Abstract
Clinical studies indicate that phenytoin prevents acute post-traumatic seizures but not subsequent post-traumatic epilepsy. We explored this phenomenon using organotypic hippocampal slice cultures as a model of severe traumatic brain injury. Hippocampal slices were cultured for up to eight weeks, during which acute and chronic electrical recordings revealed a characteristic evolution of spontaneous epileptiform discharges, including interictal spikes, seizure activity and electrical status epilepticus. Cell death exhibited an early peak immediately following slicing, and a later secondary peak that coincided with the peak of seizure-like activity. The secondary peak in neuronal death was abolished by either blockade of glutamatergic transmission with kynurenic acid or by elimination of ictal activity and status epilepticus with phenytoin. Withdrawal of kynurenic acid or phenytoin was followed by a sharp increase in spontaneous seizure activity. Phenytoin’s anticonvulsant and neuroprotective effects failed after four weeks of continuous administration. These data support the clinical findings that after brain injury, anticonvulsants prevent seizures but not epilepsy or the development of anticonvulsant resistance. We extend the clinical data by showing that secondary neuronal death is correlated with ictal but not interictal activity, and that blocking all three of these sequelae of brain injury does not prevent epileptogenesis in this in vitro model.
Keywords: epilepsy, epileptogenesis, traumatic brain injury, phenytoin, neuronal death, activity, interictal, ictal
Introduction
Traumatic brain injury (TBI) is a major cause of acquired epilepsy (Pitkanen et al., 2011). Following a latent period of months to years, recurrent spontaneous seizures occur in up to 53% of veterans with severe military head injury (Raymont et al., 2010; Salazar et al., 1985), and 17% of civilian patients with severe head injury (Annegers et al., 1998). It has been hypothesized that neuronal death and axon damage resulting from trauma (Blumbergs et al., 1995; Graham et al., 2000) initiate the process of epileptogenesis, or development of epilepsy (Ben-Ari and Dudek, 2010; Staley et al., 2005). Consequences of neuron damage could include the loss of inhibition due to death of interneurons (Cossart et al., 2001; de Lanerolle et al., 1989; Kobayashi and Buckmaster, 2003; Sloviter, 1987), and axonal sprouting (Cronin and Dudek, 1988; Okazaki et al., 1995; Sutula et al., 1989) due to deafferentation (Laurberg and Zimmer, 1981; Steward and Vinsant, 1978; Sutula and Dudek, 2007) leading to hyperexcitability (Esclapez et al., 1999; Smith and Dudek, 2001) and spontaneous seizures (Jefferys, 2003). However, seizures can also cause neuronal death. It is widely accepted that prolonged seizures (status epilepticus) result in neuronal necrosis (Meldrum, 2002; Meldrum and Brierley, 1973; Meldrum et al., 1973). The possibility that even brief, spontaneous seizures can kill neurons has been raised by MRI studies demonstrating progressive atrophy in patients with intractable epilepsy (Bernhardt et al., 2009; Fuerst et al., 2003), evidence of apoptosis in brain tissue resected for seizure control (Henshall et al., 2004) and the correlation of cortical volume loss with seizure frequency in post traumatic epilepsy (Raymont et al., 2010). It is difficult to establish causality from these observational studies. For example, cortical volume loss in patients with post traumatic epilepsy may reflect a more severely epileptogenic initial injury, or more frequent post traumatic seizures may lead to progressive volume loss. However, knowledge of causality is necessary to design rational antiepileptogenic therapies (Giblin and Blumenfeld, 2010; Pitkanen, 2010) and to know whether the benefits of seizure control include neuroprotection and / or suppression of epileptogenesis (Loscher and Brandt, 2010). The current paradigm for treatment of post-traumatic epilepsy includes treatment with anticonvulsants such as phenytoin (Chen et al., 2009; Temkin, 2009), although anticonvulsants tested to date have not demonstrated antiepileptogenic efficacy (Schierhout and Roberts, 2001; Temkin, 2001; Temkin, 2009). On the other hand, long-term monitoring studies indicate that experimental epilepsy continues to worsen after the first seizure (Williams et al., 2009), supporting the possibility that seizures themselves contribute to epileptogenesis. Open questions include whether recurrent spontaneous epileptiform activity induces neuronal death, whether such neuronal death worsens epilepsy, and whether effective anticonvulsant therapy alters the course of epilepsy.
Here, we use organotypic hippocampal slice cultures as a model of posttraumatic epileptogenesis to monitor the events following brain injury and the effects of treatment with phenytoin. We and others have previously reported that these cultures become spontaneously epileptic after a latent period in vitro (Dyhrfjeld-Johnsen et al., 2010; McBain et al., 1989). We follow both epileptogenesis and ongoing neuronal death in the same cultures using chronic electrical recordings and sequential measurement of cell death markers to test whether spontaneous ictal or interictal activity cause cell death, whether suppression of spikes or seizures is neuroprotective, and whether suppression of spikes, seizures, and ictal neuronal death have antiepileptogenic effects.
Materials and methods
All animal use protocols conformed to the guidelines of the National Institutes of Health and the Massachusetts General Hospital Center for Comparative Medicine on the use of laboratory animals.
Organotypic hippocampal slice preparation
Hippocampi were isolated from postnatal (P) day 7–8 Sprague-Dawley rats and FVB and C57BL/6 mice, cut into 350 μm slices on a McIlwain tissue chopper (Mickle Laboratory Eng. Co., Surrey, United Kingdom), and mounted in clots of chicken plasma (Cocalico Biologicals, Reamstown, PA) and thrombin (Sigma-Aldrich, St. Louis, MO) on poly-L-lysine coated glass coverslips (Electron Microscopy Sciences, Hatfield, PA). Slices were incubated in roller tubes (Nunc, Roskilde, Denmark) at 37°C in 850 μl NeurobasalA/B27 medium supplemented with 0.5 mM GlutaMAX and 30 μg/ml gentamicin (all from Invitrogen). Chronically recorded cultures were prepared according to the protocol we published earlier (Berdichevsky et al., 2008). Briefly, 350 μm P7-8 rat slices were placed in Sylgard mini-wells on multiple electrode arrays prepared in house. Slices were then incubated at 37°C in 5% CO2 humidified atmosphere in 350 μl NeurobasalA/B27 medium supplemented with 0.5mM GlutaMAX and 30 μg/ml gentamicin, with bi-weekly medium changes.
