Abstract
Biochemical and biomechanical extracellular matrix (ECM) cues have recently been shown to play a role in stimulating stem cell differentiation towards several lineages, though how they combine to induce adipogenesis has been less well studied. The objective of this study was to recapitulate both the ECM composition and mechanical properties of adipose tissue in vitro to stimulate adipogenesis of human adipose-derived stem cells (ASCs) in the absence of exogenous adipogenic growth factors and small molecules. Adipose specific ECM biochemical cues have been previously shown to influence adipogenic differentiation; however, the ability of biomechanical cues to promote adipogenesis has been less defined. Decellularized human lipoaspirate was used to functionalize polyacrylamide gels of varying stiffness to allow the cells to interact with adipose-specific ECM components. Culturing ASCs on gels that mimicked the native stiffness of adipose tissue (2 kPa) significantly upregulated adipogenic markers, in the absence of exogenous adipogenic growth factors and small molecules. As substrate stiffness increased, the cells became more spread, lost their rounded morphology, and failed to upregulate adipogenic markers. Together these data imply that as with other lineages, mechanical cues are capable of regulating adipogenesis in ASCs.
Keywords: Adipose tissue engineering, Stem cell, Cell morphology, Soft tissue biomechanics
1. Introduction
Adipose tissue engineering has recently emerged as a growing field within regenerative medicine that strives to replace lost adipose tissue due to severe burns or breast tumor resection. Unfortunately, materials currently available for adipose replacement do not mimic native adipose tissue and thus offer few cues for natural regeneration, and as such, many of these synthetically-derived materials are either encapsulated in fibrous tissue or fail to integrate with the surrounding tissue [1]. Biologically-based materials, such as hyaluronan and collagen, typically interact well with the surrounding tissue but offer few cues for adipose-specific regeneration and therefore are gradually broken down and cleared by the body with no positive remodeling [2]. Thus there remains a significant clinical need to develop materials for the treatment of burns or tumor resection that positively interact with the surrounding tissue to not only fill the void left by the damaged tissue, but also facilitate the natural remodeling and regeneration of adipose tissue.
Clinicians have begun to address this issue by offering a procedure termed “lipotransfer”, or the direct implantation of liposuctioned adipose tissue back into the subcutaneous space of a different area of the body [3, 4]. The variable outcomes of these procedures have led the field to begin mixing lipotransfer with autologous, adipose-derived adult stem cells (ASCs). These multipotent cells have been shown to possess the ability to differentiate down multiple lineages, including adipogenic, chondrogenic, osteogenic, and myogenic pathways [5]. The success of ASCs within a lipotransfer graft has been partly attributed to their ability to both encourage neovascularization and also produce new adipocytes within the implant [6]. However, adipogenic differentiation of ASCs in vitro has occurred predominantly via soluble factors within the culture media, which typically include insulin, dexamethasone and various other steroids [7]. Chemically-based differentiation protocols provide a reductionist approach to pinpoint the effect of specific induction pathways, but they likely stimulate few of the pathways utilized in vivo when ASCs differentiate. On the other hand, lipotransfer procedures with ASCs are relatively uncontrolled and outcomes can greatly vary between clinicians. Identification of materials, which could encourage ASC maturation prior to implantation to produce a more uniform population of ‘primed’ cells or which could be implanted with ASCs to guide their maturation, would improve retention and standardize outcomes of adipose defect treatments.
Because of the inability of current materials to offer encouragement for natural fat regeneration, many investigators have begun to turn to natural alternatives that better recapitulate the extracellular cues present within adipose tissue. The process of decellularization offers the possibility of isolating tissue specific extracellular matrix (ECM) components by removing the cellular content from whole organs or tissues. This produces a material composed of various proteins and proteoglycans characteristic of the tissue of interest, but devoid of the immunogenic cellular components [8]. These ECM–based materials have already been seen to stimulate stem cell differentiation for a variety of organs and facilitate constructive remodeling in vivo [9–15]. In particular, several structural proteins, such as collagen VI, have been shown to have a dramatic impact on adipose tissue metabolic function and adipocyte physiology [16]. Recently, several groups have published methods for decellularizing human adipose tissue. Adipose ECM can be prepared in a variety of forms, from solid scaffolds to injectable gels and powders, and encourages adipogenic differentiation of ASCs [17–22]. While these materials mimic the ECM composition of adipose tissue, ECM mechanical cues are also a major contributor to stem cell differentiation. Biomechanical cues can stimulate mesenchymal stem cell differentiation toward a variety of cell lineages by mimicking the stiffness of the desired tissue [23, 24]. For example, a substrate that mimics the stiffness of healthy muscle or bone encourages ASCs to express myogenic or osteogenic transcription factors, respectively, in the absence of specific exogenous growth factors [24]. ECM stiffness can even regulate differentiation in the presence of a mixture of soluble factors for osteogenic and adipogenic differentiation; softer substrates encourage bone marrow-derived mesenchymal stem cells to favor adipogenesis despite the presence of osteogenic inductive factors while stiffer substrates favor osteogenesis despite the presence of adipogenic factors [25].
These data suggest the important regulatory potential for matrix-based cues, which could be used in vitro to differentiate ASCs prior to their introduction with other tissue-engineered products in a reconstructive therapy. Thus our goal for this study was to recreate the adipose microenvironment in vitro by combining both biochemical and biomechanical adipose-specific cues to stimulate adipogenesis of ASCs. By recapitulating both the stiffness and ECM composition of adipose tissue, we can present natural stimuli to the ASCs to encourage adipogenic differentiation. Furthermore, identification of an “adipogenic stiffness” could provide an important design criteria for the future development of materials meant to encourage adipogenesis of ASCs.
