Abstract
Objective
To evaluate if there are racial differences between African-American and Caucasian women who have hysterectomy for benign conditions in terms of (1) presenting symptoms (prolapse, vaginal bleeding, pain, and known history of leiomyomas), (2) serum estradiol and testosterone levels at the visit before hysterectomy, and (3) uterine weight.
Methods
A multi-ethnic, multisite, community-based longitudinal cohort study of 3,302 women ages 42–52 at enrollment was conducted. During 9 years of follow-up, 203 African-American and Caucasian women reported a hysterectomy, 90 with evidence of uterine leiomyomas. Women were surveyed regarding their overall perceived health before and after hysterectomy, presenting symptoms, and their motivations for surgery. Serum estradiol and testosterone levels were measured. Uterine weight at time of hysterectomy and clinical pathology were determined via medical record abstraction.
Results
Previously diagnosed leiomyomas were presenting symptoms more frequently in African-American women than Caucasian women (85% vs. 63%; p = .02). African-American women had less prolapse than Caucasian women (0% vs. 10%; p = 0.04). Chronic pain was a more frequent reason for hysterectomy in African-American women than in Caucasian women (49% vs. 29%; p = .05). There were no differences between the groups in levels of estradiol or testosterone. African-American women had almost twice the uterine weight as that of Caucasian women (448 vs. 240 g; p = .0005).
Conclusion
Racial differences in frequency of hysterectomy for benign conditions are consistent with differences in presenting symptoms, where African-American women seemingly have larger, more symptomatic fibroids.
Introduction
In the United States, hysterectomy is the second most frequently performed surgical procedure for all women of reproductive age (Keshavarz, Kieke, & Marchbanks, 2002). Approximately 600,000 hysterectomies are performed annually, translating into approximately 20 million women in the United States with a history of hysterectomy (Keshavarz et al., 2002). The leading cause of hysterectomy is leiomyomas (fibroids) followed by endometriosis and uterine prolapse (Keshavarz et al., 2002; Wilcox et al., 1994).
There is a clear racial difference in hysterectomy rates for women with leiomyomas. African-American women, compared with Caucasian women, are 2–3 times more likely to have a clinical diagnosis of uterine leiomyoma (Brett, Marsh, & Madans, 1997; Day Baird, Dunson, Hill, Cousins, & Schectman, 2003; Faerstein, Szklo, & Rosenshein, 2001; Kjerulff et al., 1993; Marshall et al., 1997; Wilcox et al., 1994), are twice as likely to have a hysterectomy for leiomyomas (Keshavarz et al., 2002) and to have the hysterectomy at an earlier age (Kjerulff et al., 1993; Kjerulff, Langenberg, Seidman, Stolley, & Guzinski, 1996). Variables contributing to higher hysterectomy rates in African-American women include larger average uterine weight, larger mean size of largest leiomyoma, hematocrit <35%, and more numerous and symptomatic tumors than Caucasian women (Day Baird et al., 2003; Kjerulff et al., 1996). Nonetheless, a large, prospective, cohort study concluded that neither a higher prevalence of known risk factors nor access to health care explained the excess rate of uterine leiomyomas in African-American women age 25–44 compared with Caucasian women (Marshall et al., 1997). African-American women also experience an increased risk of 1 or more complications of operative or medical care associated with fibroids and hysterectomy, are more likely to have a hysterectomy associated hospital stay of longer than 10 days, have a higher in-hospital mortality rate associated with hysterectomy, and have a longer time interval from diagnosis to hysterectomy (Kjerulff et al., 1993; Kjerulff et al., 1996).
Androgens and estrogens have been implicated in uterine leiomyoma development. High levels of aromatase (the enzyme that converts androgens to estrogens) is present in greater abundance within fibroids than in the surrounding normal myometrium (Folkerd, Newton, Davidson, Anderson, & James, 1984; Yamamoto, Takamori, & Okada, 1984). Additionally, androgen-sensitive tissues convert testosterone to the stronger androgen 5α-dihydrotestosterone by using the enzyme 5α-reductase. This enzyme is present in higher abundance in fibroid tissues compared to myometrium or endometrium (Reddy & Rose, 1979), suggesting a role for androgens in fibroid development. To date, the differences in androgen and estrogen levels in women of varying races undergoing hysterectomy for fibroids have not been measured. Does a racial difference in androgen and estrogen levels explain the racial differences between African-American and Caucasian women who have hysterectomy for fibroids?
