Abstract
Background
Epidemiological data demonstrate an increased risk of developing incident asthma with increasing adiposity. While the vast majority of studies support the interaction between obesity and asthma, the causality is unclear.
Scope of review
This article will review the current literature supporting the presence of an obese asthma phenotype and the possible mechanisms mediating the effects of obesity on asthma.
Major conclusions
Obesity is associated with poor asthma control, altered responsiveness to medications and increased morbidity. Obesity is characterized by systemic inflammation that may result in increased airway inflammation. However, this assertion is not supported by current studies that demonstrate a lack of significant airway inflammation in obese asthmatics. In spite this observation one must consider limitations of these studies including the fact that most subjects were treated with inhaled corticosteroids that would likely alter inflammation in the lung. Thus, it remains unclear if obesity is associated with alterations in inflammation in the airways of subjects with asthma.
Hormones such as leptin and adiponectin are affected by obesity and may play a role in mediating innate immune responses and allergic responses, respectively. The role of oxidative stress remains controversial and the current evidence suggests that while oxidative stress is important in asthma, it does not fully explain the characteristics associated with this unique phenotype.
General significance
Obesity related asthma is associated with increased morbidity and differential response to asthma therapies. Understanding the mechanisms mediating this phenotype would have significant implications for millions of people suffering with asthma. This article is part of a Special Issue entitled Biochemistry of Asthma.
Keywords: Asthma, Obesity, Metabolic syndrome, Oxidative stress, Leptin, Inflammation
1. Introduction
The incidence of obesity in the United States is striking and currently 30% of the adult population is considered obese [1]. Obesity is now an epidemic with significant public health implications. Obesity is associated with an increased risk of developing diabetes, coronary artery disease and non-alcoholic steatohepatitis [2]. Numerous epidemiologic studies published during the past decade have demonstrated an increased risk of asthma associated with increasing obesity [3–5]. The effect of obesity on the occurrence of asthma is more prominent in women [6–8] and there is a dose response effect of increasing body mass index (BMI) on asthma incidence [3]. The increased risk of obesity on the occurrence of asthma is most prominent in non-allergic individuals [9,10].
Asthma is primarily characterized by T-helper (TH2) mediated inflammation, however, it has recently been recognized that non-TH2 cytokines like interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) may play a role in asthma pathogenesis [11]. Exhaled nitric oxide is an accepted marker of TH2 inflammation in asthma and its expression is increased in allergic asthma. Several studies have demonstrated an inverse relationship between obesity and exhaled nitric oxide levels [9,10,12,13] further supporting the assertion that the interaction between obesity and asthma is not primarily mediated by classical, allergic (TH2) inflammation.
Postulated mechanisms for the increased risk of asthma associated with obesity include changes in airway smooth muscle stretch due to the presence of shallow breathing associated with obesity, effects of gastroesophageal reflux, inaccurate diagnosis of asthma, sleep disordered breathing, genetic polymorphisms and the effect of systemic adipocytokines and oxidative stress on both pulmonary and extra-pulmonary inflammation [14–18].
Adipose tissue is now recognized as metabolically active, playing a role in the regulation of energy homeostasis and has significant pathological effects that result in many obesity-related diseases. The role of white adipose tissue in mediating systemic inflammation is an area of active investigation in both cardiac disease and diabetes. Adipose tissue is infiltrated by bone-marrow derived macrophages that secrete adipokines and cytokines in the systemic circulation resulting in a chronic inflammatory state [19]. Obesity is associated with increased leptin and resistin (pro-inflammatory hormones) and decreased adiponectin, a hormone with potent anti-inflammatory effects. Additionally, there are increased levels of circulating cytokines including TNF-α, interleukin (IL)-6, monocyte chemotactic protein-1 (MCP-1) and vascular endothelial growth factor (VEGF) [20,21]. These cytokines propagate inflammation and angiogenesis. Low serum IL-10 levels result in a vicious cycle of increased inflammation that is unabated. The effect of systemic inflammation on metabolic dysregulation is clear and supported by strong evidence. The role of systemic inflammation, adipocytokines and oxidative stress in mediating the obese asthma phenotype is unclear (Table 1).
Table 1.
Studies assessing the effects of obesity on asthma severity.
| Study | Design | Patient population | Analysis | Results |
|---|---|---|---|---|
| Von Mutius 2001 | Cross sectional analysis | 7505 children age 4-17 | Multivariate regression | Prevalence for asthma increased with BMI |
| Akerman 2004 | Retrospective chart analysis | 143 adults at single academic medical center | Spearman correlation coefficients | Prevalence of obesity increased with increasing asthma severity |
| Blanden 2004 | Prospective, observational surveys | 100 children age 7-17 | Central tendency, standard deviation, and standard error | Obese patients with decreased QOL compared to normal weight patients with asthma |
| Cassol 2006 | Cross sectional analysis | 4010 children age13-14 | Chi-square test and odds ratio | Positive association between obesity and both asthma prevalence and severity |
| Taylor 2008 | Cross sectional analysis | 3095 adults | Mutivariate regression analysis | Positive association between obesity and asthma severity and control |
| Mosen 2008 | Cross sectional | 1113 adults | Multiple logistic regression models | Positive association between obesity and worse asthma control and increased risk of asthma related hospitalizations |
It is becoming increasingly evident that obesity is associated with a unique asthma phenotype that is characterized by more severe disease with variable response to conventional asthma therapies. Despite this, caution must be taken when assessing obese asthmatics. It seems more plausible that obese asthmatics will fall into various phenotypes including those with allergic/atopic asthma and those with predominantly non-allergic characteristics. The latter phenotype is likely an adult onset female-predominant group with non-atopic disease [22]. More sophisticated phenotypic characterizations are essential in determining biomarkers that would improve understanding of the complex interaction between obesity and asthma.