2. Materials and Methods
2.1. Isolation of adipose stem cells
Human adipose tissue was collected from patients undergoing elective liposuction surgery at the Ranch & Coast Plastic Surgery Clinic (Del Mar, CA). All procedures involving tissue from human patients were reviewed and approved by the UCSD Institutional Review Board. Tissue was collected from seven female patients ranging in age from 34–52, with an average age of 42 ± 6. Human adipose-derived adult stem cells (ASCs) were isolated from the lipoaspirate following a previously established protocol [26]. Briefly, the lipoaspirate was digested for 20 minutes in 0.075% collagenase I (Worthington Biochemical) at 37°C. The resulting suspension was centrifuged at 5000 x g to obtain an ASC-rich pellet. The cell pellet was resuspended in 160 mM ammonium chloride buffer to lyse red blood cells and again centrifuged at 5000 x g. The new cell pellet was resuspended in growth media (DMEM/F12 plus 10% Fetal Bovine Serum, 100 I.U. penicillin, and 100 μg/mL streptomycin) and passed through a 40 μm cell strainer. The remaining cells were plated on standard tissue culture plastic overnight at 37°C and 5% CO2. After 24 hours, the non-adherent cells were removed with two rinses with 1x PBS, and then serially passaged at 70% confluence. Growth media was changed every 3–4 days. The remaining lipoaspirate was frozen at −80°C until further processing.
2.2. Adipose decellularization
Human liposuctioned adipose tissue was decellularized following our previously published protocol to isolate adipose extracellular matrix [17]. Briefly, the tissue was rinsed in 1x phosphate-buffered saline (PBS) to remove blood and free lipids. The cellular content of the tissue was then removed by rinsing with 1% sodium dodecyl sulfate (SDS). Residual SDS was removed by brief washes in DI water and then 0.01% Triton X–100. The tissue was then delipidized using a solution of 2.5 mM sodium deoxycholate with a 1:1 ratio of lipase to colipase enzymes (all chemicals were obtained from Sigma-Aldrich unless otherwise noted). The remaining white tissue was then rinsed overnight in DI water followed by a brief rinse with isopropanol. The resulting decellularized adipose ECM was then milled at room temperature using a Wiley mini-mill to produce a fine powder. To facilitate use in cell culture, the adipose ECM was reduced to a liquid form by digesting in a solution of 0.1M HCl and 1 mg/mL pepsin as previously described [17]. After digestion, the liquid adipose matrix was brought to physiological pH with sodium hydroxide on ice, then diluted to 100 μg/mL in 50 mM HEPES buffer at pH 8.5.
2.3. Fabrication of polyacrylamide gels
Polyacrylamide gels were produced using a constant 8% acrylamide solution. Glass coverslips were first activated by treating them for 10 minutes in a UV/Ozone ProCleaner (Bioforce Nanosciences) and then functionalized with 3-(Trimethoxysilyl)propyl methacrylate (20.3 mM in ethanol, 3 min). Coverslips were briefly rinsed with 100% ethanol and water and then dried. Solutions of 8% acrylamide and varying percentages of bis-acrylamide (Fisher BioReagents) were diluted in 1x PBS and the reaction was catalyzed by adding 10% ammonium persulfate and 1/1000 volume of N,N,N′,N′-tetramethylethylenediamine. This solution was then sandwiched between the methacrylate-functionalized coverslip and a DCDMS-coated glass slide. After reacting for 30 minutes, the polyacrylamide gels were rinsed three times with 1x PBS and allowed to hydrate in PBS overnight. Gels were then sterilized in a tissue culture hood by exposure to 254 nm, 30 W UV light for 4 hours. For protein attachment, the surface of the polyacrylamide gel was reacted with the photoactivated, bi-functional crosslinker Sulfosuccinimidyl 6-(4′–azido–2′-nitrophenylamino)hexanoate (sulfo-SANPAH. Pierce, 0.2 mg/mL in 50 mM HEPES pH 8.5) under 365 nm UV light with a surface intensity of 0.85 mW/cm2 for 10 minutes. Solutions of 100 μg/mL adipose matrix, in 50 mM HEPES buffer at pH 8.5, were added on top of the gels and allowed to attach for 1 hour at 37°C. To confirm protein attachment, adipose matrix was first labeled with sulfo-NHS-functionalized biotin (Pierce). After attaching the adipose matrix, the polyacrylamide gels were then reacted with neutravidin-horseradish peroxidase complex and visualized with 3,3′–Diaminobenzidine (DAB) substrate kit (Pierce).
2.4. Atomic force microscopy
Polyacrylamide gel stiffness was confirmed by atomic force microscopy (AFM; MFP3D, Asylum Research) as detailed previously [27]. Briefly, a pyramidal probe, 0.08 N/m force constant with a 35° half angle (PNP–TR–SPL, Nanoworld), was used to indent each gel in triplicate over 5 random regions of the gel to assess heterogeneity. Probe indentation velocity was fixed at 2 μm/s with the trigger force of 2 nN. Elastic moduli were determined by the Sneddon cone model with a sample Poisson ratio of 0.5 fit over a range of 10%–90% indentation force. Custom software written in Igor pro 6.22 was applied to analyze elastic modulus via Sneddon’s model [28]. Four polyacrylamide gels of each bis-acrylamide percentage were measured to determine the average elastic modulus for that group.