Multiple electrode array (MEA) fabrication
MEAs were fabricated on 3 × 2″ glass slides (Fisher Scientific) by photolithographically defining gold electrodes and contact pads. Culture wells were made by cutting the top portion of a 50 ml centrifuge tube (BD Falcon) and gluing it to the MEA with Sylgard 184 (Dow Corning). Sylgard mini-wells were prepared as described earlier (Berdichevsky et al., 2008). Gold traces connecting electrodes to the contact pads were insulated from contact with culture medium by Sylgard coating. Electrodes were left uninsulated inside the Sylgard mini-wells, creating an active electrode area of 30 μm by 750 μm, with one or two electrodes per mini-well. Each MEA had two culture wells, each containing two mini-wells for slice placement, for a total of 4 slice cultures on one MEA chip (Fig. 1A). Completed MEAs were sterilized by immersion in 70% and 100% ethanol baths for 5 minutes, and dried inside a sterile laminar flow hood.
Fig. 1.
Chronic electrical recording in rat organotypic hippocampal slices in vitro. (A), schematic of the custom MEA with four organotypic hippocampal cultures. (B), micrograph of one of the hippocampal cultures on MEA, scale bar is 200 μm. (C), raster plot of electrical activity recorded in an organotypic culture. (D), the types of activity detected with an electrode (top), and the color map of interictal activity (top trace), and ictal activity (bottom trace), each recording is 1 hour long. Arrows point to interictal spikes and ictal events shown at different time scale, trace duration is 100 seconds. (E), Incidence of ictal and interictal activity as a percentage of cultures recorded on MEAs, with age of cuture. (F), Cumulative duration of ictal activity observed per culture with age. N = 7 cultures for (E) and (F).
Drug Application
Phenytoin was dissolved in DMSO, and added to the culture medium at either 30 or 100 μM concentration. Control cultures were treated with DMSO as vehicle. Kynurenic acid (Sigma-Aldrich) was dissolved in NeurobasalA, sterilized by filtration, and added to cultures at 3 mM final concentration. For chronic application, both phenytoin and kynurenic acid (KYNA) were first added to cultures on 3rd day in vitro (DIV), and re-applied with each bi-weekly medium change.
Electrophysiological Recordings and Data Analysis
Chronic electrical recordings were carried out with rat slice cultures on MEAs, in culture medium, in tissue culture incubator with 37°C and 5% CO2 humidified atmosphere. Signals from MEAs were amplified using a 10-channel extracellular amplifier with 10 channel high-impedance head stage (EX-1000, Dagan Corporation), and digitized with a multi-channel digital acquisition board (Measurement Computing). Sampling rate was 500 Hz per channel, with one or two channels per slice. Data was recorded with dClamp customized software (available on request from the authors), and analyzed with Matlab (MathWorks). Color raster plots were created in Matlab by binning the data into 500 msec bins, and calculating the number of active (super-threshold) bins per 10 second sliding window. Threshold value was defined as five times the standard deviation of recording noise (mostly variable frequency noise from cell culture incubator heater and CO2 regulator).
For acute electrical recordings, mouse organotypic hippocampal slices were transferred to a conventional submerged chamber and continuously superfused with oxygenated ACSF (95% O2 and 5% CO2) at 32°C and a flow rate of 2.5 ml/min. ACSF contained the following compounds : 126 mM NaCl, 3.5 mM KCl, 2 mM CaCl2, 1.3 mM MgCl2, 25 mM NaHCO3, 1.2 mM NaH2PO4, and 11 mM glucose (pH 7.4). Extracellular field potential recordings were performed using tungsten microelectrodes and a low-noise multichannel amplifier (band-pass 1 Hz to 2 kHz, x 1,000). Microelectrodes were made from coated tungsten wire of 50 μM diameter. Root mean square noise level with electrodes placed in the perfusion solution was typically 3–5 μV. Simultaneous recordings of population activity and multiple unit activity were typically performed in the CA3 and CA1 pyramidal cell layer. The electrical signals were digitized using an analogue-to-digital converter (DigiData 1322A; Axon Instruments). Sampling interval per signal was 20 microseconds (2 kHz). pCLAMP 9.2 (Axon Instruments), Mini Analysis 6.03 (Synaptosoft) and Origin 7.5 SR6 (Microcal Software) programs were used for the acquisition and data analysis. Power spectrum analysis was performed after applying a Hamming window function. Power was calculated by integrating the root mean square value of the signal in frequency band from 1 to 1,000 Hz.
Propidium Iodide (PI) and SYTO 10 Staining and Image Analysis
PI imaging was carried out just before bi-weekly application of fresh drugs/medium to the mouse cultures. Sterile propidium iodide solution (1mg/ml in H20, Invitrogen), was diluted in culture medium 1:250, and applied to cultures for 1 hour before imaging. Image stacks at 10 μm intervals were acquired with a 10x objective at 0.7x zoom on an inverted confocal microscope (Zeiss), through the flat bottom of the roller-tube without compromising culture sterility. Cultures were then washed and filled with fresh medium containing appropriate drugs or vehicle, and placed back into the tissue culture incubator. Images were filtered with a 1 pixel median filter. The number of PI-positive cells in CA1 pyramidal cell layer (CA1 pcl) and the suprapyramidal blade of the dentate gyrus granule cell layer (DG gcl) was quantified with a 3D Objects Counter plugin in Fiji extension of ImageJ (NIH).
SYTO 10 (Invitrogen) staining in fixed, permeabilized mouse organotypic cultures functions analogously to a Nissl stain, with neural soma staining as brightly as the nuclei. Cultures were washed in ACSF, and fixed for 2 hours in 4% paraformaldehyde. Cultures were then permeabilized in 0.3% Triton X-100 (Sigma) in phosphate-buffered saline (PBS) for 2 hours, and incubated for 5 hours in 1:1000 SYTO 10 in PBS. Slices were then washed and mounted for confocal microscopy. Images were collected on a Leica confocal microscope with a 40x objective and appropriate filters, and processed in Fiji. The density of neurons in the same area of CA1 pyramidal layer was calculated for control and KYNA groups.
Lactate dehydrogenase (LDH) assay
LDH was measured in rat culture supernatant collected twice weekly. Measurements were carried out using LDH-Cytotoxicity Assay kit (BioVision) according to manufacturer’s protocol. Unit of LDH was defined as the amount of enzyme that catalyzes the conversion of lactate to pyruvate to generate 1.0 μmol of NADH per minute at 37° C.
Statistical Analysis
Group measures are expressed as mean ± standard deviation (s.d.) or mean ± standard error (s.e.) as indicated. The statistical significance of differences was assessed with t-test for two samples with equal or unequal variances, as appropriate. The level of significance was set at P < 0.05.
Terminology
For the purposes of this study, interictal epileptiform discharges (IEDs) were defined as paroxysmal discharges that were clearly distinguished from background activity, with an abrupt change in polarity occurring within several milliseconds (“spikiness”) and duration of less than 200 msec (Chatrian et al., 1974; Chatrian, 1974). Ictal-like events were defined as either paroxysmal discharges lasting more than 10 seconds, or sequences of at least 20 paroxysmal spikes in 10 seconds (White et al., 2010; White et al., 2006).