Previously in the Study of Women’s Health Across the Nation (SWAN), we examined more than 16,000 women and demonstrated that ethnicity was associated with hysterectomy in women age 40–55 (Powell et al., 2005). In that study, we showed that when controlling for age, education, fibroids, body mass index [BMI], marital status, smoking, geographic site, and country of education, African-American women were 1.66-fold (95% confidence interval [CI], 1.46–1.88) more likely than Caucasian women to have a hysterectomy for benign conditions (Powell et al., 2005). A diagnosis of fibroids as the indication for hysterectomy was higher for African-American women compared with Caucasian, Hispanic, and Asian-American women (Powell et al., 2005). In that study, we concluded that the higher rates of hysterectomy for benign conditions occurring in African-American women was most likely a health disparity issue where inequalities in health care between African-American women and Caucasian women leads to a different outcome.
Queries still surface as to whether the differences between African-American and Caucasian women having hysterectomies for fibroids are due to a health disparity issue or to biological/environmental factors that predispose African-American women to have larger fibroids, and hence more frequent and complicated surgeries. This study looked at women who had a hysterectomy for leiomyomas and addressed the following questions: (1) Are there racial differences in presenting symptoms (prolapse, vaginal bleeding, pain, and known history of leiomyomas) of women who have had a hysterectomy for leiomyomas? (2) Is there a difference in serum estradiol and testosterone levels at the visit before hysterectomy in African-American and Caucasian women having hysterectomy for leiomyomas? If there is a racial difference in serum estradiol and testosterone levels, does it account for the racial differences in prevalence of symptomatic fibroids? And (3) can we confirm the previously observed increase in leiomyomatous uterine weight in African-American women compared with Caucasian women?
Materials and Methods
Participants
SWAN is a multi-ethnic, multisite, community-based study of 3,302 women being followed through the menopausal transition. The design of the study has been previously described (Sowers, Crawford, & Sternfeld, 2000). Briefly, a screening survey was conducted between November 1995 and December 1997 to assess eligibility for enrollment and to collect demographic, health, reproductive, and lifestyle data. From the 16,065 women who completed this initial cross-sectional screening survey, approximately 450 eligible women were recruited for continued follow-up at each of 7 clinical sites. Women were pre- and early peri-menopausal at baseline and have provided up to 10 years of annual data as part of an 11-year study.
To be eligible for the longitudinal cohort, women had to be aged 42–52 years; have an intact uterus and at least 1 ovary; have had at least 1 menstrual period and not have used reproductive hormones in the previous 3 months; and have self-identified with 1 of the site’s designated race/ethnic groups. In addition to Caucasian women, each site recruited women from 1 specified minority group (African-American women in Pittsburgh, Pennsylvania, Boston, Massachusetts, Detroit area, Michigan, and Chicago, Illinois; Japanese women in Los Angeles, California; Chinese women in the San Francisco East Bay region [California]; and Hispanic women in Newark, New Jersey). For this paper, only African-American and Caucasian participants were considered. The Institutional Review Boards at all participating sites approved the study protocol. All participants provided informed written consent.
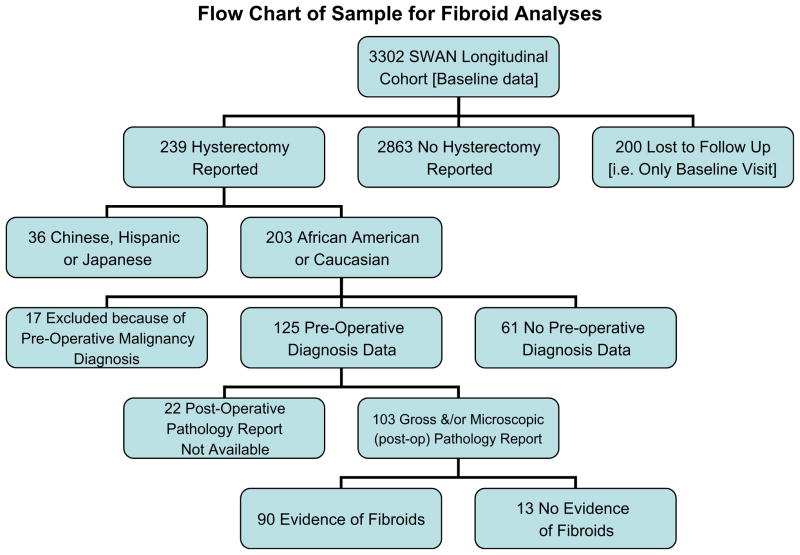
Of the 3,302 cohort women, 200 never completed a follow-up visit. From the remaining 3,102 women, 239 incident hysterectomy cases were reported over 9 years (8%), 203 of which were African-American or Caucasian women. As outlined in Figure 1, 17 participants were excluded because of a preoperative malignancy diagnosis and 125 medical records with a preoperative diagnosis were obtained. Of these records, 103 contained information on gross and/or microscopic pathology, with 90 showing evidence of uterine leiomyomas and 13 showing no evidence of uterine leiomyomas. The SWAN retention rate at the end of the 9th follow-up examination was 73% for all active participants.
