Abstract
The importance of the mass spectral product ion structure is highlighted in quantitative assays, which typically use MRM (multiple reaction monitoring), and in the discovery of novel metabolites. Estradiol is an important sex steroid whose quantitation and metabolite identification using tandem mass spectrometry has been widely employed in numerous clinical studies. Negative electrospray ionization tandem mass spectrometry of estradiol (E2) results in several product ions, including the abundant m/z 183 and m/z 169. While m/z 183 is one of the most abundant product ions used in many quantitative assays, the structure of m/z 183 has not been rigorously examined. We suggest a structure for m/z 183 and a mechanism of formation consistent with collision induced dissociation (CID) of E2 and several stable isotopes ([D4]-E2, [13C6]-E2, and [D1]-E2). An additional product ion from E2, namely m/z 169, has also been examined. MS3 experiments indicated that both m/z 183 and m/z 169 originate from only E2 [M-H]− m/z 271. These ions, m/z 183 and m/z 169, were also present in the collision induced decomposition mass spectra of other prominent estrogens, estrone (E1) and estriol (E3), indicating that these two product ions could be used to elucidate the estrogenic origin of novel metabolites. We propose two fragmentation schemes to explain the CID data and suggested a structure of m/z 183 and m/z 169 consistent with several isotopic variants and high resolution mass spectrometric measurements.
Keywords: Estradiol, Estrone, Estriol, Tandem Mass Spectrometry, Product Ion Structure
Introduction
Estrogen biology is an active area of research that continues to challenge investigators. Classically, estrogens, acting via the estrogen receptor (ER), function as growth factors responsible for female sexual organ development, regulate the menstrual cycle, and maintain bone density [1]. Estrogens have also been shown to mediate rapid, ER-dependent and independent responses, including vasodilation in coronary arteries, and activation of growth-factor-related signaling pathways in neural cells [1,2].
Estradiol is a neutral compound that cannot be examined by electrospray mass spectrometry unless it is ionized as a derivative such as dansyl chloride [3], protonated to [M+H-H2O]+ [4], or as a negative anion [M-H]−, as most approaches have employed [5–8]. CID (collision induced dissociation) experiments and tandem mass spectrometry of the negative ion generate numerous product ions and these product ions have been used to quantitate underivatized estradiol in serum, tissue samples, and drinking water [6,9–12] using reaction monitoring techniques such as multiple reaction monitoring (MRM). Little has been published regarding the structure of the product ions used in those quantitative assays in particular the two abundant product ions used in most MRM based methods, m/z 145 and m/z 183. While the former product ion, m/z 145, has been previously characterized and is well understood [5,6,8,13], the proposed structure of the very abundant product ion m/z 183 has not been rigorously established [5,7].
During development of a mass spectrometry-based quantitative assay for the main estrogens (estrone (E1), estradiol (E2), and estriol (E3)) we noticed an inconsistency between our mass transitions and the previously reported structures for two of the prominent product ions derived from E2 when using various stable isotope labeled internal standards. We sought to determine the structure of m/z 183 and m/z 169, a less abundant product ion. Our work strongly suggested that the structure of these ions were significantly different in that the estradiol carbon atoms retained in m/z 183 and m/z 169 suggested a significant degree of ring opening prior to bond cleavage that resulted in fragmentation of E2.
Materials and Methods
Deuterium oxide (99.9 atom% [D2]), estrone, β-estradiol, and estriol were purchased from Sigma-Aldrich (St. Louis, MO). The following stable isotopes were purchased from Cambridge Isotope Laboratories (Andover, MA): [13C6]-estradiol (13,14,15,16,17,18, 99 atom% [13C6]), [D4]-estradiol (2,4,16,16, 95-97 atom% [D4]), [D4]-estriol (2,4,16,17, 98 atom% [D4]), [D4]-estrone (2,4,16,16, 97 atom% [D4]), and [D1]-O-methanol (99 atom% [D1]). Sodium hydroxide-[D1] (98 atom% [D1], 40% w/w in D2O) was purchased from MSD Isotopes (Montreal Canada). All solvents and other reagents used in these studies were of the highest grade commercially available.