Results
Model of post-traumatic epileptogenesis in vitro
We recorded spontaneous activity from rat organotypic hippocampal cultures (n = 7) grown on MEAs for up to 37 DIV (Fig. 1B). All slice cultures developed epileptic activity. Raster plots were constructed from electrical data (Fig. 1C), with interictal and ictal activity pseudocolored based on spike frequency (Fig. 1D). Activity recordings reveal that organotypic cultures go through a latent period before developing epileptiform discharges, analogously to TBI patients (Annegers et al., 1998). In the organotypic cultures, the latent period lasts for up to 7 days before interictal epileptiform discharges (IEDs) develop, followed by ictal-like electrical seizures (Fig. 1E), (Dyhrfjeld-Johnsen et al., 2010). Ictal and interictal activity continued for at least 5 weeks (Fig. 1 E, F). The levels of ictal activity (seizure frequency/duration) were not constant through the five week period; there was intermittent activity clustering betwen DIV 9 to DIV 37 (Fig. 1C), similar to seizure clustering in human (Haut et al., 2002) and experimental (Kadam et al.; Williams et al., 2009) epilepsy. Some cultures had quiescent periods lasting for up to 2–3 days, terminated by eventual reappearance of ictal events (Fig. 1E, number of cultures with ictal activity fluctuates from 75% to 100% between 20 and 37 DIV, n = 7). To confirm that these results are independent of experimental variables such as rodent species or strain, and MEA culture method, we recorded activity from mouse roller-tube type cultures with metal extracellular electrodes. Recordings in these cultures revealed a similar progression from physiological activity to interictal epileptiform discharges (Fig. 2 A), followed by ictal-like discharges (Fig. 2 B). Epileptiform activity in mouse cultures persisted at least until 42 DIV (Fig. 2 C).
Fig. 2.
Epileptogenesis in mouse organotypic hippocampal slices. (A–B) Extracellular field potential recordings in the CA3 pyramidal cell layer in organotypic hippocampal slices at DIV 7 (A) and DIV14 (B). Electrical recordings revealed spontaneous epileptiform discharges, including interictal epileptiform discharges (IEDs) and ictal-like seizure activity. (C) Group data from 70 recordings (2–3 hours in each recording) from 70 organotypic hippocampal slices (n = 10 slices in each age group).
Activity dependent cell death in post-traumatic epileptogenesis
Lactate dehydrogenase (LDH) is a stable enzyme that is released into culture medium upon damage of the plasma membrane. It is a widely used marker of cell death (Bonfoco et al., 1995; Decker and Lohmann-Matthes, 1988; Korzeniewski and Callewaert, 1983). Here, we used LDH to track cell death in rat organotypic cultures from first collection of medium supernatant at 3 DIV to 27 DIV (Supplemental Fig. 1 A). Measurement of LDH activity in used culture medium revealed high levels of LDH on 0–3 DIV, dramatically lower levels of LDH between 3 and 10 DIV, and a transient increase in LDH from 10 to 20 DIV. Addition of 3mM of the ionotropic glutamate receptor antagonist kynurenic acid (KYNA) from 3 DIV to 27 DIV, significantly reduced LDH levels from 10 DIV to 20 DIV (p < 0.05, n = 3 cultures per each condition). Peak LDH levels of 14.8 ± 3.6 *10−4 units and 7.4 ± 1.4 *10−4 units occurred at 13 DIV for both epileptic and KYNA-treated cultures, respectively (mean ± standard deviation, n = 4, p < 0.05, ratio control/KYNA = 2). This finding suggested that cell death after 10 DIV was activity dependent. We examined cell death during this period in more detail with propidium iodide (PI) staining.
PI is a nucleic acid dye that is excluded from viable cells. However, it is able to enter dying or dead cells with compromised membranes and stain their nuclei. We found high numbers of PI-positive cells (2531 ± 501 cells, mean ± SD, n = 12) in rat organotypic cultures at DIV 3 (Figure 3 A, Supplemental Fig. 1B), reflecting cell death due to dissection. Dead cell counts dropped on DIV 6 in both control and KYNA-treated cultures (417 ± 124 and 119 ± 67 cells, respectively, mean ± SD, n = 6, p < 0.001, DIV3 vs. DIV6 for each group, and control vs. KYNA on DIV6). Cell death increased dramatically on DIV 9 and DIV 12 in control (epileptic) cultures. Very significant differences in numbers of dead cells were found between control and KYNA-treated cultures (on DIV 12, there were 840 ± 145 PI-positive cells in control cultures, and 186 ± 86 PI-positive cells in KYNA-treated cultures, mean ± SD, n = 6, p < 0.001, ratio control/KYNA = 4.5). Most of the PI-positive cells were concentrated in DG granule cell layer and CA3 and CA1 pyramidal cell layers (Supplemental Fig. 1, C). Similar results were obtained from mouse organotypic cultures. We found a high density of PI staining in the CA1 and in DG of mouse organotypic cultures (Fig 3C top) after 10 DIV. PI staining was largely confined to the pyramidal and granule cell layers, suggesting that the dying cells were neurons. To test whether cell death is due to seizure activity or to some other cause, we applied 3 mM of KYNA, from 3 DIV to 39 DIV (Pozzo Miller et al., 1994). KYNA dramatically reduced the amount of paroxysmal activity in slice cultures (Fig 3G, 3H), and prevented pyramidal neuron death (Fig. 3 C bottom). We quantified the number of dead, PI-positive neurons in CA1 and DG in mouse cultures from 10 DIV (when most cultures have ictal and interictal activity) to 39 DIV, and found that cell death is ongoing and progressive in controls. In KYNA-treated mouse cultures, cell death is almost completely abolished from 10 DIV and for the duration of culture (n = 7 control slices, n = 5 KYNA-treated slices, Fig. 3 D). At 15 DIV, when most mouse cultures have ictal-like epileptiform activity, there were 178 ± 175 and 15 ± 11 PI-positive cells in CA1 of control and KYNA-treated cultures, respectively (mean ± SD, n = 7 control cultures, n = 5 KYNA-treated cultures, p < 0.05, ratio control/KYNA = 11.9). In DG at 15 DIV, there were 266 ± 85 dead cells in control cultures and 49 +/− 11 dead cells in KYNA-treated controls (mean ± SD, n = 7 controls, n = 5 KYNA-treated cultures, p < 0.01, ratio control/KYNA = 5.4). In confirmation of these results, we also found that KYNA-treated cultures have significantly more surviving neurons than aged-matched controls (n = 3 slices, each condition, Fig. 3 E, F, p < 0.05).
Fig. 3.