2.5. Cell culture
For media differentiation studies, the liquid adipose matrix was added to tissue culture polystyrene wells at a concentration of 100 μg/mL and incubated at 37°C for 1 hour to allow protein adsorption, as previously described [17]. Human ASCs were then seeded in the functionalized wells in growth media at a density of 1 x 104 cells/mL and allowed to attach overnight. The next day, the media was aspirated and either fresh growth media was added, or adipogenic media (AM: DMEM/F12 plus 10% FBS, 500 μM isobutylmethyl xanthine, 1 μM dexamethasone, 10 μg/mL insulin, 200 μM indomethacin, 100 I.U. penicillin, and 100 μg/mL streptomycin). Each respective media was then changed every 2 days until completion of the study. At 48 hours after cell seeding, separate gels were stained with a Live/Dead kit (Life Technologies) to confirm cell viability, following the manufacturer’s protocol (see Supplementary Data). For stiffness differentiation studies, human ASCs were seeded in growth media on top of adipose matrix-functionalized polyacrylamide gels in a 24-well plate at a density of 1 x 104 cells per well and allowed to attach overnight. The next day the media was aspirated and either fresh growth media was added, or growth media supplemented with 0.25 μg/mL cytochalasin-D. Each respective media was then changed every day until completion of the study. All ASCs used in this study were between passages 1 and 3, and cells from at least 3 different patients were used to repeat each study. Brightfield images of the cells were taken every 2 days using a Nikon Eclipse TS100 microscope fitted with an Infinity 2 camera.
2.6. Staining
Oil Red O staining was used to visualize adipogenic differentiation. Cells were rinsed twice with 1x PBS and then fixed for 1 hour in 4% paraformaldehyde. Oil Red O was dissolved at 5 mg/mL in isopropanol and then mixed 3:2 with water to create an Oil Red O working solution. Cells were stained for 1 hour in Oil Red O working solution, and then rinsed once with 60% isopropanol and multiple washes with water. Cells cultured on tissue culture plastic were also counterstained with hematoxylin to improve visualization. Images of the cells were taken using a Nikon Eclipse TS100 microscope fitted with an Infinity 2 camera.
Cytoskeletal organization within the cells was identified using a fluorescent stain for F-actin. Cells were fixed in 4% paraformaldehyde for 10 minutes, then incubated in staining buffer (1% bovine serum albumin and 0.3% Triton X–100 in PBS) for 30 minutes to block non-specific binding. AlexaFluor 488 phalloidin was diluted 1:40 in staining buffer and added to the cells for 20 minutes. Nuclei were stained with Hoechst 33342 for 10 minutes. After rinsing, the cells were imaged on a Carl Zeiss Observer D1.
2.7. Image analysis
Brightfield images of the cells were taken every 2 days using a Nikon Eclipse TS100 microscope fitted with an Infinity 2 camera. Multiple images were taken in random locations within each well from 4 to 6 wells of each experimental condition, for a total of 12–18 images. All cells that completely fit within the field of view were analyzed. AxioVision software was used to quantitatively analyze the cells. For cell area measurements, a perimeter was drawn around the edges of each cell and the area calculated by AxioVision software. For aspect ratio calculations, the longest pair of two perpendicular lines that crossed at the nucleus were drawn. The aspect ratio was then calculated as the length of the longest line in the pair divided by the length of the shortest line.
2.8. Real Time Polymerase Chain Reaction
Gene expression was analyzed using real time polymerase chain reaction (RT–PCR). Total RNA was isolated from cells using the Qiagen RNEasy kit, following the manufacturer’s protocol. Extracted RNA was quantified using a Thermo Scientific NanoDrop 2000c. Superscript III reverse transcriptase (Life Technologies) was used to convert the RNA template into cDNA, following the manufacturer’s protocol. The reaction was conducted with an Applied Biosystems 2720 Thermal Cycler with the following temperature profile: 65°C for 5 minutes, 25°C for 5 minutes, 50°C for 50 minutes, and 70°C for 15 minutes. For PCR, cDNA was combined with SYBR Green Master Mix (Life Technologies) and 2 μM of forward and reverse primers. Three different adipogenic genes were used to assess differentiation: peroxisome proliferator-activated receptor gamma (PPARγ), C/CAAT enhancer binding protein alpha (CEBPα), and fatty acid binding protein 4 (aP2) along with the housekeeping gene gluteraldehyde phosphate dehydrogenase (GAPDH). Primer sequences are listed in Supplementary Table 1. The reaction was conducted using an Applied Biosystems 7900 HT with the following conditions: 50°C for 2 minutes, 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds followed by 1 minute at 60°C. All experimental conditions and primers were included on the same PCR plate for each repetition of the experiment. Applied Biosystems SDS 2.3 software was used to analyze amplification curves. Gene expression was first normalized to GAPDH, then to the control group (cells grown on standard tissue culture plastic in growth media), and plotted as fold change.
2.9. Statistical analysis
All data is presented as the mean ± standard error of the mean. PCR data was performed with technical duplicates and experimental triplicates. Statistical significance was assessed with Prism software, with a p-value < 0.05 considered significant. A student’s t-test was used for media differentiation studies and a one-way analysis of variance (ANOVA) for all others. A Dunnett post hoc analysis was performed for PCR data, using ASCs cultured on tissue culture plastic in only growth media as a control, and a Tukey post hoc analysis was performed for all AFM and imaging data.
