Abstract
Plasma levels of beta-endorphin (BE), an endogenous opioid analgesic, are often reported as they relate to acute and chronic pain outcomes. However, little is known about what resting plasma BE levels might reveal about functioning of the endogenous opioid antinociceptive system. This study directly examined associations between resting plasma BE and subsequent endogenous opioid analgesic responses to acute pain in 39 healthy controls and 37 individuals with chronic low back pain (LBP). Resting baseline levels of plasma BE were assessed. Next, participants received opioid blockade (8 mg naloxone i.v.) or placebo in a double-blind, randomized, crossover design. Participants then underwent two acute pain stimuli: finger pressure (FP) pain and ischaemic (ISC) forearm pain. Blockade effects (naloxone minus placebo pain ratings) were derived to index endogenous opioid analgesic function. In placebo condition analyses for both pain stimuli, higher resting BE levels were associated with subsequently greater reported pain intensity (p’s < 0.05), with this effect occurring primarily in healthy controls (BE × Participant Type interactions, p’s < 0.05). In blockade effect analyses across both pain tasks, higher resting plasma BE predicted less subsequent endogenous opioid analgesia (smaller blockade effects; p’s < 0.05). For the ISC task, these links were significantly more prominent in LBP participants (BE × Participant Type Interactions, p’s < 0.05). Results suggest that elevated resting plasma BE may be a potential biomarker for reduced endogenous opioid analgesic capacity, particularly among individuals with chronic pain. Potential clinical implications are discussed.
1. Introduction
Endogenous opioids are an important component of the antinociceptive system (Millan, 2002). Several strategies for evaluating the role of endogenous opioids in human pain modulation have been developed. One is to assay levels of beta-endorphin (BE), an endogenous mu opioid receptor agonist with significant analgesic properties (Millan, 2002). Due to ease of acquiring samples, plasma BE levels are often examined as they relate to acute and chronic pain outcomes (Cohen et al., 1982; Pickar et al., 1983; Bach et al., 1987; Falcone et al., 1993; Leonard et al., 1993; Guasti et al., 1996; Matejec et al., 2003; al’Absi et al., 2004; Bruehl et al., 2007). Other studies examine pain-related influences of BE in the central nervous system (CNS) via sampling of cerebrospinal fluid (Cleeland et al., 1984; Spaziante et al., 1990; Matejec et al., 2003). While pathways underlying antinociceptive effects of BE in the CNS are clear (Zubieta et al., 2001; Sprenger et al., 2006), peripheral analgesic actions of plasma BE are less clear (Hargreaves et al., 1987; Hargreaves et al., 1990; Dionne et al., 2001). Regardless, if plasma BE levels correspond with differences in opioid antinociceptive function, they might serve as biomarkers of endogenous antinociceptive capacity.
Differing BE levels per se do not necessarily imply corresponding differences in endogenous opioid anti-nociceptive function. For example, elevated BE levels would have little analgesic effect in the context of down-regulated opioid receptors [e.g., opioid tolerant pain patients; (Raehal and Bohn, 2005)]. Functioning of the endogenous opioid antinociceptive system can be directly assessed by examining responses to experimental acute pain stimulation in the context of placebo-controlled opioid blockade (Bruehl and Chung, 2006; France et al., 2007; Frew and Drummond, 2009). While not pragmatic for the clinical setting, this procedure nonetheless provides information on opioid antinociceptive function not obtainable through assessment of opioid levels. To date, no human studies have directly examined plasma BE levels as they relate to endogenous opioid analgesic function (i.e., opioid blockade effects on acute pain responses). Such a study might clarify the interpretation of BE levels, and potentially have implications for BE assays in the clinical context as a marker for endogenous opioid analgesic capacity.
Data from a broader study (see below) provided an opportunity to examine whether resting plasma BE might serve as a biomarker for endogenous opioid antinociceptive function. Existing literature suggested two alternatives. Three small studies reporting that higher resting pre-surgical plasma BE levels predict lower post-operative opioid analgesic requirements (Cohen et al., 1982; Pickar et al., 1983; Nader-Djalal et al., 1995) and work noting inverse associations between resting plasma BE and subsequent experimental pain sensitivity (Guasti et al., 1996) suggested that elevated resting BE levels might be a marker for more effective endogenous opioid analgesia. In contrast, clinical studies indicating that higher resting plasma BE predicts greater subsequent acute pain intensity suggested that higher BE levels might be a marker for reduced endogenous opioid analgesia (Bach et al., 1987; Leonard et al., 1993; Matejec et al., 2003). The current study tested these two alternative hypotheses.
2. Method
2.1 Design
The current study was a secondary analysis of data collected as part of a larger project regarding opioid mechanisms in the pain-related effects of anger (Bruehl et al., 2011). Given the current study aims, analyses were restricted to data from the neutral emotion condition in this larger study (see below). A double-blind, placebo-controlled, crossover design was used employing an opioid blockade methodology (Bruehl et al., 2002; Bruehl and Chung, 2006). Order of drug administration was randomized and counterbalanced. Within-subject variables were the change in acute pain intensity measures across drug conditions. Participant type [healthy vs. chronic low back pain (LBP)] was included as a between-subject variable given evidence that chronic pain may under some conditions be associated with either increased (Bruehl et al., 2010) or decreased endogenous opioid activity (Bruehl et al., 1999; Bruehl and Chung, 2006).
2.2 Participants
Participants included 37 individuals with chronic LBP and 39 healthy pain-free controls (healthy). All participants were recruited through online advertisements on the Vanderbilt email recruitment system or advertisements in local print media. General criteria for participation included age between 18 and 55; no history of cardiovascular disease, hypertension, liver or kidney disorders, post-traumatic stress disorder, diabetes, seizure disorder or opiate dependence; no use of anti-hypertensive medications; and no daily use of opioid analgesics. Additional inclusion criteria for the LBP group were chronic daily LBP of at least 3 months duration with an average past month severity of at least 3/10 (Bruehl and Chung, 2006; Bruehl et al., 2007). Due to requirements of assessment procedures, the individual conducting the laboratory sessions was not blinded to chronic pain status. All participants were asked to avoid use of as-needed opioid analgesics for 3 days prior to each study session (confirmed via urine opiate screens). Potential participants who were pregnant were excluded (confirmed by urine pregnancy screens). All participants were asked to refrain from use of any analgesic or anti-inflammatory medications (e.g., acetaminophen, ibuprofen, etc.) for 12 h prior to study participation, and to avoid use of caffeine for 3 h prior to each study session. No participants in either group were taking neuroleptic medications. Two healthy participants and three LBP participants were taking antidepressants; this difference was not significant (phi = 0.06, p > 0.10).