Activity-dependent cell death in post-traumatic epileptogenesis. (A) Time course of cell death in epileptic rat organotypic cultures. Propidium iodide uptake is a marker of cell death. Initial high counts of PI-positive cells reflect high number of dead cells following trauma (dissection). Cell death drops during the latent period, and then increases as control cultures transition from interictal to ictal activity. KYNA-treated cultures do not have epileptiform activity, and largely avoid second wave of cell death (** represents p < 0.01, *** p < 0.001, n = 6 slices in control and KYNA groups). (B) schematic representation of progression of epilepsy in organotypic cultures. (C) number of dead (PI-positive) neurons in CA1 pyramidal layer (CA1-pcl) and in the dentate gyrus granule cell layer (DG gcl) is much higher in control mouse cultures than cultures where synchronous activity has been blocked with kynurenic acid (KYNA). (D) top, evolution of cell death with time in CA1 pcl, bottom, in DG gcl in cultures (n = 7 control mouse slices, n = 5 KYNA-treated mouse slices, * represents p < 0.05. Error bars show standard deviation). (E, F) neuron density in CA1 pyramidal layer is significantly higher in chronically KYNA-treated mouse cultures than in controls at 21 DIV, standard deviation shown as error bars. (G, H) ictal-like tonic-clonic electrical seizures resume after washout of KYNA from chronically treated cultures, showing lack of effect on epileptogenesis (n = 8 cultures).
Activity blockade does not prevent epileptogenesis
KYNA suppressed almost all population activity in rat organotypic slices (Fig 3G, 3H). To test whether this suppression altered epileptogenesis, we recorded from rat organotypic slice cultures that had been chronically exposed to kynurenic acid using extracellular electrodes placed in area CA1 of 16–17 DIV cultures in culture medium, in an interface chamber. In all chronically-treated slices (n = 8), wash-out of 3 mM kynurenic acid resulted in abundant ictal-like activity (Fig. 3 G, H). We conclude that suppression of ictal and interictal activity with kynurenic acid does not prevent epileptogenesis in this model.
Anticonvulsive effect of phenytoin is concentration dependent
The anticonvulsant effects of phenytoin (30 μM and 100 μM) on spontaneous epileptiform activity in mouse organotypic hippocampal slice cultures were evaluated at DIV14-22. Electrical activity was monitored by simultaneous extracellular field potential recordings in the pyramidal cell layer of CA3 and CA1 regions.
Acute anticonvulsant effects
In control conditions, spontaneous neuronal network activity in all organotypic hippocampal slice cultures (n = 12) was characterized by interictal epileptiform discharges (IEDs) and recurrent ictal-like seizure activity (Fig. 4). In the first sub-group of slices (n = 6), application of 30 μM phenytoin for 30–60 min transiently abolished ictal-like seizure activity in 5 of 6 experiments (Fig. 4 A, C). In one of six experiments phenytoin (30 μM) reduced seizure frequency from 28 seizures / hour to 1 seizure / hour. Duration of the single ictal-like clonic seizure in the presence of phenytoin was 19 s. The mean seizure frequency decreased from 17.8 ± 4.7 seizures / hour to 0.16 ± 0.16 seizures / hour (mean ± s.e.; n = 6; p = 0.012, paired t-test). In all experiments phenytoin failed to abolish paroxysmal interictal epileptiform discharges (Fig. 4E) that strongly contribute to power of extracellular field potential activity. The mean power of extracellular field potential activity decreased to 50.4 ± 9.6% of control (p = 0.004). Seizure frequency and power rapidly rebounded after termination of drug application (Fig. 4 A–D).
Fig. 4.
Concentration dependent efficacy of Phenytoin in post-traumatic seizures. (A–B) Extracellular field potential recordings from cultured mouse hippocampal slices at DIV21 and DIV20 before (control), during and after (wash) applications of 30 μM (A) and 100 μM (B) phenytoin. Plots below show corresponding power of spontaneous electrical activity. (C–D) Mean frequency of spontaneous seizure activity and power of electrical activity before, during and after phenytoin (30 and 100 μM) application. (E) Group data of the spontaneous interictal discharges (IED) in phenytoin (30 μM) and phenytoin (100 μM) (n = 6 for each group).
In the second sub-group of age-matched slices (n = 6), application of a higher concentration of phenytoin (100 μM) for 30–60 min transiently abolished ictal-like seizures in all experiments and interictal epileptiform discharges in 3 of 6 experiments (Fig. 4 B, C, D; Fig. 5 A). The mean frequency of IEDs in the presence of 100 μM phenytoin was significantly lower than in 30 μM phenytoin (0.005 ± 0.003 Hz vs. 0.051 ± 0.01 Hz; p = 0.006). The mean power of electrical activity in the presence of 100 μM phenytoin was strongly decreased to 18.2 ± 5.4% of control (p = 0.04). Seizure frequency and power rapidly rebounded after termination of drug application (Fig. 4 A–D). Thus phenytoin exerted acute, reversible anticonvulsive effects in a model of post-traumatic seizures in vitro, and the anticonvulsive efficacy of phenytoin was strongly concentration-dependent.
Fig. 5.
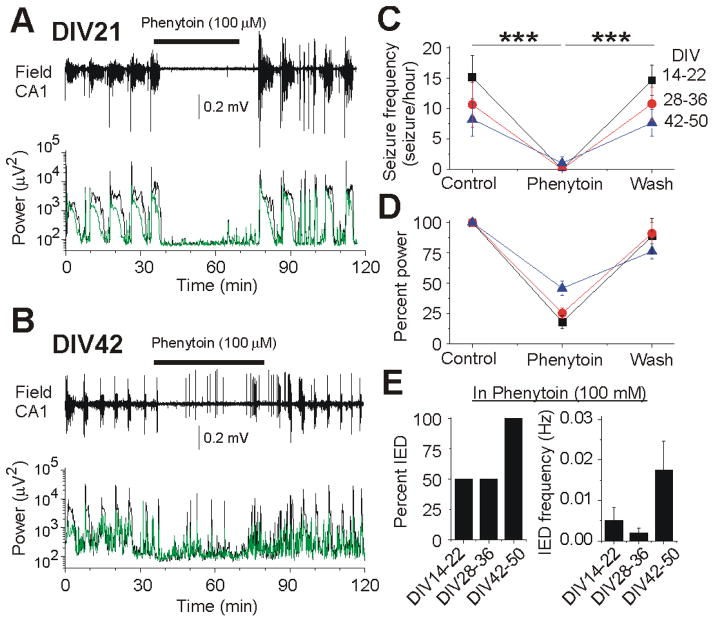
Acute administration of phenytoin suppresses seizures at all stages of epileptogenesis. (A–B) Extracellular field potential recordings from cultured mouse hippocampal slices at DIV21 and DIV42. Plots below show power of electrical activity in the CA3 and CA1 pyramidal cell layer before (control), during and after phenytoin (100 μM) application. (A) A complete anticonvulsant response to acute phenytoin including suppression of all ictal and interictal activity. Application of phenytoin abolished spontaneous interictal bursts and recurrent seizures and strongly reduced power amplitude in EEG (1–100 Hz) and fast ripple (100–500 Hz) frequency range. (B) Example of a partial anticonvulsant effect of phenytoin. Application of Phenytoin abolished recurrent seizures, but not interictal epileptiform discharges, and so only partially reduced the power amplitude. (C) Fraction of hippocampal slices in which phenytoin achieved full and partial effects on spontaneous epileptiform discharges. (D) Mean power of spontaneous electrical activity (percent of control) in EEG and fast ripple frequency range after acute application of phenytoin at each stage of epileptogenesis. (E) Summary of phenytoin effects on interictal activity at each stage.
