Abstract
Aerobic fitness is associated with better memory performance as well as larger volumes in memory-related brain regions in children, adolescents, and elderly. It is unclear if aerobic exercise also influences learning and memory functional neural circuitry. Here, we examine brain activity in 17 high-fit (HF) and 17 low-fit (LF) adolescents during a subsequent memory encoding paradigm using fMRI. Despite similar memory performance, HF and LF youth displayed a number of differences in memory-related and default mode (DMN) brain regions during encoding later remembered versus forgotten word pairs. Specifically, HF youth displayed robust deactivation in DMN areas, including the ventral medial PFC and posterior cingulate cortex, whereas LF youth did not show this pattern. Furthermore, LF youth showed greater bilateral hippocampal and right superior frontal gyrus activation during encoding of later remembered versus forgotten word pairs. Follow-up task-dependent functional correlational analyses showed differences in hippocampus and DMN activity coupling during successful encoding between the groups, suggesting aerobic fitness during adolescents may impact functional connectivity of the hippocampus and DMN during memory encoding. To our knowledge, this study is the first to examine the influence of aerobic fitness on hippocampal function and memory-related neural circuitry using fMRI. Taken together with previous research, these findings suggest aerobic fitness can influence not only memory-related brain structure, but also brain function.
INTRODUCTION
Adolescence is the transitional period between childhood and adulthood during which significant neurodevelopmental and cognitive changes occur (Casey, Jones, & Hare, 2008; Dahl, 2004; Giedd et al., 1999; Giedd, Vaituzis, et al., 1996). Given that the brain is undergoing remodeling, the adolescent period may be an especially sensitive period for environmental factors to impart their effects on brain and behavior (Marco, Macri, & Laviola, 2011; Masten, 2004; Andersen, 2003). For this reason, it is of interest to identify environmental factors that may influence normative adolescent neurodevelopment. The goal of the current study was to understand how teens’ experiences, specifically with regards to aerobic fitness, influence memory-related brain functioning. Notably, exercise levels dramatically decrease starting at age 15 (Riddoch et al., 2004), and given that aerobic exercise has been shown to impact memory performance and memory-related structure, this may be a particularly salient time to identify the influence of aerobic fitness on memory neural circuitry.
Aerobic exercise is defined as sustained activity that stimulates heart and lung function, resulting in improved bodily oxygen consumption (Armstrong & Welsman, 2007), and thus, aerobic fitness is defined as one’s “ability to deliver oxygen to the muscles and generate energy during exercise” (Armstrong & van Mechelen, 2008). An emerging body of research has shown that aerobic fitness benefits learning and memory performance in adults and aged individuals (Erickson et al., 2009, 2011; Winter et al., 2007). More recent studies have also found aerobic fitness to affect memory performance in youth. Chaddock and colleagues (Chaddock, Hillman, Buck, & Cohen, 2011; Chaddock et al., 2010) showed that less aerobically fit children (ages 9–10 years) displayed poorer performance on relational memory encoding of material, although aerobic fitness level did not correlate with nonassociative memory performance. Furthermore, aerobic fitness has been shown to relate to spatial learning in male adolescents (ages 15–18 years; Herting & Nagel, 2012). These findings are consistent with the possibility that aerobic exercise may enhance memory performance by changing the underlying neural circuitry of learning and memory processing. However, despite the beneficial effect of aerobic fitness on learning and memory performance, the neurobiological mechanisms remain unclear.
The influences of aerobic fitness on learning and memory are thought to be mediated by exercise-induced changes in brain areas that directly subserve these processes, such as the PFC and hippocampus. In rodents, aerobic exercise stimulates new cell growth and increases in neurotrophic factors in the hippocampus, and these changes are associated with improved performance on spatial memory tasks (Clark et al., 2008; Van der Borght, Havekes, Bos, Eggen, & Van der Zee, 2007; van Praag, Shubert, Zhao, & Gage, 2005). Similarly, human studies have found that aerobic exercise may also influence hippocampal structure. Aerobic fitness has been shown to relate to better visuospatial STM, and this relationship is mediated by larger hippocampal volumes in elderly adults (Erickson et al., 2009, 2011). Associations between greater aerobic fitness, larger hippocampal volumes, and better memory performance have also been reported in children (Chaddock, Hillman, et al., 2011; Chaddock et al., 2010) and adolescents (Herting & Nagel, 2012). Despite evidence for aerobic exercise-related structural changes in the brain, the impact of aerobic fitness on neural circuitry important for learning and memory has yet to be elucidated.
Although there are a number of ways to assess learning and memory using fMRI, one of the most commonly utilized and powerful task-related designs is the subsequent memory effect paradigm (Kim, 2011). In this paradigm, participants are presented with a series of stimuli to encode in the MRI scanner. After encoding, participants perform a subsequent memory task, and encoding stimuli are then sorted into trials that were later remembered versus those that were later forgotten. fMRI BOLD signal that is greater for remembered versus forgotten stimuli reflects neural activity in brain regions that contribute to successful learning, whereas BOLD signal that is greater for stimuli that are later forgotten (vs. remembered stimuli) depicts neural activity in brain areas that are thought to interfere with successful learning and memory (Kim, 2011; Rugg, Otten, & Henson, 2002). The primary advantage of using this task is that it has been readily used by the field of learning and memory (over 100+ studies, to date), and consistent patterns of memory-related brain response have emerged. This rich literature is invaluable when hypothesizing and interpreting how the neural circuitry of learning and memory may be affected by various factors.
Using this type of paradigm, encoding of successfully remembered information activates the left inferior frontal cortex, and bilateral fusiform gyrus, hippocampus, pre-motor cortex, and posterior parietal cortex (Kim, 2011). In contrast to successful encoding, areas that have been implicated in subsequent forgetting include brain regions that have previously been determined to be part of the default mode network (DMN). The DMN is a large-scale network that shows decreased activation during a variety of goal-directed tasks and is composed of the posterior cingulate cortex (pCC), medial PFC (mPFC), parietal cortices, including the TPJ, retrosplenial cortex, and angular gyrus (Fox et al., 2005; Raichle et al., 2001). On the basis of this, a hypothesis emerged suggesting that the DMN is continuously active but is suppressed when resources are required for externally focused cognitive processing, ultimately resulting in deactivation of the DMN and activation of the task-specific networks (Fox & Raichle, 2007; Raichle et al., 2001). During learning, the DMN may need to deactivate to allocate resources to areas important for encoding, such as PFC and hippocampus. Evidence for this idea comes from a number of studies showing that DMN regions, most notably the pCC and lateral parietal lobes, show greater deactivation during encoding of later remembered than forgotten items, suggesting that deactivation of the DMN is essential for processing information so that it can later be remembered (Vannini et al., 2011; Kim, Daselaar, & Cabeza, 2010; Daselaar et al., 2009; Daselaar, Prince, & Cabeza, 2004). TPJ activation for subsequently forgotten information is also seen and is thought to play a role in the orientation of attention to exogenous information (Corbetta, Patel, & Shulman, 2008). Thus, neural circuitry underlying successful memory encoding includes not only task-related activation of hippocampal and prefrontal cortices but also the engagement of attentional processes and the deactivation of the DMN.
Although the majority of subsequent memory studies have been performed with adults, a few studies have examined the neural organization of memory in childhood and adolescents. Memory-related brain activity for visual stimuli (memory encoding condition > control task) was seen in the occipital lobe, hippocampus, parahippocampus, and entorhinal cortex in youth aged 11–19 years (Menon, Boyett-Anderson, & Reiss, 2005). Ofen and colleagues (2007) also found successful memory encoding (remembered > forgotten) to be subserved by the occipital cortex, middle temporal gyrus, parahippocampus, as well as the superior parietal lobe and PFC in 8- to 24-year-olds. However, neither study reported brain activity during encoding of subsequently forgotten items (forgotten > remembered).
In the current study, we examined brain activity in 17 high-fit (HF) and 17 low-fit (LF) male adolescents aged 15–18 years during a verbal, associative subsequent memory effect fMRI paradigm. Successful verbal memory encoding is largely subserved by the left PFC and hippocampus in adults (Kim, 2011; Ofen et al., 2007; Menon et al., 2005). Moreover, the anterior hippocampus has been shown to be specifically involved in relational memory encoding (Chua, Schacter, Rand-Giovannetti, & Sperling, 2007; Giovanello, Schnyer, & Verfaellie, 2004; Lepage, Habib, & Tulving, 1998). Therefore, we hypothesized that successful memory encoding would be subserved by the left anterior hippocampus and PFC, as well as deactivation of regions of the DMN. On the basis of previous research showing a positive association between aerobic fitness and learning and memory performance in a similar sample (Herting & Nagel, 2012), we expected that HF youth would have enhanced memory performance on a verbal associative memory task compared with LF youth. Lastly, given that exercise increases new cell growth as well as a number of neurotrophic factors in the hippocampus (for a review see van Praag, 2009) that may lead to greater plasticity and excitability of the neurons, it is possible that hippocampal cells may be more efficient at learning in individuals who are more aerobically fit. Therefore, we also hypothesized less hippocampal BOLD signal activation in HF youth when successfully encoding new memories compared with their LF peers.
