Abstract
Background aims
Polarized mature dendritic cells (DC) can activate cytolytic T-lymphocyte (CTL) responses and may be a more effective clinical strategy in DC-based cancer vaccines. A subset of mature DC can down-regulate the T-cell immune response through expression of indoleamine-2,3-dioxygenase (IDO). We determined whether polarizing DC ex vivo increased IDO expression and activity.
Methods
Peripheral blood monocytes from healthy volunteers were cultured ex vivo in polarizing and non-polarizing culture conditions. DC IDO expression was detected by Western blot. IDO enzyme activity was determined by high-performance liquid chromatography (HPLC) measurement of kynurenine (K) and tryptophan (T) concentrations in culture supernatants.
Results
IDO protein was markedly increased in DC after polarization (median 1222.4%, range 331.5–2113.3%) versus non-polarized DC (median 28.3%, range 3.7–119.8%; P = 0.04). The median K/T ratio was significantly higher in polarized DC versus non-polarized DC (6.34, range 6.02–6.65, versus 0.047, range 0.004–0.541; P = 0.04). IDO protein expression correlated with enzyme activity (r = 0.80, P = 0.002).
Conclusions
DC polarizing culture conditions increased expression of IDO protein and IDO enzyme activity. DC culture and maturation methodologies may impact the effectiveness of adoptive DC therapy.
Keywords: dendritic cell; immunotherapy; indoleamine 2,3-dioxygenase; interferon-gamma; lipopolysaccharide
Introduction
Dendritic cells (DC) play a critical role in initiating T-cell responses to cancer and have been used as therapeutics in the treatment of patients with advanced cancer [1]. DC are polarized into functional subsets involved with initiation of humoral and cellular immunity. The optimal DC subtype for clinical therapy has not yet been established. DC polarized ex vivo to DC1, which activate a T-helper type-1 (Th1) and cytotoxic T-cell response, may provide a better strategy for therapeutic vaccines in human cancer [2,3]. Polarization of DC to DC1 with lipopolysaccharide (LPS) and interferon (IFN)-γ is used in clinical settings [2,4].
A subgroup of DC that expresses indoleamine 2′3′ dioxygenase (IDO), the rate-limiting enzyme in tryptophan catabolism, has an important role in immune tolerance [5,6]. IDO is transcribed and expressed as a pro-enzyme, requiring an additional proteolytic step for activation. Tryptophan, an essential amino acid, can be depleted by IDO in the local tissue micro-environment and induce T-cell apoptosis and impair normal T-cell function [5,7,8]. We therefore investigated whether established ex vivo culture conditions used to generate ‘clinical-grade’ DC preferentially induce functional IDO protein and are thus likely to enhance the immunosuppressive properties of DC.
Methods
Ex vivo DC cell culture and polarization
Two healthy male donors, after giving written informed consent to an Institutional Review Board (IRB)-approved protocol, underwent a standard leukapheresis. From the obtained leukapheresis product, monocytes were isolated by elutriation fractionation (Elutra System; Gambro BCT, Lakewood, CO, USA); 2.5 × 107 monocytes at 3–3.5 × 106 cells/mL (culture bags; VueLife, American Fluoroseal Corp., Gaithersburg, MD, USA) were cultured using six different conditions (Table I). Condition 1, without maturation factors, was used to generate immature DC. Conditions 2, 3, 4 and 6 utilized various methods for maturing DC and were derived from established ex vivo culture methods used for clinical vaccine trials. Condition 5 was a conventional method used to polarize DC to DC1 [2,9]. On the day of DC harvest (Table I), aliquots of the supernatant were collected and stored at −80°C until thawed for measurement of kynurenine and tryptophan concentrations. All DC culture condition experiments were performed in duplicate.
Table I.
