Abstract
Heart failure following a myocardial infarction continues to be a leading killer in the western world. Currently there are no therapies that effectively prevent or reverse the cardiac damage and negative left ventricular remodeling process that follows a myocardial infarction. Since the heart has limited regenerative capacity, there has been significant effort to develop new therapies that could repair and regenerate the myocardium. While cell transplantation alone was initially studied, more recently tissue engineering strategies using biomaterial scaffolds have been explored. In this review, we cover the different approaches to engineer the myocardium. These include cardiac patches, which are in vitro engineered constructs of functional myocardium, as well as injectable scaffolds that can either encourage endogenous repair and regeneration, or act as vehicles to support delivery of cells and other therapeutics.
Introduction
Several studies have shown that the heart has resident stem cell populations.1-3 These offer some innate regenerative capacity; however, in the adult heart, this is limited and cardiomyocytes lost by disease or injury cannot be efficiently replaced through endogenous repair mechanisms. Instead, fibrosis occurs and non-contractile scar tissue is created, leading to a slow decay of the contractile function of the heart. Although a complex organ such as the heart has many different components, in this review we will focus on the heart muscle, or myocardium, while we point the reader to excellent reviews focusing on tissue engineering approaches for repair of heart valves4 and conduits.5
Currently available treatment options for myocardial infarction (MI) and heart failure include the use of pharmacological agents and left ventricular (LV) assist devices. None of these approaches are regenerative in nature; they temporarily boost contractile function of the remaining cardiomyocytes, thus motivating exploration of alternative approaches through the use of tissue engineered products and biomaterials. The field of tissue engineering was formally defined in the late 1980’s, as “an interdisciplinary field that applies the principles of engineering and life sciences towards the development of biological substitutes that aim to maintain, restore or improve tissue function” 6. According to the original paradigm, functional tissue replacements were engineered using tissue cultivation in bioreactors and starting from cells, ideally autologous cells, and supporting biomaterials. Over past two decades, engineering of almost all of the tissues of the human body was achieved in laboratory settings, including skin, bone, cartilage, liver, cornea, myocardium, blood vessels, lung, gut, etc. The original paradigm was refined, as suitable for the proposed regenerative approach. For example, bioreactor cultivation was omitted if tissue functionality similar to that of the native tissue was not a prerequisite, and the body was used as a bioreactor instead.7 Although the ultimate tissue engineers are the cells themselves, and we simply provide the instructive environment for their function, it was observed that the use of biomaterials alone, tailored to recapitulate the cell niche, can result in regeneration in vivo.8
The classical tissue engineering approach has already resulted in FDA approved products of which a large number of patients benefit each year specifically, skin replacements (Dermagraft, Epicel, Apligraf) and autologous cartilage replacements (Carticel). Although these early success stories point to the importance of developing new therapies based on living cells and biodegradable materials, the long and expensive road through clinical trials leading to these FDA approvals was burdened with financial hardship and bankruptcies of early players in the field (e.g. Advanced Tissue Sciences). Tissue engineered products were also successfully applied in humans as bladder replacements,9 tracheal,10, 11 and bronchial tissues,12 as well as blood vessel substitutes.13
For the myocardium, regenerative strategies initially focused on cell transplantation without the use of biomaterials scaffolds. This approach has advanced to clinical trials; however, substantial improvements in cardiac function have yet to be seen, possibly because of the poor retention and survival of transplanted cells. In this review, we will focus on recent advances in myocardial tissue engineering, including the development of cardiac patches, defined as pieces of living tissues engineered in the laboratory to have functionality approaching that of the native heart tissue, and injectable biomaterials that can be used to attenuate pathological remodeling and drive regeneration or repair of native myocardium.
Cardiac Patches
The ideal end product of a cardiac tissue engineering approach is a functional cardiac patch of clinically relevant thickness (~1cm) to provide replacement for diseased or damaged native ventricular myocardium. Functionality is often assessed by the ability of the patch to generate active force during contraction (20-50 mN/mm2) and propagate electrical impulses (~25cm/s). The ideal cardiac patch should consist of autologous cardiomyocytes, such that minimal immune response is generated upon implantation. To achieve these requirements, different groups manipulate the microenvironment cells interact with in order to facilitate cell assembly and build a functional tissue.
According to the classical tissue engineering paradigm, cells and scaffolds are combined and cultivated in a bioreactor to reach a desired degree of functionality. Pioneering studies performed in the late 1990’s demonstrated that beating cardiac tissue can be created in a laboratory settings 14-16 and that it can be used as a cardiac patch in a rat model of myocardial infarction 17. Most cardiac tissue engineering studies use neonatal rat heart cells as a model system,18 although use of cardiomyocytes derived from human embryonic stem cells (hESC) is emerging.19-21 Both synthetic (polyglycolic acid, poly-L-lactic acid, and polyglycerol sebacate22,23) and natural (alginate,24 collagen,25 and chitosan26) biomaterials were used in scaffolds27 either in porous or fibrous form, or as hydrogels.28 Microfabrication approaches, soft lithography and patterning of synthetic materials were specifically advanced in conjunction with synthetic scaffold materials to develop tissues with a high degree of anisotropy.29-32
The advantages of pre-formed scaffolds are that they offer control of mechanical properties, have no limitations in terms of shape or size, and anisotropic structure of the scaffold can be used to control cell orientation.29 Mechanical properties of either scaffolds or hydrogels for cardiac tissue engineering depend on the ultimate envisioned application. For example, human myocardium ranges in stiffness from 20 kPa at the end of diastole up to 500 kPa at the end of systole, while the stiffness of rat myocardium ranges from 0.1-140 kPa.33-37 Our previous studies with rat heart cells demonstrated that both cardiomyocytes and cardiac fibroblasts maintained an in vivo like phenotype on substrates that matched the stiffness of native rat hearts.51 These studies are in agreement with those of Jacot et al who found that the cardiomyocytes generated highest contractile force when cultivated on substrates that matched the stiffness of the native heart.38 When the properties of hyaluronic acid hydrogels were tailored such that the substrate stiffened with time in culture to match the stiffening of the heart during development, maturation of embryonic chick cardiomyocytes was achieved.39 However, if the material is to be used to simply deliver cells into the heart and recruit cells for endogenous healing, the stiffness of the scaffold can also be at the lower end of the physiological range. If the material is to be used to artificially thicken the ventricle wall, and maintain ventricle geometry during remodeling, then it must be on the high end of the stiffness range.