3. Results
3.1. Cytoskeletal reorganization during differentiation
Preadipocytes rearrange their microfilaments during maturation to accommodate the accumulation of lipids within growing lipid vacuoles [29, 30]. In particular, a decrease in cytoskeletal protein biosynthesis and adoption of a rounded morphology have been identified during murine preadipocyte adipogenesis [31, 32]. To investigate this phenomenon with human ASCs, the cells were cultured on tissue culture plastic in either standard growth media or adipogenic media for 14 days, and morphological changes associated with adipogenesis were quantified. ASCs cultured in growth media maintained an elongated, fibroblast-like shape, characteristic of naïve ASCs (Figure 1A). However, when cultured in adipogenic media the ASCs immediately began changing shape and within just a few days had adopted a more contracted morphology (Figure 1B). By 7 days, the cells in adipogenic media still maintained the same overall area as those cultured in growth media, but had lost their characteristically elongated shape and adopted a significantly reduced cellular aspect ratio (Figure 1C,D). These morphological changes were consistent through day 14, when lipid vacuoles had developed within the intracellular space of ASCs cultured in adipogenic media. No lipid vacuoles were present in any cell cultured in growth media on tissue culture plastic. These results indicate that lipid accumulation within differentiating ASCs is preceded by a significant reduction in cellular aspect ratio, changing from an elongated shape to a more rounded morphology, despite maintenance of cell area.
Figure 1. Morphological Changes Associated with Adipogenic Media.
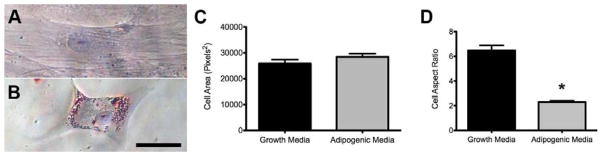
ASCs that were cultured in growth media (A) exhibited no lipid development compared to the significant lipid accumulation noted in cells cultured in adipogenic media (B) at Day 14. While no changes in cellular area were noted between the two experimental groups (C), the cells in adipogenic media did show a significantly reduced cell aspect ratio compared to those in growth media (D). Scale bar = 50 μm. * indicates p < 0.001 using a t-test to compare cells in growth media to those in adipogenic media.
3.2. Modulating cell morphology and differentiation via substrate stiffness
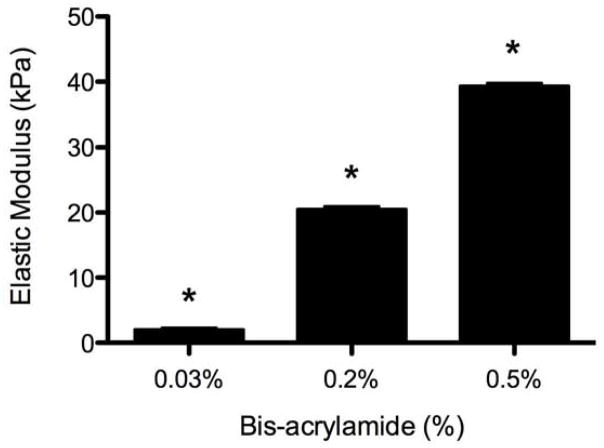
To determine if mimicking biomechanical properties of adipose tissue in vitro could induce adipogenesis in the same manner as with adipogenic media, we cultured ASCs on polyacrylamide gel substrates, which can vary stiffness over several orders of magnitude [24, 27], in the absence of chemical induction factors. Human adipose tissue has been shown to have a Young’s modulus of roughly 2 kPa [33], and thus we fabricated polyacrylamide gels using a constant 8% acrylamide monomer solution with varying bis-acrylamide monomer ranging from 0.03% to 0.5% to produce gel substrates with a stiffness range of 2 to 40 kPa (Figure 2) that remained constant across the surface of each gel. Since previous studies have shown the importance of adipose specific ECM cues, these gels were then functionalized with adipose matrix to further recapitulate the adipose microenvironment [18, 19]. After functionalization, ASCs readily adhered to the polyacrylamide gel surface (Supplementary Figure 1).
Figure 2. Polyacrylamide Gels of Varying Stiffness.
Polyacryalamide gels were produced by mixing 8% acrylamide monomer with varying concentrations of bis-acrylamide. AFM analysis revealed that the gels exhibited significantly increased elastic moduli as bis-acyrlamide concentration increased. * indicates p < 0.001 compared to all other concentrations.
