Summary
Vascular permeability is a highly coordinated process that integrates vesicular trafficking, complex junctional rearrangements, and refined cytoskeletal dynamics. In response to the extracellular environment, these three cellular activities have been previously assumed to work in parallel to regulate the passage of solutes between the blood and tissues. New developments in the area of vascular permeability, however have highlighted the interdependence between trans- and para-cellular pathways, the cross-communication between adherens and tight junctions, and the instructional role of pericytes on endothelial expression of barrier-related genes. Additionally, significant effort has been placed in understanding the molecular underpinings that contribute to barrier restoration following acute permeability events and in clarifying the importance of context-dependent signaling initiated by permeability mediators. Finally, recent findings have uncovered an unpredicted role for transcription factors in the coordination of vascular permeability and clarified how junctional complexes can transmit signals to the nucleus to control barrier function. The goal of this review is to provide a concise and updated view of vascular permeability, discuss the most recent advances in molecular and cellular regulation, and introduce integrated information on the central mechanisms involved in trans-endothelial transport.
Keywords: Adhesion molecules, endothelial cells, endothelial barrier, permeability, capillaries
Introduction
One of the main roles of endothelial cells is to function as a selective barrier between the blood stream and tissues. As sophisticated gate-keepers, endothelial cells possess a broad number of mechanisms that regulate transport of solutes, large molecules, and cells across the vessel wall. In general terms, the endothelial barrier is controlled by the combined activities of: 1) heterotypic cell associations (inflammatory cells and mural cells); 2) transcellular transport (across the endothelium) and 3) junctional complexes or intercellular junctions (paracellular tranport, i.e. between endothelial cells) (Figure 1). Together, these extracellular associations and cellular functions maintain and actively regulate transport across the endothelium. The dynamic and highly responsive control of the endothelial barrier enables macromolecular transport to be reduced or accelerated, facilitates immune surveillance and enables the deposition of matrix proteins immediately outside the vascular wall (provisional matrix) to initiate mechanisms of repair. In recent years, information related to the processes that coordinate transcellular and paracellular transport have been broadly expanded and focus has been placed on integration of these two mechanisms, as well as in understanding tissue-specific control of barrier function. In this review, we will summarise the recent conceptual advancements in both the cellular and molecular control of permeability regulation, and present these findings in the context of previous knowledge in the field.
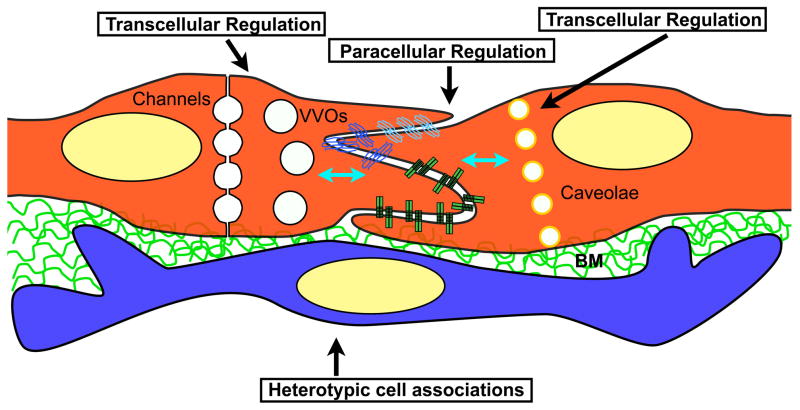
Figure 1. Pathways that regulate barrier function in endothelial cells.
Scheme shows two endothelial cells and the subendothelial space. Vascular permeability is regulated and maintained through three compartments including: paracellular junctions (adherent and junctional complexes), transcellular pathways (channels, VVOs and caveolae) and heterotypic cell interactions (usually pericytes). The three pathways are interconnected molecularly (blue arrows), however the details of this cross-talk remain largely unclear. BM: Basement membrane.
Mural cell control of vascular permeability
Historically, the majority of research on vascular permeability has been centered on transcellular regulation and endocytic transport. Nonetheless, in the last four years, a series of publications have focused on the contribution of mural cells as important instructional partners in the control of vascular permeability. These cells appear to convey tissue-specific control to endothelial barrier function. The term mural cell describes endothelial–associated cells that might not form a permanent sheath, but instead are dynamically associated with capillaries and functional participants of the “vascular unit”. Mural cells include pericytes, smooth muscle cells and macrophages. Depending on the tissue, mural cells also include astrocytes (brain) and podocytes (kidney) as per their tight association with blood vessels.
Recent evidence has brought to light the importance of pericytes in the regulation of permeability. Specifically, mice that lack pericytes showed increased permeability to water and a wide range of low- and high-molecular-mass tracers. The effect was most noticeable in the brain, indicating a stronger relevance of these cells in barrier regulation at this site. Interestingly, in adults, this in crease in permeability was mediated by endothelial transcytosis, which was reduced following activation of platelet derived growth factor (PDGFB) signalling (1).
Studies performed in developmental systems have further demonstrated a role for pericytes, and specifically PDGFRB signaling in the development of the blood brain barrier shortly after birth (2). Pericytes were shown to alter endothelial expression by suppression of molecules that increased vascular permeability (2). In particular, presence of pericytes is necessary to regulate the balance between angiopoietin-1 (high) and -2 (low) and thus control pro-permeability signals. Furthermore, through heterotypic cell interactions, pericytes instruct endothelial cell expression to suppress immune surveillance, a central feature of the blood brain barrier. Thus, absence of pericytes in Pdfrb−/− mice yield vessels with robust endothelial expression of IcamI, Alcam and Lgals3 (2). Combined, these experiments provide strong evidence that pericytes contribute to the stabilisation of the endothelial barrier, particularly in the brain, through regulation of endothelial expression and transendothelial transport to enhance barrier stability and suppress inflammation. Although there is much to be uncovered on the molecular cross-talk between endothelial cells and pericytes, the strong in vivo evidence indicating regulation of transcellular transport by pericytes points to a complex signalling circuitry that links heterotypic cell interactions with mechanisms of vesicular transport (3).