Some have suggested that pre-formed porous or fibrous scaffolds limit the active force that can be generated by the cells.40 Instead, to maximize on the development of active force, hydrogels that are remodeled by the cells can be used as a support during cardiomyocyte culture.18 In addition, scaffold-free approaches have recently emerged, where cells self-assemble into sheets or circular structures without the use of either natural or synthetic scaffolds.19, 41,42, 43 In an approach pioneered by Okano’s group, cardiomyocyte monolayers are stacked to form a functional tissue. Release of undamaged monolayers was made possible by seeding cardiac cells on poly(N-isopropylacrylamide)-grafted polystyrene dishes and then lowering the temperature to 20°C, thus inducing a hydrophobic/hydrophilic switch of the surface. After transplantation of the cell sheets onto infarcted rat hearts, cardiac performance was significantly improved and successful engraftment was observed.44
In a pioneering approach, Eschenhagen and Zimmermann generated an engineered heart tissue (EHT) by seeding a mix of collagen I, extracellular matrix proteins (Matrigel), and neonatal rat cardiomyocytes into lattices or circular molds. Upon spontaneous remodeling of the liquid reconstitution mixture and cyclic mechanical stimulation, spontaneously and synchronously contracting solid EHTs were generated after one to two weeks of cultivation.45 These studies clearly demonstrated the importance of physical stimuli in improving morphological, functional, and mechanical properties of the EHT. By stacking three of these ring-shaped constructs, consisting of neonatal rat cardiomyocytes, into a flower-like structure followed by auxotonic mechanical stimulation and implantation in a rat MI model, electrophysiological studies indicated electrical coupling with the native tissue and improved diastolic and systolic function compared to the sham-operated rats 18. This landmark study clearly demonstrated for the first time that implantation of EHT can result in functional improvement upon MI.
Alternatively, suprathreshold electrical field stimulation with monophasic pulses can be utilized to induce synchronous contractions of cardiomyocytes in porous collagen scaffolds resulting in the formation of tissue with elongated, viable cells aligned in parallel.46-48 The parameters for electrical stimulation were further optimized in a later study involving the cultivation of cardiac cells on collagen scaffolds.49 Carbon was found to be the ideal electrode material, showing the highest charge-injection capacity and yielding cardiac tissues with the best structural properties and contractile function, compared to stainless steel, titanium nitride-coated titanium and titanium. The use of biphasic pulses improved the functionality of the engineered cardiac tissues as compared to the use of monophasic pulses, the pulse type used in all previous studies.50 This was due to the enhanced cell organization as well as increased cell elongation, cell density, presence of cross-striations and connexin-43 expression. The two phases in the biphasic pulse regime act synergistically, with the first one being the conditioning sub-threshold prepulse and the second being the excitatory pulse.
Adult cardiac muscle is a highly differentiated tissue composed of cardiomyocytes and fibroblasts supported by a dense vasculature, with an average cell density of 1-10 × 108 cells/cm3. The cardiomyocytes form a three-dimensional syncytium that enables propagation of electrical signals and synchronized contraction that results in pumping of blood. Only 20-40% of the cells in the heart are cardiomyocytes, but they occupy 80-90% of the heart volume.51 Early cardiac tissue engineering work therefore focused on using mostly cardiomyocytes purified by several rounds of pre-plating.52 However, it was noted that constructs based on purified cardiomyocytes exhibited limited ability to remodel their microenvironment, consisting either of a synthetic poly(glycerol sebacate) scaffolds23 or natural hydrogels,53 thus resulting in tissues with inferior functional properties. Cardiac fibroblasts constitute most of the non-myocytes in the myocardium and their main role is to secrete the components of the extracellular matrix (ECM).54 Endothelial cells line the blood vessels of the dense myocardial vasculature and engage in cross-talk with cardiomyocytes via numerous secreted factors.55, 56 Given the lack of success with pure cardiomyocyte populations, investigators therefore started introducing fibroblasts back into cardiac cell cultures in 3-dimensional matrices 23, 53 followed by the controlled use of endothelial cells as well, to create primitive capillary sprouts 57-59.
While fibroblasts overgrow cardiomyocytes in monolayer culture, they do not overgrow in three-dimensional constructs. This might be due to the contact inhibition of a three-dimensional matrix, presence of topography that is known to inhibit proliferation60 or appropriate mechanical properties of engineered cardiac tissues. The elastic modulus of the native neonatal and adult rat heart is 6.8kPa and 25.6kPa respectively.61 Even in monolayer culture, fibroblast overgrowth was shown to be absent and elongation of cardiomyocytes was enhanced resulting in the improved contractile properties, when the cells were cultivated on polyacrylamide substrates of intermediate stiffness (22kPa and 50kPa) compared to the substrates with either low stiffness (3kPa) or high stiffness (144kPa). It is now well accepted in the field (Figure 1A), that for optimal functionality of the engineered cardiac tissues and for improved survival upon in vivo implantation, the presence of cardiomyocytes, along with endothelial cells and a mesenchymal cell type (e.g. fibroblast or mesenchymal stem cell) in a cardiac construct is critically required.19-21,62
Figure 1. Pluripotent stem cell derived cardiac patches.