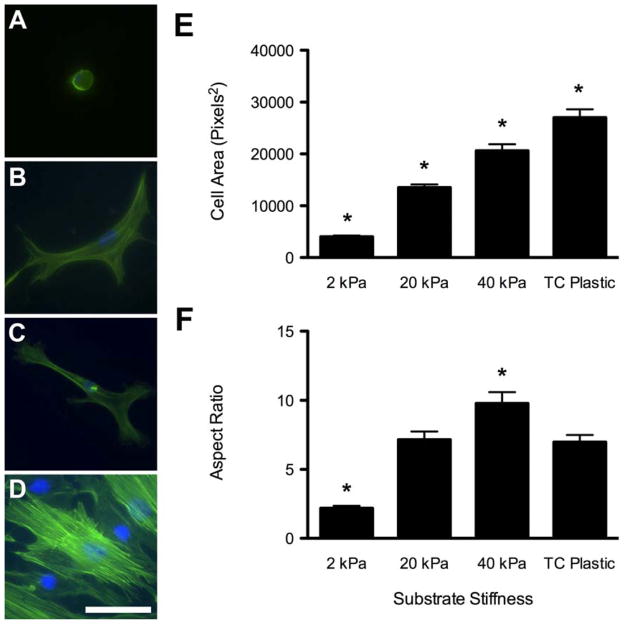
Within 24 hours of seeding, ASCs adopted drastically different morphologies that were sustained throughout the study. Cells on the soft 2 kPa gels, which mimicked the stiffness of adipose tissue, maintained a compact, rounded shape (Figure 3A). ASCs on 20 and 40 kPa gels and on tissue culture plastic demonstrated increasing levels of cellular spreading (Figure 3B–D). Each increase in substrate stiffness resulted in a significant increase in average cell area, with the ASCs cultured on tissue culture plastic having the largest area (Figure 3E). Cell aspect ratio followed a similar trend for cells on the polyacrylamide gels. The rounded shape of the ASCs on the 2 kPa gels had a significantly smaller aspect ratio than all other groups (Figure 3F). The ASCs cultured on standard tissue culture plastic, however, had a slightly smaller aspect ratio than ASCs on 40 kPa gels. These morphological differences at 24 hours were maintained throughout the 6 days of the study, at which point the cells were stained with Oil Red O to examine differentiation. Multiple cells, in each field of view using a 20x objective, stained positively for lipid vacuoles on 2 kPa gels, compared to no evidence of positive staining in any of the other groups (Figure 4A–D). This suggested adipogenic differentiation of the cells on the substrate that mimicked adipose stiffness, even in the absence of media additives. These findings were further supported by quantitative RT–PCR analysis of PPARγ, C/EBPα, and aP2 gene expression, which are early, mid, and later markers of adipogenesis, respectively (Figure 4E). ASCs cultured on 2 kPa gels in growth media exhibited significant upregulation of adipogenic markers compared to cells cultured on tissue culture plastic. Though ASCs cultured on 20 kPa gels also showed significant upregulation of C/EBPα expression compared to control cells, no other adipogenic markers were upregulated. Furthermore, gene expression for ASCs on 40 kPa gels was not statistically different from those in the control wells. These results imply that human ASCs can be directed to differentiate towards an adipogenic lineage by mechanical stimulus alone. However, it does not identify whether this encouragement was a result of just the underlying substrate stiffness or the impact of that substrate on cellular morphology.
Figure 3. Morphological Changes Associated with ASCs Cultured on Polyacrylamide Gels.
ASCs were attached and viable on polyacrylamide gels with stiffnesses ranging from 2 kPa (A), 20 kPa (B), and 40 kPa (C), and compared to those on standard tissue culture plastic (D). When cultured in growth media, the ASCs displayed increasing cell spreading as the substrate stiffness increased (A–D, Green = F-actin, Blue = nuclei). Accordingly, cell area increased with increasing stiffness (E). Cell aspect ratio also increased with substrate stiffness, with ASCs on 40 kPa gels having the largest aspect ratio and ASCs on 2 kPa gels having a significantly reduced aspect ratio (F). Scale bar = 50 μm. * indicates p < 0.01 compared to all other substrates.
Figure 4. Soft Substrates Encourage Adipogenesis of ASCs.
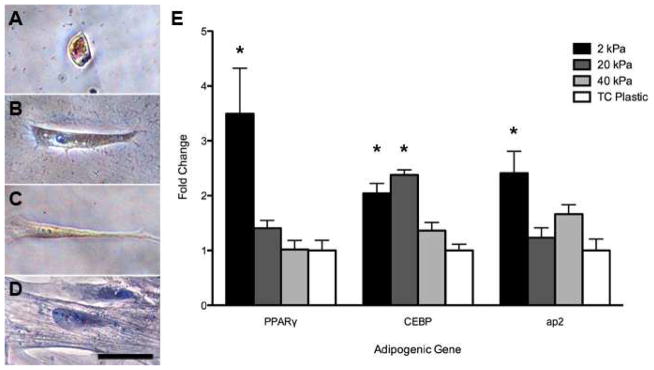
ASCs cultured in growth media for 6 days on 2 kPa gels (A) exhibited positive lipid accumulation, as identified by the red stain Oil Red O, compared to no staining observed for cells cultured on 20 kPa gels (B), 40 kPa gels (C), or tissue culture plastic (D). Examination of gene expression via PCR confirmed that ASCs on 2 kPa gels indeed expressed significantly upregulated levels of the adipogenic markers PPARγ, CEBPα, and aP2 compared to cells cultured on tissue culture plastic. Scale bar = 50 μm. * indicates p < 0.05 compared to tissue culture plastic control group.