Characteristics of both study subgroups are summarized in Table 1. Compared with LBP participants, healthy participants were significantly younger and more often female. Resting plasma BE levels did not differ between groups. Both groups were also similar in terms of general negative affect levels on the Negative Affect subscale of the Positive and Negative Affect Scales (PANAS) (Watson et al., 1988). However, LBP participants had significantly higher scores on the State Trait Anxiety Inventory (STAI) (Spielberger et al., 1970) as well as on the Beck Depression Inventory (BDI) (Beck et al., 1961), although both groups were in the non-depressed range on the latter measure.
Table 1.
Participant characteristics.
| Variable | Participant type
|
|
|---|---|---|
| Healthy control (n = 39) | LBP (n = 37) | |
| Age (years)* | 30.9 ± 8.26 | 35.2 ± 9.86 |
| Gender (% female)* | 71.8 | 48.6 |
| Race (%) | ||
| White | 87.2 | 78.4 |
| African-American | 10.3 | 8.1 |
| Ethnicity (% non-Hispanic) | 94.9 | 89.2 |
| Chronic pain duration (median, in months) | 70.2 | |
| Past month chronic pain intensity (0–100) | 44.1 ± 16.91 | |
| Resting plasma BE (ng/mL) | 1.5 ± 2.41 | 1.8 ± 2.57 |
| BDI** | 2.8 ± 3.04 | 7.5 ± 5.88 |
| STAI** | 32.1 ± 6.97 | 38.1 ± 8.38 |
| PANAS–Negative Affect | 15.4 ± 3.99 | 16.8 ± 4.20 |
Values are presented as percentages or means ± SD. BDI, Beck Depression Inventory; BE, beta-endorphin; LBP, chronic low back pain; PANAS, Positive and Negative Affect Scales; SD, standard deviation; STAI, State Trait Anxiety Inventory.
p < 0.05.
p < 0.01.
2.3 Measures
All participants completed the BDI (Beck et al., 1961), the trait form of the STAI (Spielberger et al., 1970), and the PANAS (Watson et al., 1988). These three measures are widely used and well-validated instruments for assessing various components of negative affect. They were included to permit examination of potential psychobiological pathways for hypothesized opioid findings.
The short form of the McGill Pain Questionnaire (MPQ; Melzack, 1987) was used to allow participants to describe the acute pain experienced during the laboratory pain tasks. The MPQ provides separate sub-scales assessing the sensory (MPQ-S) and affective (MPQ-A) dimensions of pain. A 100-mm visual analogue scale (VAS) measure of overall pain intensity is included on the MPQ (anchored with ‘No Pain’ and ‘Worst Possible Pain’). For purposes of the current study, this global pain intensity measure was considered as the primary pain outcome. A parallel 100-mm VAS measure of pain unpleasantness (anchored with ‘Not Unpleasant at All’ and ‘Most Unpleasant Possible’) was added as in our previous work (e.g., Bruehl et al., 2007).
2.4 Opioid blockade agent
The opioid blockade agent used in this study was naloxone, a non-selective opioid receptor antagonist with a brief half-life (1.1 h). A 20-mL dose of normal saline or an 8-mg dose of naloxone (in 20 mL saline vehicle) was infused via an automated infusion pump over a 10-min period through a venous cannula. The naloxone dosage used was chosen to insure adequate blockade of all opioid receptor subtypes given evidence for dose-dependent effects (Lewis et al., 1987).
2.5 Experimental acute pain induction
Two experimental acute pain tasks were used in this study. First, participants underwent a 1-min finger pressure (FP) pain task using a modified Forgione–Barber finger pressure pain stimulator that applied 2000 g of pressure to the dorsal surface of the second phalanx of the index finger of the dominant hand (Forgione and Barber, 1971). Participants then engaged in a forearm ischaemic (ISC) pain task based on procedures described by Maurset et al. (1992), which induces pain by a combination of muscle exercise and ischaemia. Participants were first asked to raise their dominant forearm over their head for 30 s followed by 2 min of dominant forearm muscle exercise using a hand dynamometer at 50% of his or her maximal grip strength (as determined prior to beginning the laboratory procedures). Immediately following this, a BP cuff was inflated on the participant’s dominant bicep to 200 mmHg. The cuff remained inflated until participants indicated that their pain tolerance had been reached, up to a maximum of 5 min [due to Institutional Review Board (IRB) requirements]. ISC pain threshold (time from task onset to first report that the task felt ‘painful,’ in seconds) was recorded.
2.6 Procedure
All procedures were performed at the Vanderbilt General Clinical Research Center, and were approved by the university IRB. All participants first gave written informed consent. Immediately prior to the laboratory portion of the study, participants provided demographic and background information, and completed the psychometric packet.
All participants engaged in two laboratory sessions (one under placebo and one under opioid blockade) approximately 1 week apart, at the same time of day to control for circadian rhythms. Participants remained seated upright in a comfortable chair throughout all laboratory procedures. After a 15-min seated rest period, a registered nurse under physician’s supervision placed an indwelling venous cannula in the participant’s non-dominant arm, followed by a 30-min resting adaptation period. At the end of this resting adaptation period, a 2-mL blood sample was obtained through the cannula for assessment of resting plasma BE levels. A 20-mL dose of normal saline or an 8-mg dose of naloxone (in 20 mL saline vehicle) was next infused over a 10-min period using an automated infusion pump and the cannula was then removed.
After a 10-min rest following infusion to allow peak opioid blockade activity to be achieved, participants engaged in a 5-min emotionally non-arousing interview regarding foods that they commonly eat. Participants then underwent the FP pain task, providing verbal numeric pain ratings [intra-task numeric rating scale (NRS); 0 = ‘No Pain’ and 100 = ‘Worst Possible Pain’] at 15-s intervals. Immediately upon cessation of the FP task, participants completed the MPQ to describe the acute pain experienced during the task.