Figure 1.
We included 203 African-American and Caucasian women who reported a hysterectomy: 61 did not have data on preoperative diagnoses and 17 women were excluded because of preoperative diagnoses of malignancy, leaving a sample size of 125. The initial sample size is based on the total number of women with data on preoperative diagnoses and no preoperative indication of cancer. Forty-eight percent (n = 61) of this sample is African American. The final sample size is based on total number of women with gross or microscopic/histologic pathological evidence of fibroids (n = 90).
Procedures and Measures
SWAN participants at all sites were assessed with a common protocol. Assessments included blood draw, anthropometric measurements, and interviewer- and self-administered questionnaires about health, lifestyle, and psychosocial factors. Age, race/ethnicity, education (high school degree or less, some college/vocational training, college degree or more), difficulty paying for basics (as an indicator of socioeconomic status: very hard, somewhat hard, not hard at all), total physical activity during housework and leisure activities (assessment scale previously used; Sternfeld, Ainsworth, & Quesenberry, 1999), and parity (number of live births after 20 weeks of gestation) were obtained at the baseline examination.
Annual measures included perceived health, menopausal status, exogenous hormone use, and blood assays. Participants rated their overall perceived health on a 5-point scale ranging from poor to excellent derived from the MOS SF-36 (Ware & Sherbourne, 1992). BMI (kg/m2) was calculated from measurements of weight and height, which were obtained annually with a calibrated scale and stadiometer. Waist-to-hip ratio was measured over undergarments at the narrowest part of the torso (natural waist, normally above the level of the umbilicus). For 9 of the 13 participants for whom BMI and waist-to-hip ratio at the visit the hysterectomy was reported was not available, these measures were imputed from the 2 closest visits.
A fasting blood draw was targeted to the follicular phase of the menstrual cycle (days 2 to 5). All samples were maintained at 4°C until separated and then were frozen at −80°C and shipped on dry ice to a central laboratory. Serum estradiol concentrations were measured with a modified, off-line ACS:180 (E2-6) immunoassay. Testosterone concentrations were evaluated with the ACS:180 total testosterone assay modified to increase precision in the low ranges. The intra- and interassay coefficients of variation for estradiol assays were 8.5% and 13.8%, respectively (England, Parsons, Possley, McConnell, & Midgley, 2002). For testosterone, the intraassay coefficient of variation ranged from 4.6% to 11.78% and the interassay coefficient of variation ranged from 9.7% to 11.34% (Sowers, Beebe, McConnell, Randolph, & Jannausch, 2001). Hormone assays were conducted at the University of Michigan SWAN Endocrine Laboratory using the ACS-180 automated analyzer (Bayer Diagnostics Corp, Norwood, MA).
At each annual follow-up visit, participants were asked whether or not they had had a hysterectomy. Women who reported an incident hysterectomy were then asked to consent to have their medical records requested and to complete an additional questionnaire. Physicians and medical institutions were requested to provide all information from the medical record pertinent to the hysterectomy, including pre- and postoperative notes. A single reviewer (GW) completed an abstraction form from these medical records. Included on the abstraction form were lists of presenting symptoms, preoperative diagnosis, histologic findings, uterine weight, record of uterine and ovary removal, and any operative complications. Evidence of uterine leiomyomas was verified via gross and/or microscopic findings, as described in the medical record. The participant questionnaire included questions on what influenced their decision to have a hysterectomy, alternative treatments considered and satisfaction with their experience.