Transcellular permeability
Transcellular permeability is defined as an energy-dependent trafficking of macromolecules from the luminal space to the interstitium by means of vesicular transport. This transport can occur through: (a) caveolae; (b) vesiculo-vacuolar organelles (VVOs) and/or (c) transcellular channels (4, 5) (Figure 1 and Figure 2). In this section, we will highlight the conceptual advancements made in caveolae-mediated transport, and specifically focus on the integrative links between transcellular and paracellular transport.
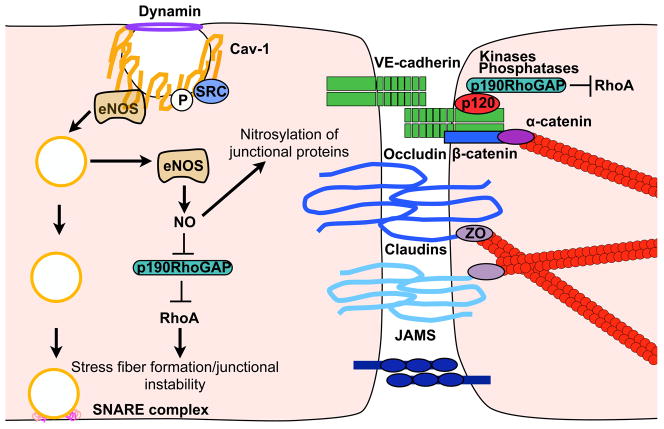
Figure 2. Cross talk between transcellular trafficking and paracellular junctional complexes.
Caveolae fission and loss of Cav-1 enhancee NOS mediated NO production. Nitrosylation of p190RhoGAP impairs inhibition of RhoA, resulting in stress fiber formation, junctional instability, and increased paracellular permeability. Direct nitrosylation of junctional proteins may also regulate junctional disassembly.
Caveolae are vesicles with high levels of caveolin-1 (Cav-1). This structural protein is critical for caveolae formation, as deletion of Cav-1 in mice results in the reduction of albumin transport (6, 7). More recently, it has become evident that along with its structural role, Cav-1 acts as a scaffold protein, recruiting Src kinase and G proteins to caveolae (8). As such, caveolae are capable of internalising cell surface macromolecular complexes and participating in cell signalling. Nonetheless, the extent to which this signalling function plays into regulation of vascular transport is yet to be clarified.
Endothelial signalling can affect transcellular trafficking through the phosphorylation of Cav-1 on Y14. Specifically src kinases have been shown to mediate Cav-1 phosphorylation downstream of growth factor signalling or upon generation of reactive oxygen species (9). While the role of phosphorylation on Cav-1 has been controversial, recent findings have shown that Cav-1 tyrosine phosphorylation is necessary for caveola biogenesis through a direct feedback loop that inactivates Erg-1 (early growth response-1) and thus enables transcription of both Cav-1 and cavin-1 (10).
Studies from Cav-1 null mice have suggested an active crosstalk between transcellular and paracellular pathways. Specifically, complete deletion, as well as, transient siRNA knockdown of Cav-1 result in an increased paracellular transport of albumin in small capillaries and veins (11, 12). This elevation in paracellular permeability, a possible compensatory mechanism for the impaired transcellular transport, was accompanied by abnormal tight junction assembly, detachment of endothelial cells from the basement membrane, and increased nitric oxide (NO) production (6, 11, 12). Endothelial nitric oxide synthetase (eNOS) inhibition restored junctional integrity in Cav-1 null mice, suggesting that eNOS-dependent redox signalling may indirectly mediate changes in paracellular permeability (11) (Figure 2). Recent elegant work has found that NO and peroxynitrile generation in the absence of Cav-1 promotes nitration of p190RhoGAP-A resulting in impaired GAP activity and subsequent RhoA activation. This increase in active RhoA is responsible herens junctions and increase in paracellular transport (13). These findings provided the missing molecular link to explain the crosstalk between Cav-1 and paracellular junctional complexes. In addition, Cav-1 may affect junctional integrity directly, through its interactions with Src, PKC, claudin-5, and actin-binding proteins, which are all involved in adherens and tight junction assembly and maintenance (8, 14). Cav-1 also binds TRPC1, a calcium transport channel important for the intracellular calcium release underlying actin-myosin remodelling (15, 16).
Consistent with the notion that Cav-1 regulates junctional complexes, it is interesting that Cav-1 levels are lower in postcapillary venules (17). Unlike arteries, venules display increased basal paracellular permeability and exhibit “unstable” junctional complexes (see discussion of constitutive VE-cadherin phosphorylation). These findings might imply that differences in Cav-1 levels may determine whether certain vascular beds are more amenable to increased transcytosis.
Together these findings provide molecular basis to the concept that transcellular vesicular trafficking, specifically through the contribution of Cav-1, regulates junctional integrity, and therefore, paracellular permeability in endothelial cells.
Paracellular permeability
As alluded to previously, maintenance of barrier function also requires the dynamic opening and closing of inter-endothelial junctions, which consist of a complex network of adherent proteins organised into adherens junctions and tight junctions. Although originally considered structural in function, it is now clear that the anchorage of adherens and tight junctions to the actin cytoskeleton allows the direct transmission of signalling events critical not only for barrier stability but also for the regulation of cell polarity, cellular movement, fluid sensing, and cell-contact inhibition (18). The distribution and predominance of junctional proteins at inter-endothelial contacts varies between different vascular beds, which suggest that junctional arrangement is unique to the functional needs of specific vascular networks (18, 19).