1.1. Obesity and asthma severity
Obesity is associated with increased asthma severity in both children and adults [10,23–26]. Taylor et al. determined that obesity is associated with increased daily asthma symptoms, missed workdays, increased rescue bronchodilator usage and increased asthma severity as determined by GINA guidelines. This association was present even after adjusting for age, gender, race, income, education level, employment status, family history and residence [27]. Obese asthmatics have a higher risk of hospitalization (OR 4.6) for acute asthma exacerbations [28] and have poorer quality of life [29]. Concurrent with more severe disease is a poor response to conventional asthma therapies.
2. Obesity and variable treatment response
Obesity is associated with decreased glucocorticoid responsiveness associated with an inability to achieve adequate asthma control both with inhaled corticosteroids and combination therapies that include inhaled corticosteroids and long acting bronchodilators [30–34](Table 2).
Table 2.
Studies assessing the response to asthma controller medications in obese asthma subjects.
| Study | Design | Patient population | Intervention | Primary endpoint | Results |
|---|---|---|---|---|---|
| Dixon 2006 | Retrospective analysis | 488 moderate asthmatics | Theophylline vs montelukast vs placebo | Asthma control | Increased asthma exacerbations in obese patients treated with theophylline but no difference in response to LTRA |
| Peters Golden 2006 | Retrospective analysis | 3073 moderate asthmatics | Beclamethasone vs montelukast vs placebo | Asthma control days | Decreased response to ICS with increasing BMI |
| Boulet and Franssen 2007 | Retrospective analysis | 1242 moderate asthmatics | fluticasone vs fluticasone/salmeterol | Asthma control | Obese patients less likely than non-obese to achieve asthma control with either |
| Sutherland 2009 | Cross sectional study | 1265 moderate asthmatics | variable | Treatment response | Overweight and obese had smaller improvements in lung function but no difference in response to LTRA |
| Sutherland 2010 | Retrospective analysis | 1052 | Fluticasone vs montelukast | FEV1 | Greater FEV1 improvement with fluticasone vs montelukast |
| Carmago 2010 | Retrospective analysis | Fluticasone/salmeterol vs montelukast | Treatment response | Greater treatment response to FP/Salmeterol than montelukast | |
| Forno 2011 | Retrospective analysis | 1041 mild to moderate asthmatics | Budesonide vs placebo or nedocromil | Lung function | Overweight and obese children had less response to budesonide |
| Farah 2011 | Cross sectional study | 49 moderate asthmatics | Budesonide | Treatment response | Asthma control, spirometry and airway inflammation improved similarly |
Multiple studies have found a blunted response to corticosteroids in overweight and obese asthmatics. Peters-Golden et al. performed a post hoc analysis of four double blind, placebo controlled studies randomizing 3073 moderate asthmatics to beclomethasone, montelukast or placebo with the primary endpoint of asthma control days. These studies demonstrated that the response to inhaled corticosteroids inversely correlated with increasing BMI. However, a similar treatment response to montelukast, a leukotriene antagonist, was seen in asthma, regardless of BMI [35].
Boulet and Franssen performed a retrospective analysis of patients enrolled in five double-blind studies that randomized 1242 moderate asthmatics to fluticasone versus fluticasone/salmeterol with asthma control as the primary outcome. They found that obese patients were less likely than non-obese patients to achieve asthma control with either fluticasone or fluticasone/salmeterol [30]. Forno et al. also performed a retrospective analysis examining 1041 children randomized to budesonide vs. placebo/nedocromil with improved lung function as the primary outcome. Overweight and obese children had less response to budesonide compared to non-overweight children [36].
The mechanisms that mediate the differential treatment response to corticosteroids are unknown. Sutherland et al. proposed decreased mitogen-activated protein kinase phosphatase-1 (MKP-1) expression in peripheral blood mononuclear cells (PBMCs) and lung cells (likely macrophages) [33]. Up regulation of MKP-1 is important in mediating the anti-inflammatory effects of dexamethasone via inactivation of pro-inflammatory signaling. Thus, they proposed that the reduced expression of MKP-1 was associated with reduced clinical corticosteroid responsiveness. Additionally, obese asthmatics also demonstrated increased PBMC TNF-α expression that correlated with increasing BMI. The presence of increased TNF-α levels may result in alterations in the inflammatory profile in the lungs of obese asthmatics and alter response to glucocorticoids.