Anticonvulsant effect of phenytoin is age-dependent
To determine whether there were age-dependent effects of phenytoin in post-traumatic seizures, the acute effects of phenytoin were compared in three groups of organotypic hippocampal slice cultures: DIV 14–22, 28–36, and 42–50 (Fig. 5). At DIV 14–22, phenytoin (100 μM) transiently abolished ictal-like seizure activity (n = 6) and interictal epileptiform discharges in 3 of 6 slices (Fig. 5 A, C, E), and depressed the power of extracellular field potential activity by 82 %. At DIV 28–36 (n = 6), the anticonvulsant effect of phenytoin (100 μM) was similar (Fig. 5 C–E). At DIV 42–50, phenytoin abolished spontaneous seizures in 3 of 4 organotypic slices and reduced seizure frequency in 1 of 4 slices. phenytoin failed to control interictal epileptiform discharges in all slices at this age group. The mean frequency of IEDs was significantly higher at DIV 42–50 compared to both DIV 14–22 and 28–36 (Fig. 5 E). As result, the mean power of extracellular field potential activity in the presence of phenytoin was less depressed compared to both DIV 14–22 and 28–36 (Fig. 5 D). Washout of phenytoin was followed by a sharp increase in seizure activity. Thus acute application of phenytoin reduced epileptiform activity at all ages and development stages of epilepsy. Phenytoin was more effective at younger ages when there was a greater proportion of ictal vs. interictal activity.
Chronic effects of phenytoin
Administration of anticonvulsants to patients with acute traumatic brain injury prevents early acute tonic-clonic seizures, but does not prevent epileptogenesis, nor is it particularly effective at suppressing late post-traumatic seizures (Raymont et al., 2010; Schierhout and Roberts, 2001; Temkin, 2001; Temkin, 2009). However, human studies to date have been compromised to some degree by injury heterogeneity and questions of compliance (Raymont et al., 2010; Temkin, 2001). In the organotypic slice culture model, blocking both ictal and interictal activity did not prevent epileptogenesis (Fig 3G, H). Such broad-spectrum blockade of neural activity has been observed to induce homeostatic upregulation of neuronal excitability (Houweling et al., 2005), which could actually promote epileptogenesis. The selective effect of phenytoin on ictal vs. interictal activity suggested that it might be possible to test whether epileptogenesis could be inhibited by blocking ictal activity but not interictal and other neuronal activity. To first establish whether phenytoin was an effective chronic anticonvulsant in this in vitro model of post-traumatic epilepsy, phenytoin (100 μM) was added to the incubation medium starting at 3 DIV (Fig. 6). After two-three weeks of incubation with phenytoin, slices were transferred to a submerged chamber and continuously superfused with ACSF containing the same concentration of phenytoin. Extracellular field potential recordings were performed in the CA3 and CA1 pyramidal cell layer (Fig. 6A). In the continued presence of 100 μM phenytoin, spontaneous seizures were observed in only one of seven organotypic hippocampal slices, indicating that phenytoin is an effective anticonvulsant for the first three weeks in culture.
Fig. 6.
Loss of anticonvulsant effect of phenytoin after chronic exposure. (A) Extracellular field potential recording from organotypic mouse hippocampal slice at DIV 20. Plot below shows corresponding power of electrical activity in 8.2 s windows. Removal of Phenytoin from incubation medium resulted was followed by a sharp increase in seizure activity. (B) Extracellular field potential recording and corresponding power of electrical activity in organotypic hippocampal slice at DIV42. Spontaneous seizures are marked by asterisks. (C–D) Frequency of seizures and power of extracellular field potential activity in the individual recordings. (E) Mean seizure frequency and changes of power of electrical activity. After four-five weeks, chronic incubation with phenytoin was less effective at controlling seizures.
Subsequent removal of phenytoin from the incubation medium was followed by a sharp increase in seizure activity (Fig. 6 A, C, E). The mean frequency of seizures increased from 0.14 ± 0.14 seizure/hour to 4.7 ± 1.9 seizure/hour (n = 7; p = 0.03). The corresponding power of extracellularly recorded population activity increased by 395 ± 84 % (p = 0.016). These data indicate that blocking seizures with phenytoin did not alter epileptogenesis in this model.
After four-five weeks of chronic incubation with phenytoin, spontaneous seizures were detected in 4 of 6 organotypic hippocampal slices (Fig. 6 B, D). After removal of phenytoin, the mean frequency of spontaneous seizures increased from 2.5 ± 1 to 5.5 ± 1.3 seizure/hour (n = 6; p = 0.023) and the corresponding power of extracellularly recorded population activity increased by 149 ± 89 % (Fig. 6 E; p = 0.19). These data demonstrate that after four-five weeks of chronic incubation, phenytoin was much less effective at controlling post-traumatic seizure activity compared to two-three weeks incubation, and again demonstrate that phenytoin exhibits no antiepileptic activity in this preparation.
Neuroprotective effect of phenytoin correlates with anticonvulsant efficacy
The late loss of anticonvulsant efficacy of phenytoin provided an opportunity to test whether the anticonvulsant effects of phenytoin correlated with a neuroprotective effect, as was observed with kynurenic acid (Fig 3 A, C–F). Neuronal death in the CA1 pyramidal layer was compared in organotypic hippocampal slice cultures under control conditions, in which they experienced spontaneous post-traumatic seizures, and in slice cultures in which seizure activity was suppressed by chronic exposure to 100 μM phenytoin (n = 5 slices, each condition, Fig. 7). From 11 DIV to 28 DIV, cultures exposed to 100 μM phenytoin demonstrated significantly reduced cell death. However, at 33 to 36 DIV, cell death in the cultures chronically treated with phenytoin increased to the point where the rate of cell death was not significantly different from age-matched controls. Cell death in KYNA-treated cultures at this time in vitro remained negligible (Fig. 3 C–D). These data demonstrate a strong correlation between the anticonvulsant efficacy of phenytoin and its neuroprotective effects: phenytoin was less effective at controlling epileptiform discharges after four weeks of chronic treatment, and was also less effective at reducing cell death, suggesting that drug-resistant epileptic activity caused cell death during the 5–6th week in vitro.
Fig. 7.