Adherens junctions are mostly formed by the clustering of homophilic calcium-dependent VE-cadherin proteins. The stability of VE-cadherin complexes between adjacent cells is regulated by phosphorylation. In fact, exposure of endothelial cells to several permeability mediators, such as VEGF, histamine and thrombin, results in tyrosine phosphorylation of VE-cadherin at Y658 and Y731, which correspond to the binding sites for p120 and β-catenin, respectively (20). Phosphorylation of VE-cadherin results in internalisation of the protein and disruption of barrier integrity resulting in vascular permeability. In addition, three other tyrosines (Y645, Y685, and Y733) and one serine (S665) have been reported to be potentially phosphorylated in vitro and participate in the regulation of permeability and leukocyte transmigration (20–23). Recently, a sophisticated study has demonstrated that phosphorylation of tyrosine residues 658 and 685 of VE-cadherin is constitutive in veins, but not in arteries. This phosphorylation is mediated by src and can be enhanced in response to bradykinin or histamine. More importantly, point mutations, Y658F and Y685F, prevent internalization of VE-cadherin and thus block vascular permeability (24). The endogenous phosphatase for VE-cadherin, VE-PTP is frequently associated with the protein and prevents VE-cadherin phosphorylation, promoting an important stabilizing role of endothelial contacts in vivo (25, 26).
Intracellularly, VE-cadherin is directly and indirectly bound to a complex network of proteins including catenins, actin binding proteins, RhoGTPases, kinases, and phosphatases, that are important for its tethering and signalling to the actin cytoskeleton (4, 18) (Figure 2). While β-catenin and plakoglobin prevent VE-cadherin proteolysis, p120-catenin alters retention of VE-cadherin at the cell surface (8, 27). Additionally, β-catenin and p120 are also critical for spatial organisation and control of the actin cytoskeleton by way of RhoGTPase activation (p190RhoGAP, Rac1, Cdc42, RhoA), and α-catenin recruitment (18). Mice engineered to express a VE-cadherin-α-catenin fusion protein developed strong stable junctions, highlighting the relevance of plasticity of cadherin- catenin complexes in the regulation of permeability (28).
Phosphorylation of other adherens junctional components also modulate the affinity of adherens junction complex components for one another, thus affecting junctional stability (8). Whether these phosphorylation events are important for barrier regulation in vivo is only beginning to be understood. Recently, the generation of a serine phosphodeficient p120 mouse demonstrated the requirement of PKCα-mediated p120 phosphorylation for p120/VE-cadherin dissociation following thrombin and lipopolysaccharide (LPS) stimulated permeability (29). Therefore, generation of phospho-mutant mice will enable dissection of the molecular events that are downstream of individual permeability mediators in vivo.
Major tight junction proteins include claudin-5, occludin, and junctional adhesion molecules (JAM). Similar to adherens junctions, phosphorylation of both tight junction proteins and their intracellular partners (ZO-1 and MAGUKS) regulate tight junction assembly and mediate changes in vascular permeability (30, 31). Although complete deletion of claudin-5 leads to early lethality shortly after birth due to blood brain barrier disruption, occluding knockout mice have no apparent defects, making its function in endothelial tight junctions less obvious (32).
While all JAM members are present in endothelial cells, only JAM-C leads to increased permeability when expressed at the cell surface of microvascular cells following stimulation with VEGF or histamine (33). Although the mechanism of JAM-C mediated permeability is still unclear, recent evidence suggests that JAM-C regulation of αVβ3 integrin localisation and activation downstream of Rap1b signalling may account for barrier breakdown (34). JAM-like molecules have also been implicated in permeability regulation, as mice null for endothelial cell-selective adhesion molecule showed reduced vascular permeability to VEGF (35).
Emerging data has indicated that adherens and tight junctions do not function independently in the regulation of barrier function, and in fact, communication between these complexes is important for permeability regulation. Akt activation downstream of VE-cadherin cell surface clustering results in nuclear expulsion of the transcription factor FoxO1. FoxO1 normally inhibits claudin-5 expression, thus translocation from the nucleus results in enhanced claudin-5 expression at tight junctions (36). Furthermore, VE-cadherin transmits shear stress signals to stabilise occluding through recruitment of Tiam1/Rac-1 and mediates reduction of occludin phosphorylation (37). These findings place VE-cadherin as a key sensor and molecular integrator of adherens and tight junctions.
Overall, this data indicates that intracellular signalling cascades and homeostasis of barrier function are more linked than previously appreciated. Furthermore, the interaction between adherens and tight junctions reveals an exquisite level of molecular regulation that is only now starting to be unraveled.
Signalling mechanisms and intracellular regulation of vascular permeability
Most mediators of permeability lead to phosphorylation of junctional proteins and reorganisation of the acto-myosin apparatus, although these consequences can occur downstream of different signal transduction pathways (Figure 3). The kinetics of these changes varies between permeability agents, as some can lead to transient and reversible effects, as in the case of thrombin and histamine, or sustained and prolonged regulation, as seen with VEGF and LPS stimulation. This section will review recent literature on some of the most well studied permeability agents and the signaling pathways that discern them.
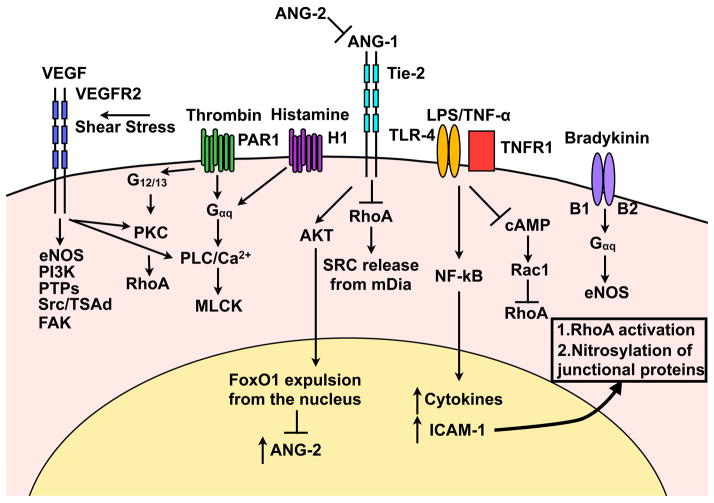
Figure 3. Signal transduction pathways that increase paracellular permeability.