METHODS
Participants
Participants included 34 eligible male youth, ages 15–18 years. Participants were recruited from the community as part of an ongoing adolescent neurodevelopment study. Inclusionary criteria for youth included being male, 15–18 years old (to ensure late-stage puberty; Herman-Giddens, 2006), and meeting either HF or LF criteria, defined below. Notably, female and male adolescents have structural and functional brain differences (Bramen et al., 2011; Lenroot & Giedd, 2010; De Bellis et al., 2001; Giedd, Snell, et al., 1996; Giedd, Vaituzis, et al., 1996), as well as differences in activity levels (Riddoch et al., 2004) and aerobic capacity (Krahenbuhl, James, & Kohrt, 1985), that may confound preliminary findings. For this reason, we chose to first examine these relationships in male adolescents alone.
Exclusionary criteria included current DSM-IV psychiatric diagnoses (Diagnostic Interview Schedule for Children Predictive Scales 4.32b; Hoven et al., 2005; Lucas et al., 2001), significant substance use (>10 lifetime alcoholic drinks or 2 drinks/occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day; Brief Lifetime Customary Drinking and Drug Use Record; Brown et al., 1998), history of psychotic disorders in biological parents (Family History Assessment Module; Rice et al., 1995), major medical condition or significant head trauma (Structured Clinical Interview; Brown, Myers, Mott, & Vik, 1994), left-handedness (Edinburgh Handedness Inventory; Oldfield, 1971), or irremovable metal. Youth and parents were each compensated $10 for completing the interviews, and youth were compensated $100 for completing behavioral tests and MRI scanning.
Group Classification
A modified version of the Youth Adolescent Activity Questionnaire (YAAQ) was administered to youth to assess participation in aerobic exercise over the past year. The YAAQ asks detailed questions about physical activity participation across all four seasons of the year as well as the number of hours per week spent doing each activity (Wolf et al., 1994). Seasonal format questionnaires, such as the YAAQ, have been shown to increase the accuracy of self-report of physical activity in adolescents (Rifas-Shiman et al., 2001). On the basis of hours of aerobic activity reported by the youth on the YAAQ, HF youth were defined as those participating in an average of ≥10 hr per week of regular, organized aerobic physical activity purposely performed to allow for improvement or maintenance of aerobic fitness, including basketball, soccer, football, track, cross country, and swimming across one or more seasons (summer, fall, winter, spring) within the past year. LF youth were defined as those individuals who had participated in ≤1.5 hr of aerobic physical activity per week over the past year. The majority of HF youth participated in an average of 10+ hr per week over the past year (n = 11), and the remaining HF that played sports more seasonally, still averaged a relatively high amount of aerobic exercise over the entire year (mean = 7.78 hr, SD = 2.4 hr, n = 6). HF youth were asked to participate in the study during the season in which they were most physically active based on their YAAQ self-report. These criteria were set forth, as significant increases in aerobic fitness have been seen in adolescents who participated in ≥10 hr of aerobic exercise per week (Lussier & Buskirk, 1977; Weber, Kartodihardjo, & Klissouras, 1976; Brown, Harrower, & Deeter, 1972), and relatively extreme categorizations (≥10 vs. ≤1.5 hr per week) maximize the likelihood of detecting group differences. On the basis of these criteria, 17 youth were considered LF and 17 were HF youth.
Aerobic Fitness Assessment
Aerobic fitness was objectively confirmed by peak aerobic uptake (VO2 peak) for each participant to confirm differences in aerobic fitness between the groups. VO2 peak is a measure of maximum capacity of an individual’s body to transport and utilize oxygen during exercise and is thought to be the most valid objective measurement of aerobic fitness (Armstrong & Welsman, 2007). VO2 peak was measured using the same computerized indirect calorimetry system (VMax Series, V6200 Autobox) during a Bruce Protocol (Bruce, Kusumi, & Hosmer, 1973). VO2 peak values were only considered valid if the participant delivered maximal effort on the test, as defined by at least one of the following the physiological criteria (Armstrong & van Mechelen, 2008): (1) oxygen consumption remained at a steady state despite an increase in workload as evidenced by a plateau in oxygen consumption, (2) heart rate reached ≥200 beats per minute, and (3) the respiratory exchange ratio ≥1.0; and/or the subjective criteria of reporting a 10 on the perceived exertion scale. Lean body mass (LBM) was determined just before aerobic testing by conducting a bioelectrical impedance test on each participant using the Body Composition Analyzer (Model 310e; Biodynamics Corp.), allowing for peak oxygen consumption to be expressed in ml/kg LBM/min.
Participant Characterization
General Intelligence
Participants were administered the two-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to estimate intellectual functioning.
Socioeconomic Status
Information was gathered on socioeconomic status (SES) by administering the Hollingshead Index of Social Position to parents as well as collecting information on total household income as part of the structured telephone interview.
Body Mass Index
Strong associations exist between sedentary lifestyle and obesity, and differences have been noted between obese and nonobese individuals using neuroimaging techniques (Carnell, Gibson, Benson, Ochner, & Geliebter, 2011). Thus, height and weight was obtained, and the Center for Disease Control and Prevention Child and Teen Calculator was used to determine body mass index (BMI; Center for Disease Control, 2011).
Pubertal Status
Although more common in girls, intensive physical exercise has been reported to delay pubertal maturation (Georgopoulos et al., 2010). Thus, pubertal status was assessed using the self-rating Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988).
Personal Lifestyle Questionnaire
The Revised Personal Lifestyle Questionnaire (Mahon, Yarcheski, & Yarcheski, 2003) was used to assess general differences in lifestyle that may also explain group differences in performance or brain response. For example, individuals who exercise more may be more conscientious about health in general, such as eating healthier and abstaining from unhealthy behaviors. Because diet (van Praag, 2009; Molteni et al., 2004) and substance abuse (Medina, Schweinsburg, Cohen-Zion, Nagel, & Tapert, 2007; Crews, Mdzinarishvili, Kim, He, & Nixon, 2006; Nagel, Schweinsburg, Phan, & Tapert, 2005; Tapert et al., 2004; Crews, Braun, Hoplight, Switzer, & Knapp, 2000; De Bellis et al., 2000; Moss, Kirisci, Gordon, & Tarter, 1994) have been shown to impact the brain and behavior, it was especially important to assess each subject’s lifestyle habits. This is a 24-item questionnaire that has six subscales, including Nutrition (4 items), Safety (3 items), Relaxation (5 items), Health Promotion (4 items), Substance Use (4 items), and Exercise (3 items). The items are measured on a 4-point scale from 1 (never) to 4 (always), with a number of items reverse-scored, resulting in higher total scores reflecting more positive health practices.
Extracurricular Activities
As another measure to assess differences in lifestyle between the groups, youth were asked the frequency in which they participated in extracurricular activities (including sports, clubs, recreational activities, etc.) on a scale of 1 (never) to 4 (at least once a week). In addition, a list of activities was obtained and the number of activities was summed for each subject. Furthermore, because exercise has been shown to be negatively associated with time spent playing video games in adolescents (Janz & Mahoney, 1997), average time spent playing video games was assessed. Subjects reported the console type used (e.g., PlayStation, etc.), the average number of hours on each console type per day (i.e., sessions), and the number of sessions per console type per week. Number of hours and number of sessions were summed across console types and multiplied together to assess the average number of hours of video games played per week.
Image Acquisition
Images were acquired on a 3.0-T Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1-weighted MP-RAGE scanning sequence (inversion time = 900 msec, flip angle = 10°, echo time = 3.58 msec, repetition time [TR] = 2300 msec, acquisition matrix = 256 × 240, resolution = 1 mm3). Whole-brain functional images were collected in the axial plane, oblique to the AC–PC, using a T2*-weighted echo-planar BOLD interleaved sequence (TR = 2000 msec, echo time = 30 msec, field of view = 240 mm, flip angle = 90°, 33 slices no gap, resolution = 3.75 mm3). Stimuli were viewed through a mirror mounted on the head coil and responses made through a button box.
Verbal Associative Memory Encoding Task
A subsequent memory paradigm was used and consisted of two phases: encoding in the MRI scanner and a subsequent memory recognition test outside the scanner (Figure 1). For encoding, a rapid event-related design subsequent memory word pair fMRI task was used to assess intentional verbal associative memory encoding. Stimuli consisted of two unrelated words presented horizontally in white font against a black background. A total of 231 word pairs were presented for 2.75 sec each, followed by 0.25 sec of visual fixation across three runs using E-prime software (Psychology Software Tools, Pittsburgh, PA). Words were implemented from a published subsequent memory paradigm by Kuhl, Shah, DuBrow, and Wagner (2010) and, to stay consistent with the published paradigm, were randomly combined to construct the novel pairs. Fixation stimuli consisted of a white crosshair presented against a black background. Each word pair was then followed by an additional varying period of fixation varying from 0 to 10 sec using an optimized (created by optseq2 from Freesurfer; surfer. nmr.mgh.harvard.edu/optseq) jittered event-related design (Dale, 1999). Participants were instructed to “learn which word pairs are presented together, as you will be tested on them later.” In addition to learning word pairs, they were told to make a subjective decision about whether or not the words “fit” together with a button press for “yes, the words fit together,” or “no, the words do not fit together.” These instructions ensured the participants attended to both words and processed them associatively.