Statistical Methods
Standard χ2, Fisher exact, Student t-test, and Wilcoxon tests were used as appropriate to compare African American and Caucasian women who reported a hysterectomy (n = 125) and who had evidence of uterine leiomyomas (n = 90). The sample of 125 women was used to generally compare African-American and Caucasian women having hysterectomy. The sample of 90 women was used to answer the 3 objectives of this paper (racial differences in symptoms, serum estradiol and testosterone levels, and uterine weight). Analyses included evaluation of general characteristics, presenting symptoms, uterine weight, and participants’ perceptions. Data were extracted from the regularly scheduled annual visit, the medical record, and the participant questionnaire completed at a visit after the hysterectomy. Results did not change when limited to the 4 sites that recruited African-American women; thus, findings from the full sample are reported. All analyses were computed using SAS (Version 9.1, SAS Institute, Inc., Cary, NC).
Exploratory multivariable logistic regression models were used to assess whether frequency of leiomyomas by race/ethnicity was independent of other factors by examining the 103 available pathology reports. Because of the limited number of women without evidence of fibroids (n = 13), covariates were restricted to age at hysterectomy and BMI. Further, although the model fit was evaluated via the Hosmer and Lemeshow goodness-of-fit statistic (Hosmer and Lemeshow, 1989), results of these models were interpreted with caution given the large confidence intervals.
Results
In examining women with hysterectomy and available preoperative data (n = 125), African-American women, compared with Caucasian women, had their hysterectomy at a younger age (48 vs. 50 years; p = .0008), were less physically active, and had higher BMI and WHR values (Table 1). At study entry, African-American women, compared with Caucasian women were also younger (baseline age 45.2 vs. 46.7; p = .003) and showed greater BMI (33.1 vs. 26.8; p < 0.0001) and WHR at the visit before the hysterectomy (0.83 vs. 0.80; p = .02; data at study entry are not presented in tables). Self-perceived health among African-American women before surgery, relative to the Caucasian women, was poorer (fair/poor health, 20% vs. 9%), although this difference did not remain significant in the year after surgery (fair/poor health, 17% vs. 7%).
Table 1.
General Characteristics of Women With hysterectomy and Available Preoperative Diagnosis Data by Race (n = 125)
| Characteristics | African American (n = 61) | Caucasian (n = 64) | p |
|---|---|---|---|
| Age at hysterectomy (y), mean ± SD | 48.0 ± 3.0 | 50.0 ± 3.3 | .0008 |
| BMI at visit after hysterectomy, mean ± SD | 33.9 ± 7.3 | 27.8 ± 6.5 | <.0001 |
| Baseline physical activity, mean ± SD (range, 3–15) | 7.1 ± 1.7 | 8.1 ± 1.8 | .004 |
| Waist to hip ratio at visit after hysterectomy (n = 61 ± 60) | 0.84 ± 0.07 | 0.80 ± 0.08 | .01 |
| Parity (number of children), mean ± SD | 2.4 ± 1.8 | 1.8 ± 1.5 | .08 |
| Overall health at visit before hysterectomy, n (%)* | .03 | ||
| Excellent | 4 (7) | 15 (26) | |
| Very good | 17 (31) | 19 (33) | |
| Good | 22 (41) | 19 (33) | |
| Fair/poor | 11 (20) | 5 (9) | |
| Overall health at visit after hysterectomy, n (%)* | .16 | ||
| Excellent | 6 (10) | 13 (21) | |
| Very good | 24 (41) | 24 (39) | |
| Good | 19 (32) | 20 (33) | |
| Fair/poor | 10 (17) | 4 (7) |
Data not available for all participants.
Table 2 presents a comparison between the African-American and Caucasian women with hysterectomy and a postoperative confirmation of leiomyoma from the pathology report (n = 90). Data from the medical record indicated that previously diagnosed leiomyoma was a presenting symptom more frequently in the African-American women than in the Caucasian women (85% vs. 63%; p = .02). In contrast, Caucasian women were more likely to have prolapse (10% vs. 0%; p = .04). A comparison of uterine weight in the 2 groups revealed that, in African-American women, the uteri weighed almost twice as much as the Caucasian women’s uteri (448 vs. 240 g; p = .0005). There were no racial differences in serum estradiol and testosterone levels in women who had hysterectomy for fibroids. This similarity in endogenous hormones remained when ethnicities were subdivided into pre-menopausal/early perimenopausal and late perimenopausal/postmenopausal groups.
Table 2.