Chemicals and cytokines used in the stated culture conditions.
| Cytokines added | ||||||
|---|---|---|---|---|---|---|
| Condition | Addition at day 0, 3, 6 | Day (6/7) | Day (5/6/7) | Day of harvest |
||
| 1 | Immature DC | GM-CSF (500 IU/mL) | IL-4 (20 ng/mL) | 9 | ||
| 2 | Mature DC | GM-CSF (500 IU/mL) | IL-4 (20 ng/mL) | TNF-α (50 ng/mL) (6) | 9 | |
| 3 | Mature DC | GM-CSF (500 IU/mL) | IL-4 (20 ng/mL) | TNF-α (50 ng/mL) (7) | PGE2 (1 µg/mL) (7) | 8 |
| 4 | Mature DC | GM-CSF (1000 IU/mL) | IL-4 (40 ng/mL) | TNF-α (50 ng/mL) (6) | PGE2 (1 µg/mL) (6) | 7 |
| 5 | Polarized DC | GM-CSF (500 IU/mL) | IL-4 (20 ng/mL) | LPS (250 ng/mL) (6) | IFN-γ (0.5 µg/mL) (5) | 7 |
| 6 | Mature DC | GM-CSF (500 IU/mL) | IL-4 (20 ng/mL) | TNF-α (50 ng/mL) (6) | Sunitinib (100 ng/mL) (6) | 9 |
Suppliers: IL-4, TNF-α (R&D Systems, Minneapolis, MN, USA), GM-CSF (Berlex, Bayer Healthcare, Seattle, WA, USA), IFN-γ, PGE2 (Sigma Aldrich, St. Louis, MO, USA), LPS (List Lab, Gainesville, FL, USA) and sunitinib (Pfizer, New York, NY, USA).
Western blot for IDO protein
For Western blot analysis, lysate from 1.6 × 105 cells/lane was loaded on a 12% polyacrylamide gel (Pierce, Rockford, IL, USA). After electrophoresis and blotting, nitrocellulose membranes were first exposed to anti-human IDO antibody (Ab) (1:1000; Chemicon, Temecula, CA, USA) for 1 h and then exposed to horseradish peroxidase secondary Ab (1:3000 goat anti-mouse IgG–HRP; BioRad, Hercules, CA, USA) for 1 h. Membranes were incubated for 1 min at room temperature with chemiluminescence solution (Pierce). Protein concentration was determined by densitometry. IFN-γ-treated HeLa cells and day zero monocytes were used as a positive control and negative control, respectively, for IDO protein. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control (rabbit polyclonal anti-GAPDH at 1:2000; Abcam, Cambridge, MA, USA).
Measurement of tryptophan and kynurenine concentrations in cell culture media
A modification of the high-performance liquid chromatography (HPLC) method described by Widner et al. [10] was used. DC culture medium (100 µL) was deproteinized with 25 µL 2 m trichloroacetic acid and an equal volume of internal standard 3-nitro-tyrosine (10 µm) was added. Following centrifugation, separation (100-µL sample) was achieved using a Phenomenex Ultrasphere ODS column (4.6 × 150 mm; Torrance, CA, USA) protected by a precolumn. The mobile phase (7% acetonitrile in 50 mm sodium acetate, pH 4) had a flow rate of 0.5 mL/min. A UV/vis detector (360 nm) was used to monitor kynurenine and 3-nitro-tyrosine and in series and downstream of this was placed a fluorescence detector (excitation 286, emission 366 nm) to monitor tryptophan. The assay was linear over the concentration range 0.2–45 µm for both kynurenine and tryptophan.
Statistical analysis
Summary data were expressed as the mean values from the two healthy donors (Tables II and III). The Mann–Whitney rank sum test was used to test for differences between Western blot IDO protein expression and kynurenine/tryptophan (K/T) ratios between different culture conditions. The test uses median values and data range (Table II). The association between IDO enzyme activity and protein expression was explored using scatter plots and the Pearson moment correlation.
Table II.