Figure 1.
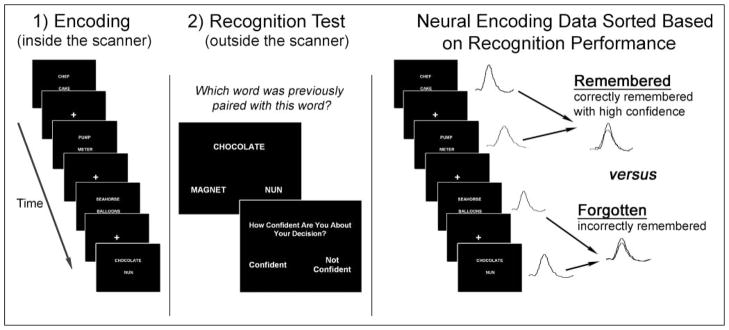
Verbal associative subsequent memory fMRI task. (1) Neural activity is recorded during encoding of word pairs in the MRI scanner. (2) Approximately 20 min following encoding, participants complete a recognition memory task outside the MRI. Neural responses from encoding are then sorted according to whether the item was later remembered or forgotten and then used to examine memory-related neural circuitry.
For the subsequent memory test, participants completed a self-paced 20-min postscan association recognition memory test outside the scanner. The association recognition memory test included 231 trials presented on a computer via E-prime software (Psychology Software Tools, Pittsburgh, PA), where participants were given the first word presented from each of the 231 word pairs and two words presented underneath it and were instructed to “choose which word was correctly paired with the presented word in the scanner.” Out of the two word choices presented for each trial, one was always the correctly paired word and the other was a foil with equal familiarity (i.e., another word that presented as part of a different word pair in the scanner). After every trial, participants were asked if they had high or low confidence in their decision. All participants received the same order of word pairs for encoding and the subsequent memory tests. By using a subsequent association recognition memory test, we were able to classify neural response of trials during encoding that were later remembered (correctly identified association on subsequent memory test) versus those that were later forgotten (incorrect identified association on subsequent memory test). Furthermore, by assessing subjective confidence on recognition performance, we were able to further classify correct responses into high-confidence correct and low-confidence correct. The assumptions for these classifications are that high-confidence correct associations reflect those word pairs that the individual had a strong memory of whereas the low-confidence correct associations may reflect a sense of either familiarity or correct guessing. Therefore, because the root of low-confidence correct associations may be more ambiguous, group differences in associative memory were examined between encoding trials that were later remembered correctly with high confidence (“remembered”) versus those associations that were later incorrectly identified on subsequent memory test (“forgotten”; Figure 1). Accuracy and RT were assessed during the recognition task. In addition, Type 2 signal discriminability (d′) was calculated p(high confidence|correct) − p(high confidence|incorrect) for each participant to assess above chance performance (Clarke, Birdsall, & Tanner, 1959).
Image Preprocessing
Data were processed and analyzed using Analysis of Functional Neuroimages (AFNI; Cox, 1996). The first three TRs of each scan were excluded to allow for steady-state magnetization. Preprocessing included slice timing correction, motion correction, coregistration of functional to anatomical images, and spatial smoothing using a Gaussian filter (FWHM = 6 mm kernel). To further reduce movement-related confounds, root mean square (RMS) of within-run motion across the six motion parameters were calculated for each subject and TRs showing greater than 2.5 mm or 2.5° in any of the six parameters were removed from the subsequent analyses. Importantly, all RMS values were low (<1), and RMS values between groups were not statistically different (U = 134, z = −0.36, p = .73). Next, functional masks were created to mask out nonbrain, and time series data were normalized to the time series mean.
To determine if aerobic fitness in adolescents has a global impact on brain activity or does so with some degree of regional specificity, we examined the amplitude and shape of the BOLD signal in a hypothetical control region, the primary visual cortex. To this end, an exploratory analysis was performed where the BOLD time course for the task was modeled with nine individual tent functions that were evenly spaced from onset of the stimulus (0 sec) to 12 sec poststimulus, without assuming a particular shape (Cox, 1996). Bilateral primary visual cortex ROIs were created (4 mm diameter spheres centered at Talairach coordinates ±17, −95, 8), and time courses of the hemodynamic response function (HRF) for later remembered and forgotten word pairs were extracted and plotted for each group. The results showed no significant differences in the peak amplitude or the time course for the HRF (ps ≥ .13). Given these results, a gamma-variate HRF was assumed for examining group-related differences in BOLD response during memory encoding. Using a deconvolution process, regressors representing high-confidence correct (remembered), low-confidence correct, and incorrect (forgotten) word pair stimuli were modeled with stimulus times corresponding to the onset of stimuli presentation, with the duration of the event coded as the length of each stimuli convolved with a gamma-variate HRF (Cohen, 1997). Brain response during encoding of low-confidence correct stimuli was included in the model but was not analyzed. The estimated baseline in this model was composed of the BOLD signal from the entire time course, linear drift, unmodeled fixation periods, and regressors of no interest (e.g., six motion parameters; Cox, 1996). Contrasted images for average percent signal change of remember versus baseline, forgotten versus baseline, and remember versus forgotten were created. Functional data sets were resampled into 3 mm3 voxels and transformed into standard Talairach coordinates (Talairach & Tournoux, 1988) before group-level comparisons.
Hippocampal Regions of Interests (ROIs)
Bilateral hippocampal ROIs were manually traced on each individual’s high-resolution anatomical image based on a previously established method (Herting & Nagel, 2012). The tracer (M.H.) was blind to participant characteristics and established high intra- and interrater reliability (all intraclass correlations ≥.95) on an independent sample using this previously published protocol before tracing. Bilateral hippocampi were traced on contiguous coronal slices, perpendicular to the AC–PC plane using AFNI, and were confirmed in axial and sagittal view. ROIs were resampled to match the functional data (3 mm3 voxels).
Susceptibility Artifact Assessment
Regions of the medial-temporal lobe are especially susceptible to signal loss because of its relative location near the petrous bone (Ojemann et al., 1997). To assess hippocampal signal-to-noise ratios (SNRs), hippocampal ROIs were used to extract signal from the original T2*-weighted EPIs. SNRs were then estimated by dividing each voxel’s mean intensity by its standard deviation across time and extracted from the hippocampal ROIs for each subject. All subjects had adequate hippocampal SNR (mean = 99.18, SD = 28.5) to detect an expected 1–2% change in the MR signal based on the fMRI paradigm design (Parrish, Gitelman, LaBar, & Mesulam, 2000). Groups did not differ in estimated SNR values, t(32) = −0.98, p = .33.
Analyses
All statistical analyses were carried out using PASW 18 (Chicago, IL). Normality was verified on all variables, and transformations were used when appropriate. When data continued to violate normality, nonparametric tests were employed. Independent t tests were used to examine participant demographics, aerobic fitness, BMI, as well as lifestyle differences between the two groups. Group differences in demographic data (ps ≤ .09) were used as covariates when exploring memory behavior and brain activity between the groups. To determine if memory behavior was different from chance, one-sample t tests were used to examine d′ from zero for each group. To examine memory recognition performance (accuracy and RT), separate 2-within (Recognition [remembered vs. forgotten], Confidence [high vs. low]), 2-between (Group) ANCOVA was used. Follow-up regression analyses were also used to determine if VO2 peak values were related to the dependent variables of interest while controlling for puberty and SES.
Brain response was examined using AFNI with both whole-brain and ROI approaches. To assess overall task-related activity, individual whole-brain one-sample t tests were used to examine the remembered versus forgotten contrast for each group. Results were voxelwise (p < .01) and cluster-corrected (number of voxels ≥ 60) for multiple comparisons and mapped onto surface using Caret software (Van Essen et al., 2001). Whole-brain ANCOVA analysis was used to examine group differences in subsequent memory (remembered vs. forgotten) brain activity. These results were also corrected for multiple comparisons at both the voxelwise (p < .01) and cluster level (number of voxels ≥ 48). To further assess group differences, mean percent signal change in each of the simple contrasts (remembered vs. baseline and forgotten vs. baseline) was extracted for each participant from significant clusters and exported into PASW.
A priori analyses were performed to examine group differences in brain response in the bilateral anterior hippocampus. A bilateral anterior group-level hippocampal mask was created by summing the hand-drawn ROIs across all participants and extracting the anterior one-half. ANCOVA was then used to examine group differences in memory encoding (remembered vs. forgotten) brain activity bilaterally in the anterior hippocampus. For this a priori ROI analysis, p < .05, with a cluster correction of ≥ 15 voxels, was considered significant. Post hoc regression analyses were also performed to examine how VO2 peak related to subsequent memory brain activity for the simple contrasts in both whole-brain and ROI-based clusters (remembered vs. baseline and forgotten vs. baseline) while controlling for puberty and SES.