Racial Comparison of Women With Hysterectomy and Postoperative Confirmation of Leiomyomas (n = 90)
| African American (n = 48) | Caucasian (n = 42) | p | |
|---|---|---|---|
| Estradiol (pg/mL) at visit before hysterectomy, median [IQR]; n = 42 ± 37 | 51.6 [33.4–88.9] | 47.9 [22.5–162.9] | .61 |
| Testosterone (ng/dL) at visit before hysterectomy, median [IQR]; n = 42 ± 37 | 33.8 [19.7–49.9] | 33.3 [23.8–40.3] | .88 |
| From medical record | |||
| Presenting symptoms | |||
| Prolapse | 0 (0) | 4 (10) | .04 |
| Problems with vaginal bleeding | 34 (74) | 24 (60) | .17 |
| Leiomyomas (previously diagnosed) | 39 (85) | 25 (63) | .02 |
| Acute pelvic pain | 4 (9) | 0 (0) | .12 |
| Chronic pelvic pain | 12 (26) | 12 (30) | .69 |
| Uterine weight (g), median [IQR] | 448 [272–794] | 240 [142–440] | .0005 |
| From self-report | |||
| Influenced decision to have hysterectomy | |||
| Acute pain | 9 (19) | 4 (10) | .24 |
| Chronic pain | 23 (49) | 12 (29) | .05 |
| Problems with bleeding | 38 (81) | 28 (67) | .13 |
| Leiomyomas | 44 (94) | 29 (69) | .005 |
| Health care provider recommendation | 34 (72) | 33 (79) | .50 |
| Primary motivation for hysterectomy | .003 | ||
| Chronic pain | 11 (23) | 1 (2) | |
| Problem with bleeding | 18 (38) | 15 (36) | |
| Leiomyomas | 11 (23) | 6 (14) | |
| Health care provider recommendation | 1 (2) | 5 (12) | |
| Other (e.g. acute pain, prolapse, other) | 6 (13) | 15 (36) | |
| Felt hysterectomy took care of problem | 46 (100) | 37 (97) | .45 |
| Quality of life better/much better after 3 mo | 26 (55) | 24 (57) | .72 |
All values in table are n [range] or n (%).
Multivariable logistic regression models showed that of those with available pathology reports (n = 103), African-American women had a higher odds of evidence of leiomyoma than Caucasian women (odds ratio [OR], 6.97; 95% CI, 1.22–39.81; p = .03) after controlling for age at hysterectomy (OR, 1.03; 95% CI, 0.84–1.26) and BMI (OR, 0.96; 95% CI, 0.88–1.05), which were not significantly different.
Data from the self-report questionnaire, assessed after surgery, indicated that chronic pain was a more frequent reason for hysterectomy among African-American women than among Caucasian women (49% vs. 29%; p = .05). There was no differential encouragement to have the surgery by the health care provider in 1 group relative to the other. When women were asked to choose the 1 primary motivation for having the surgery, the African-American women, relative to the Caucasian women, more frequently chose chronic pain (23% vs. 2%; p = .003). In contrast, the Caucasian women, relative to the African-American women, more frequently chose “Other (e.g., acute pain, prolapse)” (36% vs. 13%). Self-reported quality of life 3 months after the hysterectomy was similar between the groups.
Discussion
African-American women with fibroids were more symptomatic (chronic pain), and this was a primary motivator for operative management. Furthermore, African-American women underwent hysterectomy at an earlier age, supporting the observation that symptoms were more severe in the African-American population requiring earlier operative management. This interpretation is supported by a comparative study where African-American women having larger uteri and more numerous leiomyomas also had a higher incidence of a hematocrit <35%, severe pain, constipation, and stomach aches (Kjerulff et al., 1996). Prolapse was reported more frequently in the Caucasian population. It is plausible that prolapse was the reason for hysterectomy in these Caucasian patients with asymptomatic concurrent incidental fibroids. Multiple studies have shown that prolapse and incontinence are more frequent in Caucasians compared with African Americans (Bump, 1993; Howard, Delancey, Tunn, & Ashton-Miller, 2000; Rortveit et al., 2007). Overall, the data suggest that hysterectomies for fibroids in the African-American population were more likely due to severe symptomatic fibroids, but in Caucasian women with hysterectomies, fibroids were more likely to be incidental.
