002;86:1–39. doi: 10.1016/s0065-230x(02)86001-8. [DOI] [PubMed] [Google Scholar]
- Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- Meyer RG, Korn S, Micke P, Becker K, Huber C, Wolfel T, et al. An open-label, prospective phase I/II study evaluating the immunogenicity and safety of a ras peptide vaccine plus GM-CSF in patients with non-small cell lung cancer. Lung Cancer. 2007;58:88–94. doi: 10.1016/j.lungcan.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Miled N, Yan Y, Hon WC, Perisic O, Zvelebil M, Inbar Y, et al. Mechanism of two classes of cancer mutations in the phosphoinositide 3-kinase catalytic subunit. Science. 2007;317:239–242. doi: 10.1126/science.1135394. [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Peoples GE, Singletary SE. Breast cancer vaccines: promise for the future or pipe dream? Cancer. 2007;110:1677–1686. doi: 10.1002/cncr.22978. [DOI] [PubMed] [Google Scholar]
- Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61:274–278. doi: 10.1097/00007890-199601270-00019. [DOI] [PubMed] [Google Scholar]
- Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–6539. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- Routh JC, Ashley RA, Sebo TJ, Lohse CM, Husmann DA, Kramer SA, et al. B7-H1 expression in Wilms tumor: correlation with tumor biology and disease recurrence. J Urol. 2008;179:1954–1959. doi: 10.1016/j.juro.2008.01.056. discussion 1959–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51:279–285. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- Slovin SF. Pitfalls or promise in prostate cancer immunotherapy-which is winning? Cancer J. 2008;14:26–34. doi: 10.1097/PPO.0b013e318161bffa. [DOI] [PubMed] [Google Scholar]
- Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Dong H, Kwon ED. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007;13:709s–715s. doi: 10.1158/1078-0432.CCR-06-1868. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.