After completion of these pain ratings, participants then engaged in the ISC pain task. Intra-task NRS pain ratings (identical to those obtained for the FP task) were obtained at 30-s intervals during the ISC task. Participants then rated the acute pain immediately following the ISC task using the MPQ. Because of ceiling effects due to the high proportion of participants reaching the 5-min tolerance limit (more than 60% of participants in both drug conditions), valid analyses of ISC task tolerance as an outcome were not possible.
2.7 BE assays
BE assay procedures were identical to our past work (Bruehl et al., 2007). Blood samples (in purple-top Vacutainer tubes with ethylenediaminetetraacetic acid) were immediately stored on ice. Within 30 min of collection, samples were processed in a cool centrifuge (0–4 °C) at 3000 rpm for 15 min. Plasma was then extracted and stored at −70 °C until assays were conducted. Plasma BE levels were determined using a commercially available enzyme immunoassay kit following standard published procedures (Phoenix Pharmaceuticals, Belmont, CA, USA). The detection limit was 0.1 ng/mL, with 0% cross reactivity with metenkephalin, alpha-melanocyte stimulating hormone or adrenocorticotropic hormone.
2.8 Statistical analysis
All analyses were conducted using the PASW 18 statistical package (SPSS, Inc., Chicago, IL, USA). Primary analyses consisted of two sets of hierarchical regressions, the first focusing on placebo condition pain ratings and the second focusing on measures of endogenous opioid analgesic function. To serve as an index of opioid analgesic activity, opioid blockade effects were derived reflecting the difference between placebo condition and naloxone condition acute pain ratings as in our prior work (e.g., Bruehl et al., 2002; Bruehl and Chung, 2006). These blockade effects were derived so that positive values reflected increased pain in the naloxone condition relative to placebo (i.e., evidence for endogenous opioid analgesia). Preliminary analyses of these blockade effect measures using t-tests indicated that naloxone significantly altered (increased) acute pain ratings only in the LBP subgroup, for VAS ratings of FP task pain intensity and unpleasantness (p’s < 0.05). Resting plasma BE levels for the placebo condition session were used in placebo condition analyses to permit examination of their association with subsequent pain responses in that condition, whereas mean (pre-drug) resting plasma BE levels across both drug conditions were used in analyses of associations with the blockade effect variables.
Regression analyses for placebo condition and blockade effect values were conducted on the primary pain outcome measure (VAS intensity) as well as several secondary outcome measures (MPQ-S, MPQ-A, VAS unpleasantness and intra-task NRS). In both sets of regression analyses, independent predictors of interest were participant type (healthy vs. LBP; dummy coded) and resting plasma BE. Main effects were entered jointly first, followed by the two-way (multiplicative) interaction of these variables in the next step. A dummy-coded assay set variable was entered into the first step of the hierarchical regression models above as a control for possible differences between assay plates. Because of significant differences across participant types in age and gender distribution that could confound both placebo and blockade effect analyses, and presence of significant drug order effects in preliminary blockade effect analyses, these variables were also entered as control variables in the first step of relevant regression models. Significant interactions in these regression analyses were followed up with simple effects analyses: examination of associations between BE and opioid blockade effect outcomes by participant type. To address the possibility that significant findings in primary blockade effect analyses were influenced by extreme outliers, significant analyses were rerun using winsorized blockade effect measures [at three standard deviations (SDs) above or below the mean as appropriate; Barnett and Lewis, 1994]. All significant effects in the original analyses remained significant and all beta values remained virtually unchanged in these analyses using winsorized data, suggesting that outliers did not have undue influence on the results of primary analyses described below. Correlation coefficients (r) are presented for all analyses as an index of effect size.
3. Results
3.1 Plasma BE and placebo condition pain responses
3.1.1 FP pain task
Mean pain ratings by drug condition and participant type are presented in Table 2. Regression analyses of FP task placebo condition data for the primary pain outcome (VAS intensity) revealed only one significant effect: Participant type exhibited a significant main effect [beta = 0.26; t(75) = 2.22, p < 0.03; r = 0.25], with LBP participants reporting higher pain intensity levels. Resting BE levels were not a significant predictor of FP task VAS intensity (p > 0.10). A main effect for participant type like that above was also found for the secondary FP task VAS unpleasantness measure [beta = 0.26; t(75) = 2.21, p < 0.03; r = 0.25], again with no significant effect noted for BE levels (p > 0.10).
Table 2.
Mean (±SD) pain task ratings across drug conditions and participant types.
| Variable | Placebo
|
Naloxone
|
||
|---|---|---|---|---|
| Healthy control | LBP | Healthy control | LBP | |
| FP-MPQ-S | 10.5 ± 6.55 | 11.3 ± 5.77 | 10.5 ± 6.19 | 11.2 ± 5.52 |
| FP-MPQ-A | 1.1 ± 1.79 | 1.2 ± 1.34 | 1.0 ± 1.77 | 1.3 ± 1.45 |
| FP-VAS intensity | 46.6 ± 24.54 | 53.3 ± 22.63 | 45.7 ± 20.76 | 57.2 ± 21.68 |
| FP-VAS unpleasantness | 51.2 ± 24.92 | 57.6 ± 22.51 | 47.8 ± 20.01 | 61.5 ± 24.79 |
| FP-intra-task NRS | 45.1 ± 25.47 | 47.7 ± 19.91 | 46.0 ± 23.73 | 48.8 ± 19.92 |
| ISC threshold (s) | 26.5 ± 32.25 | 31.1 ± 43.25 | 37.6 ± 49.68 | 40.6 ± 47.85 |
| ISC-MPQ-S | 8.6 ± 6.33 | 10.7 ± 5.11 | 8.4 ± 5.95 | 9.6 ± 4.82 |
| ISC-MPQ-A | 1.6 ± 2.02 | 1.4 ± 1.19 | 1.4 ± 2.09 | 1.4 ± 1.69 |
| ISC-VAS intensity | 43.2 ± 26.33 | 50.4 ± 21.9 | 43.0 ± 25.47 | 46.4 ± 25.68 |
| ISC-VAS unpleasantness | 52.6 ± 25.36 | 54.5 ± 22.01 | 53.7 ± 24.89 | 49.0 ± 26.91 |
| ISC-intra-task NRS | 44.8 ± 28.47 | 43.3 ± 18.72 | 42.3 ± 27.44 | 42.0 ± 17.33 |
FP, finger pressure task; ISC, ischaemic task; LBP, chronic low back pain; MPQ, McGill Pain Questionnaire-Sensory (S) and Affective (A) subscales; NRS, numeric rating scale; SD, standard deviation; VAS, visual analogue scale.