VEGF activation of VEGFR2 initiates several downstream signalling cascades leading to adherens protein internalisation, calcium release, and stress fiber formation. Thrombin and histamine, via G-protein coupled receptors, results in RhoA activation, calcium release and the development of stress fibers. Ang-2 inhibits the barrier stabilising effect of Ang-1 thus making the barrier vulnerable to permeability enhancing agents. LPS and TNF-α signalling result in NF-κB nuclear translocation where increased ICAM-1 expression leads to RhoA activation and NO-mediated nitrosylation of junctional proteins. Bradykinin promotes eNOS signalling.
1. Inflammatory mediators
Histamine, thrombin, and bradykinin exposure result in a transient increase in vascular permeability followed by barrier stabilisation. Thrombin signalling through its receptor, PAR-1, yields a transient increase in vascular permeability which is followed by an equally rapid restoration. PAR-1 activates several downstream G proteins, which promotes intracellular calcium release, RhoA-dependent activation of myosin light chain kinase and cell contraction (38). Rho activation leads to stress fiber assembly and cell contraction, a mechanism that is responsible for enhancement of permeability. Histamine also promotes permeability through calcium release and myosin light chain kinase activation, but in addition, mediates src-dependent phosphorylation of adherens and tight junction proteins. Conversely, bradykinin, acting through B1 and B2 receptors, results in an eNOS/iNOS dependent increase in permeability, although it is unclear whether nitrosylation of junctional proteins following increased NO production leads to barrier destabilisation (4, 39).
Long-term mediators of permeability such as LPS and tumour necrosis factor (TNF)-α, result in nuclear factor (NF)-κB transcriptional expression of cytokines and leukocyte adhesion molecules. ICAM-1 cell surface activation results in RhoA directed stress fiber formation as well as increased NO production, which further potentiates increased permeability (39).
2. Vascular endothelial growth factor (VEGF)
VEGF induces vascular permeability by several mechanisms, including junctional remodelling, induction of fenestrae, and VVOs (40). VEGF concurrently activates multiple signalling pathways downstream of VEGFR2 that have been implicated in vascular permeability. These include PLC-dependent intracellular calcium release, src kinase-mediated phosphorylation/internalisation of junctional proteins, RhoGTPase activation, cytoskeletal rearrangement, and eNOS signalling (41). More recently, in vivo data has demonstrated the requirement for VE-PTP/VE-cadherin dissociation (26, 42) and FAK-dependent β-catenin phosphorylation (43) in VEGF-mediated permeability. Furthermore, the T-cell-specific adapter, TSAd was found to be essential for src activation and subsequent phosphorylation of junctional proteins downstream of VEGFR2 (44). The contribution of eNOS signalling upon VEGFR2 activation has remained elusive, but recent data suggest that nitrosylation of β-catenin by NO may be an additional mechanism of junctional destabilisation by mediating the dissociation of β-catenin from VE-cadherin (45).
Although there have been great strides in understanding the molecular players that coordinate permeability downstream of VEGF, the complexity of the signalling networks has made it difficult to understand how all of these pathways interact to control barrier function. Furthermore, quantitative assessment of each of these signalling pathways in vivo has not been obtained. It is possible that subsets of downstream mediators are activated in distinct vascular beds or under different physiological contexts upon VEGF exposure. With regards to angiogenesis, the in vivo presentation of VEGF isoforms (in the context of matrix or soluble) results in differential signal transduction outputs (46, 47). It is likely that similar nuances partake in the regulation of vascular permeability by VEGF.
3. Angiopoietin (Ang)/Tie receptor signalling
Tie receptors and their ligands (Ang1–4) are critical regulators of vascular maturation and quiescence (48). Tie-2 is constitutively phosphorylated upon binding to Ang-1 in mature vessels. In fact, Ang-1 secretion from perivascular cells is important for maintaining vascular stability and endothelial cell adhesion while inhibiting vascular permeability (49, 50). Ang-1/Tie-2 signalling has been shown to inhibit VEGF-mediated vascular permeability via several downstream signalling cascades. These include p190RhoGAP driven cytoskeletal modulation (51), sequestration of Src from VEGFR2 by the RhoGTPase effector protein mDia (52), inhibition of calcium release (53), and phosphorylation of eNOS by atypical PKC-zeta (54).
One of the main questions in understanding Ang-1 signalling is how it can orchestrate both vascular remodelling and quiescence by signalling through the same receptor. Recent evidence suggests that Ang-1 stimulation leads to differential Tie-2 localisation and signalling depending on whether endothelial cells have engaged cell-cell contacts or not. Homotypic cell interactions between endothelial cells trigger recruitment of Tie2 to cell-cell contacts upon Ang-1 exposure leading to enhanced vascular stability following Akt-mediated eNOS phosphorylation. In contrast, migrating endothelial cells displayed Dok-R phosphorylation and Tie-2 recruitment to the cell rear (55, 56).
Endothelial produced Ang-2 is considered to be the natural antagonist of Ang-1 activity by inhibiting phosphorylation of Tie-2 (48). Thus, Ang-2 sensitises the endothelium to both growth factors and inflammatory mediators, which increase vascular destabilization (57). The mechanism of Ang-2 action is not fully understood, but recent evidence suggests that Ang-2 regulates Tie-2 interaction with αVβ3 integrin, resulting in FAK activation and consequent integrin internalisation and degradation (58). Although both Ang-1 and Ang-2 mediate Tie-2 clustering at cell-cell contacts, their differential signalling may explain their opposing effects on vascular stability. Several groups have also demonstrated that Ang-2 can act as a partial agonist of Tie-2 signalling through Tie-2 phosphorylation (59, 60) and can enhance barrier function following endothelial stress (61). Generation of mice with endothelial specific deletion of Ang-2 will help to address its physiological role in vivo and enable a better understanding of the homeostatic functions of Ang-2 in the endothelium.