3.3. Substrate stiffness stimulates adipogenesis by reducing cellular aspect ratio
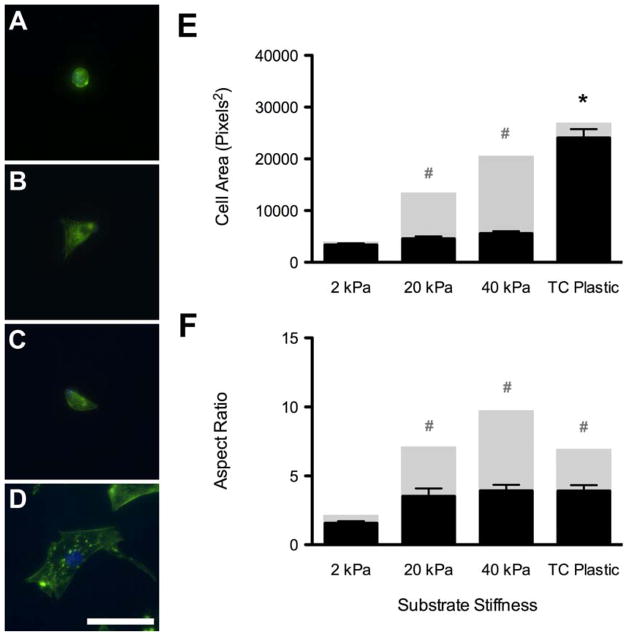
To determine the extent to which cytoskeletal-mediated morphology regulates adipogenesis, we supplemented growth media with cytochalasin–D, which inhibits actin polymerization [32]. ASCs were first allowed to attach to polyacrylamide gel substrates in growth media, and were then cultured in cytochalasin-supplemented growth media for 6 days. Within 6 hours of adding the cytochalasin-supplemented growth media, the cells on all polyacrylamide gels adopted a more rounded morphology, regardless of the underlying stiffness (Figure 5A–C). Even cells that were cultured on tissue culture plastic began retracting their extensions (Figure 5D). After 6 days in the cytochalasin-supplemented growth media, the actin filaments of all cells exhibited punctuated intracellular staining, in contrast to the bright filaments seen in cells cultured with normal growth media (Figure 3). Inspecting cell morphology also revealed that addition of cytochalasin-D significantly reduced the cellular area and aspect ratio of cells on 20 kPa and 40 kPa gels compared to the same gels in normal growth media (Figure 5E,F). However, cytochalasin-treated cells on tissue culture plastic exhibited a reduction in aspect ratio but no change in cell area (Figure 5E,F). Oil Red O staining of these cells suggested the cytochalasin treatment also caused an increase in adipogenesis for all of the cells. Positive lipid vacuoles were identified in cells from all groups that received the cytochalasin-supplemented growth media (Figure 6A–D). Quantitative RT–PCR analysis showed induction of PPARγ, C/EBPα, and aP2 expression for cytochalasin-treated cells compared to ASCs cultured in normal growth media on tissue culture plastic. Cytochalasin-treated ASCs on 2 kPa gels showed further upregulation of both PPARγ and aP2 compared to all other substrates (Figure 6E). Collectively, this data supports the assertion that mimicking the soft stiffness of adipose tissue can induce the adipogenic differention of adult stem cells through restricting the aspect ratio of the cell.
Figure 5. Effect of Cytochalasin-D on Cell Morphology.
Addition of the actin polymerization inhibitor, cytochalasin–D, caused the cells to adopt a more rounded morphology on 2 kPa (A), 20 kPa (B), and 40 kPa (C) gels and tissue culture plastic (D), and disrupted intracellular actin filaments (A–D, Green = F-actin, Blue = nuclei). Disrupting these actin filaments with cytochalasin–D resulted in significantly reduced cellular area for cells on 20 and 40 kPa gels compared to growth media values, but had no effect on ASCs on tissue culture plastic (E; black columns; cytochalasin–D media; grey columns: normal growth media). More importantly, however, it caused a significant reduction in cell aspect ratio for ASCs on 20 and 40 kPa gels and those cultured on standard tissue culture plastic compared to values in growth media (F, black columns; cytochalasin–D media; grey columns: normal growth media). There were no significant differences in aspect ratio between substrates in cytochalasin media. Scale bar = 50 μm. * indicates p < 0.001 compared to other groups in cytochalasin-supplemented media. # indicates p < 0.001 compared to same group in normal growth media.
Figure 6. Decreased Aspect Ratio Stimulates Adipogenesis.
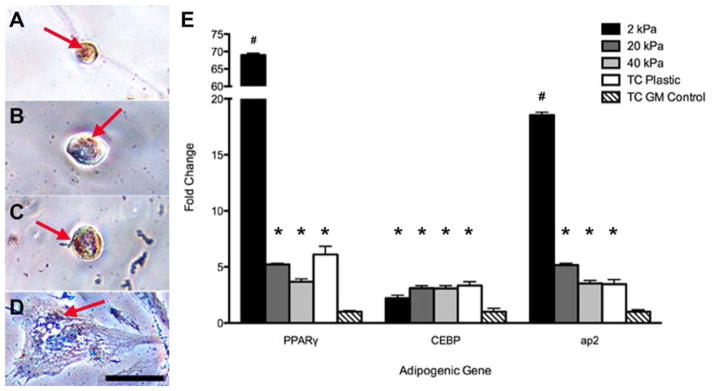
ASCs cultured in cytochalasin–D for 6 days exhibited a more rounded morphology and as a result, stained positively for lipid accumulation via Oil Red O (arrows) regardless of the underlying substrate stiffness of 2 kPa (A), 20 kPa (B), 40 kPa (C), or standard tissue culture plastic (D). Investigating the gene expression also revealed their adipogenic nature, as these cells exhibited significantly higher levels of all three adipogenic markers PPARγ, CEBPα, and aP2 compared to control cells cultured on tissue culture plastic in growth media. Scale bar = 50 μm. * indicates p < 0.05 compared to tissue culture plastic control group; # indicates p < 0.01 compared to all groups.