Other postulated mechanisms for the variable response to therapy include the assertion that obesity has effects on asthma control mediated by obesity related changes in lung mechanics [37]. Additional proposed mechanisms include the potential role of vitamin D deficiency on glucocorticoid responsiveness. Vitamin D deficiency is more common in obese individuals as demonstrated by Sutherland et al. who found an inverse relationship between vitamin D levels and BMI [38]. More importantly, low vitamin D levels are associated with decreased glucocorticoid responsiveness in asthma [38].
The role of leukotriene (LT) antagonists in the treatment of obese patients with asthma is unclear. Increased 5-lipooxgenase activating protein and LTB4 levels have been demonstrated in the adipose tissue of obese mice [39]. In addition, macrophage infiltration and free fatty acid secretion is increased in parallel to changes in 5-lipooxygenase activating protein levels [39]. Adipose tissue incubated with 5-lipoxygenase products results in increased nuclear factor kappa beta (NFκB) activation and secretion of pro-inflammatory cytokines including TNF-α, MCP-1 and IL-6, an effect that is ameliorated by the addition of 5-lipoxygenase activating protein inhibitors [39,40]. Leung et al. noted increased exhaled nitric oxide and urinary LTB4 levels in children with asthma. However these inflammatory markers were not affected by obesity status [41].
The role of leukotrienes and 5-lipooxygenase in asthma is well accepted and the beneficial effects of therapy with 5-lipooxygenase inhibitors and leukotriene antagonists are clear [42–44]. However, the role of leukotrienes and 5-lipooxygenase in modulating the effects of obesity in asthma remains controversial and warrants further investigation. If these mediators are prominent contributors to inflammation in the lungs of obese asthmatics, then there would be potential treatment implications. Specifically, leukotriene antagonists and 5-lipooxygenase inhibitors would be considered more targeted treatments in obese asthmatics and thus would be more widely used in this population.
Human studies focused on delineating the impact of leukotrienes and 5-lipooxygenase inhibitors on asthma control in obese asthmatics are all retrospective and have yielded conflicting results. Prior studies noted improvements in asthma control with leukotriene antagonist therapy in obese asthmatics, without any differential effects of obesity on response to therapy with leukotrienes [31]. However, a recent retrospective analysis by Sutherland et al. suggested that fluticasone was more effective than montelukast at achieving asthma control, irrespective of body mass index [45,46]. Combination therapy with fluticasone and salmeterol resulted in greater treatment response (based on FEV1, asthma symptom scores and albuterol usage) than montelukast in obese asthmatics. Conversely, in a study by Camargo et al., lean asthma subjects had a greater treatment response to combination therapy and montelukast as defined by improvement in FEV1, asthma symptom scores and albuterol usage as compared to obese asthmatic subjects [46].
Although there are conflicting data regarding the role of 5-lipooxygenase and leukotrienes in the pathogenesis of asthma in obese persons, there is some scientific rationale for potential effects of these mediators in the obesity-asthma interaction. Therefore, further research is required to determine the role of these mediators in affecting both asthma pathogenesis and response to treatment in obese asthmatics. Prospective studies comparing effectiveness of the addition of leukotriene antagonists and 5-lipooxygenase inhibitors to inhaled corticosteroid therapy on achieving and maintaining asthma control in obese asthmatics are warranted.
Thus far, there is mounting evidence that supports the assertion that obesity results in a unique asthma phenotype that requires further characterization and more importantly, a focus on alternative therapies [47].
3. Metabolic dysregulation, systemic and airway inflammation and asthma
The National Heart Lung Blood Institute and American Heart Association define the metabolic syndrome as a syndrome that includes abdominal obesity, artherogenic dyslipidemia, elevated blood pressure, insulin resistance or impaired glucose tolerance, a pro-inflammatory state and a pro-thrombotic state [48]. The metabolic syndrome is associated with an increased risk of diabetes, cardiovascular disease, sleep apnea, asthma and certain malignancies. Leone et al. found an association between impaired lung function and metabolic syndrome regardless of BMI and predominately due to central adiposity [49]. This association persisted after exclusion of patients with a prior history of lung disease. Similar results were reported in prior studies that demonstrated an association between lung function impairment and metabolic syndrome [50,51]. The authors did not specifically focus on asthma in this cohort; however prior studies have found an increased risk of asthma associated with obesity. One can postulate that common pathways underlie lung function impairments and development of metabolic syndrome.