Barrier stabilisation
Barrier restoration is critical for maintenance of basal permeability and recovery following exposure to acute inflammatory events, yet our understanding of how this process occurs at the molecular level has remained elusive. Here, we discuss some of the mediators of barrier stability and their known mechanisms of action (Figure 4).
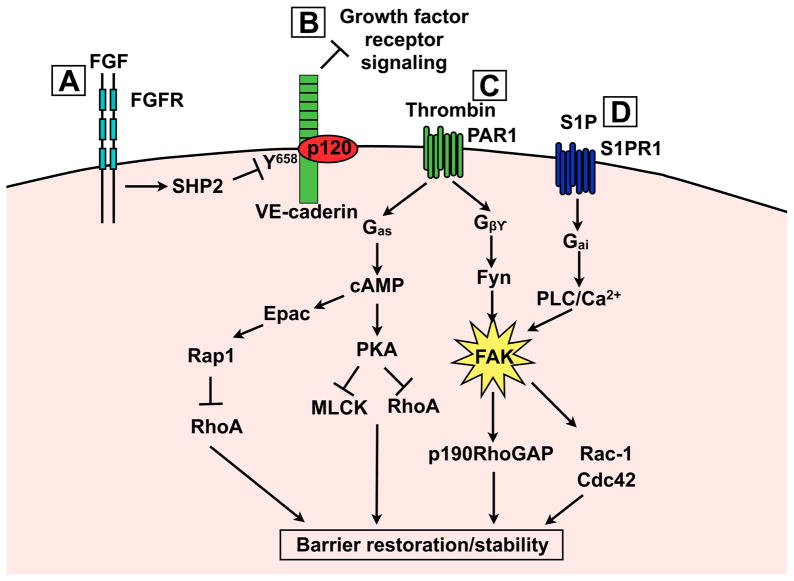
Figure 4. Signalling mechanisms leading to enhanced barrier stability and restoration.
A) FGF signalling increases basal barrier function through SHP2 phosphatase mediated p120/VEcadherin complex stabilisation. B) VE-cadherin can inhibit growth factor receptors that normally enhance permeability. C) Gβγ and Gas signal transduction results in FAK, Epac/Rap1 and PKA activation. All three of these targets coordinate to increase cortical actin and stabilise junctional complexes. D) S1P signalling leads to FAK phosphorylation via a PLC-dependent mechanism. Differential phosphorylation and cellular recruitment of FAK may explain how thrombin and S1P regulate FAK by distinct mechanisms.
Although the mechanisms of barrier breakdown following thrombin have been well studied, the actual process whereby the endothelial barrier is stabilised quickly thereafter has only begun to be understood. Recent evidence has demonstrated that a G protein downstream of thrombin activation Gβ1, increases barrier stabilisation by redistribution of focal adhesion kinase (FAK) to adherens junctions following Fyn-induced phosphorylation of FAK (62) (Figure 4 C). How FAK becomes targeted to adherens junctions remains to be clarified.
Signalling via cAMP also contributes to the regulation of barrier function. Increases in cAMP levels downstream of the G protein, Gαs, reduces vascular leakage through activation of protein kinase A (PKA) and the guanine exchange factor, Epac (Figure 4 C). Epac mediated Rap1 activation results in increased junctional adhesions and reorganisation of actin filaments (63). Emerging evidence on Rap1 suggests it has a cooperative association with VE-cadherin as they can both modulate each other’s responses (64,65). Interestingly, Rap1 can increase KRIT-1 targeting to endothelial cell-cell junctions, suppressing stress fibre formation and stabilizing the barrier. Thus, defects in Rap1 signalling downstream of mutated KRIT-1 protein may explain the loss of vascular integrity seen in cerebral cavernous malformations (66).
More recently, fibroblast growth factor (FGF) has been found to play an important role in adherens junction integrity. Absence of FGF signalling was found to reduce expression of the phosphatase, SHP2, resulting in increased phosphorylation of VE-cadherin, impairing its ability to bind p120 catenin (67, 68) (Figure 4 A). VEcadherin itself can affect barrier stability by inhibiting growth factor signalling pathways including VEGF, TGFB, and PDGF, which promote permeability following angiogenic responses (19) (Figure 4 B).
Another emerging and potent barrier stabilising factor is sphingosine-1-phosphate (S1P). S1P circulates at high levels in the blood and signals through the G-coupled protein receptor S1P1 to mediate cortical actin organisation via a number of downstream targets including Rac-1, cortactin, FAK, paxillin, and actinin 1 and 4 (8, 38, 69) (Figure 4 D). Two recent studies have unequivocally demonstrated the effect of S1P in barrier stability in vivo. Pharmacological or genetic blockade of the S1P signalling axis results in adherens junctions destabilisation, permeability and in some cases angiogenesis (70, 71).
Unlike thrombin, S1P signals exclusively through the G protein Gi. Gi activation leads to PLC-dependent calcium release, which is necessary for FAK phosphorylation (38). FAK activation is required for barrier integrity, as impairment of FAK function leads to increased endothelial permeability and subsequently abrogates S1P barrier enhancement (38, 69, 72). Although S1P signals through a different G protein cascade, Fyn activation of FAK as seen downstream of thrombin, may also play a role in S1P signalling.
Interestingly, FAK has been shown to both preserve and disrupt the endothelial barrier (43, 73, 74). This dual ability has been proposed to be regulated by other events including: differential posttranslational modifications, spatial/temporal activation, cellular localisation, or association with binding partners (38, 75). Recent evidence in support of this, demonstrated that alternative phorylation and cellular localisation of FAK contribute to the differential mode of barrier restoration seen following thrombin and S1P stimulation (62, 69, 76).