Western blot results.
| IDO intensity/ GAPDH intensity (% of positive control) | ||||||
|---|---|---|---|---|---|---|
| Median and data range for condition 1, 2, 3, 4, 6 versus 5 (%) |
IDO intensity/GAPDH intensity ratio | |||||
| Condition | Donor | Mean ± SD | Mean ± SD | |||
| 1 | 1 | 119.8 | 61.8 ± 82.1 | 28.3 (3.7–119.8) | 0.246 | 0.13 ± 0.16 |
| 2 | 3.7 | 0.021 | ||||
| 2 | 1 | 34.9 | 21.1 ± 19.4 | 0.071 | 0.05 ± 0.03 | |
| 2 | 7.5 | 0.028 | ||||
| 3 | 1 | 26.4 | 16.2 ± 14.4 | 0.056 | 0.04 ± 0.02 | |
| 2 | 6.0 | 0.031 | ||||
| 4 | 1 | 82.0 | 56.1 ± 36.7 | 0.192 | 0.14 ± 0.07 | |
| 2 | 30.1 | 0.089 | ||||
| 5 | 1 | 2113.3 | 1222.4 ± 1259.91 | 1222.4 (331.5–2113.3) | 4.375 | 2.77 ± 2.271 |
| 2 | 331.5 | 1.170 | ||||
| 6 | 1 | 54.8 | 30.2 ± 34.9 | 0.151 | 0.09 ± 0.09 | |
| 2 | 5.5 | 0.023 | ||||
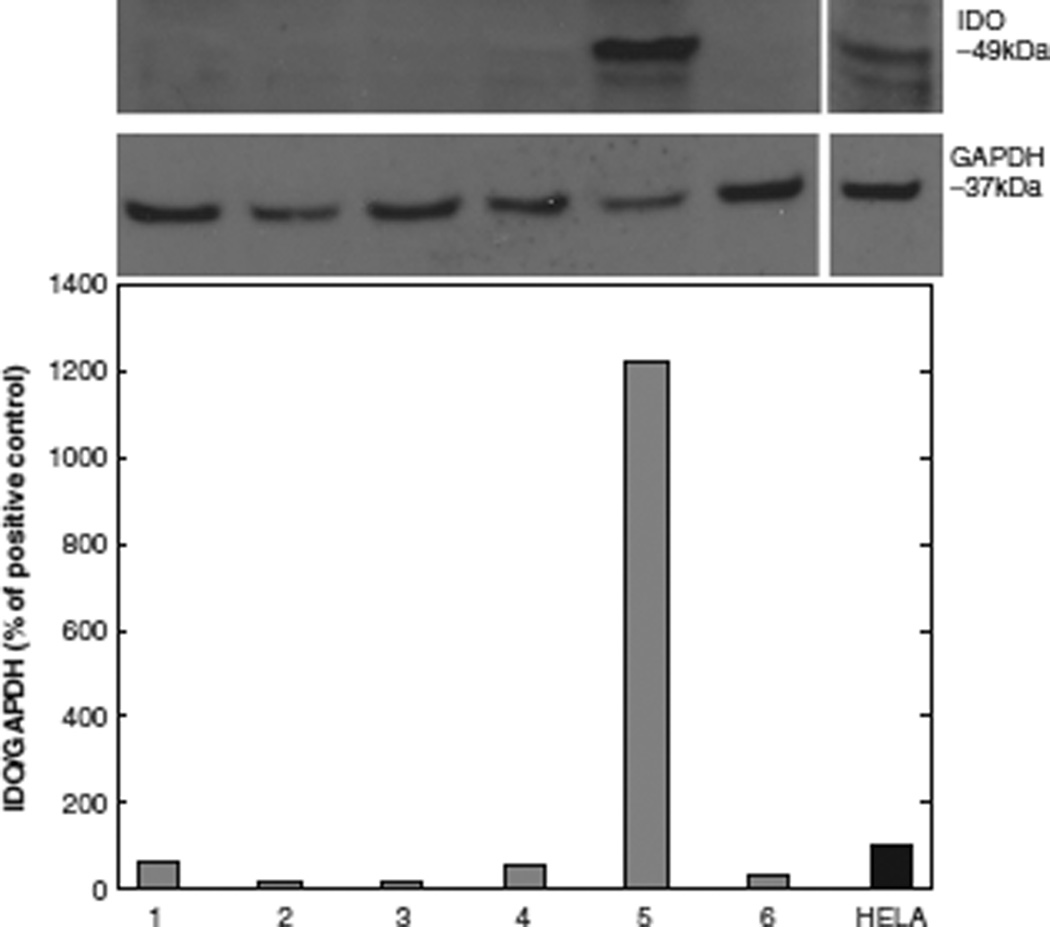
IDO protein expression in non-polarizing (1, 2, 3, 4, 6) and polarizing (5) DC culture conditions was measured densitometrically. It is shown as a ratio (IDO/GAPDH) in the two right-hand columns and as a percentage of the positive control (IFN-γ-stimulated HeLa cells) in the two middle columns. Mean values ± SD have been calculated for two healthy donors. DC in condition 5 were polarized to DC1 and showed a markedly elevated IDO protein expression compared with non-polarized culture conditions (Mann-Whitney rank sum test, 1P = 0.04).