Deuterium Exchange Reactions
Unlabeled steroid (100 μg) dissolved in either methanol or ethanol was evaporated to dryness under vacuum, resuspended with 60% MeOD, 39.5% D2O, and 0.5% NaOD and left at room temperature overnight. Before the sample was infused into the electrospray ion source, the lines were flushed with 60% MeOD/ 40% D2O to minimize deuterium back exchange. The unlabeled precursor ion served as an internal control for the deuterated precursor ion.
Mass Spectrometry
Low mass resolution experiments (MS, MS/MS, MS3, and precursor ion scan) were performed on either a 5500 QTRAP (AB Sciex, Framingham, MA) or LTQ Linear Ion Trap (Thermo Finnigan, Waltham, MA). MS/MS experiments utilized CE = 55 (collision energy)-the same CE used in our quantitative assay. MS3 experiments utilized multiple excitation energies with a representative spectra displayed; excitation energy was ramped from the lowest setting to the maximum setting or until no product ion was detected. High mass accuracy experiments (MS and MS/MS) were performed using a Synapt G2-S (Waters, Milford, MA) with the lock mass feature. Measured exact mass for steroid-derived ions were compared to theoretical monoisotopic masses. Unless otherwise stated, samples-dissolved in 80% methanol, 0.2% ammonium hydroxide and 2% acetonitrile-were infused into the electrospray ion source.
Results
E2 m/z 183
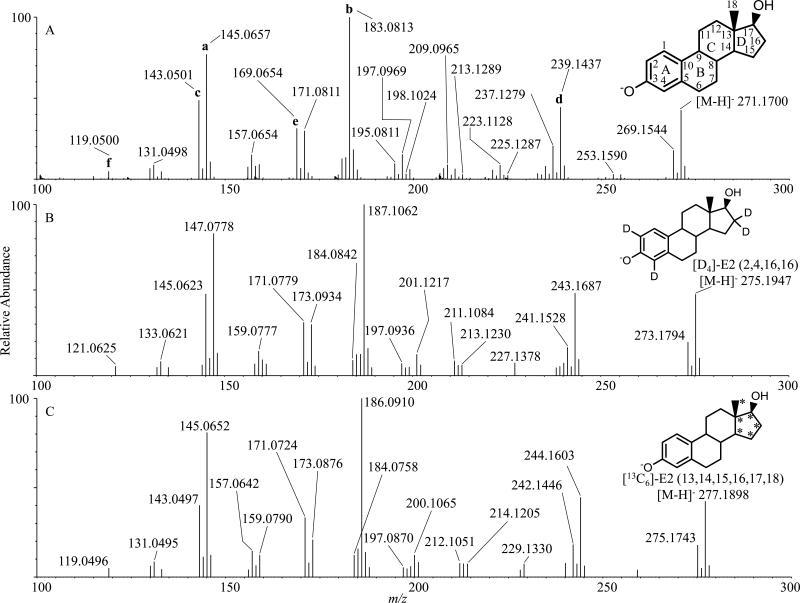
The electrospray ionization of estradiol in the negative ion mode resulted in the molecular anion [M-H]− m/z 271 [5,6,8]. The collisional activation of this [M-H]− resulted in numerous product ions-including m/z 119 (f), m/z 143 (c), m/z 145 (a), m/z 169 (e), m/z 183 (b), and m/z 239 (d) (Figure 1A) [5–8,13]; the standard steroid carbon (C1-C18) and ring nomenclature (A-D) for product ion descriptions displayed on precursor ion [M-H]− m/z 271 E2 is used for structural descriptions. The previously published structures of the most abundant ions are summarized in Supplementary Scheme 1.
Figure 1.