RESULTS
One participant’s parent (LF) chose not to disclose total household income, and one participant (HF) did not complete the Personal Lifestyle Questionnaire, resulting in pairwise missing data for these measures. Participant characteristics, aerobic fitness, and body composition results can be found in Table 1. The groups were matched on age and IQ and were not significantly different on a number of lifestyle behaviors, including nutrition, relaxation, health promotion, safety, substance use, frequency and number of extracurricular activities, and video game habits. The groups were also matched on BMI. Although both groups came from households that made above the national income average, the HF had an overall higher SES and median household income, as reported by their parents. Self-report of pubertal maturation was also different between the groups, with HF being less mature compared with LF. To account for these differences, SES and puberty were covaried for in all subsequent analyses. VO2 peak testing was used to objectively measure aerobic fitness and aerobic differences expected between the groups based on self-report of aerobic exercise participation. VO2 peak values were significantly different between the groups, confirming better aerobic fitness in HF youth.
Table 1.
Participant Characteristics
| HF | LF | ||
|---|---|---|---|
| Demographics | |||
| n | 17 | 17 | |
| Age | 16.6 (.8) | 16.2 (.8) | t(32) = 1.36, p = .19 |
| % White | 82.4 | 82.4 | |
| IQa | 117.1 (11.8) | 118.0 (8.1) | t(26.1) = 0.26, p = .79 |
| SESb | 18.3 (5.9) | 26.5 (12.9)* | t(22.6) = 2.39, p = .03 |
| Median household incomeb (thousands) | 130 | 90c | |
| Pubertyd | 3.06 (.4) | 3.3 (.3)* | U = 80, z = 2.24, p = .026 |
| Aerobic Fitness | |||
| Aerobic Activity (hr/week over past year)e | 11.3 (3.4) | .26 (.5) | t(16.6) = 11.33, p < .001 |
| Aerobic Activity (hr/week in season scanned)e | 12.6 (3.8) | .23 (.5) | t(16.5) = 13.39, p < .001 |
| VO2 peak (ml/kg LBM/min) | 77.7 (10.5) | 67.0 (7.4)** | t(32) = 3.41, p = .002 |
| Body Composition | |||
| BMIf | 21.6 (2.9) | 22.4 (4.4) | t(25.72) = 0.67, p = .51 |
| Lifestyle | |||
| Nutritionc,g | 12.0 (1.0) | 12.4 (1.5) | t(31) = 0.77, p = .45 |
| Relaxationc,g | 15.2 (2.2) | 15.4 (2.2) | t(31) = 0.22, p = .83 |
| Health promotionc,g | 13.7 (1.3) | 13.0 (2.3) | U = 117, z = 0.70, p = .51 |
| Safetyc,g | 14.2 (1.3) | 15.0 (1.2) | U = 86.5, z = 1.8, p = .07 |
| Substance usec,g | 11.6 (.5) | 11.3 (1.0) | U = 122, z = 0.58, p = .63 |
| Video game habits (hr/week) | 6.0 (5.9) | 8.4 (9.6) | U = 127, z = 0.60, p = .56 |
| Extracurricular activities | |||
| Frequency | 4 (0) | 3.5 (1.0) | U = 110.5, z = 2.09, p = .25 |
| Number | 2.9 (1.3) | 2.3 (1.3) | U = 109, z = 1.28, p = .23 |
Means and standard deviations unless otherwise noted.
Wechsler Abbreviated Scale of Intelligence.
Hollingshead Index of Social Position.
n = 16 because of missing data.
Pubertal Development Scale.
Youth Adolescent Activity Questionnaire.
Body Mass Index.
Personal Lifestyle Questionnaire.
p < .05.
p < .01.
Task Performance
During encoding, both groups showed similar task performance. Both groups had a high percentage of “no fit” responses, supporting the purposely chosen unrelated nature of the word pairs used as stimuli (HF: 84 ± .029%; LF: 82.8 ± .034%; U = 136.5, z = −0.28, p = .77). Groups also did not differ on RT during encoding (HF: 1586 ± 44.9 msec; LF: 1590 ± 52.8 msec; U = 127, z = −0.60, p = .56).
On the recognition task, HF and LF youth also showed similar memory performance (Table 2). Both groups showed d′ that was significantly above chance (HF: t(16) = 10.8, p < .001, Cohen’s d = 5.4; LF: t(16) = 12.4, p < .001, Cohen’s d = 6.2); although d′ was not significantly different between the groups, t(32) = 0.94, p = .36, Cohen’s d = .32. ANCOVA showed no main effect for accuracy [recognition: F(1, 30) = 2.13, p = .16, partial η2 = .066; confidence: F(1, 30) = 1.83, p = .19, partial η2 = .057]. Furthermore, the effect of group was not significant for accuracy, F(1, 30) = 0.63, p = .43, partial η2 = .02, nor were there any significant two-way interactions [recognition: F(1, 30) = 0.15, p = .69, partial η2 = .005; confidence: F(1, 30) = 0.15, p = .70, partial η2 = .005] or a three-way interaction with group status, F(1, 30) = 0.45, p = .51, partial η2 = .015. The main effects of recognition, F(1, 30) = 0.953, p = .34, partial η2 = .03; confidence, F(1, 30) = 2.13, p = .16, partial η2 = .07; and group, F(1, 30) = 0.19, p = .67, partial η2 = .006 on RTs were not significant. In addition, two-way interactions [recognition: F(1, 30) = 1.77, p = .19, partial η2 = .056; confidence: F(1, 30) = 0.01, p = .91, partial η2 = .000] and three-way interaction, F(1, 30) = 1.69, p = .20, partial η2 = .053, with group were not significant. A positive relationship was seen between encoding response and recognition performance for both groups, with a significant correlation between number of word pairs found to “fit” together and high-confidence memory performance (Spearman’s ρ = .63, p < .001; HF: ρ = .67, p < .003; LF: ρ = .60, p = .01). No significant relationship was seen between VO2 peak and performance (ps > .05).
Table 2.
Subsequent Memory Task Performance for Each Group
| HF | LF | |
|---|---|---|
| Overall accuracy | 78.12 (9.7) | 80.18 (10.03) |
| % High-confidence correct | 56.67 (15.4) | 62.31 (17.1) |
| % Low-confidence correct | 22.04 (8.8) | 17.86 (10.5) |
| % High-confidence incorrect | 7.13 (7.6) | 6.78 (6.5) |
| % Low-confidence incorrect | 14.14 (6.9) | 13.05 (8.4) |
| d′ | 49.5 (18.9) | 55.5 (18.5) |
| High-confidence correct RT | 2819.7 (635.7) | 2624.6 (759.2) |
| Low-confidence correct RT | 4279.4 (1928.9) | 3698.7 (1368.2) |
| High-confidence incorrect RT | 3873.8 (1590.0) | 3215.7 (901.1) |
| Low-confidence incorrect RT | 4457.6 (1969.0) | 3793.5 (1688.0) |
Means (SDs).
Verbal Associative Encoding Brain Activity
Whole Brain
Individual groups showed similar overall patterns of brain activity in the remembered versus forgotten contrast (Figure 2 and Table 3). Both groups showed greater activation during the encoding of word pairs later remembered compared with those later forgotten in a number of brain regions important for verbal memory encoding, including the left inferior, middle, and superior frontal gyri, as well as in left temporal lobe, including the parahippocampal gyrus and hippocampus. LF adolescents also showed significant activation to remembered versus forgotten word pairs in additional regions, including the right hippocampus, bilateral BG, and right cerebellum. For both groups, the pattern of activity during unsuccessful encoding of word pairs was seen in key default mode regions as well as regions involved in attention, such as the TPJ. Both groups showed greater activity in the right inferior and superior parietal lobules, middle frontal gyrus, pCC, and supramarginal gyrus during encoding of forgotten word pairs (Table 3).
Figure 2.
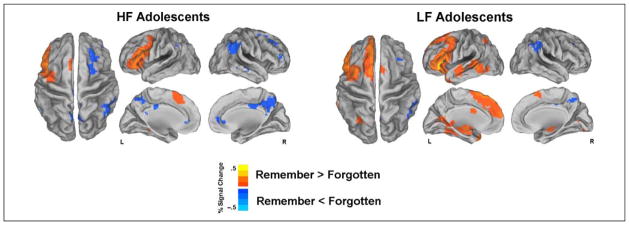
Individual whole-brain voxelwise t maps for remembered versus forgotten for each group (p < .01, corrected for multiple comparisons).
Table 3.