Loss of neuroprotective effect of chronic phenytoin administration. Mouse slice cultures chronically exposed to 100 μM phenytoin from 3DIV. At 14 DIV, phenytoin significantly reduces neuronal death in CA1 pyramidal layer. At 36 DIV, when phenytoin loses efficacy as an anticonvulsant, cell death in cultures chronically treated with phenytoin is not significantly different from controls (n = 5 slices, each condition, error bars show standard deviation, * represents p < 0.05)
Discussion
This study demonstrates clear relationships between ongoing seizure activity and accelerated neuronal death in vitro that was prevented by successful anticonvulsant therapy. However, prevention of ictal and interictal activity and ictal cell death did not prevent epileptogenesis. The temporal definition of status epilepticus, a medical emergency comprised of unremitting seizures, remains controversial (Lowenstein et al., 1999; Shinnar and Hesdorffer, 2010) because the duration of seizure activity that causes neuronal injury is not precisely known. It is more difficult to study the relationship between spontaneous seizures and neuronal death using data from patients or animal models, where sequential measurements of cell death are generally infeasible (Rocha et al., 2007). Causality is even more difficult to address. For example, an insult to the brain could cause both progressive neuron death and epilepsy, in which case there is correlation, but not causation. On the other hand, progressive seizure-induced cell death could lead to medically intractable epilepsy due to either disinhibition as a consequence of ongoing loss of interneurons, or progressively more intense recurrent axon sprouting as a consequence of continued loss of principal cells (Sutula and Dudek, 2007); in this case, neuronal death would be causally related to worsening epilepsy. Untangling the causes and effects in the relationship between seizures, neuronal death, and epileptogenesis is important to understand the progression of epilepsy and assess the neuroprotective role of anticonvulsant medications. It is also important to understand the effects that intractable epilepsy may have on the brain.
The organotypic hippocampal slice culture is a robust, rapid model of post-traumatic epileptogenesis (Dyhrfjeld-Johnsen et al., 2010). The data presented here indicate that the model is species-independent. Advantages of this preparation include homogenous injury, consistent epileptogenesis, and dramatically improved experimental accessibility: organotypic cultures easily lend themselves to continuous recordings of electrical activity, chronic imaging of neuronal activity and death, and chronic application of drugs during the course of epileptogenesis. The key features of human and experimental in vivo epileptogenesis are reproduced in this model: latency (Annegers et al., 1998; Raymont et al., 2010; Williams et al., 2009) electrographic spiking prior to the onset of spontaneous seizures (White et al., 2010), clustering of seizure activity (Haut et al., 2002; Williams et al., 2009), suppression of seizures but not interictal spikes by anticonvulsants (Duncan, 1987) and the gradual development of anticonvulsant resistance (Berg, 2008).
We have used the organotypic hippocampal slice culture model to address the relationship between spontaneous seizures and neuronal death. The process of brain slicing creates a worst-case axonal shear injury, as all afferent and efferent axons entering the plane of the slice are severed. Dissection of hippocampal slices causes significant amount of neuronal injury in the slice volume (up to 100 μm thick) closest to the cutting planes (Kirov et al., 1999). Dissection of the slice then represents a very severe trauma with attendant neuronal death and deafferentation. The initial trauma is followed by a recovery period, where counts of dead cells fall rapidly to insignificant levels in healthy cultures (Pozzo Miller et al., 1994). However, after 7–10 DIV, LDH levels and counts of dead (PI-positive) cells in the pyramidal layer and granule cell layer begin to increase again. We hypothesize that this second wave of neuronal death is caused by spontaneous, post-traumatic epileptiform discharges that we have shown to begin at this time in vitro. Slices in which epileptiform activity was blocked by KYNA had dramatically reduced PI counts and increased neuronal density, indicating that glutamatergic activity was necessary for the secondary neuronal death in this preparation, and suggesting that epileptiform activity was inducing the neuronal death. The experiments in which chronic phenytoin exposure provided neuroprotection and anticonvulsant effects for the first four weeks in vitro, followed by loss of seizure control and accelerated neuronal death, further support the hypothesis that ongoing seizure activity causes neuronal death in this preparation. These results support the progression of epileptogenesis as shown in Figure 3 B: initial trauma, modeled by dissection in organotypic cultures, is accompanied by cell death. A latent period with little seizure activity or cell death then occurs approximately from 3 to 7 DIV. However, active epileptogenesis during the latent period culminates in the appearance of interictal and then ictal-like activity after 7 DIV, followed by dramatically increased activity-dependent cell death.
There are many questions regarding ictal neuronal death that can be addressed in future studies using this preparation. The critical temporal pattern of seizure activity that induces neuronal death was not resolved by these studies. Does 15 minutes of seizure activity within 1 hour induce neuronal death? This question will require resolving both the numerator, e.g. the critical amount of seizure activity (15 minutes in this example), and the denominator, that is the amount of time needed for the neuron to recover, which in this example is 1 hour. Similarly, we do not know the type of neurons that die, whether the proportion of principal cells to interneurons is constant in the face of ongoing ictal neuronal death, and whether this ongoing neuronal death contributes to the temporal pattern of seizures observed, including seizure clustering (e.g. Figure 1C, E). All these questions have important implications for clinical epilepsy management.
Neuroprotective agents administered acutely after status epilepticus do not prevent epileptogenesis (Loscher and Brandt, 2010). However, recent experimental data indicate that epileptogenesis develops gradually, and continues to evolve long after the first spontaneous seizure (Williams et al., 2009). Here we demonstrate that reduction of ongoing ictal neuronal death induced by spontaneous seizure activity does not inhibit epileptogenesis. Washout of KYNA from chronically-treated cultures revealed a strongly epileptic network exhibiting ictal-like seizure activity. While this could potentially represent a rebound effect from the washout of glutamate antagonists (Segal, 1994), the development of epilepsy in the continued presence of phenytoin demonstrates that epileptogenesis under these conditions is not a rebound phenomenon. It is important to note that these experiments were not designed to test whether phenytoin had modulatory effects on epileptogenesis. For example, phenytoin might delay the onset of spontaneous seizures, or change the rate of increase in seizure frequency or duration, or even reduce the frequency or severity of seizures in fully-developed epilepsy, although the last outcome is unlikely in light of the late loss of anticonvulsant efficacy. In the undercut cortex model of brain trauma, silencing activity during a critical period after the lesion prevented hyperexcitability (Graber and Prince, 2004). Our results are not directly comparable with those of Graber and Prince, because they did not study epileptogenesis directly in the undercut cortex, but rather the development of hyperexcitability; epilepsy and seizures were not part of their model.