Transcriptional mechanisms of barrier regulation
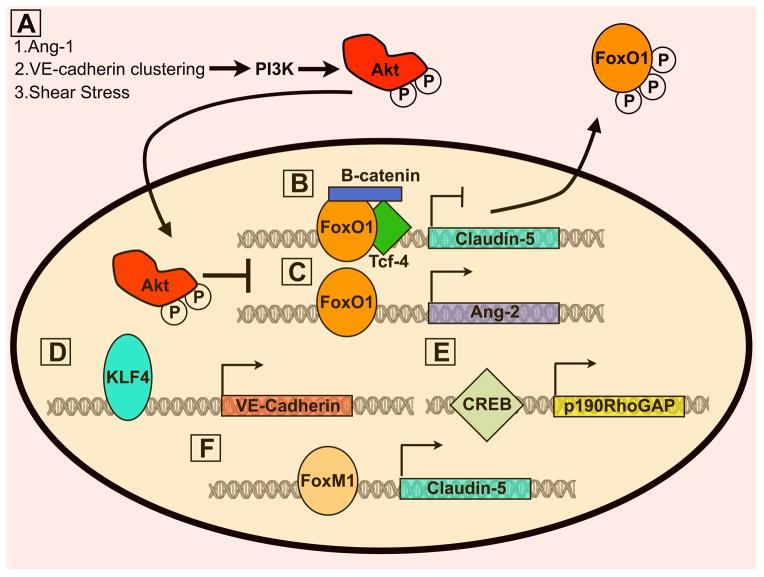
While most events in the regulation of barrier function are nontranscriptional in nature, evidence that transcriptional activation/repression is also required has recently been revealed (Figure 5). Both shear stress and Ang-1 signalling to Akt mediates endothelial quiescence by FoxO1 phosphorylation and subsequent exclusion from the nucleus (Figure 5 A). FoxO1 target genes include Ang-2 and genes important for matrix remodelling and migration. Thus, inhibition of FoxO1 is important for restricting the expression of barrier destabilising proteins (77, 78) (Figure 5 C). In addition, interaction of FoxO1 with β-catenin and Tcf was found to transcriptionally repress claudin-5 expression. VE-cadherin sequestration of β-catenin from the nucleus inhibits its association with FoxO1, enabling claudin-5 expression and junctional stability (36) (Figure 5 B). Conversely, another forkhead member, FoxM1, positively regulates β-catenin expression (Figure 5 F). Surprisingly basal permeability was not affected following endothelial deletion of FoxM1 in vivo; however, barrier stability could not be restored following thrombin treatment (69).
Figure 5. Transcriptional regulation of vascular permeability.
A) Akt phosphorylation by several stimuli results in FoxO1 phosphorylation and nuclear translocation. Subsequently, FoxO1 target genes including claudin-5 (B) and Ang-2(C) are expressed and repressed, respectively. Claudin-5 inhibition by FoxO1 requires complex formation between β-catenin and Tcf. Therefore, VE-cadherin/β-catenin complex formation is important for claudin-5 expression as it prevent β-catenin translocation to the nucleus. D) KLF4 stabilises the barrier by enhancing expression of VE-cadherin. E) CREB upregulates p190RhoGAP, which is important for inhibiting RhoA activation at adherens junctions. F) Both estrogen receptor (not depicted) and FoxM1 increase claudin-5 expression, thus promoting barrier stability.
The Krüppel-like family member, KLF4, directly binds the VEcadherin promoter and upregulates its expression. Basal permeability is increased following KLF4 knockdown in vitro and in mouse lung microvasculature (79) (Figure 5 D). Analogously, KLF2 also stabilises barrier function, as heterozygous loss of KLF2 in mice leads to increased basal permeability and exacerbated barrier disruption upon addition of histamine and H2O2. How KLF2 mediates barrier function and whether this requires transcriptional activation of KLF2 was not determined (80).
In addition to junctional proteins, elements of the cytoskeleton and regulation of its dynamics are essential to the initiation and restoration of vascular permeability. Along these lines, the contribution of small GTPases, as means of controlling contractility and dynamics of the cytoskeleton, have received significant attention (81–83). Transcriptional regulation of RhoGTPases was found to be coordinated by factors that regulate multiple aspects of the permeability response. For example, CREB (cAMP response element binding) directly regulates p190RhoGAP, a RhoA inhibitor important for barrier stabilisation (Figure 5 E). In support of this, in vivo expression of endothelial dominant negative CREB enhanced basal permeability and exacerbated the response to thrombin and LPS (84).
Recently, two nuclear hormone receptors, Nur77 and estrogen receptor, have also been implicated in barrier regulation. Nur77 is increased upon exposure to VEGF, histamine, and serotonin resulting in barrier destabilisation through the downregulation of several adherent junctional components. Transcriptional activity of Nur77 was found to be required, but whether Nur77 directly binds to the promoters of these adhesion molecules was not addressed (85). Interestingly, estrogen signalling through the estrogen receptor directly upregulates claudin-5 expression and thus, possibly is important for barrier stability and restoration (86). It is apparent from these results that regulation of barrier function cannot simply be explained by transient signalling events. Additional research on transcriptional mediation may reveal further insight into the complexities of permeability regulation.
Acknowledgments
The authors wish to acknowledge funding from the National Institutes of Health, NHLBI, RO1 HL74455-01 to MLIA, the Ruth L. Kirschstein National Research Service Award (T32HL69766 to L.M.G), the American Heart Association Predoctoral Fellowship (AHA-11PRE7300043 to L.M.G), and funding from the Iris Cantor- UCLA Women’s Health Center Executive Advisory Board.
Footnotes
Conflicts of Interest
None declared.