Within and Between-group Results for Remembered versus Forgotten Contrast
| Peak Anatomic Location | Regions Included | Number of Voxels | x | y | z |
|---|---|---|---|---|---|
| Remembered vs. Forgotten Within Groups | |||||
| HF | |||||
| Remembered > forgotten | |||||
| L inferior frontal gyrus | L middle frontal gyrus; L BA 9, 11, 45, 46, 47 | 941 | −50 | 29 | −7 |
| L superior frontal gyrus | L medial frontal gyrus; L BA 6, 8 | 96 | −5 | 20 | 66 |
| L middle temporal gyrus | L fusiform gyrus, parahippocampus; L BA 37 | 67 | −50 | −47 | −10 |
| Forgotten > remembered | |||||
| R superior parietal lobule | L superior parietal lobule, bilateral precuneus, cuneus, posterior and midcingulate cortex; bilateral BA 7, 31 | 1942 | 5 | −68 | 57 |
| R medial frontal gyrus | R superior, middle, and medial frontal gyrus; R BA 8, 10; bilateral anterior cingulate gyrus; bilateral BA 32 | 1006 | 2 | 59 | 12 |
| R inferior temporal gyrus | R middle temporal gyrus; R BA 21 | 135 | 65 | −20 | −19 |
| L IPL | L supramarginal gyrus; L BA 40 | 83 | −56 | −50 | 42 |
| LF | |||||
| Remembered > forgotten | |||||
| L inferior frontal gyrus | L middle frontal gyrus; L BA 11, 44, 45, 46, 47 | 1439 | −53 | 23 | −1 |
| L fusiform gyrus | L superior and middle temporal gyrus, middle occipital gyrus, hippocampus, parahippocampus; L BA 21, 22 | 663 | −59 | −56 | −16 |
| L superior frontal gyrus | L medial frontal gyrus; L BA 6, 8, 9, 10 | 598 | −8 | 17 | 66 |
| L posterior cingulate gyrus | L parahippocampus, cuneus; L BA 30 | 160 | −2 | −56 | 9 |
| L caudate | L putamen | 140 | −11 | 5 | 9 |
| R cerebellum (declive) | R pyramis, R uvula | 116 | 11 | −80 | −13 |
| L middle frontal gyrus | L BA 21 | 74 | −59 | −2 | −10 |
| R caudate | R putamen | 70 | 14 | 5 | 15 |
| R hippocampus | R parahippocampus, amygdala | 66 | 20 | −17 | −13 |
| L precuneus | L superior parietal lobule; L BA 7 | 62 | −26 | −71 | 51 |
| Forgotten > remembered | |||||
| R IPL | R supramarginal gyrus; R BA 40 | 461 | 47 | −56 | 48 |
| R postcentral gyrus | R BA 31; bilateral precuneus, posterior cingulate gyrus; bilateral BA 7 | 267 | 5 | −53 | 72 |
| R middle frontal gyrus | R BA 8, 9 | 82 | 35 | 32 | 48 |
| Remembered vs. Forgotten Between Groups | |||||
| HF < LF | |||||
| L superior temporal gyrus | L middle temporal gyrus; L BA 38 | 98 | −29 | 14 | −25 |
| L ventral mPFC | L BA 10 | 74 | −5 | 62 | 12 |
| R pCC | Bilateral pCC/posterior midline | 62 | 2 | −50 | 21 |
| R IPL | R precuneus; R BA 7 | 55 | 35 | −44 | 60 |
| R superior frontal gyrus | R middle frontal gyrus; R BA 6 | 54 | 8 | 26 | 57 |
Peak location, regions included, voxel number, and peak Talairach Coordinates are provided for each cluster. R = right; L = left; BA = Brodmann’s area.
Despite these similarities, between-group analyses of the remembered versus forgotten contrast revealed significant group differences in BOLD response (Figure 3 and Table 3). LF youth showed greater BOLD activity in the right superior and middle frontal gyri as well as in several DMN regions, including left superior temporal gyrus, left ventral mPFC, bilateral pCC, and right inferior parietal lobule/precuneus (IPL), when compared with HF youth. To further understand these differences, mean percent signal change in the remembered versus baseline and the forgotten versus baseline contrast was extracted. Furthermore, exploratory analyses were performed to determine if percent signal change in these clusters related to performance. Results revealed two distinct patterns driving these group differences. In the right superior and middle frontal gyri, LF adolescents had significantly higher activation during encoding of remembered word pairs compared with HF youths (e.g., Figure 3A, green subplot), whereas groups did not significantly differ in activation in this region when encoding later forgotten word pairs. Furthermore, percent signal for remembered versus baseline was significantly related to high-confidence memory performance in LF youth (Spearman’s Rho = .493, p = .045); this relationship was not seen in HF youth (Spearman’s Rho = .048, p = .87). The remaining four clusters showed a different pattern (Figure 3B). Consistent with the fact that these four clusters overlapped with typical DMN areas (Fox et al., 2005; Raichle et al., 2001), HF youth showed strong deactivation in these regions during successful encoding and less deactivation when encoding word pairs that were subsequently forgotten. LF youth, however, had less deactivation in these regions compared with HF youth during successful encoding but had similar or even greater deactivation compared with HF youth during encoding later forgotten stimuli (e.g., Figure 3B, blue subplot). Effect size calculations showed that these differences were large in magnitude between the groups (Cohen’s d’s ≥ 1.24), and the effect size of group difference in these clusters was significantly larger than the effect size in the visual control region (z statistics ≥ 2.02, ps < .05). No significant relationships were seen between successful memory encoding BOLD response and memory performance in these four clusters for either group (ps > .05).
Figure 3.
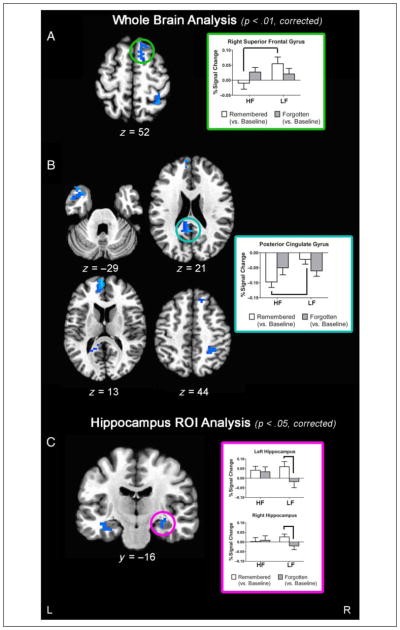
Between-group t maps of areas where LF adolescents show greater brain activity than HF adolescents for remembered versus forgotten contrast. Whole-brain analysis results are driven by both (A) greater activation for remembered (vs. baseline) (green subplot) and (B) reduced deactivation for remembered (vs. baseline) in LF compared with HF youth. (C) Hippocampus ROI analyses show greater bilateral activation for remembered versus forgotten contrast in LF compared with HF adolescents, which is largely driven by greater activation during remembered (vs. baseline) and greater deactivation during forgotten (vs. baseline) encoding (pink subplot).
No significant results were found when using VO2 peak to predict brain activity in the remembered versus baseline or forgotten versus baseline contrasts across all subjects (ps > .05). Exploratory follow-up analyses were performed to determine if VO2 peak explained the relationship between group and BOLD signal, and all five clusters remained significant between the groups after controlling for VO2 peak.
Hippocampal ROIs
Between-group analyses revealed bilateral increased BOLD response for the remembered versus forgotten contrast in the LF youth compared with HF youth (left cluster: 19 voxels; x = −32, y = 17, z = −19, Cohen’s d = 1.02; right cluster: 15 voxels; x = 29, y = 17, z = −16, Cohen’s d = .88; Figure 3C). Simple contrasts showed that HF adolescents significantly activated the left hippocampus during encoding of later remembered word pairs (vs. baseline), whereas the LF group showed bilateral increases in hippocampal activity during encoding of remembered word pairs (vs. baseline). LF youth also showed significantly greater activation in both the right and left hippocampus during the encoding of later remembered versus forgotten stimuli. HF youth, however, did not show a difference in BOLD response in the left or right hippocampus when encoding later remembered versus forgotten word pairs (Figure 3C, pink subplot). Group effect sizes were larger for both the hippocampal ROIs compared with the visual control regions, albeit these did not reach statistical significance (z statistics: left: z = 1.60, p = .11; right: z = 1.34, p = .18). Follow-up analyses examining the relationship between VO2 peak and hippocampal ROI BOLD signal were not significant (ps > .05), and percent signal change in the left and right hippocampus during successful memory encoding did not significantly relate to memory performance in either group (ps > .05).