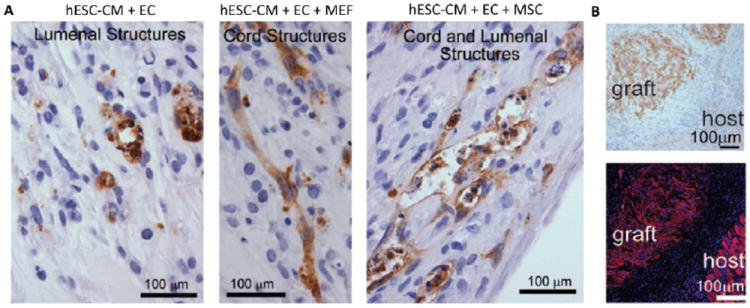
A) Vascularization of cardiac patches based on cardiomyocytes (CM) derived from human embryonic stem cells (hESC) can be enhanced by co-culture with endothelial cells (EC) and either mouse embryonic fibroblasts (MEFs) or mesenchymal stem cells (MSC). B) Human iPSC based cardiac patch can be implanted onto the heart of an athymic rat and persist for one week. Top β-myosin heavy chain staining; bottom, α-actinin, showing the graft–host interface. From Circ Res,62 with permission. CM= cardiomyocytes; EC = endothelial cells; MEFs = mouse embryonic fibroblasts; MSC = mesenchymal stem cell.
These technological advances of recent years enabled generation of tissue with sophisticated architecture and functional properties approaching those of native rat hearts. However, our understanding of the molecular mechanisms orchestrating this functional assembly of cells on different scaffolds and in different bioreactors remains underdeveloped, thus warranting further work. For example, Dvir and Cohen reported that Erk 1/2 cascade was activated in cardiomyocytes cultivated in the presence of interstitial pulsatile flow, leading to physiological cell hypertrophy. The cross-talk between the non-myocytes and cardiomyocytes in engineered heart tissue was also demonstrated to be mediated by secreted VEGF165 that directly acted on cardiomyocytes to enhance the expression of the gap junctional protein connexin-43, thus leading to improved functional properties of the tissue.
Working towards the ideal cardiac patch, one is faced with a critical question of the autologous source of cardiomyocytes. Adult cardiomyocytes are considered to be terminally differentiated and have a limited ability to proliferate, thus they cannot be expanded to sufficient numbers (millions/patient) from cardiac biopsies. Therefore, stem cells must be used as a source of cardiomyocytes. Although bone marrow derived cells, such as mesenchymal stem cells (MSCs), can express cardiac markers by immunostaining and polymerase chain reaction upon treatment with specific cytokines, or in co-culture with differentiated cardiomyocytes, they cannot develop active force during contraction and they cannot exhibit ventricular action potentials.63 Three-dimensional cultivation of MSCs was reported to enhance expression of cardiac markers in these cells compared to the two-dimensional cultures, however development of active force was not reported.64, 65 In the past year, resident cardiac stem cells, either c-kit+ (SCIPIO, ClinicalTrials.gov identifier NCT00474461) or those derived from cardiospheres (CADUCEUS, NCT00893360) demonstrated rather promising functional improvements in Phase I clinical studies as well as the restoration of viable tissue per MRI imaging, presumably due to their ability to give rise to cardiomyocytes in addition to vascular cells.66, 67 However, it is yet to be demonstrated if these cells can give rise to millions of cardiomyocytes that are required for cardiac tissue engineering during in vitro culture.
Pluripotent stem cells such as ESC or induced pluripotent stem cells (iPSC) have the advantage of being able to give rise to bona fide cardiomyocytes and the ability to be expanded to sufficient numbers (millions/patient) using existing technologies. The discovery of human iPSCs68 and the ability to generate cardiomyocytes from them69 offers the possibility to provide unlimited numbers of autologous cardiomyocytes for cell therapy without ethical concerns raised with the use of hESCs. Studies from a number of groups have conclusively shown that it is possible to generate cardiomyocytes from mouse70 and human ESCs71 and iPSCs.69 Cardiac patches based on both hESC and iPSC have already been generated and implanted in rodent models (Figure 1B).62 The most efficient and reproducible protocols to date are those that have replicated the signaling pathways that regulate lineage commitment in the early embryo. With this approach, the earliest stages of cardiovascular development in ESC differentiation cultures were mapped, identifying a multipotent cardiovascular progenitor that displays the capacity to generate cardiac and vascular progeny.70, 71 In mouse Flk1, and in humans KDR expression can be used to enrich for cardiac specified mesoderm.72 When isolated from the differentiated embryoid bodies (EBs) and cultured as a monolayer, these progenitors generate contracting cardiomyocytes.70, 71 As these progenitors differentiate, they progress through the developmental stages thought to be involved in the establishment of the cardiovascular lineages in vivo, for which specific cytokines are required. The combination of activin A and BMP4 on Days 1-4 of EB differentiation induces a primitive-streak-like population and mesoderm development. Subsequent application of WNT inhibitor DKK1 and KDR ligand VEGF165 significantly enhances the differentiation of KDR+ progenitors into cardiomyocytes,70, 71 while bFGF is added to support continued expansion of cardiovascular lineages. These protocols are now being adapted to growth factor-free conditions with the use of small molecules such as glycogen synthase kinase 3 inhibitors and chemical inhibitors of WNT signaling.73 Interestingly, the same protocols can be used to derived cardiomyocytes from parthenogenic cells. It was recently demonstrated that engineered heart muscle can be generated from cardiomyocytes based on mouse parthenogenetic cells and that the engineered muscle can contributed to the contractile function of the infarcted heart of the cell donor (Figure 2).74
Figure 2. Cardiac patches derived from mouse parthenogenetic cells.
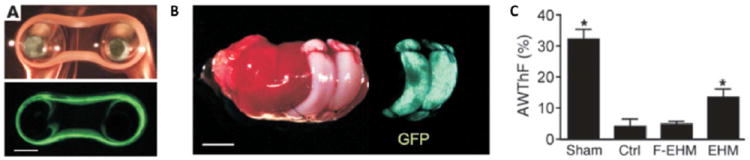
A) Patches were conditioned by mechanical stimulation. B) They were used to repair infarcted myocardium of the mouse donors. C) Patch implantation resulted in the improvement of anterior wall thickening at diastole. From J Clin Invest,74 with permission.