It is well established that growth of uterine leiomyomas occur during the reproductive years and growth inhibition and regression occur with both GnRH agonist treatment and at menopause, suggesting that fibroids are steroid responsive. Previously, studies demonstrated higher estrogen, aromatase, 5α-reductase, and androgen activity in leiomyoma tissue (Folkerd et al., 1984; Reddy & Rose, 1979; Yamamoto et al., 1984). An observation in support of the theory that higher androgens are involved in leiomyoma development is that African-American women with polycystic ovarian syndrome have a higher incidence of uterine leiomyomas compared with African-American women without polycystic ovarian syndrome (Wise, Palmer, Stewart, & Rosenberg, 2007). Hence, sex steroids (both tissue specific and serum) are of great interest when studying leiomyoma development. We demonstrated that there were no racial differences in serum estradiol or testosterone levels at the annual study visit before hysterectomy in women with leiomyomas. Post hoc analyses revealed that even with our limited sample size we had sufficient power (94% for estradiol, 84% for testosterone) to detect clinically meaningful differences between groups. Thus, larger, more symptomatic fibroids in African-American women who have had a hysterectomy for fibroids do not seem to be related to these circulating steroids.
We confirm that uterine weight at hysterectomy was significantly higher in African-American women compared with Caucasian women. The racial difference in uterine weight is a theme that resonates throughout the literature. A comparative study population in Maryland, consisting of 409 African-American women and 836 Caucasian women, demonstrated that the average uterine weight for African-American women with leiomyomas was 420.8 g and 319.1 g for Caucasian women, that 56% of African-American women but only 36% of Caucasian women had 7 or more leiomyomas, and that African-American women had a mean size of largest leiomyoma 5.2 ± 2.9 cm compared with 4.6 ± 3.2 cm in Caucasian women (Kjerulff et al., 1996). Larger leiomyomas have been associated with complications of hysterectomy, including risk of blood loss, blood transfusions, vaginal cuff cellulitis, urinary tract infection, pyelonephritis, fever, nonpelvic venous thrombosis, pelvic hematoma, wound infection, bowel obstruction, and hospital readmission (Hillis, Marchbanks, & Peterson, 1996).
Multiple factors influence leiomyoma development. There are several biological differences between African-American and Caucasian women with leiomyomata that predispose African-American women to fibroids. These include estrogen receptor-α PP polymorphism and catechol-O-methyltransferase polymorphism Val/Val genotype (Al-Hendy & Salama, 2006a, 2006b). An epidemiologic study of African-American and Caucasian sister pairs, of similar socioeconomic status, diagnosed with uterine leiomyomas demonstrated that African-American women were diagnosed 5.3 years younger and more likely to report severe disease, suggesting an underlying genetic predisposition to uterine leiomyomas in African-American women (Huyck et al., 2008). High BMI, diets rich in red meat/fats and low in fiber, low physical activity levels, and increased WHRs have all been associated with uterine leiomyoma development (Baird, Dunson, Hill, Cousins, & Schectman, 2007; Chiaffarino et al., 1999; Faerstein et al., 2001; Ross et al., 1986; Terry et al., 2007; Wise et al., 2005). Wise et al. (2005) showed that the incidence of uterine leiomyomata increased with a BMI up to 32.4 kg/m2, but was not significantly different at BMI ≥ 32.5 kg/m2. However, Marshall et al. (1997) conducted a multivariate analysis controlling for confounders like BMI, and still found a higher-incidence of uterine leiomyoma in the African-American group compared with the White, Hispanic, and Asian groups. Hence, the exact role of body habitus on the incidence of uterine leiomyoma remains unclear. Our data point to baseline racial differences in BMI, baseline physical activity, and WHR. These are known differences, but presently there is no reason to assume causality.
We have demonstrated that the perceived overall health of African-American women before hysterectomy was significantly poorer compared with the Caucasian women. This may reflect the patients’ perceptions of health as a result of the burden with fibroids or may be a true disparity issue. Our study is limited by not controlling for concurrent morbidities, which could influence a patient’s overall perceived health. For example, an African-American woman with poorly controlled hypertension and diabetes might have a poorer perceived health regardless of her symptoms related to her fibroids. We nevertheless conclude that, in our study, women with larger and more symptomatic fibroids are likely to be sicker and consequently may be more likely to report a poorer health status. This is consistent with previous reports (Spies et al., 2002; Williams, Jones, Mauskopf, Spalding, & DuChane, 2006).
There are several strengths and limitations of this study. The diverse cohort of women in this study was taken from 7 study sites and numerous medical facilities across the country where different surgeons and pathologists evaluated these cases. Hence, the results of the study have strong external validity. Serum estradiol and testosterone levels before hysterectomy were measured in this study. There are only a few limited studies that evaluate sex steroids and leiomyomas. This is the first assessment of sex steroids as a possible factor of racial differences between African-American and Caucasian women who have hysterectomies for fibroids. The African-American women presented at a younger age at study entry, and had hysterectomy for fibroids at an earlier age. This earlier presentation at study entry could be interpreted as a confounder for hysterectomy at an earlier age. However, it is more an indication that the fibroids were larger and more symptomatic in the African-American group leading to an earlier presentation. It is this burden of symptoms and size that led to the hysterectomy for fibroids at an earlier age in the African-American group.