Exploratory Context-dependent Correlation Analyses
Given that the hippocampus has been shown to decouple from the DMN to enable successful memory encoding (Huijbers, Pennartz, Cabeza, & Daselaar, 2011; Vannini et al., 2011), the reduced DMN deactivation reported above may implicate that segregation between the hippocampus and DMN may be impaired in LF youth during successful memory encoding. In hopes of better understanding, the above differences between the groups, context-dependent changes in functional correlations were examined using AFNI (Chen, 2011). This analysis was used to determine whether group differences were seen between hippocampal functional connectivity (correlated activity) with DMN regions during trials of successful memory encoding (remembered) relative to the non-encoding context of the baseline condition. For each participant, a physiological regressor was made by removing the linear trend, extracting the average time series data from the seed regions (left and right hippocampal ROIs) and de-convolving it with a gamma-variate HRF. The encoding condition (remembered vs. baseline) was used as the psychological regressor. The psychophysiological interaction regressor was created by multiplying the deconvolved HRF time series (physiological regressor) with a vector coded for the contextual contrast of interest (psychological regressor). Separate deconvolution regression analyses were then performed for the left and right hippocampal seed regions, using a model that included the original regressors of interest and no interest, as well as the deconvolved hippocampal time course and the psychophysiological interaction regressor. ANCOVA was used to assess the fit of the interaction regressor between the two groups within the DMN regions previously shown to be significantly different between the groups (pCC, mPFC, IPL). Multiple comparison correction was performed (voxelwise threshold of p < .005; cluster threshold of ≥8 voxels).
Results were similar for the left and right hippocampus and showed that correlation between the BOLD response in the hippocampus and the mPFC, as well as the hippocampus and right IPL, differed between the groups during successful encoding of new memories (Table 4). In other words, functional connectivity between the hippocampus and mPFC and IPL during remembered versus baseline conditions differed between HF and LF youth. To further understand these findings, functional connectivity during remembered versus baseline were investigated for each group separately. HF youth showed a strong negative coupling in BOLD response between the hippocampus and both DMN brain regions during successful memory encoding (vs. baseline), whereas LF youth showed positive coupling (Table 4).
Table 4.
Left and Right Hippocampal Context-dependent Correlation Analyses Between-group Results
| Location | Number of Voxels | x | y | z | Interaction Fit Coefficient
|
|
|---|---|---|---|---|---|---|
| HF | LF | |||||
| Regions Showing Group Differences in Left Hippocampal Connectivity during Remembered vs. Baseline Conditions | ||||||
| Right IPL | 23 | 35 | −44 | 60 | −4.3 ± 11.0 | 2.3 ± 9.1 |
| Left mPFC | 12 | −5 | 56 | 15 | −7.7 ± 10.3 | 1.5 ± 15.2 |
| Regions Showing Group Differences in Right Hippocampal Connectivity during Remembered vs. Baseline Conditions | ||||||
| Left mPFC | 17 | −5 | 53 | 18 | −5.6 ± 9.3 | 8.2 ± 15.4 |
| Right IPL | 15 | 35 | −44 | 54 | −3.7 ± 8.4 | 6.0 ± 11.7 |
Peak location, voxel number, and peak Talairach Coordinates are provided for each cluster. Mean psychophysiological interaction fit coefficients (±SD) for each group are also reported; negative fit coefficient = negative coupling of BOLD response with hippocampus, positive fit coefficient = positive coupling in BOLD response with hippocampus; R = right; L = left.
DISCUSSION
The current study examined subsequent memory neural circuitry in adolescents, as well as how aerobic fitness affects memory-related brain activity. Overall, encoding of remembered (vs. forgotten) word pairs was subserved by the left PFC and hippocampus, whereas greater brain activity was seen in DMN regions during encoding of later forgotten (vs. remembered) stimuli. Aerobic fitness did not appear to influence memory performance in this study, as no significant difference was seen when comparing recognition memory between HF and LF adolescents. However, differences were seen in brain response between the groups. LF youth showed increased BOLD response in the right superior frontal gyrus and bilateral hippocampi during encoding of subsequently remembered versus forgotten word pairs compared with HF youth. Furthermore, LF youth showed less deactivation in DMN regions and had reduced negative functional connectivity between bilateral hippocampus and DMN regions, including the mPFC and IPL during successful memory encoding.
A meta-analysis of 74 adult studies found that successful verbal associative encoding requires activation of the left inferior frontal gyrus and left hippocampus (Kim, 2011). Consistent with these findings, the current study showed that both HF and LF adolescents had greater activation of the left inferior frontal gyrus and the left hippocampus during encoding of later remembered (vs. forgotten) word pairs. Furthermore, the engagement of the PFC and hippocampus during encoding of remembered items is similar to previously published results in 8- to 24-year-olds (Ofen et al., 2007). Recent research also attests to the importance of DMN deactivation for successful encoding, as unsuccessful encoding has been demarcated by less deactivation in key default regions, like the pCC (Kim, 2011; Vannini et al., 2011; Daselaar et al., 2004, 2009). This idea was supported in the current study as encoding of later forgotten word pairs was hallmarked by increased task-related BOLD response in DMN areas (as opposed to deactivation), including the mPFC, pCC, and lateral parietal cortices, as well as the TPJ. Although, to our knowledge, this is the first study to report subsequent forgetting effects in adolescents, these patterns mirror the adult literature (Kim, 2011; Daselaar et al., 2004, 2009; Daselaar, Fleck, & Cabeza, 2006), suggesting that DMN deactivation is important for successful memory encoding in adolescents as well as adults.
Of the 231 word pairs shown, participants remembered approximately 60% on the postscan recognition memory test. Somewhat surprisingly, however, aerobic fitness did not relate to memory performance, as there were no group differences in accuracy on the recognition task. This did not confirm our hypothesis and failed to echo previous work finding greater associative memory performance in higher-fit compared with aerobically lower-fit individuals (Chaddock, Hillman, et al., 2011; Erickson et al., 2009, 2011; Chaddock et al., 2010). The dissimilarities between our study and previous research may be because of the relatively small sample size of the given study, which may have had insufficient power to detect a difference in memory performance between the groups. Alternatively, it is possible that the apparent disparities in the literature may be a function of the tasks used to assess learning and memory. Whereas the current study examined verbal associative encoding, differences in performance between HF and LF individuals were previously seen for verbal item memory (Winter et al., 2007), visual item and relational memory (Chaddock, Hillman, et al., 2011; Chaddock et al., 2010), and visual short-term delayed match to sample (Erickson et al., 2009, 2011). Furthermore, work from our laboratory has shown that aerobic fitness in adolescents significantly predicted spatial learning but not spatial memory on a virtual Morris water task, and no significant relationships were seen between aerobic fitness and performance during word list learning and memory (Herting & Nagel, 2012). Therefore, it is possible that aerobic exercise may not have ubiquitous effects on memory. Research is needed to determine whether different types of memory are influenced to different degrees by exercise and if these relationships change with age.
Despite the groups not showing significant differences in performance, distinct patterns of memory encoding neural circuitry were apparent between HF and LF youth. First, LF youth showed greater bilateral anterior hippocampal BOLD response to encoding later remembered versus forgotten word pairs compared with HF youth. To our knowledge, this is the first study to examine how exercise relates to hippocampal function. Although fMRI does not allow us to determine the molecular and cellular mechanisms that might underlie these findings, differences in hippocampal function may result from exercise-induced changes in neurogenesis and growth factors that have been well documented in animals (for a review, see van Praag, 2008). In particular, aerobic exercise in HF youth may lead to increases in hippocampal neurogenesis and synaptic plasticity, which may ultimately result in more efficient hippocampal neurons, as depicted by less BOLD activity during successful memory encoding in HF than LF youth. If true, this hypothesis may help to explain why LF youth showed greater hippocampal activation during encoding of remembered versus forgotten word pairs compared with HF peers.
In terms of patterns of activation, hemispheric differences were also seen. Whereas HF youth showed unilateral activation in left hippocampus and superior and middle frontal gyrus, which mirrored previous reports in adults (Kim, 2011), LF youth displayed bilateral BOLD response in these areas during successful versus unsuccessful encoding. In addition, BOLD signal in the right superior and middle frontal gyrus was positively related to better performance for LF youth; this relationship was not seen in HF youth. Although the scarcity of published research on subsequent memory effects in adolescents limits interpretation, recruitment of the adjacent hemispheric homologue has been repeatedly viewed as a compensatory mechanism to allow groups to obtain similar performance (e.g., children vs. adults, Moses et al., 2002; Gaillard et al., 2000; patients vs. controls, Jensen et al., 2011; Thiel et al., 2006; young adults vs. aging, Dolcos, Rice, & Cabeza, 2002). Thus, the engagement of the right PFC during successful memory encoding and its positive relationship to performance may suggest that LF youth may need to recruit additional brain regions to perform similarly to HF youth. To confirm if this bilateral activation is indeed compensatory, future work should examine brain activity in HF and LF youth while varying task demands.