Table III.
K/T ratio measured in culture supernatants.
| (K/T)/105 cells | |||
|---|---|---|---|
| Condition | Donor | Mean ± SD | |
| 1 | 1 | 0.004 | 0.02 ± 0.02 |
| 2 | 0.033 | ||
| 2 | 1 | 0.040 | 0.05 ± 0.02 |
| 2 | 0.064 | ||
| 3 | 1 | 0.033 | 0.03 ± 0.01 |
| 2 | 0.023 | ||
| 4 | 1 | 0.541 | 0.38 ± 0.23 |
| 2 | 0.210 | ||
| 5 | 1 | 6.015 | 6.33 ± 0.451 |
| 2 | 6.654 | ||
| 6 | 1 | 0.053 | 0.08 ± 0.04 |
| 2 | 0.113 | ||
Mean values ± SD were calculated for two donors. Polarization of DC with LPS and IFN-γ (condition 5) resulted in a marked elevation of K/T ratio (Mann-Whitney rank sum test, 1P = 0.04).
Results
Increased IDO protein concentration after DC polarization
IDO protein expression is shown in Table II and Figure 1. In contrast to non-polarizing conditions, polarization of DC to DC1 with LPS and IFN-γ resulted in significantly elevated median IDO protein expression, expressed as a percentage of the positive control (Mann–Whitney statistic, unpolarized conditions versus polarized condition, median 28.3%, range 3.7–119.8%, versus 1222.4%, range 331.5–2113.3%, respectively, P = 0.04). Neither the maturation of DC with tumor necrosis factor (TNF)-α and prostaglandin E2 (PGE2) (conditions 2–4), nor the addition of the Vascular Endothelial Growth Factor Receptor (VEGFR)/Platelet Derived Growth Factor Receptor (PDGFR) tyrosine kinase inhibitor sunitinib (condition 6), increased IDO protein expression. Sunitinib was incorporated in these studies to inhibit the tyrosine kinase-mediated receptor signaling known to be involved in DC maturation and function.
Figure 1.
IDO protein expression in DC after polarizing and non-polarizing culture conditions (unpolarized, 1, 2, 3, 4, 6; polarized, 5), expressed as an IDO/GAPDH ratio as the percentage of positive control (IFN-γ-stimulated HeLa cells = 100%). Western blot IDO protein expression was measured densitometrically. Mean values of two healthy donors are shown as bar graphs. A representative immunoblot is shown above the graph for IDO and GAPDH loading control.