Negative ESI MS/MS Q-TOF of infused 10 ng/μl A E2, B [D4]-E2, and C [13C6]-E2. TOF lock mass corrections and calibration was performed on leucine enkephalin ([M-H]− 554 and the CID fragment at 179), followed by CID at Collision Energy (CE) 55-the same CE used in our quantitative assay. A The structures of published product ions (a-f) are indicated and displayed in Supplementary Scheme 1; the carbons (C1-C18) in estradiol have been indicated along with the ring nomenclature (A-D) for reference in structural discussions. B [D4]-E2 (2,4,16,16) CID spectra. Note the shift of m/z 183 (A) to m/z 187 indicating that all 4 deuterium molecules (2 from A-ring and 2 from D ring) are in the m/z 183 product ion. Also note the shift from m/z 169 (A) to m/z 171 most likely due to the presence of the 2 A-ring deuteriums on C2 and C4. C [13C6]-E2 (13, 14, 15, 16, 17, 18) CID spectra. Note the shift of m/z 183 (A) to m/z 186 indicating 3 carbons from the D-ring are in the m/z 183 product ion structure. Also note the shift from m/z 169 (A) to m/z 171 indicating two carbons from the D ring are part of the final product ion structure.
When several of the isotopic variants of E2 were examined, two notable product ions were not consistent with previously suggested structures, m/z 183 and m/z 169. For the second most abundant product ion, m/z 183, the isotopic variant data suggested a more complex origin for this ion. The only structure proposed for this ion [7] retained carbon atoms 1-10, 11, 12 and 14 (Supplementary Scheme 1 b) but this structure was inconsistent with the [D4]-E2 and [13C6]-E2 which shifted to m/z 187 and m/z 186 respectively (Figures 1B and 1C, Table 1). These isotopic variants required inclusion of carbon atom 16 and possibly 14 and 15. The exact mass of m/z 183.0813 had a calculated elemental composition of C13H11O (theoretical m/z 183.0810, 1.6 ppm, Supplementary Table 1) suggesting a highly unsaturated product ion likely delocalizing the anionic charge over many carbon atoms. The [D4]-E2 spectra suggested that this ion retained the A-ring as well as carbon atom 16 where two deuterium atoms were present. The [13C6]-E2 spectrum was consistent with only three of the six labeled carbon atoms in the D-ring being retained in the structure and therefore splitting of the D-ring in the ion fragmentation mechanism. The data from the exchangeable proton on the C17 alcohol ([D1]-E2, Supplementary Figure 1) suggested that this deuteron was being partially exchanged with a proton during formation of m/z 183 at some stage of the complex fragmentation mechanism that split the D-ring leading to a highly stabilized m/z 183 from m/z 271.
Table 1.
Product Ions Derived from MS/MS of E2 Acquired with High Accuracy Tandem Mass Spectrometry
| Precursor Ion1 | m/z (intensity)2 |
|||||
|---|---|---|---|---|---|---|
| a | b | c | d | e | f | |
| E2 (m/z 271.1700) | 145.0657 (77) | 183.0813 (100) | 143.0501 (49) | 239.1437 (44) | 169.0654 (31) | 119.0500 (5) |
| [D4]-E23 (m/z 275.1947) | 147.0778 (83) | 187.1062 (100) | 145.0623 (48) | 243.1687 (48) | 171.0779 (31) | 121.0625 (6) |
| [13C6]-E24 (m/z 277.1898) | 145.0652 (81) | 186.0910 (100) | 143.0497 (40) | 244.1603 (45) | 171.0724 (33) | 119.0496 (5) |
| [D1]-E25 (m/z 272.1762) | 145.0653 (85) 146.0707 (26) |
183.0808 (100) 184.0865 (47) |
143.0498 (57) 144.0548 (16) |
239.1435 (55) 240.1474 (12) |
169.0653 (37) 170.0709 (12) |
119.0498 (8) |
CID performed on this ion. Full MS/MS mass spectra in Figures 1A, 1B, 1C, and Supplementary Figure 1.