In addition to PFC and hippocampus, group differences were also found in deactivation patterns within DMN regions. HF youth showed greater deactivation than LF youth in key DMN regions, such as the mPFC and pCC, as well as the IPL for remembered versus forgotten word pairs. This difference was primarily driven by less deactivation in the remembered (vs. baseline) condition in LF youth. Exploratory context-dependent functional connectivity further showed that HF youth showed strong negative correlations between bilateral hippocampus and mPFC and right IPL, suggesting the hippocampus is inversely cooperating with the DMN during successful memory encoding. These findings converge with existing literature suggesting that hippocampal activation and strong deactivation of the DMN co-occur to allow for successful memory encoding (Kim, 2011; Vannini et al., 2011; Daselaar et al., 2004, 2009). Interestingly, LF youth did not show this strong negative correlation in BOLD response between the hippocampus and mPFC and the right IPL. This result may reflect poorer cooperation between the hippocampus and DMN during encoding of memories in LF youth. However, neither directionality nor cause and effect with regard to brain and behavior can be resolved from this type of analysis. Context-dependent correlation analyses could either reflect that encoding new memories influences functional connectivity between the hippocampus and DMN or, alternatively, that the hippocampus influences the effect that the psychological state of memory encoding has on DMN activity. Nonetheless, together, these findings suggest that the hippocampus and DMN regions show altered connectivity in LF youth when encoding later remembered word pairs when compared with their HF peers.
Given that DMN deactivation is thought to be essential for successful encoding and is not seen in populations with or at-risk for impaired memory performance (Pihlajamaki et al., 2009; Miller et al., 2008), it is somewhat surprising that LF youth display this pattern of deactivation during the successful encoding of new memories and also are able to obtain similar task performance to their HF peers. There are a number of possible explanations. First, although it is possible that HF and LF adolescents differ in their intentional encoding strategies, on self-report, the groups reported similar levels of motivation, task difficulty, as well as comparable strategies, reducing this likelihood. Another possibility may be that the activation of the right hippocampus and PFC allow LF youth to successfully encode new memories despite poor DMN deactivation. Specifically, the engagement of the right hemisphere in LF youth may occur as a result of poor inverse cooperation between the hippocampal and DMN. Another possibility is that atypical DMN deactivation does not lead to poor memory performance in LF youth, because additional compensatory processes occur during the retrieval process. That is, the current study focused on how aerobic fitness relates to brain response during successful memory encoding. However, a successful memory requires not only encoding of new information, but also storage, consolidation, and retrieval. LF youth may, therefore, show differences in brain response during encoding that may be compensated for at later stages of memory processing, ultimately resulting in adequate task performance. Lastly, although deactivation was of a smaller magnitude than seen in HF youth, LF adolescents might have sufficient DMN deactivation to encode an adequate amount of information to perform well on a relatively STM recognition task. For example, it is possible that differences in memory performance would be seen using a longer memory retrieval delay or a more difficult type of memory test (e.g., free recall). More research is warranted to examine how exercise influences the neural mechanisms underlying other stages of memory, such as retrieval, as well as various types of memory performance (free recall, recognition, etc.).
Beyond these speculative functional explanations, the finding that LF youth show atypical patterns of DMN deactivation is in agreement with previous research showing that aerobic exercise influences DMN activity in the elderly. Voss and colleagues (2010) reported that decrements in functional connectivity between DMN regions, occurring with normal aging, are mitigated following 12 months of aerobic training. They also found exercise to restore functional differentiation between brain networks in elderly individuals (Voss, Prakash, et al., 2010). Although similar research on DMN functional connectivity and exercise has not been published in younger populations, our results implicate that aerobic fitness may also affect the integrity of the DMN, as well as the functional distinction between DMN and task-positive networks in younger populations. Future research will determine if the intrinsic organization and functional connectivity of the DMN is influenced by aerobic exercise in adolescents.
Overall, this study provides an important extension of previous research examining how aerobic exercise impacts learning and memory across the lifespan. However, a number of limitations must be mentioned. Here, we’ve shown several distinct differences in memory-related neural circuitry between LF and HF adolescents. In such studies, there is often concern that other non-exercise-related factors might account for group differences, such as nutrition, lifestyle factors, or environmental enrichment that may coexist with exercise. In the current study, we assessed these variables and, to the best of our ability, matched groups. Because both groups reported similar lifestyle behaviors, including participation in extracurricular activities, the functional neural differences reported appear most parsimoniously explained by differences in aerobic fitness. However, these lifestyle measures were brief and based on self-report (questionnaire), so it is impossible to entirely rule out nonexercise factors as potential confounds. It will be important to replicate these findings, using a longitudinal aerobic exercise intervention study, to confirm the influence of aerobic fitness on memory encoding in youth.
Second, although a number of additional studies have used fMRI technology to study exercise-induced changes in cognitive function (Chaddock, Erickson, et al., 2011; Voss et al., 2011; Voss, Erickson, et al., 2010; Voss, Prakash, et al., 2010), animal studies have shown that exercise can influence mitochondria (Steiner, Murphy, McClellan, Carmichael, & Davis, 2011) and angiogenesis (Van der Borght et al., 2009), which could presumably change the shape and amplitude of the BOLD signal. Interestingly, however, previous work examining the effects of acute exercise on factors that could influence the BOLD signal (i.e., CBF and cerebral oxygen to glucose uptake) suggest that these effects are largely transient, with the values returning to pre-exercise levels within 60 min or less after completion of aerobic exercise (Williamson, Querry, McColl, & Mathews, 2009; Dalsgaard, Ide, Cai, Quistorff, & Secher, 2002). Furthermore, a study examining 12 weeks of aerobic training (1 hr/4 times per week) on human hippocampal blood flow revealed specific, rather than global effects, which mapped onto changes seen in neural plasticity in rodents (Pereira et al., 2007), suggesting that aerobic fitness does not lead to global changes in blood flow.
The current study also tried to reduce as well as assess this potential confound. Given that blood flow changes can be seen during and immediately following exercise (Williamson et al., 2009; Dalsgaard et al., 2002), participants were not allowed to exercise for 1 hr before scanning. This method was used to try to eliminate acute blood flow and behavioral changes seen with exercise, because cognitive benefits have been reported immediately following a single bout of exercise (Coles & Tomporowski, 2008; Hillman, Erickson, & Kramer, 2008), and to eliminate the effects of general increases in arousal that accompany performing and recovering from aerobic exercise (Hopkins, Davis, Vantieghem, Whalen, & Bucci, 2012). In addition, we attempted to rule out global blood flow changes by examining group differences in the HRF in a control region. The effect sizes for group were significantly larger for the memory-related brain activity in the five cortical regions (d’s ≥ 2.02) when compared with the visual cortex (d = .21). In addition, the effect sizes were larger in the hippocampus (d’s = .88 and 1.02) compared with visual cortex; albeit these did not reach statistical significance. Together, these findings argue against the idea that exercise-induced global differences in fMRI signal is the driving factor for the reported differences in memory-related brain activity.
Certain additional limitations of the current study are important to note. First, because the current subsequent memory task resulted in strikingly similar associative encoding neural circuitry as seen in adults (Kim, 2011), and the few studies performed in youth have reported similar memory-related neurocircuitry (ages 8–24, Ofen et al., 2007; ages 11–19, Menon et al., 2005), we have interpreted the influence of aerobic exercise on memory-related processes using the adult literature as a reference for expected subsequent memory effects in adolescents. However, neurodevelopmental changes have been reported in PFC and hippocampal activation during memory encoding (Ofen et al., 2007; Menon et al., 2005), so it is currently unclear what patterns of activation are typical for this age range. More research is needed to characterize memory encoding processes in adolescents, which is ultimately necessary to help clarify the impact of aerobic exercise on brain function during this sensitive time period. In addition, although we did attempt to reduce the acute effects of exercise by having participants refrain from exercise for 1 hr before scanning, we did not directly assess the duration between each subject’s last bout of physical activity and testing. Although the literature suggests that persistence of exercise-induced physiological and cognitive changes is relatively short (Coles & Tomporowski, 2008; Hillman et al., 2008), the research in this area is scarce, and it is possible that physiological changes may possibly last longer. Thus, the timing of data acquisition is a possible limitation of the current study, and it remains a challenge for future studies aimed at teasing apart the duration of exercise-induced changes in cognitive and physiological systems.
In summary, LF youth show a number of distinct differences in brain activity in PFC, hippocampus, and DMN when encoding new memories, compared with their HF peers. We show that, in addition to previous research on exercise-induced volumetric changes in regions important for learning and memory, aerobic fitness levels influence memory-related functional neural circuitry in youth. Moving forward, it will be important to clarify the functional implications for these atypical patterns of activation in LF adolescents during memory encoding, as well as replicate these preliminary findings using an exercise intervention design. Given previous work showing exercise to benefit memory in children, adults, and elderly, future research is also needed to extend these findings to different populations to determine if aerobic exercise can influence learning and memory neural processes across the lifespan.
Acknowledgments
This research was supported by the National Institutes of Health (F31AA019866-Herting; R01 AA017664-Nagel; K08 NS052147-Nagel), the Dana Foundation (Nagel), the Oregon Clinical and Translational Research Institute, the OHSU Tartar Trust Research Fellowship (Herting), American Psychological Association Science Directorate’s Dissertation Research Award (Herting), and ARCS Foundation, Inc., Portland Chapter (Herting). A special thanks to Madison Stroup, Khadiya Chinnarath, Jill Waldman, Stephanie Sasse, Jenny Peraza, and Kristen Seghete for their assistance in data collection and data entry. Thank you to Dr. Jacob Raber, Dr. Joel Nigg, and Dr. Diane Elliot for their time and guidance with data analyses and Dr. Elliot for her help with aerobic fitness testing.