IDO enzyme activity after polarizing and non-polarizing culture conditions
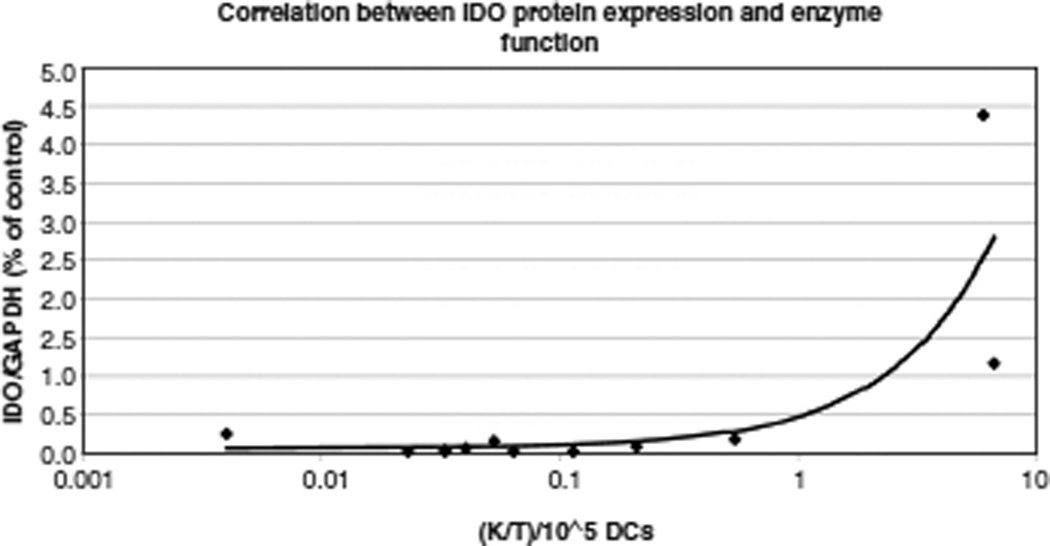
tryptophan is metabolized initially by IDO to N-formylkynurenine, which is then catabolized by a formamidase to kynurenine. Thus the K/T ratio provides an indirect measurement of IDO enzyme activity. Compared with the median K/T ratio in non-polarized culture conditions (median 0.047, range 0.004–0.54), polarization resulted in a markedly higher K/T ratio (median 6.34, range 6.02–6.65; Mann–Whitney statistic, P = 0.04; Table III). We also observed a positive correlation between IDO protein expression and IDO enzyme activity (Pearson moment correlation, r = 0.80, P = 0.002), as shown in Figure 2.
Figure 2.
Densitometrically determined intensity of IDO protein expression/GAPDH protein expression (y-axis) versus K/T-ratio (x-axis, logarithmic scale). Trend line and Pearson correlation coefficient show a positive correlation between the variables (Pearson r = 0.80, P = 0.002).
Discussion
In our study, the LPS and IFN-γ ex vivo DC polarizing culture condition significantly increased IDO protein content, whereas the other culture conditions tested did not. These findings differ from those in a prior study that reported induction of IDO protein with PGE2 and induction of IDO enzymatic activity with PGE2 + TNF-α maturation signals [11]. There are several methodologic differences between the studies that may help explain the divergent data, including the culture conditions, such as the concentration of tryptophan, time of exposure to PGE2 and TNF, timing of the measurement of IDO protein, Ab specificity for IDO isoforms 1 and 2, and the specifics of the assays used to measure kynurenine. Our data may also explain further the ability of PGE2 and TNF exposure to generate activating and not suppressive DC, as reported by others [12].
Moreover, we show that in the experimental conditions in our studies the IDO protein content was paralleled by increased IDO enzymatic activity. Growing DC in LPS and IFN-γ therefore leads to marked up-regulation of functional IDO in our studies. We also investigated the effects of sunitinib, a VEGFR/PDGFR tyrosine kinase inhibitor used for molecularly targeted therapies in advanced renal cancer, on DC IDO protein expression and enzyme activity, and found no significant effect.