References
- 1.Armulik A, Genové G, Mäe M, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 2.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Mehta D. Signalling Mechanisms Regulating Endothelial Permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 6.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M008340200. [DOI] [PubMed] [Google Scholar]
- 7.Schubert W, Frank PG, Razani B, et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–48622. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 8.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Hu G, Zhang X, et al. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ Res. 2009;105:676–685. doi: 10.1161/CIRCRESAHA.109.201673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi B, Bastiani M, Strgnell SS, et al. Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. J Cell Biol. 2012;199:425–435. doi: 10.1083/jcb.201207089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schubert W, Frank PG, Woodman SE, et al. Microvascular hyperpermeability in caveolin-1 (−/−) knock-out mice. Treatment with a specific nitric-oxide synthase inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J Biol Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- 12.Miyawaki-Shimizu K, Predescu D, Shimizu J, et al. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L405–413. doi: 10.1152/ajplung.00292.2005. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui MR, Komarova YA, Vogel SM, et al. Caveolin-1-eNOS signalling promotes p190RhoGAP-A nitration and endothelial permeability. J Cell Biol. 2011;30:841–850. doi: 10.1083/jcb.201012129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minshall RD, Sessa WC, Stan RV, et al. Caveolin regulation of endothelial function. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1179–1183. doi: 10.1152/ajplung.00242.2003. [DOI] [PubMed] [Google Scholar]
- 15.Sundivakkam PC, Kwiatek AM, Sharma TT, et al. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiatek AM, Minshall RD, Cool DR, et al. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 17.Marmon S, Hinchey J, Oh P, et al. Caveolin-1 Expression Determines the Route of Neutrophil Extravasation through Skin Microvasculature. Am J Pathol. 2009;174:684–692. doi: 10.2353/ajpath.2009.080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejana E, Tournier-Lasserve E, Weinstein BM. The Control of Vascular Integrity by Endothelial Cell Junctions: Molecular Basis and Pathological Implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Dejana E, Giampietro C. Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol. 2012;19:218–223. doi: 10.1097/MOH.0b013e3283523e1c. [DOI] [PubMed] [Google Scholar]
- 20.Potter MD, Barbero S, Cheresh DA. Tyrosine phosphorylation of VE-cadherin prevents binding of p120- and beta-catenin and maintains the cellular mesenchymal state. J Biol Chem. 2005;280:31906–31912. doi: 10.1074/jbc.M505568200. [DOI] [PubMed] [Google Scholar]
- 21.Baumeister U, Funke R, Ebnet K, et al. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 23.Turowski P, Martinelli R, Crawford R, et al. Phosphorylation of vascular endothelial cadherin controls lymphocyte emigration. J Cell Sci. 2008;121:29–37. doi: 10.1242/jcs.022681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orsenigo F, Giampietro C, Ferrari A, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun. 2012;3:1208. doi: 10.1038/ncomms2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vestweber D, Winderlich M, Cagna G, et al. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2009;19:8–15. doi: 10.1016/j.tcb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Broermann A, Winderlich M, Block H, et al. Dissociation of VE-PTP from VE-cadherin is required for leukocyte extravasation and for VEGF-induced vascular permeability in vivo. J Exp Med. 2011;208:2393–2401. doi: 10.1084/jem.20110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vandenbroucke E, Mehta D, Minshall R, et al. Regulation of Endothelial Junctional Permeability. Ann NY Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 28.Schulte D, Kuppers V, Dartsch N, et al. Stabilising the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenbroucke St Amant E, Tauseef M, et al. PKCα Activation of p120-Catenin Serine 879 Phospho-Switch Disassembles VE-Cadherin Junctions and Disrupts Vascular Integrity. Circ Res. 2012;111:739–749. doi: 10.1161/CIRCRESAHA.112.269654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signalling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuse M. Knockout animals and natural mutations as experimental and diagnostic tool for studying tight junction functions in vivo. Biochim Biophys Acta. 2009;1788:813–819. doi: 10.1016/j.bbamem.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Orlova VV, Economopoulou M, Lupu F, et al. Junctional adhesion molecule-C regulates vascular endothelial permeability by modulating VE-cadherin-mediated cell-cell contacts. J Exp Med. 2006;203:2703–2714. doi: 10.1084/jem.20051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Stankovic M, Lee BP, et al. JAM-C induces endothelial cell permeability through its association and regulation of {beta}3 integrins. Arterioscler Thromb Vasc Biol. 2009;29:1200–1206. doi: 10.1161/ATVBAHA.109.189217. [DOI] [PubMed] [Google Scholar]
- 35.Wegmann F, Petri B, Khandoga AG, et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 37.Walsh TG, Murphy RP, Fitzpatrick P, et al. Stabilisation of brain microvascular endothelial barrier function by shear stress involves VE-cadherin signalling leading to modulation of pTyr-occludin levels. J Cell Physiol. 2011;226:3053–3063. doi: 10.1002/jcp.22655. [DOI] [PubMed] [Google Scholar]
- 38.Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res. 2012;83:31–44. doi: 10.1016/j.mvr.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chavez A, Smith M, Mehta D. New insights into the regulation of vascular permeability. Int Rev Cell Mol Biol. 2011;290:205–248. doi: 10.1016/B978-0-12-386037-8.00001-6. [DOI] [PubMed] [Google Scholar]
- 40.Weis SM, Cheresh DA. Pathophysiological consequences of VEGF-induced vascular permeability. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- 41.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nottebaum AF, Cagna G, Winderlich M, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205:2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen XL, Nam JO, Jean C, et al. VEGF-Induced Vascular Permeability Is Mediated by FAK. Dev Cell. 2012;22:146–157. doi: 10.1016/j.devcel.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Z, Li X, Massena S, et al. VEGFR2 induces c-Src signalling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med. 2012;209:1363–1377. doi: 10.1084/jem.20111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibeault S, Rautureau Y, Oubaha M, et al. S-Nitrosylation of beta-Catenin by eNOS-Derived NO Promotes VEGF-Induced Endothelial Cell Permeability. Mol Cell. 2010;39:468–476. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Lee SS, Jilani SM, Nikolova GV, et al. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen TT, Luque A, Lee S, et al. Anchorage of VEGF to the extracellular matrix conveys differential signalling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 49.Wong AL, Haroon ZA, Werner S, et al. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–574. doi: 10.1161/01.RES.81.4.567. [DOI] [PubMed] [Google Scholar]