Obtained on a Waters Synapt G2-S (< 5 ppm mass accuracy, Supplementary Table 1)
(2,4,16,16-[D4])-Estradiol
(13,14,15,16,17,18-[13C6])-Estradiol
(17-O[D1])-Estradiol
MS3 experiments of m/z 271→269, m/z 271→253, m/z 271→239, m/z 271→225, m/z 271→213, m/z 271→198, did not yield an abundant m/z 183 suggesting that m/z 183 formed directly from the [M-H]− m/z 271 without a significant product ion of intermediate mass-to-charge ratio (Supplementary Figure 2).
E1 and E3 m/z 183
To determine whether m/z 183 was unique to E2 or whether it could serve as a diagnostic product ion for other estrogen species, we performed CID of E1, [D4]-E1, E3, and [D4]-E3 (Supplementary Figure 3). CID of E2 resulted in a similar abundance of m/z 145 and m/z 183 (Figure 1A, Table 1). In comparison, CID of E1 resulted in mostly m/z 145 with m/z 183 present but significantly less abundant. CID of E3 resulted in m/z 171 as the most abundant product ion, m/z 145 as the second most abundant ion and m/z 183 present but in low relative abundance. The m/z 183 from E1 and E3 were shifted to m/z 187 with the [D4] labeled compound suggesting the same structure of m/z 183 to that from E2. The [D4] labeling on E3-(2,4,16,17) differs from E1/2-(2,4,16,16) and suggests the deuterium on C17 rearranges such that it is present in m/z 187; this mechanism will not be addressed here. The MS3 spectra, m/z 271/269/287→183→, are indistinguishable (Supplementary Figure 4A, B, C). Together, we conclude that the product ion m/z 183 observed in CID of E1, E2, and E3 were identical and characteristic of estrogens. The differential abundance of m/z 183 in E2 and the presence of distinct dominant product ions in E1 (m/z 145) and E3 (m/z 171) should permit specific identification, characterization, and quantitation of members of the estrogen family and elucidation of the origins of their metabolites.
m/z 169
Our data partially conflicted with the structure of m/z 169 [8]. Our [D4]-E2 CID spectra supported the reported structure with C2 and C4, both on the A-ring, being present in the final structure because of the observed shift in 2 Da. However, our [13C6]-E2 data conflicted with the reported structure of m/z 169 which has C11 and C12 in the final structure [8]. The [13C6]-E2 CID data demonstrated a mass shift of 2 Da indicating that two of the [13C]-carbons in the D-ring were present in m/z 169 and we suggest that C14 and C15 from the C and D-rings are retained in the final structure of m/z 169. The CID of E2 in the ion trap mass spectrometer did not yield a very abundant ion at m/z 169 (Supplementary Figure 2) and none of the selected ions were found to generate this ion in any significant abundance except [M-H]− m/z 271.
To determine whether m/z 169 was unique to E2 or could be found in the estrogens, CID spectra of E1, [D4]-E1, E3, and [D4]-E3 (Supplementary Figure 3) were acquired. M/z 169 was present in E1 (Supplementary Figure 3A) and E3 (Supplementary Figure 3C) and like E2, m/z 169 was not very abundant. M/z 169 shifted to m/z 171 in both [D4]-E1 (Supplementary Figure 3B) and [D4]-E3 (Supplementary Figure 3D) reflecting the same mass shift observed in [D4]-E2 (Figure 1B). To further examine the nature of m/z 169 and ascertain whether m/z 169 observed from E1/2/3 were structurally similar or not, we performed MS3 of m/z 269/271/287→169→ (Supplementary Figure 4D, E, F). The spectra were indistinguishable, and with the data presented, we concluded that m/z 169 from E1, E2, and E3 behaved identically in CID and MS3 experiments. Along with m/z 183, m/z 169 could be used to indicate whether an unknown metabolite originated from one of the estrogens presented here.