Analyses of other secondary placebo condition FP task pain outcomes revealed several significant interactions. The Participant Type × BE interaction model for MPQ-A ratings was significant [t(75) = −2.25, p < 0.03]. This interaction was due to a marginally significant positive association between BE levels and MPQ-A ratings in healthy participants [beta = 0.32; t(38) = 1.72, p < 0.10; r = 0.27] that was absent in LBP participants [beta = −0.12; t(36) = −0.65, p > 0.10; r = −0.11]. Placebo condition FP-MPQ-S ratings revealed a similar pattern. Higher resting plasma BE was associated with significantly higher MPQ-S ratings in healthy participants [beta = 0.40; t(38) = 2.15, p < 0.04; r = 0.33], but not significantly associated in LBP participants {beta = −0.19; t(36) = −1.00, p > 0.10; r = −0.16; Participant Type × BE interaction [t(75) = −1.90, p < 0.07]}. Main effect and interaction models for the placebo FP task NRS pain measure were non-significant (p’s > 0.10).
3.1.2 ISC pain task
Examination of the primary ISC task pain outcome revealed a significant main effects model, with higher resting plasma BE levels associated with significantly higher placebo condition VAS pain intensity ratings [beta = 0.28; t(75) = 2.24, p < 0.03; r = 0.25]. A similar non-significant trend was noted for the secondary ISC task VAS unpleasantness measure [beta = 0.23; t(75) = 1.69, p < 0.10; r = 0.19].
Examination of other secondary pain outcomes for the ISC task revealed a pattern similar to the FP task. A significant Participant Type × BE interaction was noted for ISC-MPQ-A ratings [t(75) = −2.46, p < 0.02]. This effect was derived from a significant positive association between resting BE and placebo MPQ-A ratings in healthy participants [beta = 0.38; t(38) = 2.08, p < 0.05; r = 0.32] that was non-significant in the LBP participants [beta = −0.13; t(36) = −0.68, p > 0.10; r = −0.01]. A similar non-significant Participant Type × BE interaction trend was found for ISC placebo MPQ-S ratings [t(75) = −1.69, p < 0.10]. In healthy participants, a non-significant positive association between plasma BE and MPQ-S ratings was noted [beta = 0.30; t(38) = 1.58, p < 0.13; r = 0.25], with a non-significant negative association in LBP participants [beta = −0.14; t(36) = −0.74, p > 0.10; r = −0.12]. All other main effect tests (including participant type) failed to reach statistical significance (p’s > 0.10).
In summary, the placebo condition findings above indicate that for the primary pain outcome measure, elevated BE was associated with greater subsequent acute pain responsiveness only for the ISC task, and this effect did not differ as a function of chronic pain status. However, for secondary pain outcomes, elevated resting plasma BE was associated with greater subsequent acute pain responsiveness on both pain tasks, with these effects appearing most prominent among individuals without chronic pain.
3.2 Plasma BE and opioid blockade effects
3.2.1 FP pain task
All Participant Type × BE interaction models for the FP task failed to reach the criterion of statistical significance (p’s > 0.10). Nonetheless, the main effects model for the primary FP task VAS intensity blockade effect outcome revealed that, across both participant groups, higher resting plasma BE was a significant predictor of smaller opioid blockade effects [beta = −0.29; t(73) = −2.36, p < 0.03; r = −0.27]. Analyses of secondary FP task blockade effect outcomes portrayed a similar pattern, with significant main effects noted for VAS unpleasantness [beta = −0.32; t(73) = −2.74, p < 0.009; r = −0.31] and intra-task NRS blockade effect measures [beta = −0.34; t(73) = −2.67, p < 0.01; r = −0.30], with a similar non-significant trend for MPQ-S blockade effects [beta = −0.23; t(73) = −1.74, p < 0.09; r = −0.20]. A scatterplot of mean resting plasma BE levels by NRS blockade effects is presented in online supplemental materials. All of the effects above, for both primary and secondary outcomes, indicated an association between elevated resting (pre-pain) plasma BE and less subsequent endogenous opioid analgesia during the FP task that did not depend on participant type. Controlling for outliers using winsorized data did not alter this pattern of findings.
3.2.2 ISC pain task
For the primary ISC task VAS intensity blockade effect measure, a significant Participant Type × BE interaction was noted [t(73) = −2.30, p < 0.03]. This interaction resulted from no association between BE and blockade effects in healthy controls [beta = 0.10; t(35) = 0.49, p > 0.10; r = 0.09], but a significant inverse association in LBP participants [beta = −0.43; t(36) = −2.48, p < 0.02; r = −0.40]. The comparable interaction was likewise significant for the secondary VAS unpleasantness blockade effect outcome [t(72) = −2.45, p < 0.02]. As for the primary measure, this latter interaction was the result of no association in healthy controls [beta = 0.01; t(35) = 0.06, p > 0.10; r = 0.01], but a significant inverse association in LBP participants [beta = −0.44; t(36) = −2.61, p < 0.02; r = −0.40]. To illustrate this latter interaction graphically as recommended by Aiken and West (1991), the regression equations computed for healthy control and LBP participants were solved for hypothetical resting plasma BE values (−1 SD and +1 SD from the mean BE value). These values are displayed in Fig. 1. Endogenous opioid analgesia (positive blockade effects) was evident only for LBP participants with lower resting plasma BE levels. The Participant Type × BE interaction model for the ISC pain threshold measure was also significant [t(61) = −2.77, p < 0.009], resulting from no association between BE and blockade effects in healthy controls [beta = −0.11; t(28) = −0.47, p > 0.10; r = −0.09], but a significant link between elevated BE and smaller blockade effects in LBP participants [beta = −0.52; t(32) = −2.56, p < 0.02; r = −0.41]. Main effects models for primary and secondary ISC task blockade effect outcomes were all non-significant (p’s > 0.10).
Figure 1.