The presence of systemic inflammation as characterized by an elevated C-reactive peptide (CRP) level is well accepted as part of the metabolic syndrome [52]. The effect of systemic inflammation on airway inflammation in asthma is controversial. Most studies have found no association between obesity and increased airway inflammation in asthma [53–56]. A prospective study by Sutherland et al., demonstrated increased IL-1β, IL-5, IL-6 and IL-8 in sputum supernatants of asthmatics, however no differences were noted between obese and lean asthmatics leading the authors to conclude that the obese asthma phenotype is not a result of changes in airway inflammation [57]. Dixon et al. studied a cohort of patients with asthma who underwent bariatric surgery. There was a significant improvement in asthma symptoms and lung function with weight loss but no change in airway inflammation as characterized by bronchoalveolar lavage and induced sputum cell counts [58]. These studies suggest that airway inflammation and classical TH-2 driven inflammation does not mediate the variable response to therapy, increased symptoms and decreased asthma control seen in obese asthmatics. Although these results are interesting, the majority of subjects included by in both studies were taking an inhaled corticosteroid and this may have altered the inflammatory profile in the lung. The report by Dixon et al. did not include the measurements of cytokines in bronchoalveolar lavage fluid and therefore differences in inflammation may not have been elucidated. The compartments of the lung sampled by sputum and bronchoalveolar lavage differ making comparisons between the two studies more challenging. Despite the findings in these two studies, conclusions regarding whether inflammation in asthmatics differs based on obesity should be reserved until further evidence is obtained.
4. Oxidative stress in obesity and the effect on asthma
Oxidative stress is characterized by the presence of increased reactive oxygen species (ROS) either as a result of increased production of ROS or decreased amounts of antioxidants present. Reactive oxygen species create a variety of pathologic changes in the airways including increased airway reactivity and increased mucous production, factors that have important implications in asthma. Obesity is associated with increased oxidative stress and systemic inflammation [59,60]. Increased systemic or airway oxidative stress may be a potential mechanism by which obesity results in increased asthma severity. Assessment of oxidative stress can be made through direct measurement of reactive oxygen species or indirect measurement of the oxidative products in either plasma or exhaled breath condensate (EBC).
Asthma is associated with increased exhaled breath condensate levels of malondialdehyde (MDA) and reduced glutathione, both demonstrated in children [61,62]. Glutathione, in its reduced form, protects airway epithelial cells from free radicals while MDA is formed due to the action of reactive oxygen species on membrane phospholipids and is a marker of oxidative stress. Plasma MDA levels are increased and glutathione levels are decreased in children with asthma, with the highest levels of oxidant stress occurring in children with more severe disease [63]. Epithelial lining fluid levels of anti-oxidants are significantly lower in children with severe asthma indicating the presence of oxidative stress [64]. These alterations in oxidative stress occur independent of BMI and appear to be indicative of disease activity. Exhaled breath 8-isoprostane levels are increased in the children with asthma, however the effect of obesity was not delineated [65]. Oxidant–antioxidant imbalance plays an important role in asthma; however, the effects of obesity are unknown in children.
In adults, the available data are contradictory. The current studies reveal distinct differences in plasma and exhaled levels of oxidative markers that are not concordant and thus difficult to interpret. Increased plasma 8-isoprostane levels have been noted in asthma. However this association is not present after adjusting for obesity. This suggests that the elevated plasma levels are a consequence of obesity rather than asthma [66]. Conversely, Komakula et al. demonstrated an association between increased exhaled 8-isoprostane levels and BMI but only in asthmatics [67]. Interestingly, the level of exhaled 8-isoprostanes did not differ between asthmatics and normal controls. The asthma subjects demonstrated an inverse correlation between exhaled nitric oxide (NO) and BMI. In this study, serum levels of adipokines (leptin and adiponectin) did not correlate with changes in markers of oxidative stress. The authors proposed that obesity was associated with increased oxidative stress, but only in asthma. The decreased exhaled NO levels were thought to result from changes in baseline NO redox metabolism and conversion of the NO present in the airway to reactive nitric oxygen species [67]. Furthermore, the authors postulated that increased oxidative stress in the lung might be due to increased leptin levels that occur with obesity. Leptin has been shown to increase oxidative stress in endothelial cells by increasing NFκB activation in an oxidant dependent manner [68]. The role of leptin in inducing increased oxidative stress in the lungs of obese asthmatics is unknown.
The current data support the assertion that asthma is associated with increased oxidative stress. However, it is unclear if the presence of increased airway oxidative stress is a consequence of changes in systemic oxidative stress seen in obesity. To determine the association between airway and systemic oxidative stress, Holguin et al. measured serum and exhaled 8-isoprostane levels in a cohort of moderate to severe adult asthmatics. The presence of obesity, but not asthma, was associated with increased exhaled 8-isoprostane levels. Conversely, plasma levels of 8-isoprostanes were higher in asthmatics but there was no effect of obesity noted [18]. In addition, there was no correlation between exhaled and plasma 8-isoprostane levels [18]. Based on these data, the authors inferred that while asthma increases systemic oxidative stress and obesity increases airway oxidative stress there was no synergism between plasma and exhaled 8-isoprostane levels.
The discordance in the exhaled as compared to plasma level of 8-isoprostanes has led to challenges in delineating the role of oxidative stress in obese asthmatics and more importantly, the use of 8-isoprostanes as a reliable marker of variability in oxidative stress. It is plausible that oxidative stress may not be a causative factor but rather may modulate asthma severity and alter response to medications. The authors further inferred that although baseline levels of inflammation did not differ, response to exacerbating factors might be more robust in obese asthmatics with propagation of oxidative stress that results in more severe symptoms and prolonged episodes of poor asthma control [18]. Conclusions regarding the role of oxidative stress in obese asthmatics are difficult to make given the conflicting data that are available at this time.