Suppression of interictal spikes did not prevent epileptogenesis in this model. This is contrary to our previous hypothesis in which we proposed that neurons that fire together, wire together just as during development (Staley et al. 2005). The lack of effect of interictal spike suppression in organotypic slices may indicate that this hypothesis is incorrect. Alternatively, the lack of effect of spike suppression may be a consequence of the differences between the slice model and epileptogenesis in vivo. For example, in vitro there are fewer targets available for axon sprouting and subsequent synaptogenesis. In the intact, 3-dimensional nervous system, far more targets are available for axon sprouting, and synapses onto these targets might not result in epilepsy, whereas this is not the case in the organotypic slice preparation. In the absence of nonepileptogenic targets, the role of interictal spikes as a guidance mechanism may be minimal. While the degree of deafferentation in this preparation is more severe than seen in the brain as a whole after traumatic brain injury, local areas of diffuse axonal injury can be as severe as in the organotypic slice (Warner et al., 2010). Further, the incidence of epilepsy in the most severely injured humans approaches 80% (Jennett et al., 1973), which is comparable to our preparation. The degree of the deafferentation induced by slicing is so severe that it may induce robust recurrent sprouting independently of epileptiform activity (Buckmaster, 2004). In other words, it is possible that in this preparation all targets for axon sprouting promote epileptogenesis, and the same might be true for patients with severe brain injury. Although the persistent survival of interneurons in this preparation (Dyhrfjeld-Johnsen et al., 2010) should provide “antiepileptic” targets for axons sprouting from principal cells, there may be a limit to the number of inputs that the surviving interneurons can accept. Standard culture media for organotypic slices are complex mixtures of salts, amino acids and proteins empirically developed to maximize early neuronal survival (Gahwiler et al., 1997). Some of these components might be sufficiently epileptogenic to obscure the antiepileptogenic effects of the blockade of interictal and ictal activity and ictal cell death. Epileptogenic substances such as iron that are included in culture media are also thought to contribute to epileptogenesis after hemorrhagic brain injuries (Pitkanen et al., 2011; Willmore et al., 1978). Thus the results presented here are likely to be most applicable to severe brain injury with pronounced initial neuronal loss and hemorrhage, and may be less applicable to milder head injuries (Annegers et al., 1998; Raymont et al., 2010). The antibiotic gentamicin does not have an effect on epileptogenesis at 30 μg/ml (Grondahl and Langmoen, 1993) at the concentration at which it was added to our culture medium, and is thus unlikely to affect our results.
The late development of seizure activity despite the ongoing presence of phenytoin has also been observed in human posttraumatic epilepsy (Temkin, 2001). One possibility is that ongoing recurrent axon sprouting in the organotypic slice (and injured human brain) eventually create such massively positive feedback loops that anticonvulsant effects are not sufficient to prevent seizures (Esclapez et al., 1999). Alternatively, ongoing interneuron loss might disinhibit the network to the point that anticonvulsants were no longer effective, despite the substantial capacity of surviving interneurons to compensate for such loss (Zhang et al., 2009). However, although chronically applied anticonvulsant became ineffective, the same anticonvulsant was efficacious when applied acutely to slice cultures of the same age as those that began seizing despite chronic phenytoin application. This indicates that the ongoing presence of phenytoin contributed to the loss of anticonvulsant efficacy. This loss of efficacy may reflect homeostatic alterations in the excitability of individual neurons or their connectivity (Houweling et al., 2005), and provides a useful new model for the development of anticonvulsant resistance. It also raises the possibility that anticonvulsant exposure contributes to the development of medical intractability.
Conclusions
Organotypic hippocampal cultures reproduce many of the salient features of severe post-traumatic epilepsy, and are therefore a good model to study epileptogenesis and effects of anticonvulsant and antiepileptogenic drugs in vitro. We found substantial evidence that spontaneous recurrent electrical seizures resulting from initial trauma cause secondary neuronal death. We also found that prevention of spontaneous electrical seizures has a neuroprotective, but not an antiepileptogenic effect. Finally, chronic treatment with phenytoin, while delaying occurrence of spontaneous seizures and attendant neuronal death, resulted in the eventual development of intractable seizures and loss of the neuroprotective effect.
Supplementary Material
Highlights.
Organotypic hippocampal cultures are an in vitro model of epileptogenesis
Model enables longitudinal studies of seizures and cell death
Spontaneous seizures induce ongoing neuronal death
Preventing spontaneous seizures prevents ongoing neuronal death
Blocking seizure activity and neuronal death does not prevent epileptogenesis
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) and Epilepsy Foundation (EFA).
Abbreviations
- MEA
multiple electrode array
- PI
propidium iodide
- KYNA
kynurenic acid
- LDH
lactate dehydrogenase
- DIV
days in vitro
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–4. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Dudek FE. Primary and secondary mechanisms of epileptogenesis in the temporal lobe: there is a before and an after. Epilepsy Curr. 2010;10:118–25. doi: 10.1111/j.1535-7511.2010.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky Y, Sabolek H, Levine JB, Staley KJ, Yarmush ML. Microfluidics and multielectrode array-compatible organotypic slice culture method. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT. The natural history of mesial temporal lobe epilepsy. Curr Opin Neurol. 2008;21:173–8. doi: 10.1097/WCO.0b013e3282f36ccd. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–54. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12:565–72. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–6. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS. Prolonged infusion of tetrodotoxin does not block mossy fiber sprouting in pilocarpine-treated rats. Epilepsia. 2004;45:452–8. doi: 10.1111/j.0013-9580.2004.67103.x. [DOI] [PubMed] [Google Scholar]
- Chatrian G, Bergamini L, Dondey M, Klass D, Lennox-Buchthal M, Peterson I. Glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Chatrian GE. Report of the Committee on Terminology. Proceedings of the General Assembly. The VIIIth International Congress of Electroencephalography and Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol. 1974;37:521–553. [Google Scholar]
- Chen JW, Ruff RL, Eavey R, Wasterlain CG. Posttraumatic epilepsy and treatment. J Rehabil Res Dev. 2009;46:685–96. doi: 10.1682/jrrd.2008.09.0130. [DOI] [PubMed] [Google Scholar]