Cardiomyocytes derived by spontaneous or directed differentiation protocols are generally considered immature, thus efforts are under way to develop methods that would enhance maturation state of these cells. Prolonged time in culture, up to 100 days, on stiff substrates (gelatin coated glass cover slips) was reported to enhance maturation of pluripotent stem cell derived cardiomyocytes.75 In addition, electrical stimulation of cells in monolayers76 or mechanical stimulation of cells encapsulated in collagen gels62, 77 was reported to enhance signs of maturation. The use of electrical stimulation in conjunction with stretch as a mimic of cardiac load in cardiac patches might be required to induce terminal differentiation in human pluripotent stem cell derived cardiomyocytes. In addition, incorporation of the native decellularized heart extracellular matrix78, 79 into cardiac patches, may further enhance cell maturation.
Over years, rodent models such as rat or mouse were utilized for studies of integration of cardiac patches in MI models.80-82 These models were often criticized as unsuitable due to the large differences in the resting heart rates between hESC derived cardiomyocytes and rodent ventricular myocytes. Laflamme and colleagues recently conclusively demonstrated through Ca2+ imaging studies that hESC derived cardiomyocytes can electrically couple and suppress arrhythmias in the hearts of guinea pig upon MI induced by cryoinjury.83
The engraftment and survival of transplanted engineered cardiac tissues are limited by the lack of vasculature in the ischemic area. Thus, sophisticated structures created during in vitro electro-mechanical conditioning can rapidly disappear upon implantation. In the context of cardiac tissue engineering, angiogenic scaffolds containing angiogenic growth factors84-87 or precisely designed porous structures88 were used to enhance cell survival and angiogenesis upon implantation. Other strategies for inducing rapid vascularization of cardiac tissues include the modular approach89 and co-culture with endothelial cells and fibroblasts.19-21, 90 In vivo, angiogenesis involves endothelial cell proliferation and sprouting followed by connection of extended cellular processes and subsequent lumen propagation through vacuole fusion. This process was mimicked by engineering an organized capillary network anchored by an artery and a vein.91 The network was generated by inducing directed capillary sprouting from vascular explants on micropatterned polydimethyl siloxane substrates containing a thymosin β4-hydrogel (Figure 3). The capillary outgrowths connected between the parent explants by day 21, a process that was accelerated to 14 days by application of soluble VEGF and hepatocyte growth factor. Cardiac tissues engineered around the resulting vasculature exhibited improved functional properties, cell striations, and cell-cell junctions compared with tissues without prevascularization and allowed for easy network removal. Shimizu and colleagues used a resected vascular bed with connectable artery and vein to vascularize cardiac tissues by stacking cardiac cell sheets around a vascular bed.92 They also demonstrated the tissues could be implanted by direct anastomosis of the vascular bed.
Figure 3. Engineering of vascularized myocardium by the control of topography and presentation of angiogenic growth factors.
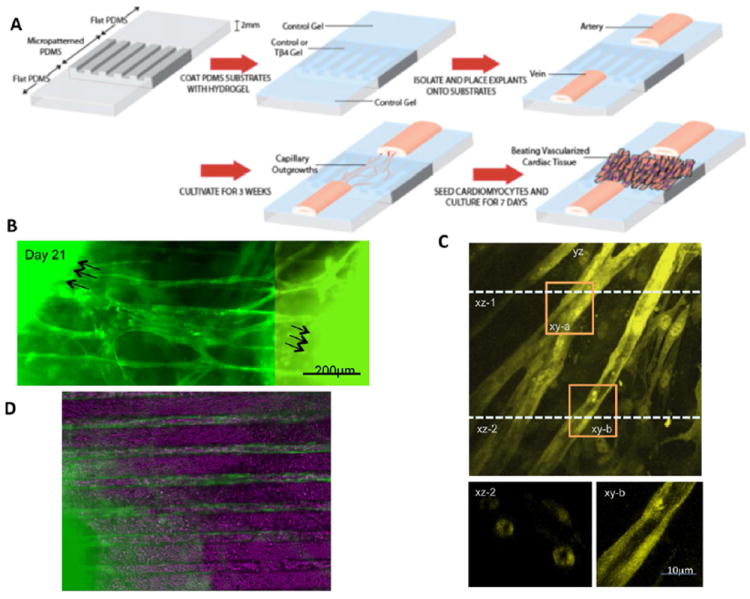
A) A polydimethylsiloxane stamp with topographical cues (grooves and ridges) is coated with collagen:chitosan hydrogel desired for controlled release of an angiogenic factor thymosin b4. The topographical cues guide the outgrowth of capillaries from an artery and vein, resulting in a microvessel bed around which cardiomyocytes are seeded. B) Microvascular outgrowths are connected by Day 21. C) Confocal micrographs indicate the presence of open lumens. D) Vascularized cardiac tissue. From PNAS,91 with permission.
Injectable Scaffolds
While injectable biomaterial scaffolds do not allow for control over cell and biomaterial organization afforded by in vitro engineered and patch based approaches, research in this area has rapidly expanded in recent years.93 One of the main benefits for injectable materials is the potential to be delivered via catheter without the need for a surgical based procedure and general anesthesia. Injectability also allows the material to be delivered directly inside the infarct wall to the site of damage. These injectable materials are typically hydrogels, which are water swollen crosslinked polymer networks derived from either natural or synthetic sources. They are liquid outside the body and then gel into a semi-solid form upon injection in vivo. This gelation can be triggered a number of ways depending on how the hydrogel is crosslinked (i.e. covalent bonds, ionic interactions, or physical entanglements) and its chemical makeup, which can respond to environmental triggers such as temperature and pH.