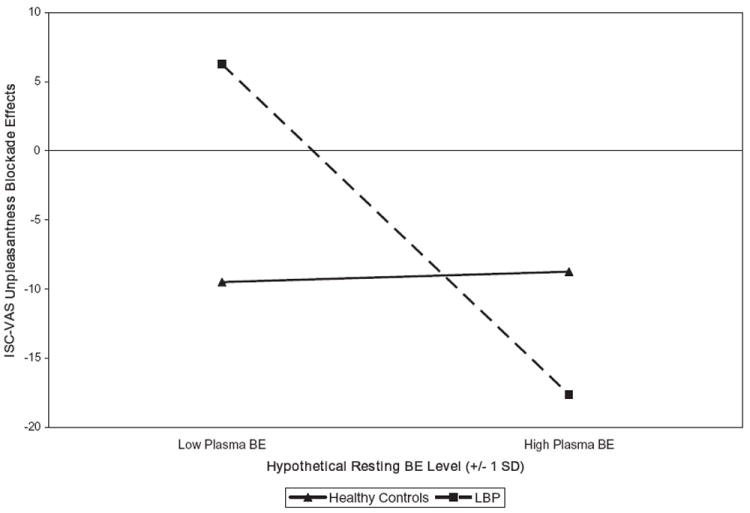
Associations between resting plasma beta-endorphin (BE) levels and ischaemic (ISC) task visual analogue scale (VAS) unpleasantness opioid blockade effects in healthy controls and low back pain (LBP) participants. BE values plotted are hypothetical values representing one standard deviation (SD) below and above the sample mean. Larger positive blockade effects indicate greater endogenous opioid analgesia.
3.3 Are resting BE levels a surrogate marker for affect-related opioid system activation?
Release of BE from the pituitary into circulation is part of the systemic stress response [Hypothalamic-Pituitary-Adrenal axis activation (HPA) axis activation; Solomon, 1999; Hook et al., 2009]. While no pure measure of life stress was included in this study, measures of trait and state negative affect were. This provided an opportunity to explore whether associations between elevated resting plasma BE and lower endogenous opioid analgesia might be accounted for by opioid-related influences of elevated negative affect (e.g., Kennedy et al., 2006). First, correlations were examined between resting plasma BE and BDI scores (r = −0.10, p > 0.10), STAI scores (r = −0.05, p > 0.10) and PANAS–Negative Affect scores (r = −0.12, p > 0.10), all of which were non-significant. Second, the significant analyses above revealing main effects of resting BE on opioid blockade effects were rerun, entering BDI, STAI and PANAS–Negative Affect scores in the first step to statistically remove variance in blockade effects attributable to negative affect. These analyses indicated that for the primary FP task VAS intensity outcome [beta = −0.32; t(73) = −2.71, p < 0.009] as well as secondary FP pain measures including MPQ-S [beta = −0.27; t(73) = −2.15, p < 0.04], FP-VAS unpleasantness [beta = −0.36; t(73) = −3.08, p < 0.004] and FP mean intra-task NRS [beta = −0.34; t(73) = −2.73, p < 0.009], the pattern of results previously described was essentially unchanged. Overall, it did not appear that negative affect could account for observed inverse associations between resting plasma BE and degree of endogenous opioid analgesia.
4. Discussion
Endogenous opioids are relevant to understanding responses to both acute (Buchsbaum et al., 1977; Gracely et al., 1983; Anderson et al., 2002) and chronic pain (Maixner et al., 1995; Bruehl et al., 1999; Bragdon et al., 2002; Bruehl et al., 2004). BE is a key analgesic endogenous opioid that has been the focus of numerous studies, often via assessment of plasma levels (Cohen et al., 1982; Pickar et al., 1983; Bach et al., 1987; Leonard et al., 1993; Guasti et al., 1996; Bragdon et al., 2002; Matejec et al., 2003; al’Absi et al., 2004; Bruehl et al., 2007). Surprisingly little is known about what information resting plasma BE levels provide regarding functioning in the endogenous opioid antinociceptive system. Previous studies have not directly examined associations between resting plasma BE and functional measures of endogenous opioid antinociceptive activity. Prior work focusing on plasma BE and pain-related outcomes variously suggested these associations might be positive (Cohen et al., 1982; Pickar et al., 1983), negative (Bach et al., 1987; Leonard et al., 1993; Matejec et al., 2003) or non-existent (Sheps et al., 1995; Tordjman et al., 2009). Interpretation of these studies was hindered by frequently small sample sizes [e.g., n = 9 (Cohen et al., 1982); n = 17 (Matejec et al., 2003)]. The primary aim of this study was to directly evaluate associations between resting plasma BE and opioid blockade-derived indices of opioid antinociceptive function in a relatively large sample, including chronic pain patients.
Placebo condition results indicated that for all significant associations across both pain tasks, higher resting plasma BE levels were associated with greater subsequent pain responsiveness. For secondary pain outcomes only, significant Participant Type × BE interactions suggested that this positive resting BE/pain intensity link was restricted primarily to the healthy participant group. Significant blockade effect analyses across both acute pain stimuli and on both primary and secondary outcomes suggested that associations between higher resting BE and greater placebo pain intensity might be related to lower endogenous opioid analgesia during acute pain stimulation. Although results for the FP task suggested these links between elevated resting BE and reduced opioid analgesic function were similar regardless of chronic pain status, significant Participant Type × BE interactions for primary and two secondary ISC task blockade effect measures indicated that these effects were most prominent among the chronic pain subgroup. These latter findings suggest that elevated resting plasma BE levels may be particularly relevant as a biomarker for reduced endogenous opioid antinociceptive function in chronic pain patients.
On the face of it, an association between higher circulating BE and lower subsequent endogenous opioid analgesia appears counter-intuitive. However, to the extent that resting plasma BE levels might also reflect tonic activation of CNS opioid pathways, the results of this study could be due to the influence of opioid receptor down-regulation. Specifically, higher BE levels could be associated with compensatory down-regulation of opioid receptors, in which context, pain-evoked release of endogenous opioids would have less analgesic efficacy (i.e., smaller opioid blockade effects). Such down-regulation of mu opioid receptors in response to elevated BE levels has been demonstrated in animals (Petraschka et al., 2007). This interpretation must be considered in light of previous findings that BE levels assessed contemporaneously in plasma and cerebrospinal fluid (CSF) do not necessarily correspond (Kosten et al., 1987; Bach et al., 1992; Baker et al., 1997). This would not be surprising given their different sources: BE in circulation derives largely from the pituitary (Solomon, 1999), whereas BE in the CNS derives from the hypothalamus, periaqueductal gray and other brain regions (Basbaum and Fields, 1984; Pilcher et al., 1988; Zubieta et al., 2001; Sprenger et al., 2006).