Previous reports have claimed that immature as well as fully matured DC have the ability to express IDO [11]. We did not find significant IDO expression, nor evidence of IDO enzyme activity, in immature DC cultured with interleukin (IL)-4 and granulocyte–macrophage (GM) colony-stimulating factor (CSF) culture conditions. IDO mRNA expression follows a defined time–course and has been shown to be down-regulated at 36 h after initial up-regulation [13]. Therefore, we cannot exclude the possibility that IDO protein expression in matured DC was already decreased at the time-point of cell harvest in our experiments. If this were the case, polarization with LPS and IFN-γ would have appeared to maintain IDO protein expression, while maturation alone did not. IFN-γ may be the key regulator of IDO expression in our present study. It is known that IFN-γ is capable of inducing IDO protein expression in human peripheral blood mononuclear cells (PBMC) ex vivo via signal transducer and activator of transcription 1 (STAT1) and IFN-regulatory factor 1 (IRF1)-dependent induction of IDO [14]. As observed by others [13], we noted considerable interindividual variation in IDO protein expression (Table II).
IDO has a critical impact on T-cell numbers and function. Tissue micro-environmental depletion of tryptophan via the IDO pathways renders T cells more susceptible to apoptosis [7]. Various tryptophan down-stream metabolites, e.g. kynurenine and quinolinate, are directly toxic to T cells [15]. Most importantly, it has been shown that IDO, induced in DC in ex vivo culture conditions, maintained its activity when those DC were reinfused into patients [13]. Finally, IDO-expressing, tolerizing DC have also been shown to induce CD4+ T-regulatory cells [3,16].
The clinical extrapolation of the data from our current study would be that adoptive transfer of LPS plus IFN-γ polarized DC may lead to low response rates and systemic immunologic hyporesponsiveness because of the expression of IDO and thus the increased likelihood of therapeutic failure of such DC-based cancer immune therapies. Further careful consideration and analysis of the different conditions of ex vivo DC culture is recommended prior to initiating clinical trials with cellular DC vaccine therapies.
Acknowledgments
Supported, in part, by NIH grant RO1 CA5648 and Cancer Center Support Grant NIH CA 23108.
Footnotes
Declaration of Interest: The authors report no coflict of interest. The author alone are responsible for the content and writing of the paper.
References
- 1.Ranieri E, Gigante M, Storkus WJ, Gesualdo L. Translational mini-review series on vaccines: dendritic cell-based vaccines in renal cancer. Clin Exp Immunol. 2007;147:395–400. doi: 10.1111/j.1365-2249.2006.03305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dohnal AM, Graffi S, Witt V, Eichstill C, Wagner D, Ul-Haq S, et al. Comparative evaluation of techniques for the manufacturing of dendritic cell-based cancer vaccines. J Cell Mol Med. 2009 Jan;13(1):125–35. doi: 10.1111/j.1582-4934.2008.00304.x. Epub 2008 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007 Jan;30(1):75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 4.Ten BA, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25:7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Hwu P, Du MX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 7.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 9.Huttner KG, Breuer SK, Paul P, Majdic O, Heitger A, Felzmann T. Generation of potent anti-tumor immunity in mice by interleukin-12-secreting dendritic cells. Cancer Immunol Immunother. 2005;54:67–77. doi: 10.1007/s00262-004-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 11.Braun D, Longman RS, Albert ML. A two-step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic-cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drause P, Singer E, Darley PI, Klebensberger J, Groettrup M, Legler DF. Prostaglandin E2 is a key factor for monocyte-derived dendritic cell maturation: enhanced T cell stimulatory capacity despite IDO. J Leukoc Biol. 2007;82:1106–1114. doi: 10.1189/jlb.0905519. [DOI] [PubMed] [Google Scholar]
- 13.Wobser M, Voigt H, Houben R, Eggert AO, Freiwald M, Kaemmerer U, et al. Dendritic cell based antitumor vaccination: impact of functional indoleamine 2,3-dioxygenase expression. Cancer Immunol Immunother. 2007;56:1017–1024. doi: 10.1007/s00262-006-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon-gamma-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–17252. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- 15.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faunce DE, Terajewicz A, Stein-Streilein J. Cutting edge: in vitro-generated tolerogenic APC induce CD8+ T regulatory cells that can suppress ongoing experimental autoimmune encephalomyelitis. J Immunol. 2004;172:1991–1995. doi: 10.4049/jimmunol.172.4.1991. [DOI] [PubMed] [Google Scholar]