Similar to patch based approaches, injectable biomaterials were initially explored to improve the poor cell survival seen with the typical cellular cardiomyoplasty technique by delivering cells in a viscous liquid that could prevent ejection from the beating heart and then forming a temporary ECM to prevent anoikis. This concept was first demonstrated with fibrin glue, which is used commercially as a sealant. This dual component material contains thrombin, which enzymatically cleaves fibrinogen into fibrin monomers that subsequently assemble into a fibrous fibrin network. Given that fibrin is the body’s natural scaffold for wound healing and fibrin glue contains residual growth factors, this material has been used as a scaffold for a variety of tissue engineering applications. In a rat MI model, survival of injectable skeletal myoblasts was significantly increased when delivered in fibrin glue.94 This work has been corroborated by several subsequent studies showing increased survival of bone marrow cells,95 marrow-derived cardiac stem cells,96 and adipose-derived stem cells.97, 98 Several other injectable materials have now also been shown to improve survival of other cell types, such neonatal cardiomyocytes, embryonic stem cell and induced pluripotent stem cell derived cardiomyocytes, bone marrow derived mesenchymal stem cells, and adipose derived stem cells. These materials include protein based materials such as Matrigel99, synthetic materials, such as polyethylene glycol (PEG)100 and poly(N-isoproylacrylaminde) (PNIPAAm)101 based polymers and self-assembling peptide nanofibers,102 and even polysaccharides such as chitosan,103 which do not have inherent cell adhesion domains.
While these injectable scaffolds have been repeatedly shown to increase cell survival, one of the intriguing findings from the original fibrin experiment was that the control group, fibrin alone (without cells), also preserved cardiac function post-MI similar to groups that contained cells,104 demonstrating that a material alone could prevent negative LV remodeling and that cells may not be necessary. Since that time, numerous other injectable materials have been shown to preserve or improve various measures of cardiac function in animal models, including alginate, collagen, decellularized tissues, hyaluronic acid (HA), Matrigel, chitosan, keratin, and synthetic materials such as self assembling peptides or polymers containing PEG or PNIPAAm.93, 105-108 Notably, alginate has progressed through small and large animal pre-clinical studies to clinical trials. Leor, Cohen, and colleagues originally tested the calcium crosslinked seaweed derived polysaccharide in rodent models showing that injection of the material one week following MI resulted in increased scar thickness and decreased end-diastolic and systolic areas compared to the saline control as well as preserved cardiac function after 8 weeks as measured by fractional shortening. When injected two months post-MI, alginate increased scar thickness over the 8 week follow-up and improved diastolic properties (E/A ratio) compared to the control, but LV areas and fractional shortening were not statistically different.109 They followed this work with a large animal study that was the first to transition injectable materials from a direct epicardial injection procedure to a catheter-based procedure in a MI model. In a porcine model, they delivered the material via transcoronary infusion (Figure 4), taking advantage of the damaged and permeable infarct vasculature as well as the high calcium concentration of the acute infarct to crosslink the material in place (Figure 5).110 Given the non-thrombogenicity of alginate, this approach did not result in downstream embolization or ischemia. When delivered three to four days post-MI, the material significantly increased myofibroblasts in the infarct, increased infarct thickness, reduced end-systolic and end-diastolic areas, and decreased the E/A ratio compared to the saline control, but there was no significant difference in fractional shortening.110 This work led to the first clinical trial with a catheter-deliverable biomaterial for treating MI (Safety and Feasibility of the Injectable BL-1040 Implant, NCT00557531). This phase 1/2 study looked at the safety of transcoronary infusion of alginate in acute MI patients who had undergone successful revascularization. A subsequent phase 2 clinical trial is currently underway (A Placebo Controlled, Multicenter, Randomized, Double Blind Trial to Evaluate the Safety and Effectiveness of IK-5001 for the Prevention of Remodeling of the Ventricle and Congestive Heart Failure After Acute Myocardial Infarction - Preservation I Trial, NCT01226563), also in revascularized acute MI patients, with a primary outcome measure of LV end-diastolic volume index.
Figure 4. Delivery approaches for injectable biomaterial scaffolds.
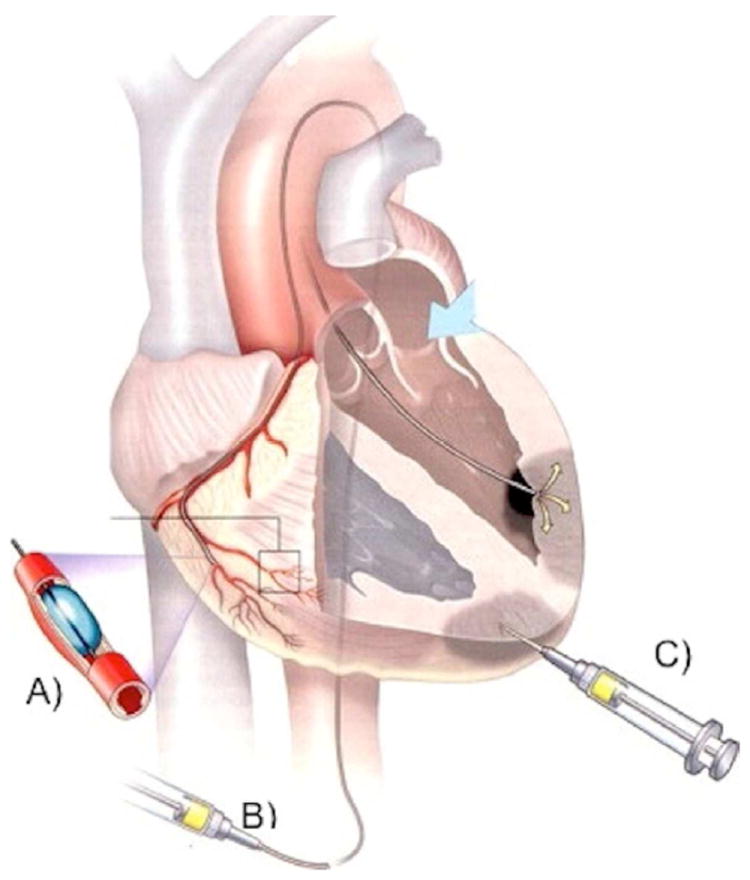
Similar to cell injections, biomaterial scaffolds can be delivered via transcoronary infusion (A), which involves infusion of the material across the leaky coronary vessels acutely post-MI, transendocardial injections (B), which involves catheter placement inside the chamber of the left ventricle and injection across the endocardium, or direct epicardial injection (C), which requires a surgical approach to access the epicardium. Both transendocardial and direct epicardial injection involve puncture with a needle and targeted injection into the infarct area. From Heart Lung Circ,132 with permission.