5. The role of adipokines in obesity related asthma
Obesity is associated with increased serum leptin levels that may be associated with increased inflammation in the airways of obese asthmatics [11]. Leptin has been shown to regulate T-cell proliferation and activation, to recruit and activate macrophages and promote angiogenesis [55,69]. Studies assessing the role of leptin in human asthma are limited. Guler et al. noted that serum leptin was predictive of asthma in boys, even after adjusting for BMI [70]. Other studies reveal higher leptin levels in asthmatic women compared to non-asthmatic women. However, adjusting for serum leptin levels does not affect the association between BMI and asthma [71]. One can postulate that leptin, a potent pro-inflammatory hormone, may promote increased non-TH2 airway inflammation, a mechanism that has not been studied extensively in humans. In mouse models of asthma, leptin augments airways hyperresponsiveness (AHR) but a lack of leptin does not completely attenuate AHR indicating that leptin does not entirely explain the presence of increased AHR in obese mice [72–74]. Leptin also augments ozone-induced inflammation in obese mice [73].
Recently, several studies in humans have not demonstrated significant differences in inflammation in the airways of obese asthmatics in spite differences in disease characteristics [57,58]. However, as previously discussed, there are limitations with these studies that affect our ability to draw concrete conclusions about the presence of inflammation in the airways of obese asthmatics. We propose that leptin may play both a pro-inflammatory and immune modulating role in obese asthmatics, thus contributing to this unique phenotype (Fig. 1).
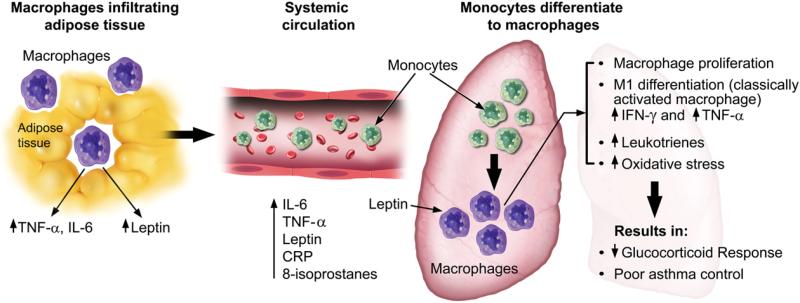
Fig. 1.
Adipose tissue in obese subjects produces pro-inflammatory cytokines that result in systemic inflammation with increased circulating levels of IL-6, TNF-α, and leptin. Circulating monocytes are recruited into the lung where they differentiate into macrophages. Leptin plays a key role in macrophage proliferation and differentiation in the lung with subsequent secretion of pro-inflammatory cytokines. Additionally obese asthmatics may have increased oxidative stress and leukotriene synthesis. These alterations in pathophysiology result in poor asthma control and decreased glucocorticoid response.
Adiponectin is a 30 kDa protein with an N-terminal collagenase domain followed by a C-terminal globular domain. It exists in both low molecular weight and high molecular weight forms [75]. Adiponectin has primary effects on energy metabolism and its effects are anti-diabetic in nature. It also has many anti-inflammatory effects and inhibits the production of the pro-inflammatory cytokines IL-6 and TNF-α [76] while inducing the anti-inflammatory cytokines IL-1 receptor antagonist and IL-10 [77–79]. Serum adiponectin and IL-10 levels are lower in obesity [80] and increase with weight loss [81–84]. Interestingly, adiponectin can have pro-inflammatory effects depending on the stimulus present [21]. Adiponectin markedly attenuates allergen induced airway inflammation in mice [85] and adiponectin deficient mice demonstrate greater eosinophilia [86]. In premenopausal women serum adiponectin levels are protective against the development of asthma [87]. A study assessing the effects of allergen challenge on serum adiponectin levels in asthmatics did not reveal any correlation leading the authors to conclude that adiponectin may not play a role in the acute allergen response in humans [88]. Adiponectin resistance and deficiency may play a role in propagating unabated inflammation in the obese patient with asthma. However, further studies are warranted to determine its role in humans. The data obtained from mouse models are promising but lack of translation to human obesity is a concern and is most likely due to a complex interaction of several pro and anti-inflammatory adipokines.
One potential way to evaluate the role of adiponectin on asthma control in obese asthmatics would be to evaluate the impact of thiazolidinediones (TZDs) on asthma control. TZDs increase serum adiponectin levels [75] and the higher adiponectin levels could have potential anti-inflammatory effects in the lung. Furthermore, mechanistic studies in humans are needed to determine the role of adiponectin in ameliorating inflammation in various cell types obtained from the lungs of asthmatics.
6. Effects of weight loss on obese asthmatics
The obese asthma phenotype can be reversed by weight loss with improvements in lung function, asthma control and asthma severity with decreased medication utilization and hospitalizations [16,58,89–92]. Even modest dietary alterations can result in decreased markers of oxidative stress and inflammation in overweight patients with moderate asthma [93]. Prospective studies to determine the etiology of these improvements in asthma are necessary and will provide invaluable insights into the pathophysiology of asthma in obesity. Moreover, despite the lack of a clear mechanism, the beneficial effects of weight loss appear to the unequivocal and thus weight management strategies should be included in the management of the obese asthmatic.