Discussion
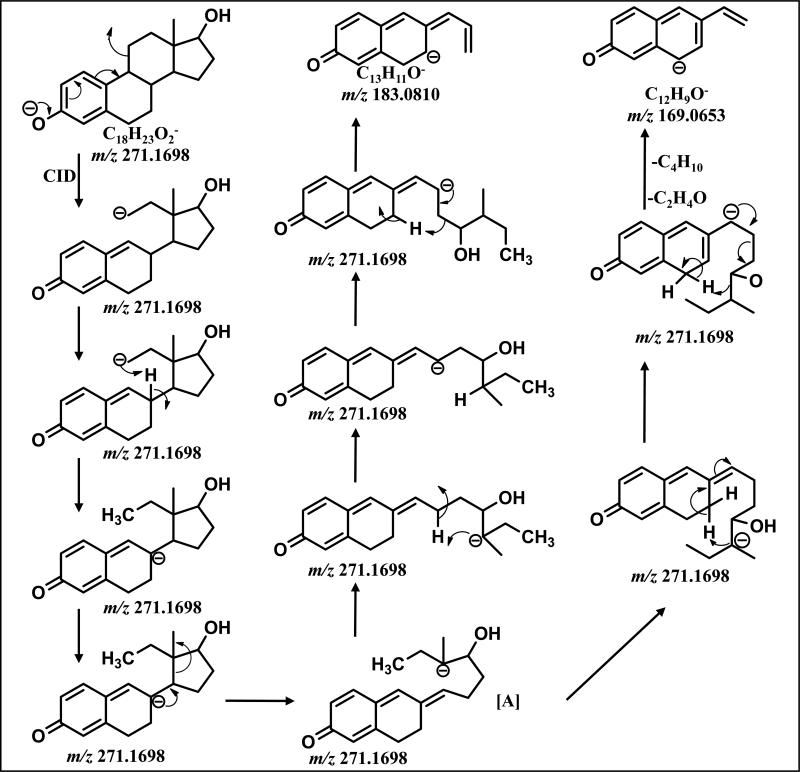
A mechanism for the collisionally induced decomposition of the [M-H]− from E2 (m/z 271) consistent with all isotope labeling data led to the proposed structure of m/z 183 (Scheme1). An initial charge-driven-rearrangement resulted in the initial cleavage of C9-C11 as an unstable primary anion that had opened the C-ring. This intermediate methylene anion could be stabilized with a transfer of the negative charge to C8, a more stable delocalized anion. The C13-C14 bond could then break in a charge driven event followed by proton rearrangement to yield a further delocalized anionic site at D-ring C15. This would set up a final charge driven carbon-carbon bond cleavage (C16-C17) releasing a saturated group (C5H12O). Our mechanism is consistent with the shift of 3 Da with CID of [13C6]-E2 (m/z 183→m/z 186), the shift in 4Da with CID of [D4]-E2 (m/z 183→m/z 187), and the lack of significant product ions in MS3 experiments (m/z 271→183).
Scheme 1.
Proposed Fragmentation Mechanism of m/z 271 to form m/z 183 and m/z 169
The proposed structure of m/z 169 was derived from the collisionally induced decomposition of the [M-H]− from E2 (m/z 271) and consistent with isotope CID data (Scheme 1). Intermediate [A] can undergo a rotation about C14-C15 which allows the anion on C13 to transfer its charge to C7 resulted in a negative charge at C14. Proton rearrangement set up two concerted final charge driven carbon-carbon bond cleavages (C15-C16, C13-C17) releasing (C4H10) and (C2H4O) and leaving a highly delocalized and stable anion m/z 169. Our mechanism is consistent with the shift in 2 Da with CID of [D4]-E2 (m/z 169→m/z 171) and CID of [13C6]-E2 (m/z 169→m/z 171) as well as the lack of significant product ions in MS3 experiments (m/z 271→169).
The importance for determining the structure of product ions is twofold. In mass spectrometric based quantitative assays, it is critical to know the identity of the product ions and their origins in relation to isotopic variants used as internal standards. Such information is also of great value when novel compounds are being structurally characterized. High mass accuracy measurements can aid in the identification of the elemental composition of a compound but cannot reveal significant structural information. The unexpected and extensive rearrangement of the C and D rings of E2 prior to fragmentation would be a valuable behavior for structurally related steroids.