Figure 5. Transcoronary delivery of alginate.
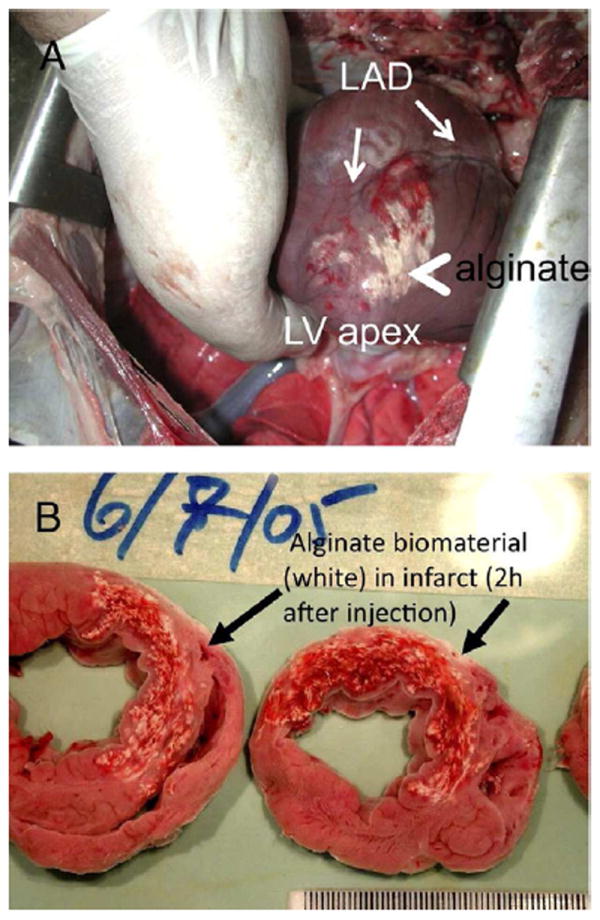
Alginate, which is crosslinked with Ca2+, was delivered via a catheter-based transcoronary infusion in a porcine MI model. Given the leaky vasculature and high extracellular Ca2+ in an acute MI, the material is able to pass through the vasculature and gel once inside the tissue. The white material is visible on the epicardial surface of the infarct (A) and in tissue slices (B). From JACC,110 with permission.
Myocardial matrix, which is hydrogel derived from decellularized cardiac ECM, has also been shown to be deliverable via catheter in a large animal MI model.111 In this case, the materials is delivered via a percutaneous transendocardial approach, which can be a preferred method of catheter delivery112-114 because it increases retention and does not require coronary access, which may be compromised in the relevant patient population. Myocardial matrix is fabricated by first decellularizing porcine myocardium, and then processing it into a liquid form through partial enzymatic digestion. This liquid form then self-assembles into a porous and fibrous scaffold once injected in tissue,115 and degrades by 3 weeks.8 The material was first tested in a rat MI model where delivery 2 weeks post-MI preserved LV volumes and ejection fraction 4 weeks post-injection.111 When the tissue was examined one week post-injection, at a time when the material is still present in the tissue, increased areas of cardiomyocytes surviving within the infarct were found compared to the control; small numbers of c-kit+ stem cells and proliferating cardiomyocytes were also observed within the scaffold. Recently, the myocardial matrix hydrogel was tested following transendocardial delivery in a porcine MI model.8 When injected via catheter two weeks post-MI, the hydrogel significantly improved end-diastolic and end-systolic volumes and both regional and global cardiac function as measured by global wall motion index scores and ejection fraction, respectively. Improvement in end-systolic volume, in particular, was the most pronounced;8 this parameter is known to be the best clinical predictor of survival post-MI.116, 117 This finding may have been the result of an observed increase in cardiac muscle at the endocardium (Figure 6), which was accompanied by foci of neovascularization, and decreased infarct fibrosis compared to controls.8
Figure 6. Increased cardiac muscle using an acellular myocardial matrix hydrogel.
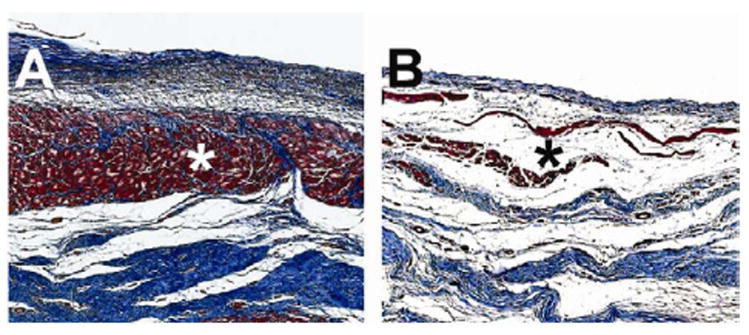
In a porcine myocardial infarction model, percutaneous transendocardial injections of a decellularized myocardial extracellular matrix based hydrogel were performed 2 weeks post-MI. Three months following injection, there was significantly more cardiac muscle (*) found at the endocardium following matrix treatment (A) compared to controls (B). Images are of trichrome stained heart sections, where muscle is stained red and collagen is stained blue. From Science Translational Medicine,8 with permission.