4. Discussion
The field of tissue engineering has recently emphasized designing materials that closely mimic the specific microenvironment of a damaged tissue in order to stimulate natural regeneration, a trend that has carried over into adipose tissue engineering. Within just the past 4 years, several groups have developed methods to extract ECM components from human adipose tissue. Presenting this complex combination of adipose-specific proteins has already been seen to foster the development of ASCs toward an adipogenic lineage [18, 19, 34]. We hoped to expand on these results by not only recreating the biochemical cues of the adipose microenvironment, but also simulate the biomechanical properties of the tissue as well. Using a polyacrylamide gel system we were able to produce substrates that were both at and above the native stiffness of adipose tissue. We kept the acrylamide monomer concentration constant at 8% to minimize the potential variance of available protein binding sites between the different stiffness gels. As a result, we investigated the influence of three distinct substrate stiffnesses, 2 kPa, 20 kPa, and 40 kPa, on ASC adipogenesis and compared those results to cells cultured on tissue culture plastic, a substantially stiffer substrate and the standard platform for in vitro cell culture.
The substrates used in this study were functionalized with components isolated from decellularized human lipoaspirate to allow the cells to directly interact with adipose ECM components while still sensing the stiffness of the underlying substrate. After just 6 days in this recreated adipose microenvironment, we were able to induce significant differentiation of ASCs, without the need for any chemical additives in the media. When cultured on adipose-relevant 2 kPa substrates, ASCs showed elevated expression of early, mid, and late adipogenic transcription factors, and also began accumulating intracellular lipid vacuoles. It is possible that proteins adsorbed to tissue culture plastic are presented to the cells in a different conformation than those that were chemically attached to the polyacrylamide gels. However, the adipogenic gene expression of cells on the 40 kPa substrates was not significantly different than that of cells cultured on tissue culture plastic, suggesting that the polyacrylamide gel system itself was not responsible for changes in adipogenesis. As seen here, by mimicking the biochemical and biomechanical cues associated with adipose tissue, we were able to provide cues for adipogenic differentiation.
We began to elucidate how ASCs were induced toward an adipogenic lineage by quantifying the morphological changes associated with their differentiation. The immediate consequence of culturing ASCs on softer substrates was a noticeable reduction in cellular area. A recent study by Guvendiren and Burdick detailed a similar observation, where soft substrates facilitated the adoption of a smaller cell area for bone marrow-derived stem cells (MSCs). When cultured with a chemically-modified differentiation media, the MSCs also expressed a higher percentage of adipogenesis than those on stiffer substrates [25]. However, as seen in our studies, simply reducing the cellular area alone did not appear to be the controlling mechanism for inducing adipogenesis. When using adipogenic media, ASCs undergoing adipogenesis maintained a similar cell area to undifferentiated cells, but showed a significantly reduced cellular aspect ratio, indicating that the aspect ratio may be a more significant morphological change associated with adipogenic differentiation. This was further explored in our polyacrylamide gel studies, where the ASCs cultured on 20 kPa and 40 kPa substrates, despite having significantly reduced average cellular area compared to cells on tissue culture plastic, did not express lipid accumulation when cultured in growth media. Accordingly, cells that were on the 20 kPa and 40 kPa substrates had either similar or slightly larger cellular aspect ratios, respectively, compared to cells cultured on tissue culture plastic. Only ASCs that were cultured on the 2 kPa substrates, which demonstrated both significantly reduced cellular area and aspect ratio, exhibited lipid accumulation and upregulated gene expression of all three adipogenic markers. Based on these results, the ASCs either needed to be below a certain threshold cellular area or have a significantly reduced aspect ratio, or both, in order to spontaneously differentiate down an adipogenic lineage.
We further investigated these controlling mechanisms by using an inhibitor of actin polymerization to decouple the mechanical influence on the cells. Adding cytochalasin–D to the growth media encouraged all cells to adopt a more rounded morphology, regardless of the underlying substrate stiffness, and begin accumulating lipids. A similar result has been seen when cytochalasin–D was added to murine preadipocytes by facilitating a more rounded morphology [32]. Strikingly, ASCs on tissue culture plastic only saw a reduction in aspect ratio as a result of the cytochalasin, a similar result to what was seen when adding adipogenic media. These ASCs on tissue culture plastic showed positive lipid staining and upregulated expression of all three adipogenic genes despite having the same cellular area as ASCs that did not receive cytochalasin–D, highlighting that the difference in aspect ratio could be the stimulus for adipogenic differentiation. Recently, a study by Yao et al also suggested that a reduced aspect ratio could encourage adipogenesis of neonatal rat MSCs on micropatterned surfaces [35]. This adds further support to the assertion that reduced aspect ratio appears to be the driving force for mechanically stimulating adipogenesis of ASCs. McBeath et al proposed a possible pathway for adipogenic differentiation of MSCs beginning with the influence of soluble differentiation factors, followed by an inhibition of the RhoA–ROCK pathway. They determined the influence of mechanical forces worked downstream of soluble media additives but could still be overridden through inhibition of RhoA–Kinase (ROCK) and thus decreasing intracellular tension [36]. Our research adds to this model by suggesting that these mechanical forces consist of both a reduction in cellular area and, more importantly, a reduction in cellular aspect ratio in order to minimize cellular tension and drive adipogenic differentiation.
Several studies have indicated that pre-committing ASCs toward an adipogenic lineage may improve function once implanted in vivo [37, 38]. With the increasing use of ASCs within the clinic to augment autologous lipotransfer procedures, the ability to encourage adipogenesis of ASCs prior to implantation without having to use soluble chemical factors could be an advantage. Pre-treating ASCs on an adipose-mimicking substrate could prove to be a translationally-relevant method for priming the cells for adipogenic function once injected into the patient. We have demonstrated here a method for inducing adipogenesis of human-derived ASCs, a potentially autologous cell source, without the use of any chemical additives or steroids. Overall, this study offers insight into the influence of both ECM composition and mechanical properties on adipogenic differentiation of ASCs, as well as suggests that future materials for adipose tissue engineering should emulate adipose biomechanical properties to encourage de novo adipogenesis of ASCs.