- Cossart R, Dinocourt C, Hirsch JC, Merchan-Perez A, De Felipe J, Ben-Ari Y, Esclapez M, Bernard C. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nat Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- Cronin J, Dudek FE. Chronic seizures and collateral sprouting of dentate mossy fibers after kainic acid treatment in rats. Brain Res. 1988;474:181–4. doi: 10.1016/0006-8993(88)90681-6. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–95. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–9. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Antiepileptic drugs and the electroencephalogram. Epilepsia. 1987;28:259–66. doi: 10.1111/j.1528-1157.1987.tb04216.x. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Berdichevsky Y, Swiercz W, Sabolek H, Staley KJ. Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis. J Clin Neurophysiol. 2010;27:418–24. doi: 10.1097/WNP.0b013e3181fe0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408:449–60. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fuerst D, Shah J, Shah A, Watson C. Hippocampal sclerosis is a progressive disorder: a longitudinal volumetric MRI study. Ann Neurol. 2003;53:413–6. doi: 10.1002/ana.10509. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–7. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Giblin KA, Blumenfeld H. Is epilepsy a preventable disorder? New evidence from animal models. Neuroscientist. 2010;16:253–75. doi: 10.1177/1073858409354385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber KD, Prince DA. A critical period for prevention of posttraumatic neocortical hyperexcitability in rats. Ann Neurol. 2004;55:860–70. doi: 10.1002/ana.20124. [DOI] [PubMed] [Google Scholar]
- Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–51. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- Grondahl TO, Langmoen IA. Epileptogenic effect of antibiotic drugs. J Neurosurg. 1993;78:938–43. doi: 10.3171/jns.1993.78.6.0938. [DOI] [PubMed] [Google Scholar]
- Haut SR, Swick C, Freeman K, Spencer S. Seizure clustering during epilepsy monitoring. Epilepsia. 2002;43:711–5. doi: 10.1046/j.1528-1157.2002.26401.x. [DOI] [PubMed] [Google Scholar]
- Henshall DC, Schindler CK, So NK, Lan JQ, Meller R, Simon RP. Death-associated protein kinase expression in human temporal lobe epilepsy. Ann Neurol. 2004;55:485–94. doi: 10.1002/ana.20001. [DOI] [PubMed] [Google Scholar]
- Houweling AR, Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex. 2005;15:834–45. doi: 10.1093/cercor/bhh184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. Models and mechanisms of experimental epilepsies. Epilepsia. 2003;44(Suppl 12):44–50. doi: 10.1111/j.0013-9580.2003.12004.x. [DOI] [PubMed] [Google Scholar]
- Jennett B, Teather D, Bennie S. Epilepsy after head injury. Residual risk after varying fit-free intervals since injury. Lancet. 1973;2:652–3. doi: 10.1016/s0140-6736(73)92488-4. [DOI] [PubMed] [Google Scholar]
- Kadam SD, White AM, Staley KJ, Dudek FE. Continuous electroencephalographic monitoring with radio-telemetry in a rat model of perinatal hypoxia-ischemia reveals progressive post-stroke epilepsy. J Neurosci. 30:404–15. doi: 10.1523/JNEUROSCI.4093-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Sorra KE, Harris KM. Slices have more synapses than perfusion-fixed hippocampus from both young and mature rats. J Neurosci. 1999;19:2876–86. doi: 10.1523/JNEUROSCI.19-08-02876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23:2440–52. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–20. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- Laurberg S, Zimmer J. Lesion-induced sprouting of hippocampal mossy fiber collaterals to the fascia dentata in developing and adult rats. J Comp Neurol. 1981;200:433–59. doi: 10.1002/cne.902000310. [DOI] [PubMed] [Google Scholar]
- Loscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein DH, Bleck T, Macdonald RL. It’s time to revise the definition of status epilepticus. Epilepsia. 1999;40:120–2. doi: 10.1111/j.1528-1157.1999.tb02000.x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Boden P, Hill RG. Rat hippocampal slices ‘in vitro’ display spontaneous epileptiform activity following long-term organotypic culture. J Neurosci Methods. 1989;27:35–49. doi: 10.1016/0165-0270(89)90051-4. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog Brain Res. 2002;135:3–11. doi: 10.1016/S0079-6123(02)35003-9. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Brierley JB. Prolonged epileptic seizures in primates. Ischemic cell change and its relation to ictal physiological events. Arch Neurol. 1973;28:10–7. doi: 10.1001/archneur.1973.00490190028002. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Vigouroux RA, Rage P, Brierley JB. Hippocampal lesions produced by prolonged seizures in paralyzed artificially ventilated baboons. Experientia. 1973;29:561–3. doi: 10.1007/BF01926665. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV. Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol. 1995;352:515–34. doi: 10.1002/cne.903520404. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Therapeutic approaches to epileptogenesis--hope on the horizon. Epilepsia. 2010;51(Suppl 3):2–17. doi: 10.1111/j.1528-1167.2010.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Bolkvadze T, Immonen R. Anti-epileptogenesis in rodent post-traumatic epilepsy models. Neurosci Lett. 2011 doi: 10.1016/j.neulet.2011.02.033. [DOI] [PubMed] [Google Scholar]
- Pozzo Miller LD, Mahanty NK, Connor JA, Landis DM. Spontaneous pyramidal cell death in organotypic slice cultures from rat hippocampus is prevented by glutamate receptor antagonists. Neuroscience. 1994;63:471–87. doi: 10.1016/0306-4522(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Raymont V, Salazar AM, Lipsky R, Goldman D, Tasick G, Grafman J. Correlates of posttraumatic epilepsy 35 years following combat brain injury. Neurology. 2010;75:224–9. doi: 10.1212/WNL.0b013e3181e8e6d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha LL, Lopez-Meraz ML, Niquet J, Wasterlain CG. Do single seizures cause neuronal death in the human hippocampus? Epilepsy Curr. 2007;7:77–81. doi: 10.1111/j.1535-7511.2007.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, Jabbari B, Vance SC, Grafman J, Amin D, Dillon JD. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 1985;35:1406–14. doi: 10.1212/wnl.35.10.1406. [DOI] [PubMed] [Google Scholar]
- Schierhout G, Roberts I. Anti-epileptic drugs for preventing seizures following acute traumatic brain injury. Cochrane Database Syst Rev. 2001:CD000173. doi: 10.1002/14651858.CD000173. [DOI] [PubMed] [Google Scholar]
- Segal MM. Endogenous bursts underlie seizurelike activity in solitary excitatory hippocampal neurons in microcultures. J Neurophysiol. 1994;72:1874–84. doi: 10.1152/jn.1994.72.4.1874. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Hesdorffer DC. Pediatric status epilepticus: should the diagnostic evaluation change? Neurology. 2010;74:624–5. doi: 10.1212/WNL.0b013e3181d0ce5b. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–6. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. J Neurophysiol. 2001;85:1–9. doi: 10.1152/jn.2001.85.1.1. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–6. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Steward O, Vinsant SL. Collateral projections of cells in the surviving entorhinal area which reinnervate the dentate gyrus of the rat following unilateral entorhinal lesions. Brain Res. 1978;149:216–22. doi: 10.1016/0006-8993(78)90601-7. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–30. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–63. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–24. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–3. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Warner MA, Youn TS, Davis T, Chandra A, Marquez de la Plata C, Moore C, Harper C, Madden CJ, Spence J, McColl R, Devous M, King RD, Diaz-Arrastia R. Regionally selective atrophy after traumatic axonal injury. Arch Neurol. 2010;67:1336–44. doi: 10.1001/archneurol.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Edward Dudek F, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51:371–83. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–66. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–12. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore LJ, Sypert GW, Munson JV, Hurd RW. Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science. 1978;200:1501–3. doi: 10.1126/science.96527. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamawaki R, Wen X, Uhl J, Diaz J, Prince DA, Buckmaster PS. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–56. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.