There are numerous other materials, which have been tested in rodent and a few large animal MI models following direct epicardial delivery; these studies have been described in detail in several reviews.93, 105-108 Only a few studies have, however, sought to address the mechanism by which these materials improved cardiac function. Burdick, Gorman, and colleagues have begun to examine how material properties, such as mechanical properties and degradation, play a role using methacrylated HA, which is a naturally derived polysaccharide found in the ECM. They first developed two versions of crosslinked HA, one with a compressive modulus of 43 kPa (MeHA high) and another with a modulus of 7.7 kPa (MeHA low), which is more similar to myocardium.118 In an ovine model, injections were performed 30 min after onset of ischemia, and the animals were followed-up at 2 and 6 weeks post-MI. The stiffer material was able to significantly reduce infarct expansion with trends towards lower LV volumes; ejection fraction and cardiac output were, however, not changed with either hydrogel compared to the control, which received no injection. Histological analysis did not find increased macrophage or myofibroblasts infiltration that has been seen with other materials.118 In a subsequent study, this group further examined MeHA hydrogels that were designed to degrade by functionalizing the HA with hydroxyethyl methacrylate, which contains hydrolytically cleavable ester bonds.119 The new hydrogels matched the initial mechanical properties of low and high MeHA hydrogels from the previous study with degradation times of approximately 3 and 10 weeks, respectively. The hydrogels were again injected immediately post-MI in an ovine model. Eight weeks post-MI, the non-degradable gels were able to provide a greater increase in infarct wall thickness, and the nondegradable stiffer material significantly reduced end-systolic volume. There were, however, no significant differences again among the groups in terms of ejection fraction or cardiac output. Both degradable hydrogels resulted in increased inflammation, while all of the hydrogels increased the density of blood vessels compared to the control, which again received no injection.119
The exact mechanism by which injectable materials improve cardiac function is still under debate. Several studies have suggested that injectable biomaterials act as a mechanical wall support by thickening the infarct wall and reducing wall stress, which should prevent negative LV remodeling.118, 120, 121 A recent study, however, suggests that the bioactivity of injectable biomaterials may be playing a greater role. Rane et al. compared the injection of a non-degradable PEG to saline one week post MI in a rat model. PEG is well known to be inert, and there was no difference in neovascularization or inflammatory response compared to the saline control. Injection of the polymer was able to increase infarct wall thickness, but despite this response, LV volumes continued to expand and ejection fraction continued to decline 6 weeks post-injection. This study indicates that passive wall thickening is insufficient to prevent negative LV remodeling and the decline in cardiac function, suggesting that bioactivity of materials may result in the improvement in cardiac function seen with other injectable biomaterials. Similar results were found by Dobner et al., who injected a non-degradable PEG-vinyl sulfone hydrogel immediately post-MI and found a decrease in end-diastolic diameter at two and four weeks post-MI but not at 13 weeks.122
In addition to cells, injectable materials can also be used to deliver other therapeutics such as growth factors and genes to further improve cardiac function and/or cell survival. By entrapping proteins, genes, or small molecules in a biomaterial scaffold, release is prolonged as the therapeutic diffuses out of the scaffold. The material can be tuned to provide a controlled release my modulating crosslinking, porosity, hydrophobicity, etc. These therapeutics can also be bound to the material, typically through ionic interactions or covalent bonds, which allow release upon material degradation or cell infiltration. In general all of these result in increased efficacy of the therapeutic. Attachment to a material can also increase protein activity, such as with growth factors, which are typically bound to the native ECM. A variety of growth factors, including bFGF, VEGF, PDGF, IGF-1, SDF-1, TGF-β1, and HGF, have been delivered to date. Similarly, plasmids encoding VEGF and PTN (pleiotrophin) have also been delivered in injectable biomaterials to improved transfection efficiency.93 A few of these studies have been performed in more translational large animal models. For example, Hseih et al. recently showed that delivery of VEGF in self-assembling peptide nanofibers prolongs its delivery for 14 days, which resulted in increased fractional shortening in a porcine model. Interestingly, free VEGF also increased capillary density, but delivery in the peptide scaffold significantly increased arteriole and artery density, suggesting that arteriogenesis rather than angiogenesis may be more important for treating MI. To improve cell survival, growth factors as well as a more complex mixture of small molecules were combined with Matrigel in what was termed a pro-survival cocktail.123 This mixture contained several components designed to target different mechanisms by which transplant cell death occurs, and were only effective in improving survival of injected hESC derived cardiomyocytes when combined together.123
Future Directions
The work in cardiac tissue engineering has shown promising results in numerous small animal and a few large animal models. As the field moves forward, it is important to keep clinical translation in mind, which is the ultimate goal for all of this research. One key point to consider in these studies is how the animal model relates to the clinical situation. For example, many studies will apply the treatment, whether a cardiac patch or injectable scaffold, immediately following coronary ligation. This is however unlikely to be clinically relevant given that implantation of either a patch or injectable biomaterial could not occur in this timeframe in patients. It is therefore critical to carefully consider delivery timing in future studies as the immediate infarct is a different environment than the 1 week or 1 month old infarct. This should also be specific to the intended patient population whether acute MI, which is prior to negative LV remodeling and scar tissue formation, or heart failure, at which point a scar has fully formed.
In terms of cardiac patches, the main efforts are focused on selecting an appropriate cell source and promising pre-clinical work in relevant animal models has been demonstrated. For example, it is now conclusively demonstrated that hESC-derived cardiomyocytes delivered into an infarcted heart of a guinea pig, in a hydrogel designed to provide pro-survival factors, can couple with the native myocardium.83 Guinea pig was selected as the resting heart rates were in the upper physiological range of human cardiomyocytes. Human iPSC derived cardiomyocytes were also cultivated as cell sheets and implanted into the infarcted hearts of minipigs. In this model, relatively few human cardiomyocytes persisted and the underlying functional improvements were ascribed to paracrine effects, specifically higher levels of VEGF and bFGF released from the cell sheet group compared to the sham.124 These differences in outcome, point to the importance of a careful selection of the animal model, together with the appropriate immunosupression regime, as well as the careful selection of the pro-survival factors in the delivery matrix.
Regardless, a promising clinical application of a cell sheet-based cardiac patch has been demonstrated. A patient with idiopathic cardiomyopathy, who failed to improve after being placed on a left ventricular assist device for a year, was treated by implantation of cell sheets based on autologous skeletal myoblasts.125 A total of 20 cell sheets were implanted at 5 sites at the patient’s heart, resulting in a recovery of the left ventricular ejection fraction to 50% and removal of the patient from the assist device. It remains to be demonstrated if similar benefits will be observed in larger patient populations.