References
- Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Armstrong N, van Mechelen W. Paediatric exercise science and medicine. 2. New York: Oxford University Press; 2008. [Google Scholar]
- Armstrong N, Welsman JR. Aerobic fitness: What are we measuring? Medicine and Sport Science. 2007;50:5–25. doi: 10.1159/000101073. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Harrower JR, Deeter MF. The effects of cross-country running on pre-adolescent girls. Medicine and Sport Science. 1972;4:1–5. [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Applied & Preventive Psychology. 1994;3:61–73. [Google Scholar]
- Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. American Heart Journal. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: Current knowledge and future directions. Obesity Reviews. 2011;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control. Child and teen BMI calculator. 2011 Sep; Retrieved from apps.nccd.cdc.gov/dnpabmi/
- Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Research. 2010;1358:172–183. doi: 10.1016/j.brainres.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, Pontifex MB, et al. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biological Psychology. 2011;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Hillman CH, Buck SM, Cohen NJ. Aerobic fitness and executive control of relational memory in preadolescent children. Medicine and Science in Sports and Exercise. 2011;43:344–349. doi: 10.1249/MSS.0b013e3181e9af48. [DOI] [PubMed] [Google Scholar]
- Chen G. Context-dependent correlation analysis. 2011 Retrieved April 12, 2012, from afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html.
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clarke FR, Birdsall TG, Tanner J. Two types of ROC curves and definitions of parameters. The Journal of the Acoustical Society of America. 1959;31:629–630. [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coles K, Tomporowski PD. Effects of acute exercise on executive processing, short-term and long-term memory. Journal of Sports Sciences. 2008;26:333–344. doi: 10.1080/02640410701591417. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, an International Journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcoholism, Clinical and Experimental Research. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. Epub 2005 Nov 2014. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Science. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK, Ide K, Cai Y, Quistorff B, Secher NH. The intent to exercise influences the cerebral O(2)/carbohydrate uptake ratio in humans. Journal of Physiology. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: Recollection, familiarity, and novelty. Journal of Neurophysiology. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in Human Neuroscience. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, et al. Hippocampal volume in adolescent-onset alcohol use disorders. The American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, et al. Sex differences in brain maturation during childhood and adolescence. Cerebral Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: Right hemisphere decline or asymmetry reduction. Neuroscience and Biobehavioral Reviews. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19:1030–1039. doi: 10.1002/hipo.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, USA. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Georgopoulos NA, Roupas ND, Theodoropoulou A, Tsekouras A, Vagenakis AG, Markou KB. The influence of intensive physical training on growth and pubertal development in athletes. Annals of the New York Academy of Sciences. 2010;1205:39–44. doi: 10.1111/j.1749-6632.2010.05677.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: Ages 4–18 years. Journal of Comparitive Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in United States children: The secular trend toward earlier development. International Journal of Andrology. 2006;29:241–246. doi: 10.1111/j.1365-2605.2005.00575.x. discussion 286–290. [DOI] [PubMed] [Google Scholar]
- Herting MM, Nagel BJ. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behavioural Brain Research. 2012;233:517–525. doi: 10.1016/j.bbr.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: Exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Davis FC, Vantieghem MR, Whalen PJ, Bucci DJ. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience. 2012;215:59–68. doi: 10.1016/j.neuroscience.2012.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoven CW, Duarte CS, Lucas CP, Wu P, Mandell DJ, Goodwin RD, et al. Psychopathology among New York City public school children 6 months after September 11. Archives of General Psychiatry. 2005;62:545–552. doi: 10.1001/archpsyc.62.5.545. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6:e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz KF, Mahoney LT. Maturation, gender, and video game playing are related to physical activity intensity in adolescents: The Muscatine Study. Pediatric Exercise Science. 1997;9:353–363. [Google Scholar]
- Jensen EJ, Hargreaves I, Bass A, Pexman P, Goodyear BG, Federico P. Cortical reorganization and reduced efficiency of visual word recognition in right temporal lobe epilepsy: A functional MRI study. Epilepsy Research. 2011;93:155–163. doi: 10.1016/j.eplepsyres.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. Neuroimage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kim H, Daselaar SM, Cabeza R. Overlapping brain activity between episodic memory encoding and retrieval: Roles of the task-positive and task-negative networks. Neuroimage. 2010;49:1045–1054. doi: 10.1016/j.neuroimage.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl GS, James SS, Kohrt WM. Developmental aspects of maximal aerobic power in children. Exercise and Sport Sciences Reviews. 1985;13:503–538. [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Neuroscience. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain and Cognition. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, et al. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Lussier L, Buskirk ER. Effects of an endurance training regimen on assessment of work capacity in prepubertal children. Annals of the New York Academy of Sciences. 1977;301:734–747. doi: 10.1111/j.1749-6632.1977.tb38243.x. [DOI] [PubMed] [Google Scholar]
- Mahon NE, Yarcheski TJ, Yarcheski A. The revised Personal Lifestyle Questionnaire for early adolescents. Western Journal of Nursing Research. 2003;25:533–547. doi: 10.1177/0193945903253000. [DOI] [PubMed] [Google Scholar]
- Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: Evidence from animal models. Neurotoxicity Research. 2011;19:286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- Masten AS. Regulatory processes, risk, and resilience in adolescent development. Annals of the New York Academy of Sciences. 2004;1021:310–319. doi: 10.1196/annals.1308.036. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Research, Cognitive Brain Research. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences, USA. 2008;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Moses P, Roe K, Buxton RB, Wong EC, Frank LR, Stiles J. Functional MRI of global and local processing in children. Neuroimage. 2002;16:415–424. doi: 10.1006/nimg.2002.1064. [DOI] [PubMed] [Google Scholar]
- Moss HB, Kirisci L, Gordon HW, Tarter RE. A neuropsychologic profile of adolescent alcoholics. Alcoholism, Clinical and Experimental Research. 1994;18:159–163. doi: 10.1111/j.1530-0277.1994.tb00897.x. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parrish TB, Gitelman DR, LaBar KS, Mesulam MM. Impact of signal-to-noise on functional MRI. Magnetic Resonance in Medicine. 2000;44:925–932. doi: 10.1002/1522-2594(200012)44:6<925::aid-mrm14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences, USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, O’Keefe K, Bertram L, Tanzi RE, Dickerson BC, Blacker D, et al. Evidence of altered posteromedial cortical fMRI activity in subjects at risk for Alzheimer disease. Alzheimer Disease & Associated Disorders. 2009;24:28–36. doi: 10.1097/WAD.0b013e3181a785c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, et al. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism, Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Riddoch CJ, Bo Andersen L, Wedderkopp N, Harro M, Klasson-Heggebo L, Sardinha LB, et al. Physical activity levels and patterns of 9- and 15-yr-old European children. Medicine and Science in Sports and Exercise. 2004;36:86–92. doi: 10.1249/01.MSS.0000106174.43932.92. [DOI] [PubMed] [Google Scholar]
- Rifas-Shiman SL, Gillman MW, Field AE, Frazier AL, Berkey CS, Tomeo CA, et al. Comparing physical activity questionnaires for youth: Seasonal vs annual format. American Journal of Preventive Medicine. 2001;20:282–285. doi: 10.1016/s0749-3797(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: Evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. Journal of Applied Physiology. 2011;111:1066–1071. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Three-dimensional coplanar stereotaxic atlas of the human brain proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Thiel A, Habedank B, Herholz K, Kessler J, Winhuisen L, Haupt WF, et al. From the left to the right: How the brain compensates progressive loss of language function. Brain and Language. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behavioral Neuroscience. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, et al. Physical exercise leads to rapid adaptations in hippocampal vasculature: Temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19:928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. Journal of the American Medical Informatics Association. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: Past and future directions. Neuromolecular Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: Something to chew on. Trends in Neurosciences. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, O’Brien J, O’Keefe K, Pihlajamaki M, Laviolette P, Sperling RA. What goes down must come up: Role of the posteromedial cortices in encoding and retrieval. Cerebral Cortex. 2011;21:22–34. doi: 10.1093/cercor/bhq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Chaddock L, Kim JS, Vanpatter M, Pontifex MB, Raine LB, et al. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash RS, Chaddock L, Malkowski E, Alves H, et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience. 2010;2:1–17. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G, Kartodihardjo W, Klissouras V. Growth and physical training with reference to heredity. Journal of Applied Physiology. 1976;40:211–215. doi: 10.1152/jappl.1976.40.2.211. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- Williamson JW, Querry R, McColl R, Mathews D. Are decreases in insular regional cerebral blood flow sustained during postexercise hypotension? Medicine and Science in Sports and Exercise. 2009;41:574–580. doi: 10.1249/MSS.0b013e31818b98c8. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of Learning and Memory. 2007;87:597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. International Journal of Epidemiology. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]