- 50.Thurston G, Rudge JS, Ioffe E, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 51.Mammoto T, Parikh SM, Mammoto A, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 52.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 53.Jho D, Mehta D, Ahmmed G, et al. Angiopoietin-1 Opposes VEGF-Induced Increase in Endothelial Permeability by Inhibiting TRPC1-Dependent Ca2 Influx. Circ Res. 2005;96:1282–90. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 54.Oubaha M, Gratton JP. Phosphorylation of endothelial nitric oxide synthase by atypical PKC zeta contributes to angiopoietin-1-dependent inhibition of VEGF-induced endothelial permeability in vitro. Blood. 2009;114:3343–3351. doi: 10.1182/blood-2008-12-196584. [DOI] [PubMed] [Google Scholar]
- 55.Saharinen P, Eklund L, Miettinen J, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 56.Fukuhara S, Sako K, Minami T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 57.Scharpfenecker M, Fiedler U, Reiss Y, et al. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 58.Thomas M, Felcht M, Kruse K, et al. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalisation and degradation. J Biol Chem. 2010;285:23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan HT, Khankin EV, Karumanchi SA, et al. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signalling in the endothelium. Mol Cell Biol. 2009;29:2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harfouche R, Hussain SN. Signalling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol. 2006;291:H1635–1645. doi: 10.1152/ajpheart.01318.2005. [DOI] [PubMed] [Google Scholar]
- 61.Daly C, Pasnikowski E, Burova E, et al. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci USA. 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knezevic N, Tauseef M, Thennes T, et al. The G protein betagamma subunit mediates reannealing of adherens junctions to reverse endothelial permeability increase by thrombin. J Exp Med. 2009;206:2761–2777. doi: 10.1084/jem.20090652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cullere X, Shaw SK, Andersson L, et al. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 64.Fukuhara S, Sakurai A, Sano H, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signalling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 66.Glading A, Han J, Stockton RA, et al. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol. 2007;179:247–254. doi: 10.1083/jcb.200705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murakami M, Nguyen LT, Zhuang ZW, et al. The FGF system has a key role in regulating vascular integrity. J Clin Invest. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatanaka K, Lanahan A, Murakami M, et al. Fibroblast Growth Factor Signalling Potentiates VE-Cadherin Stability at Adherens Junctions by Regulating SHP2. PLoS One. 2012;7:e37600. doi: 10.1371/journal.pone.0037600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun X, Shikata Y, Wang L, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77:304–313. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung B, Obinata H, Galvani S, et al. Flow-regulated endothelial S1P receptor-1 signalling sustains vascular development. Dev Cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaengel K, Niaudet C, Hagikura K, et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell. 2012;23:587–599. doi: 10.1016/j.devcel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Shikata Y, Birukov KG, Garcia JG. S1P induces FA remodelling in human pulmonary endothelial cells: role of Rac, GIT1, FAK, and paxillin. J Appl Physiol. 2003;94:1193–1203. doi: 10.1152/japplphysiol.00690.2002. [DOI] [PubMed] [Google Scholar]
- 73.Guo M, Wu MH, Granger HJ, et al. Focal adhesion kinase in neutrophil-induced microvascular hyperpermeability. Microcirculation. 2005;12:223–232. doi: 10.1080/10739680590905251. [DOI] [PubMed] [Google Scholar]
- 74.Lee YH, Kayyali US, Sousa AM, et al. Transforming growth factor-beta1 effects on endothelial monolayer permeability involve focal adhesion kinase/Src. Am J Respir Cell Mol Biol. 2007;37:485–493. doi: 10.1165/rcmb.2006-0439OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belvitch P, Dudek SM. Role of FAK in S1P-regulated endothelial permeability. Microvasc Res. 2012;83:22–30. doi: 10.1016/j.mvr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shikata Y, Birukov KG, Birukova AA, et al. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodelling: role of Src and GIT. FASEB J. 2003;17:2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 77.Daly C, Wong V, Burova E, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18:1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dixit M, Bess E, Fisslthaler B, et al. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc Res. 2008;77:160–168. doi: 10.1093/cvr/cvm017. [DOI] [PubMed] [Google Scholar]
- 79.Cowan CE, Kohler EE, Dugan TA, et al. Kruppel-Like Factor-4 Transcriptionally Regulates VE-Cadherin Expression and Endothelial Barrier Function. Circ Res. 2010;107:959–966. doi: 10.1161/CIRCRESAHA.110.219592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Z, Natesan V, Shi H, et al. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haidari M, Zhang W, Chen Z, et al. Myosin light chain phosphorylation facilitates monocyte transendothelial migration by dissociating endothelial adherens junctions. Cardiovasc Res. 2011;92:456–465. doi: 10.1093/cvr/cvr240. [DOI] [PubMed] [Google Scholar]
- 82.David S, Ghosh CC, Mukherjee A, et al. Angiopoietin-1 Requires IQ Domain GTPase-Activating Protein 1 to Activate Rac1 and Promote Endothelial Barrier Defense. Arterioscler Thromb Vasc Biol. 2011;31:2643–2652. doi: 10.1161/ATVBAHA.111.233189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng J, He F, Zhang C, et al. Protein kinase Ca signals P115RhoGEF phosphorylation and RhoA activation in TNF-a-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation. 2011;8:28. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chava KR, Tauseef M, Sharma T, et al. Cyclic AMP response element binding (CREB) protein prevents endothelial permeability increase through transcriptional controlling p190RhoGAP expression. Blood. 2012;119:308–319. doi: 10.1182/blood-2011-02-339473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao D, Qin L, Bourbon PM, et al. Orphan nuclear transcription factor TR3/Nur77 regulates microvessel permeability by targeting endothelial nitric oxide synthase and destabilising endothelial junctions. Proc Natl Acad Sci USA. 2011;108:12066–12071. doi: 10.1073/pnas.1018438108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burek M, Arias-Loza PA, Roewer N, et al. Claudin-5 as a novel estrogen target in vascular endothelium. Arterioscler Thromb Vasc Biol. 2010;30:298–304. doi: 10.1161/ATVBAHA.109.197582. [DOI] [PubMed] [Google Scholar]