7. Conclusions
Numerous studies have demonstrated a strong association between obesity and asthma however the direction of causality is unclear. The current evidence supports the finding that obese asthmatics have more severe disease and variable treatment response. Additionally, it is becoming increasingly evident that there is heterogeneity within this population of obese asthmatics. There may in fact be several distinct obese asthma phenotypes based on the presence or absence of atopy. The interaction between obesity and asthma is complex and there are several potential mechanisms that mediate the recognized clinical phenotype. Further studies are desperately needed to delineate the mechanism that mediates the clinical phenotype and more importantly, to help guide future therapeutic targets for this unique, but common asthma phenotype.
Footnotes
This article is part of a Special Issue entitled Biochemistry of Asthma.
References
- 1.CDC Obesity trends in the United States [Google Scholar]
- 2.N. NIH Obesity Education Initiative, Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. 1998:98–4083. [PubMed] [Google Scholar]
- 3.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibi H, et al. The relationship between asthma and obesity in children: is it real or a case of over diagnosis? J. Asthma. 2004;41(4):403–410. doi: 10.1081/jas-120026097. [DOI] [PubMed] [Google Scholar]
- 5.Flaherman V, Rutherford GW. A meta-analysis of the effect of high weight on asthma. Arch. Dis. Child. 2006;91(4):334–339. doi: 10.1136/adc.2005.080390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camargo CA, Jr., et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch. Intern. Med. 1999;159(21):2582–2588. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Sex specificity of asthma associated with objectively measured body mass index and waist circumference: the Humboldt study. Chest. 2005;128(4):3048–3054. doi: 10.1378/chest.128.4.3048. [DOI] [PubMed] [Google Scholar]
- 8.Coogan PF, et al. Body mass index and asthma incidence in the Black Women's Health Study. J. Allergy Clin. Immunol. 2009;123(1):89–95. doi: 10.1016/j.jaci.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Dales R, Jiang Y. The association between obesity and asthma is stronger in nonallergic than allergic adults. Chest. 2006;130(3):890–895. doi: 10.1378/chest.130.3.890. [DOI] [PubMed] [Google Scholar]
- 10.von Mutius E, et al. Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examination Study III. Thorax. 2001;56(11):835–838. doi: 10.1136/thorax.56.11.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canoz M, et al. The relationship of inflammatory cytokines with asthma and obesity. Clin. Invest. Med. 2008;31(6):E373–E379. doi: 10.25011/cim.v31i6.4924. [DOI] [PubMed] [Google Scholar]
- 12.De Winter-de Groot KM, et al. Exhaled nitric oxide: the missing link between asthma and obesity? J. Allergy Clin. Immunol. 2005;115(2):419–420. doi: 10.1016/j.jaci.2004.11.025. [see comment] [DOI] [PubMed] [Google Scholar]
- 13.von Mutius E. Is asthma really linked to atopy? Clin. Exp. Allergy. 2001;31(11):1651–1652. doi: 10.1046/j.1365-2222.2001.01272.x. [DOI] [PubMed] [Google Scholar]
- 14.Boulet LP, et al. Deep inspiration avoidance and airway response to methacholine: Influence of body mass index. Can. Respir. J. 2005;12(7):371–376. doi: 10.1155/2005/517548. [DOI] [PubMed] [Google Scholar]
- 15.Aaron SD, et al. Overdiagnosis of asthma in obese and nonobese adults. Can. Med. Assoc. J. 2008;179(11):1121–1131. doi: 10.1503/cmaj.081332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss ST. Obesity: insight into the origins of asthma. Nat. Immunol. 2005;6(6):537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- 17.Shore SA. Obesity and asthma: possible mechanisms. J. Allergy Clin. Immunol. 2008;121(5):1087–1093. doi: 10.1016/j.jaci.2008.03.004. quiz 1094–5. [DOI] [PubMed] [Google Scholar]
- 18.Holguin F, Fitzpatrick A. Obesity, asthma, and oxidative stress. J. Appl. Physiol. 2010;108(3):754–759. doi: 10.1152/japplphysiol.00702.2009. [DOI] [PubMed] [Google Scholar]
- 19.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauner H. Secretory factors from human adipose tissue and their functional role. Proc. Nutr. Soc. 2005;64(2):163–169. doi: 10.1079/pns2005428. [DOI] [PubMed] [Google Scholar]
- 21.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005;115(5):911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 22.Holguin F, et al. Obesity and asthma: an association modified by age of asthma onset. J. Allergy Clin. Immunol. 2011;127(6):1486–1493. e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J. Asthma. 2004;41(5):521–526. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- 24.Shore SA. Obesity and asthma: implications for treatment. Curr. Opin. Pulm. Med. 2007;13(1):56–62. doi: 10.1097/MCP.0b013e3280110196. [DOI] [PubMed] [Google Scholar]
- 25.Cassol VE, et al. Obesity and its relationship with asthma prevalence and severity in adolescents from southern Brazil. J. Asthma. 2006;43(1):57–60. doi: 10.1080/02770900500448597. [DOI] [PubMed] [Google Scholar]
- 26.Carroll CL, et al. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr. Crit. Care Med. 2006;7(6):527–531. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 27.Taylor B, et al. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63(1):14–20. doi: 10.1136/thx.2007.082784. [DOI] [PubMed] [Google Scholar]