Release of BE from the pituitary into circulation is part of the systemic stress response (HPA axis activation; Solomon, 1999; Hook et al., 2009), and elevated emotional arousal is often associated with HPA activation (Schlotz et al., 2006; Bosch et al., 2009; Weinstein et al., 2010). Elevated BE in the current study could have been a reflection of greater tonic activation of stress systems, which might have reduced central opioid analgesic function via receptor down-regulation as previously described. This possibility was tested indirectly by examining associations between BE and several negative affect measures. Findings that general negative affect and plasma BE levels were not significantly correlated suggest that BE levels in this study were not simply a marker of affect-related arousal. Moreover, statistical control of negative affect measures did not alter the associations between BE and opioid antinociceptive function.
In summary, the current findings indicate that elevated resting plasma BE levels are associated with lower endogenous opioid analgesia. These findings were consistent across two different experimental acute pain stimuli and both primary and secondary pain outcomes. The key difference across pain stimuli was not whether inverse associations between plasma BE levels and opioid analgesia exist, but rather, whether these links are altered by the presence of chronic pain. The current findings, if replicated, may suggest that a reinterpretation of past work regarding the impact of chronic pain on endogenous opioid systems is in order. For example, a previous review (Bruehl et al., 1999) reported that chronic pain patients frequently exhibit lower resting plasma and CSF levels of endogenous opioids (including BE) when compared with healthy pain-free controls. This pattern was originally interpreted as being consistent with impaired endogenous opioid function in chronic pain patients, although none of these studies directly assessed opioid function. The current results suggest the possibility that previous findings of reduced endogenous opioid levels might indicate the opposite, i.e., relatively greater endogenous opioid analgesic function in chronic pain patients relative to healthy controls. This effect might be expected if the presence of chronic nociceptive input resulted in compensatory upregulation of endogenous opioid systems. Consistent with this notion, the only prospective data available (an intensive single case study) suggest that endogenous opioid analgesic systems are indeed upregulated, at least during initial transition from the pain-free state to chronic pain of relatively brief duration (Bruehl et al., 2010). On the other hand, this pattern would not be consistent with a previous report that chronic back pain, in combination with a positive parental history of chronic pain, was associated with reduced endogenous opioid analgesia compared with pain-free controls (Bruehl and Chung, 2006).
Whether individuals with chronic pain have impaired or upregulated endogenous opioid systems, interventions that increase endogenous opioid activity may in either case have clinical applications. For example, both exercise (McCubbin et al., 1992; Boecker et al., 2008) and relaxation training (McCubbin et al., 1996) can activate endogenous opioid systems and thereby might enhance analgesia in chronic pain patients. This possibility is supported by recent animal work indicating that aerobic exercise training in a rat neuropathic pain model significantly reduced neuropathic pain symptoms by enhancing endogenous opioid activity (Stagg et al., 2011).
Findings of the current study also suggest the potential clinical utility of assessing plasma BE values. For example, if large normative datasets were available as a reference, higher resting plasma BE values might identify individuals most likely to have exaggerated pain responses and inadequate endogenous antinociceptive activity. These individuals might consequently require more aggressive pain management in the post-surgical or other clinical contexts. The fact that inverse associations between resting BE and opioid analgesic function in this study were particularly prominent in individuals with chronic pain suggests possible clinical utility of this measure in the context of chronic pain management as well. Finally, given the likelihood that effects of exogenous opioids (both desired and undesired) would be influenced by individual differences in baseline status of opioid systems (e.g., mu opioid receptor function), it is intriguing to consider whether resting plasma BE levels might predict responses to exogenous opioid analgesics in the clinical context. To our knowledge, this issue has never been addressed in the chronic pain population.
The current study has several potential limitations. While BE in the CNS could directly inhibit pain responses via activation of opioid receptors in pain-relevant brain regions and the spinal cord (Zubieta et al., 2001; Sprenger et al., 2006), analgesic pathways for circulating BE are less clear (Basbaum and Fields, 1984). Experimental inhibition of pituitary BE release into circulation increases acute pain responses (Hargreaves et al., 1987, 1990), and potential peripheral sites of analgesic action for BE have been demonstrated in the context of chronic pain (Dionne et al., 2001). However, the current study design cannot determine peripheral versus central origin of the observed opioid analgesia, but rather only permits concluding that plasma BE levels were inversely associated with degree of overall endogenous opioid analgesia.
Another limitation is that this study was restricted to examining resting baseline BE. On the one hand, this is advantageous because BE levels were not confounded by subsequent study manipulations, including forearm exercise (as part of the ISC task), which might themselves have altered opioid levels. On the other hand, some studies of pain- or stress-induced changes in BE as they relate to pain outcomes (Nader-Djalal et al., 1995; al’Absi et al., 2004; Bruehl et al., 2007) suggest that increases in BE may parallel decreases in perceived pain, as might be expected. Findings of the current study therefore do not imply that greater pain-induced release of plasma BE would be associated with lower endogenous opioid analgesia. It should be noted that while forearm exercise during the ISC task procedure would not have affected resting BE levels because they were obtained prior to the ISC task, this brief forearm exercise could potentially have influenced ISC task blockade effects via exercise-induced opioid activation (e.g., Boecker et al., 2008). Although possible, the fact that FP task results (obtained prior to any forearm exercise) showed inverse correlations between resting BE and opioid blockade effects similar to those noted for the ISC task would argue against this being a significant confound in the current study. Nonetheless, the possibility that differences in overall aerobic conditioning at the time of the laboratory sessions might have influenced opioid levels or blockade effect results cannot be ruled out (McCubbin et al., 1992).
A final potential limitation is that the individual conducting the laboratory sessions was not blinded to chronic pain status. As a result, drug condition by chronic pain status interactions that were observed could in theory have been influenced by expectancy effects with regard to the latter. However, influence of chronic pain-related expectancies on blockade effect results would likely be limited by the fact that drug condition was double blinded.
In summary, the current study found that elevated resting plasma BE levels were associated with greater subsequent acute pain responsiveness. This effect appears to be related to associations between elevated resting BE and lower subsequent endogenous opioid analgesia. The link between higher resting plasma BE and lower opioid analgesia was more prominent among individuals with chronic pain. If replicated, it may prove valuable to explore the potential clinical utility of plasma BE as a biomarker for endogenous opioid function, and possibly as a predictor of responses to and risks associated with exogenous opioid analgesics.