Supplementary Material
Acknowledgment
This work was supported in part by grants from the National Institutes of Health HD058155 (NS), ES 022172 (RCM) and Lipid Maps Collaborative Grant GM069338 (RCM), and Colorado Clinical and Translational Sciences Institute RR025780.
References
- 1.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and Actions of Estrogens. The New England Journal of Medicine. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 2.Kelly MJ, Rønnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Frontiers in neuroendocrinology. 2012;33:376–87. doi: 10.1016/j.yfrne.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai SS, Welch MJ. Development and Evaluation of a Reference Measurement Procedure for the Determination of Estradiol-17B in Human Serum Using Isotope-Dilution Liquid Chromatography-Tandem Mass Spectrometry. Analytical chemistry. 2005;77:6359–6363. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- 4.Vanderford BJ, Pearson R. a, Rexing DJ, Snyder S. a. Analysis of endocrine disruptors, pharmaceuticals, and personal care products in water using liquid chromatography/tandem mass spectrometry. Analytical chemistry. 2003;75:6265–74. doi: 10.1021/ac034210g. [DOI] [PubMed] [Google Scholar]
- 5.Croley TR, Hughes RJ, Koenig BG, Metcalfe CD, March RE. Mass spectrometry applied to the analysis of estrogens in the environment. Rapid communications in mass spectrometry : RCM. 2000;14:1087–93. doi: 10.1002/1097-0231(20000715)14:13<1087::AID-RCM992>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Magi E, Scapolla C, Di Carro M, Liscio C. Determination of endocrine-disrupting compounds in drinking waters by fast liquid chromatography-tandem mass spectrometry. Journal of mass spectrometry : JMS. 2010;45:1003–11. doi: 10.1002/jms.1781. [DOI] [PubMed] [Google Scholar]
- 7.Gentili A, Perret D, Marchese S, Mastropasqua R, Curini R, D. C. Analysis of Free Estrogens and their Conjugates in Sewage and River Waters by Solid-Phase Extraction then Liquid Chromatography- Electrospray-Tandem Mass Spectrometry. Chromatographia. 2002;56:25–32. [Google Scholar]
- 8.Rannulu NS, Cole RB. Novel fragmentation pathways of anionic adducts of steroids formed by electrospray anion attachment involving regioselective attachment, regiospecific decompositions, charge-induced pathways, and ion-dipole complex intermediates. Journal of the American Society for Mass Spectrometry. 2012;23:1558–68. doi: 10.1007/s13361-012-0422-y. [DOI] [PubMed] [Google Scholar]
- 9.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clinica chimica acta; international journal of clinical chemistry. 2006;372:76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Pouech C, Tournier M, Quignot N, Kiss A, Wiest L, Lafay F, Flament-Waton M-M, Lemazurier E, Cren-Olivé C. Multi-residue analysis of free and conjugated hormones and endocrine disruptors in rat testis by QuEChERS-based extraction and LC-MS/MS. Analytical and bioanalytical chemistry. 2012;402:2777–88. doi: 10.1007/s00216-012-5723-2. [DOI] [PubMed] [Google Scholar]
- 11.Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman J-M. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2012;893-894:57–62. doi: 10.1016/j.jchromb.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Chiuminatto U, Gosetti F, Dossetto P, Mazzucco E, Zampieri D, Robotti E, Gennaro MC, Marengo E. Automated Online Solid Phase Extraction Ultra High Performance Liquid Chromatography Method Coupled with Tandem Mass Spectrometry for Determination of Forty-Two Therapeutic Drugs and Drugs of Abuse in Human Urine. Analytical chemistry. 2010;82:5636–5645. doi: 10.1021/ac100607v. [DOI] [PubMed] [Google Scholar]
- 13.Bourcier S, Poisson C. Elucidation of the decomposition pathways of protonated and deprotonated estrone ions: application to the identification of photolysis products. Rapid Communications in Mass Spectrometry. 2010;24:2999–3010. doi: 10.1002/rcm.4722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.