5. Conclusions
We have shown here that an in vitro recreation of adipose stiffness can stimulate adipogenesis of human adult stem cells without the use of chemical media additives. Analyzing the morphological changes associated with differentiation revealed that stimulation was not only the result of a reduced cellular area, but more importantly, the restriction of cell aspect ratio. Investigating the materials currently available on the market today for repair of lost adipose tissue reveals a relative paucity of any materials that attempt to mimic the adipose extracellular environment. Consequently, there exists a significant need for the application of engineering principles to the field of adipose tissue engineering in order to design materials that will recreate the adipose microenvironment and thus offer the possibility of encouraging natural adipogenesis of either endogenously recruited or exogenously supplied ASCs.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Paul Chasan and Ranch & Coast Plastic Surgery for providing access to waste adipose tissue. Funding for this project was provided in part by the NIH Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant numbers 1-DP2-OD004309-01 and 1-DP2-OD006460-01. D.A.Y. would also like to acknowledge the support of the NSF Graduate Research Fellowship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast Surg. 2003;27:354–66. doi: 10.1007/s00266-003-3022-1. discussion 67. [DOI] [PubMed] [Google Scholar]
- 2.Young DA, Christman KL. Injectable biomaterials for adipose tissue engineering. Biomedical materials. 2012;7:024104. doi: 10.1088/1748-6041/7/2/024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier J, Glasgold R, Glasgold M. Autologous fat grafting: Long-term evidence of its efficacy in midfacial rejuvenation. Arch Facial Plast Surg. 2009;11:24–8. doi: 10.1001/archfacial.2008.518. [DOI] [PubMed] [Google Scholar]
- 4.Cohen G, Treherne A. Treatment of facial lipoatrophy via autologous fat transfer. J Drugs Dermatol. 2009;8:486–9. [PubMed] [Google Scholar]
- 5.Zuk P, Zhu M, Ashjian P, De Ugarte D, Huang J, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mole Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toledo LS, Mauad R. Fat injection: A 20-year revision. Clin Plast Surg. 2006;33:47–53. vi. doi: 10.1016/j.cps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 8.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Valentin JE, Turner NJ, Gilbert TW, Badylak SF. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–84. doi: 10.1016/j.biomaterials.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: The promise of decellularized matrices. J Card Trans Res. 2010;3:478–86. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–14. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 15.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: A potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16:2017–27. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, et al. Metabolic dysregulation and adipose tissue fibrosis: Role of collagen VI. Mol Cell Biol. 2009;29:1575–91. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Poon CJ, Pereira E, Cotta MV, Sinha S, Palmer JA, Woods AA, Morrison WA, et al. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013;9:5609–20. doi: 10.1016/j.actbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Wu I, Nahas Z, Kimmerling KA, Rosson GD, Elisseeff JH. An injectable adipose matrix for soft-tissue reconstruction. Plast Recon Surg. 2012;129:1247–57. doi: 10.1097/PRS.0b013e31824ec3dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JS, Yang H–J, Kim BS, Kim JD, Kim JY, Yoo B, et al. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2–7. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Turner AEB, Flynn LE. Design and characterization of tissue-specific extracellular matrix-derived microcarriers. Tissue Eng Part C, Methods. 2012;18:186–97. doi: 10.1089/ten.TEC.2011.0246. [DOI] [PubMed] [Google Scholar]
- 23.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 24.Choi YS, Vincent LG, Lee AR, Dobke MK, Engler AJ. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials. 2012;33:2482–91. doi: 10.1016/j.biomaterials.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Comms. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 26.Bernacki S, Wall M, Loboa E. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol. 2008;86:257–78. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 27.Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;Chapter 10(Unit 10.6) doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- 28.Sneddon IN. The relation between load and penetrtion in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- 29.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 30.Kuri-Harcuch W, Wise LS, Green H. Interruption of the adipose conversion of 3t3 cells by biotin deficiency: Differentiation without triglyceride accumulation. Cell. 1978;14:53–9. doi: 10.1016/0092-8674(78)90300-8. [DOI] [PubMed] [Google Scholar]
- 31.Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3t3 adipocytes. Cell. 1982;29:53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- 32.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3t3-adipocytes. Cell. 1983;35:657–66. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 33.Comley K, Fleck NA. A micromechanical model for the young’s modulus of adipose tissue. Int J Solids Struct. 2010;47:2982–90. [Google Scholar]
- 34.Turner AEB, Yu C, Bianco J, Watkins JF, Flynn LE. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials. 2012;33:4490–9. doi: 10.1016/j.biomaterials.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Peng R, Ding J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials. 2013;34:930–9. doi: 10.1016/j.biomaterials.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 36.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 37.Itoi Y, Takatori M, Hyakusoku H, Mizuno H. Comparison of readily available scaffolds for adipose tissue engineering using adipose-derived stem cells. J Plast Reconstr Aesthet Surg. 2010;63:858–64. doi: 10.1016/j.bjps.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 38.Choi YS, Cha SM, Lee YY, Kwon SW, Park CJ, Kim M. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophysical Res Commun. 2006;345:631–7. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.