One of the limitations of our assessment of symptoms is that the questionnaire completed by participants could have been done shortly after or many years after hysterectomy. However, this limitation may have worked to our advantage in terms of symptoms, because women are more likely to remember their worst symptoms before hysterectomy. Despite the large geographic area of the selected cohort of women, the study is limited by a small number of women who had pathology proven leiomyomatous uteri (n = 90). Further, the small number of women without documented uterine leiomyomas (n = 13) limited our ability to utilize multivariable analyses. It is also unfortunate that data were not available for all participants; nonetheless, post hoc comparisons between women with complete data (n = 103) versus those without (n = 83 [61 ± 22]) showed no differences between these groups. Larger studies that compare fibroids in women with and without hysterectomy should be done to substantiate our findings.
The study is also limited by the fact that we do not know which patients had supracervical versus total hysterectomy. Although the weight of the cervix should be taken into consideration, it is unlikely that the cervical weight could be a significant contribution to an already higher uterine weight in patients with heavy leiomyomatous uteri as seen in the African-American cohort. Furthermore, the early and perimenopausal Caucasian women with prolapse (and minimal to no leiomyomas in their uteri) more likely had cervical weight that had an appreciable contribution to the total uterine weight. Despite this, there was still a significant difference in overall uterine weight between the 2 races.
In conclusion, symptoms at presentation differed between African-American and Caucasian women. Severe symptomatic fibroids were the reason for hysterectomy in most African-American women and prolapse with potentially incidental asymptomatic fibroids occurred only in Caucasian women. Estrogen and testosterone blood levels did not differ between the 2 races, suggesting that these steroids were not responsible for the racial differences in women who have hysterectomy for fibroids. We confirmed that leiomyomatous uterine weight is significantly higher in African-American women having hysterectomies for fibroids compared with Caucasian women. African-American women seemed predisposed to larger and more symptomatic fibroids requiring operative management at an earlier age than Caucasian women. Based on our findings, we conclude that the difference in frequency of hysterectomy between African-American and Caucasian women is most likely related to the racial differences in leiomyoma disease rather than health disparity.
Acknowledgments
Clinical Centers
University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 – 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark –Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office
National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory
University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center
New England Research Institutes, Watertown, MA -Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee
Chris Gallagher, chair; Susan Johnson, chair. The authors thank the study staff at each site and all the women who participated in SWAN.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Biographies
Gerson Weiss, MD, is Professor and Chair of the Department of Obstetrics, Gynecology, and Women’s Health at New Jersey Medical School–UMDNJ. He is the Principal Investigator of the SWAN study at the New Jersey Medical School site.
Dorette Noorhasan, MD, is a Reproductive Endocrinology and Infertility Fellow in the Department of Obstetrics, Gynecology, and Women’s Health at New Jersey Medical School–UMDNJ. She is an Investigator of SWAN research.
Laura L. Schott, PhD, is a Statistician in the Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh. She is a Statistician in SWAN research.
Lynda Powell, PhD, is a Professor in the Department of Preventive Medicine, Rush-Presbyterian-St. Luke’s Medical Center. She is the Prinicipal Investigator of the SWAN study at Rush University Medical Center site.
John F. Randolph, Jr., MD, is a Professor in the Department of Obstetrics and Gynecology at the University of Michigan Health System. He is the Co-Principal Investigator of the SWAN study at the University of Michigan site.