Supplementary Material
Scatterplot of mean resting plasma betaendorphin levels by finger pressure (FP) task NRS opioid blockade effects. Larger positive blockade effects indicate greater endogenous opioid analgesia.
Acknowledgments
The authors gratefully acknowledge the contribution of Raymond Johnson to this project, as well as the assistance of the research nurses of the Vanderbilt General Clinical Research Center.
Funding sources
This research was supported by the National Institutes of Health (NIH) grants NS050578 and NS046694, NICHD grant P30 HD15052 awarded to the Vanderbilt Kennedy Center for Research on Human Development, and Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, et al. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Anderson WS, Sheth RN, Bencherif B, Frost JJ, Campbell JN. Naloxone increases pain induced by topical capsaicin in healthy human volunteers. Pain. 2002;99:207–16. doi: 10.1016/s0304-3959(02)00103-3. [DOI] [PubMed] [Google Scholar]
- Bach FW, Fahrenkrug J, Jensen K, Dahlstrøm G, Ekman R. Plasma beta-endorphin during clinical and experimental ischaemic pain. Scand J Clin Lab Invest. 1987;47:751–8. [PubMed] [Google Scholar]
- Bach FW, Langemark M, Secher NH, Olesen J. Plasma and cerebrospinal fluid beta-endorphin in chronic tension-type headache. Pain. 1992;51:163–8. doi: 10.1016/0304-3959(92)90257-C. [DOI] [PubMed] [Google Scholar]
- Baker DG, West SA, Orth DN, Hill KK, Nicholson WE, Ekhator NN, et al. Cerebrospinal fluid and plasma beta-endorphin in combat veterans with post-traumatic stress disorder. Psychoneuroendocrinology. 1997;22:517–29. doi: 10.1016/s0306-4530(97)00053-x. [DOI] [PubMed] [Google Scholar]
- Barnett V, Lewis T. Outliers in statistical data. 3. Chichester: John Wiley & Sons; 1994. [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock JE, Erbough JK. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Boecker H, Sprenger T, Spilker ME, Henriksen G, Koppenhoefer M, Wagner KJ, et al. The runner’s high: opioidergic mechanisms in the human brain. Cereb Cortex. 2008;18(11):2523–31. doi: 10.1093/cercor/bhn013. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EJ, Carroll D, Goedhart AD, Anane LA, van Zanten JJ, et al. A general enhancement of autonomic and cortisol responses during social evaluative threat. Psychosom Med. 2009;71:877–85. doi: 10.1097/PSY.0b013e3181baef05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, et al. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–37. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Chont M. Interacting effects of trait anger and acute anger arousal on pain: the role of endogenous opioids. Psychosom Med. 2011;73:612–9. doi: 10.1097/PSY.0b013e318227cb88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Burns JW, Chung OY, Ward P, Johnson B. Anger and pain sensitivity in chronic low back pain patients and pain-free controls: the role of endogenous opioids. Pain. 2002;99:223–33. doi: 10.1016/s0304-3959(02)00104-5. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY. Parental history of chronic pain may be associated with impairments in endogenous opioid analgesic systems. Pain. 2006;124:287–94. doi: 10.1016/j.pain.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Burns JW, Diedrich L. Trait anger expressiveness and pain-induced beta-endorphin release: support for the opioid dysfunction hypothesis. Pain. 2007;130:208–15. doi: 10.1016/j.pain.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Chont M. Chronic pain-related changes in endogenous opioid analgesia: a case report. Pain. 2010;148:167–71. doi: 10.1016/j.pain.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehl S, Chung OY, Ward P, Johnson B. Endogenous opioids and chronic pain intensity: interactions with level of disability. Clin J Pain. 2004;20:283–92. doi: 10.1097/00002508-200409000-00002. [DOI] [PubMed] [Google Scholar]
- Bruehl S, McCubbin JA, Harden RN. Theoretical review: altered pain regulatory systems in chronic pain. Neurosci Biobehav Rev. 1999;23:877–90. doi: 10.1016/s0149-7634(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MD, Davis GC, Bunney WE. Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature. 1977;270:620–2. doi: 10.1038/270620a0. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Shacham S, Dahl JL, Orrison W. CSF B-endorphin and the severity of pain. Neurology. 1984;34:378–80. doi: 10.1212/wnl.34.3.378. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Pickar D, Dubois M, Bunney WE., Jr Stress-induced plasma beta-endorphin immunoreactivity may predict postoperative morphine usage. Psychiatry Res. 1982;6(1):7–12. doi: 10.1016/0165-1781(82)90032-4. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Lepinski AM, Gordon SM, Jaber L, Brahim JS, Hargreaves KM. Analgesic effects of peripherally administered opioids in clinical models of acute and chronic inflammation. Clin Pharmacol Ther. 2001;70(1):66–73. doi: 10.1067/mcp.2001.116443. [DOI] [PubMed] [Google Scholar]
- Falcone C, Guasti L, Ochan M, Codega S, Tortorici M, Angoli L, et al. Beta-endorphins during coronary angioplasty in patients with silent or symptomatic myocardial ischemia. J Am Coll Cardiol. 1993;22(6):1614–20. doi: 10.1016/0735-1097(93)90585-o. [DOI] [PubMed] [Google Scholar]
- Forgione AG, Barber TX. A strain gauge pain stimulator. Psychophysiology. 1971;8:102–6. doi: 10.1111/j.1469-8986.1971.tb00441.x. [DOI] [PubMed] [Google Scholar]
- France CR, al’Absi M, Ring C, France JL, Harju A, Wittmers LE. Nociceptive flexion reflex and pain rating responses during endogenous opiate blockade with naltrexone in healthy young adults. Biol Psychol. 2007;75:95–100. doi: 10.1016/j.biopsycho.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Opposite effects of opioid blockade on the blood pressure-pain relationship in depressed and non-depressed participants. Pain. 2009;142:68–74. doi: 10.1016/j.pain.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Wolskee PJ, Deeter WR. Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature. 1983;306:264–5. doi: 10.1038/306264a0. [DOI] [PubMed] [Google Scholar]