- 28.Mosen DM, et al. The relationship between obesity and asthma severity and control in adults. J. Allergy Clin. Immunol. 2008;122(3):507–511. e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 29.Blandon Vijil V, et al. Quality of life in pediatric patients with asthma with or without obesity: a pilot study. Allergol. Immunopathol. 2004;32(5):259–264. doi: 10.1016/s0301-0546(04)79252-6. [DOI] [PubMed] [Google Scholar]
- 30.Boulet LP, Franssen E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 2007;101(11):2240–2247. doi: 10.1016/j.rmed.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Dixon AE, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J. Asthma. 2006;43(7):553–558. doi: 10.1080/02770900600859123. [DOI] [PubMed] [Google Scholar]
- 32.Camargo CA, Jr., et al. Effect of increased body mass index on asthma risk, impairment and response to asthma controller therapy in African Americans. Curr. Med. Res. Opin. 2010;26(7):1629–1635. doi: 10.1185/03007995.2010.483113. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland ER, et al. Body mass and glucocorticoid response in asthma. Am. J. Respir. Crit. Care Med. 2008;178(7):682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland ER, et al. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J. Allergy Clin. Immunol. 2009;123(6):1328–1334. e1. doi: 10.1016/j.jaci.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters-Golden M, et al. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 36.Forno E, et al. Decreased response to inhaled steroids in overweight and obese asthmatic children. J. Allergy Clin. Immunol. 2011;127(3):741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farah CS, et al. Obesity Is a Determinant of Asthma Control, Independant of Inflammation and Lung Mechanics. Chest. 2011;140(3):659–666. doi: 10.1378/chest.11-0027. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, et al. Vitamin D levels, lung function, and steroid response in adult asthma. Am. J. Respir. Crit. Care Med. 2010;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horrillo R, et al. 5-lipoxygenase activating protein signals adipose tissue inflammation and lipid dysfunction in experimental obesity. J. Immunol. 2010;184(7):3978–3987. doi: 10.4049/jimmunol.0901355. [DOI] [PubMed] [Google Scholar]
- 40.Chakrabarti SK, et al. Evidence for activation of inflammatory lipoxygenase pathways in visceral adipose tissue of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2011;300(1):E175–E187. doi: 10.1152/ajpendo.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung TF, et al. The relation between obesity and asthmatic airway inflammation. Pediatr. Allergy Immunol. 2004;15(4):344–350. doi: 10.1111/j.1399-3038.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel SE. Leukotriene receptor antagonists and related compounds. Can. Respir. J. 1999;6(2):189–193. doi: 10.1155/1999/676798. [DOI] [PubMed] [Google Scholar]
- 43.Drazen J. Clinical pharmacology of leukotriene receptor antagonists and 5-lipoxygenase inhibitors. Am. J. Respir. Crit. Care Med. 1998;157(6 Pt 2):S233–S237. discussion S247-8. [PubMed] [Google Scholar]
- 44.Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma trea1tment. Clin. Exp. Allergy. 2010;40(12):1732–1741. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland ER, et al. Comparative effect of body mass index on response to asthma controller therapy. Allergy Asthma Proc. 2010;31(1):20–25. doi: 10.2500/aap.2010.31.3307. [DOI] [PubMed] [Google Scholar]
- 46.Camargo CA, Jr., et al. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J. Asthma. 2010;47(1):76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 47.Lugogo NL, Kraft M, Dixon AE. Does obesity produce a distinct asthma pheno-type? J. Appl. Physiol. 2010;108(3):729–734. doi: 10.1152/japplphysiol.00845.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundy SM, et al. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109(4):551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 49.Leone N, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am. J. Respir. Crit. Care Med. 2009;179(6):509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 50.Lin WY, et al. Insulin resistance, obesity, and metabolic syndrome among non-diabetic pre- and post-menopausal women in North Taiwan. Int. J. Obes. (Lond.) 2006;30(6):912–917. doi: 10.1038/sj.ijo.0803240. [DOI] [PubMed] [Google Scholar]
- 51.Fimognari FL, et al. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(7):760–765. doi: 10.1093/gerona/62.7.760. [DOI] [PubMed] [Google Scholar]
- 52.Grundy SM, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 53.Todd DC, et al. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clin. Exp. Allergy. 2007;37(7):1049–1054. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 54.van Veen IH, et al. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63(5):570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 55.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am. J. Respir. Crit. Care Med. 2006;174(2):112–119. doi: 10.1164/rccm.200602-231PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lessard A, et al. Obesity and asthma: a specific phenotype? Chest. 2008;134(2):317–323. doi: 10.1378/chest.07-2959. [DOI] [PubMed] [Google Scholar]
- 57.Sutherland TJ, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am. J. Respir. Crit. Care Med. 2008;178(5):469–475. doi: 10.1164/rccm.200802-301OC. [DOI] [PubMed] [Google Scholar]
- 58.Dixon A. Pathophysiological Changes in Asthma Induced by Weight Loss. Proc. Am. Thorac. Soc. 2009:6. [Google Scholar]
- 59.Keaney JF, Jr., et al. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler. Thromb. Vasc. Biol. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 60.Steffes MW, et al. Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: the Coronary Artery Risk Development in Young Adults Study. Obesity (Silver Spring) 2006;14(2):319–326. doi: 10.1038/oby.2006.41. [DOI] [PubMed] [Google Scholar]