References
- Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. Journal of the Society for Gynecologic Investigation. 2006a;13:136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertility & Sterility. 2006b;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. Association of physical activity with development of uterine leiomyoma. American Journal of Epidemiology. 2007;165:157–163. doi: 10.1093/aje/kwj363. [DOI] [PubMed] [Google Scholar]
- Brett KM, Marsh JV, Madans JH. Epidemiology of hysterectomy in the United States: demographic and reproductive factors in a nationally representative sample. Journal of Womens Health. 1997;6:309–316. doi: 10.1089/jwh.1997.6.309. [DOI] [PubMed] [Google Scholar]
- Bump RC. Racial comparisons and contrasts in urinary incontinence and pelvic organ prolapse. Obstetrics and Gynecology. 1993;81:421–425. [PubMed] [Google Scholar]
- Chiaffarino F, Parazzini F, La Vecchia C, Chatenoud L, Di Cintio E, Marsico S. Diet and uterine myomas. Obstetrics and Gynecology. 1999;94:395–398. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. American Journal of Obstetrics and Gynecology. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clinical Chemistry. 2002;48:1584–1586. [PubMed] [Google Scholar]
- Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: A practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. American Journal of Epidemiology. 2001;153:1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- Folkerd EJ, Newton CJ, Davidson K, Anderson MC, James VH. Aromatase activity in uterine leiomyomata. Journal of Steroid Biochemistry. 1984;20:1195–1200. doi: 10.1016/0022-4731(84)90366-2. [DOI] [PubMed] [Google Scholar]
- Hillis SD, Marchbanks PA, Peterson HB. Uterine size and risk of complications among women undergoing abdominal hysterectomy for leiomyomas. Obstetrics and Gynecology. 1996;87:539–543. doi: 10.1016/0029-7844(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, Inc; 1989. [Google Scholar]
- Howard D, Delancey JO, Tunn R, Ashton-Miller JA. Racial differences in the structure and function of the stress urinary continence mechanism. Obstetrics and Gynecology. 2000;95:713–717. doi: 10.1016/s0029-7844(00)00786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck KL, Panhuysen CI, Cuenco KT, Zhang J, Goldhammer H, Jones ES, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. American Journal of Obstetrics and Gynecology. 2008;198:168, e161–169. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarz H, Kieke B, Marchbanks P. Hysterectomy surveillance—United States 1994–1999. Morbidity and Mortality Weekly Report Surveillance Summaries. 2002;51:1–8. [PubMed] [Google Scholar]
- Kjerulff KH, Guzinski GM, Langenberg PW, Stolley PD, Moye NE, Kazandjian VA. Hysterectomy and race. Obstetrics and Gynecology. 1993;82:757–764. [PubMed] [Google Scholar]
- Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. Journal of Reproductive Medicine. 1996;41:483–490. [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstetrics and Gynecology. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- Powell LH, Meyer P, Weiss G, Matthews KA, Santoro N, Randolph JF, Jr, et al. Ethnic differences in past hysterectomy for benign conditions. Womens Health Issues. 2005;15:179–186. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Reddy VV, Rose LI. delta 4-3-Ketosteroid 5 alpha-oxido-reductase in human uterine leiomyoma. American Journal of Obstetrics and Gynecology. 1979;135:415–418. doi: 10.1016/0002-9378(79)90716-6. [DOI] [PubMed] [Google Scholar]
- Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstetrics and Gynecology. 2007;109:1396–1403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]
- Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT. Risk factors for uterine fibroids: Reduced risk associated with oral contraceptives. British Medical Journal (Clinical Research Edition) 1986;293:359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Beebe JL, McConnell D, Randolph J, Jannausch M. Testosterone concentrations in women aged 25–50 years: Associations with lifestyle, body composition, and ovarian status. American Journal of Epidemiology. 2001;153:256–264. doi: 10.1093/aje/153.3.256. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Crawford SL, Sternfeld B. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. Lobo, Kelsey, Marcus. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. [Google Scholar]
- Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstetrics and Gynecology. 2002;99:290–300. doi: 10.1016/s0029-7844(01)01702-1. [DOI] [PubMed] [Google Scholar]
- Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Preventive Medicine. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- Terry KL, De Vivo I, Hankinson SE, Spiegelman D, Wise LA, Missmer SA. Anthropometric characteristics and risk of uterine leiomyoma. Epidemiology. 2007;18:758–763. doi: 10.1097/EDE.0b013e3181567eed. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstetrics and Gynecology. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Williams VS, Jones G, Mauskopf J, Spalding J, DuChane J. Uterine fibroids: A review of health-related quality of life assessment. Journal of Womens Health (Larchmont) 2006;15:818–829. doi: 10.1089/jwh.2006.15.818. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Spiegelman D, Harlow BL, Stewart EA, Adams-Campbell LL, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16:346–354. doi: 10.1097/01.ede.0000158742.11877.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Stewart EA, Rosenberg L. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertility & Sterility. 2007;87:1108–1115. doi: 10.1016/j.fertnstert.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Takamori K, Okada H. Estrogen biosynthesis in leiomyoma and myometrium of the uterus. Hormone and Metabolic Research. 1984;16:678–679. doi: 10.1055/s-2007-1014884. [DOI] [PubMed] [Google Scholar]