- Guasti L, Cattaneo R, Daneri A, Bianchi L, Gaudio G, Regazzi MB, et al. Endogenous beta-endorphins in hypertension: correlation with 24-hour ambulatory blood pressure. J Am Coll Cardiol. 1996;28:1243–8. doi: 10.1016/S0735-1097(96)00312-9. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Flores CM, Dionne RA, Mueller GP. The role of pituitary beta-endorphin in mediating corticotropin-releasing factor-induced antinociception. Am J Physiol. 1990;258:E235–42. doi: 10.1152/ajpendo.1990.258.2.E235. [DOI] [PubMed] [Google Scholar]
- Hargreaves KM, Schmidt EA, Mueller GP, Dionne RA. Dexamethasone alters plasma levels of beta-endorphin and postoperative pain. Clin Pharmacol Ther. 1987;42:601–7. doi: 10.1038/clpt.1987.206. [DOI] [PubMed] [Google Scholar]
- Hook V, Funkelstein L, Toneff T, Mosier C, Hwang SR. Human pituitary contains dual cathepsin L and prohormone convertase processing pathway components involved in converting POMC into the peptide hormones ACTH, alpha-MSH, and beta-endorphin. Endocrine. 2009;35:429–37. doi: 10.1007/s12020-009-9163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Swift C, Carney MK, Ferdinands L. Beta endorphin levels in CSF during methadone maintenance. Life Sci. 1987;41:1071–6. doi: 10.1016/0024-3205(87)90623-0. [DOI] [PubMed] [Google Scholar]
- Leonard TM, Klem SA, Asher MA, Rapoff MA, Leff RD. Relationship between pain severity and serum beta-endorphin levels in postoperative patients. Pharmacotherapy. 1993;13:378–81. [PubMed] [Google Scholar]
- Lewis J, Mansour A, Khachaturian H, Watson SJ, Akil H. Opioids and pain regulation. Pain Headache. 1987;9:129–59. [PubMed] [Google Scholar]
- Maixner W, Sigurdsson A, Fillingim RB, Lundeen T, Booker DK. Regulation of acute and chronic orofacial pain. In: Fricton JR, Dubner R, editors. Orofacial pain and temporomandibular disorders. New York: Raven Press; 1995. pp. 85–102. [Google Scholar]
- Matejec R, Ruwoldt R, Bödeker RH, Hempelmann G, Teschemacher H. Release of beta-endorphin immunoreactive material under perioperative conditions into blood or cerebrospinal fluid: significance for postoperative pain? Anesth Analg. 2003;96:481–6. doi: 10.1097/00000539-200302000-00034. [DOI] [PubMed] [Google Scholar]
- Maurset A, Skoglung LA, Hustveit O, Klepstad P, Oye I. A new version of the ischemic tourniquet pain test. Meth Find Exp Clin Pharmacol. 1992;13:643–7. [PubMed] [Google Scholar]
- McCubbin JA, Cheung R, Montgomery TB, Bulbulian R, Wilson JF. Aerobic fitness and opioidergic inhibition of cardiovascular stress reactivity. Psychophysiology. 1992;29:687–97. doi: 10.1111/j.1469-8986.1992.tb02047.x. [DOI] [PubMed] [Google Scholar]
- McCubbin JA, Wilson JF, Bruehl S, Ibarra P, Carlson CR, Norton JA, et al. Relaxation training and opioid inhibition of blood pressure response to stress. J Consult Clin Psychol. 1996;64:593–601. doi: 10.1037//0022-006x.64.3.593. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short form of the McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Nader-Djalal N, de Leon-Casasola OA, Peer GL, Vladutiu AO, Lema MJ. The influence of preoperative concentrations of beta-endorphin and met-enkephalin on the duration of analgesia after transurethral resection of prostate. Anesth Analg. 1995;81:591–5. doi: 10.1097/00000539-199509000-00030. [DOI] [PubMed] [Google Scholar]
- Petraschka M, Li S, Gilbert TL, Westenbroek RE, Bruchas MR, Schreiber S, et al. The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience. 2007;146:1795–807. doi: 10.1016/j.neuroscience.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickar D, Cohen MR, Dubois M. The relationship of plasma cortisol and beta-endorphin immunoreactivity to surgical stress and postoperative analgesic requirement. Gen Hosp Psychiatry. 1983;5:93–8. doi: 10.1016/0163-8343(83)90106-8. [DOI] [PubMed] [Google Scholar]
- Pilcher WH, Joseph SA, McDonald JV. Immunocytochemical localization of pro-opiomelanocortin neurons in human brain areas subserving stimulation analgesia. J Neurosurg. 1988;68:621–9. doi: 10.3171/jns.1988.68.4.0621. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–91. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Schulz P, Hellhammer J, Stone AA, Hellhammer DH. Trait anxiety moderates the impact of performance pressure on salivary cortisol in everyday life. Psychoneuroendocrinology. 2006;31:459–72. doi: 10.1016/j.psyneuen.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Sheps DS, Ballenger MN, De Gent GE, Krittayaphong R, Dittman E, Maixner W, et al. Psychophysical responses to a speech stressor: correlation of plasma beta-endorphin levels at rest and after psychological stress with thermally measured pain threshold in patients with coronary artery disease. J Am Coll Cardiol. 1995;25:1499–503. doi: 10.1016/0735-1097(95)00045-6. [DOI] [PubMed] [Google Scholar]
- Solomon S. POMC-derived peptides and their biological action. Ann N Y Acad Sci. 1999;885:22–40. doi: 10.1111/j.1749-6632.1999.tb08663.x. [DOI] [PubMed] [Google Scholar]
- Spaziante R, Merola B, Colao A, Gargiulo G, Cafiero T, Irace C, et al. Beta-endorphin concentrations both in plasma and in cerebrospinal fluid in response to acute painful stimuli. J Neurosurg Sci. 1990;34:99–106. [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Sprenger T, Valet M, Boecker H, Henriksen G, Spilker ME, Willoch F, et al. Opioidergic activation in the medial pain system after heat pain. Pain. 2006;122:63–7. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, et al. Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–8. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Botbol M, Brailly-Tabard S, Perez-Diaz F, Graignic R, et al. Pain reactivity and plasma beta-endorphin in children and adolescents with autistic disorder. PLoS One. 2009;4:e5289. doi: 10.1371/journal.pone.0005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol Psychol. 2010;84:228–34. doi: 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplot of mean resting plasma betaendorphin levels by finger pressure (FP) task NRS opioid blockade effects. Larger positive blockade effects indicate greater endogenous opioid analgesia.