- 61.Dut R, et al. Oxidative stress and its determinants in the airways of children with asthma. Allergy. 2008;63(12):1605–1609. doi: 10.1111/j.1398-9995.2008.01766.x. [DOI] [PubMed] [Google Scholar]
- 62.Sackesen C, et al. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008;122(1):78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 63.Ercan H, et al. Oxidative stress and genetic and epidemiologic determinants of oxidant injury in childhood asthma. J. Allergy Clin. Immunol. 2006;118(5):1097–1104. doi: 10.1016/j.jaci.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Fitzpatrick AM, et al. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J. Allergy Clin. Immunol. 2009;123(1):146–152. e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baraldi E, et al. Increased exhaled 8-isoprostane in childhood asthma. Chest. 2003;124(1):25–31. doi: 10.1378/chest.124.1.25. [DOI] [PubMed] [Google Scholar]
- 66.Sood A, et al. Obesity-asthma association: is it explained by systemic oxidant stress? Chest. 2009;136(4):1055–1062. doi: 10.1378/chest.09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komakula S, et al. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir. Res. 2007;8:32. doi: 10.1186/1465-9921-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bouloumie A, et al. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13(10):1231–1238. [PubMed] [Google Scholar]
- 69.Sierra-Honigmann MR, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 70.Guler N, et al. Leptin: does it have any role in childhood asthma? J. Allergy Clin. Immunol. 2004;114(2):254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 71.Sood A, Ford ES, Camargo CA., Jr. Association between leptin and asthma in adults. Thorax. 2006;61(4):300–305. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shore SA, et al. Pulmonary responses to subacute ozone exposure in obese vs. lean mice. J. Appl. Physiol. 2009;107(5):1445–1452. doi: 10.1152/japplphysiol.00456.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shore SA, et al. Effect of leptin on allergic airway responses in mice. J. Allergy Clin. Immunol. 2005;115(1):103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J. Appl. Physiol. 2010;108(3):735–743. doi: 10.1152/japplphysiol.00749.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pajvani UB, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 76.Masaki T, et al. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40(1):177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 77.Kumada M, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109(17):2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 78.Wolff B, et al. Identifying the concepts contained in outcome measures of clinical trials on four internal disorders using the International Classification of Functioning, Disability and Health as a reference. J. Rehabil. Med. 2004;44(Suppl):37–42. doi: 10.1080/16501960410015407. [DOI] [PubMed] [Google Scholar]
- 79.Wulster-Radcliffe MC, et al. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem. Biophys. Res. Commun. 2004;316(3):924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 80.Hotta K, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50(5):1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 81.Milan G, et al. Resistin and adiponectin expression in visceral fat of obese rats: effect of weight loss. Obes. Res. 2002;10(11):1095–1103. doi: 10.1038/oby.2002.149. [DOI] [PubMed] [Google Scholar]
- 82.Kern PA, et al. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 83.Engeli S, et al. Association between adiponectin and mediators of inflammation in obese women. Diabetes. 2003;52(4):942–947. doi: 10.2337/diabetes.52.4.942. [DOI] [PubMed] [Google Scholar]
- 84.Jung SH, et al. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J. Nutr. Biochem. 2008;19(6):371–375. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Shore SA, et al. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J. Allergy Clin. Immunol. 2006;118(2):389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Medoff BD, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am. J. Respir. Cell Mol. Biol. 2009;41(4):397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sood A, et al. Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63(10):877–882. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sood A, et al. Effect of specific allergen inhalation on serum adiponectin in human asthma. Chest. 2009;135(2):287–294. doi: 10.1378/chest.08-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Spivak H, et al. Weight loss and improvement of obesity-related illness in 500 U.S. patients following laparoscopic adjustable gastric banding procedure. Am. J. Surg. 2005;189(1):27–32. doi: 10.1016/j.amjsurg.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 90.Stenius-Aarniala B, et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320(7238):827–832. doi: 10.1136/bmj.320.7238.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maniscalco M, et al. Weight loss and asthma control in severely obese asthmatic females. Respir. Med. 2008;102(1):102–108. doi: 10.1016/j.rmed.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 92.Eneli IU, Skybo T, Camargo CA., Jr. Weight loss and asthma: a systematic review. Thorax. 2008;63(8):671–676. doi: 10.1136/thx.2007.086470. [DOI] [PubMed] [Google Scholar]
- 93.Johnson JB, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007;42(5):665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]