Going forward, one of the main areas where patch based cardiac tissue engineering can contribute rapidly is drug testing and target validation. Promising micro-tissue based platforms are being developed for this application.126 For example, Eschenhagen and colleagues have screened compounds in a 24-well platform of EHTs derived from hESC derived cardiomyocytes subjected to mechanical stimulation, with force of contraction as a key outcome.77, 127 The ability to obtain iPSCs from patients with different cardiac genetic abnormalities and derive human cardiomyocytes presents a unique opportunity to build cardiac disease models in a dish, in an engineered tissue platform that is amenable to measurements of electrophysiological properties and contractile force. Towards this goal, iPSCs from patients harboring genetic cardiac mutations have been generated and differentiated into cardiomyocytes including cells from Timothy,128 long QT,129 and LEOPARD130 syndrome and dilated cardiomyopathy patients.131 However, these studies were limited by their use of cell monolayers.
In the case of injectable scaffolds, there are also additional issues that should be considered. While all injectable materials can be delivered via a needle and syringe, this does not necessarily translate to cardiac catheter delivery. To be truly minimally invasive, these scaffolds should be capable of delivery via a percutaneous procedure that does not require an incision, surgery, or general anesthesia. For the heart, this is currently through either transcoronary infusion or transendocardial injections (Figure 4). In both cases the material must remain liquid in the catheter, for potentially over an hour, and in the blood stream upon potential leakage, and only gel once inside the intended delivery location. Therefore, many of the quick gelling injectable systems are not compatible with these delivery methods. Both of these approaches also require the material to be hemocompatible as it will leak into the systemic circulation, particularly with the former approach. Ideally, the material will also be biodegradable upon injection, remaining in the heart only during the necessary treatment window. Permanently implantable materials are typically surrounded by a fibrous capsule as a result of the typical wound healing response, and also open up the possibilities for a chronic inflammatory response. Lastly, with injection into the heart, there is always the potential for arrhythmogenesis. While no studies have demonstrated an increase in arrhythmias, this is an ongoing concern that should be carefully monitored.
Conclusions
Overall, significant progress has been made over the past decade and a half with various approaches to cardiac tissue engineering. Both cardiac patches and injectable biomaterial scaffolds have progressed into a few early clinical trials, and it is anticipated that more will follow in the next few years. It is likely that biomaterial only based approaches will reach the clinic faster; however, with continued advances in stem cell biology, achieving a functional contractile patch may be achievable.
Acknowledgments
Radisic lab is supported by funding from the following sources: ORF-GL2 grant, NSERC Strategic Grant (STPGP 381002-09), NSERC-CIHR Collaborative Health Research Grant (CHRPJ 385981-10), NSERC Discovery Grant (RGPIN 326982-10) and Discovery Accelerator Supplement (RGPAS 396125-10). Christman lab is supported by the National Institutes of Health (Common Fund and NHLBI), the Wallace H. Coulter Foundation, and the American Heart Association.
List of Abbreviations
- EBs
embryoid bodies
- EHT
engineered heart tissue
- HA
hyaluronic acid
- hESC
human embryonic stem cells
- iPSC
induced pluripotent stem cells
- LV
left ventricular
- MI
myocardial infarction
- PEG
polyethylene glycol
- PNIPAAm
poly(N-isoproylacrylaminde)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433(7026):647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95(9):911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 4.Schoen FJ. Heart valve tissue engineering: Quo vadis? Curr Opin Biotechnol. 2011;22(5):698–705. doi: 10.1016/j.copbio.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Kurobe H, Maxfield MW, Breuer CK, Shinoka T. Concise review: Tissue-engineered vascular grafts for cardiac surgery: Past, present, and future. Stem Cells Transl Med. 2012;1(7):566–571. doi: 10.5966/sctm.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godbey WT, Atala A. In vitro systems for tissue engineering. Ann N Y Acad Sci. 2002;961:10–26. doi: 10.1111/j.1749-6632.2002.tb03041.x. [DOI] [PubMed] [Google Scholar]
- 7.Stevens MM, Marini RP, Schaefer D, Aronson J, Langer R, Shastri VP. In vivo engineering of organs: The bone bioreactor. Proc Natl Acad Sci U S A. 2005;102(32):11450–11455. doi: 10.1073/pnas.0504705102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, KO L, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach J, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science Translational Medicine. 2013;5:173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 10.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 11.Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, Cochrane L, Jephson C, Janes S, Beaumont NJ, Cogan T, Bader A, Seifalian AM, Hsuan JJ, Lowdell MW, Birchall MA. Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet. 2012;380(9846):994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jungebluth P, Alici E, Baiguera S, Le Blanc K, Blomberg P, Bozoky B, Crowley C, Einarsson O, Grinnemo KH, Gudbjartsson T, Le Guyader S, Henriksson G, Hermanson O, Juto JE, Leidner B, Lilja T, Liska J, Luedde T, Lundin V, Moll G, Nilsson B, Roderburg C, Stromblad S, Sutlu T, Teixeira AI, Watz E, Seifalian A, Macchiarini P. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: A proof-of-concept study. Lancet. 2011;378(9808):1997–2004. doi: 10.1016/S0140-6736(11)61715-7. [DOI] [PubMed] [Google Scholar]
- 13.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L’Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: A multicentre cohort study. Lancet. 2009;373(9673):1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 14.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Woil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson E. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: A new heart model system. FASEB Journal. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 15.Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G. Cardiac tissue engineering: Cell seeding, cultivation parameters and tissue construct characterization. Biotechnology and Bioengineering. 1999;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Li R-K, Yau TM, Weisel RD, Mickle DAG, Sakai T, Choi A, Jia Z-Q. Construction of a bioengineered cardiac graft. Journal of Thoracic and Cardiovascular Surgery. 2000;119:368–375. doi: 10.1016/S0022-5223(00)70193-0. [DOI] [PubMed] [Google Scholar]
- 17.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S. Bioengineerred cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102(suppl III):III56–III61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12(4):452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 19.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106(39):16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100(2):263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 21.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16(1):115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 22.Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Biomimetic approach to cardiac tissue engineering: Oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12(8):2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M, Park H, Martens TP, Salazar-Lazaro JE, Geng W, Wang Y, Langer R, Freed LE, Vunjak-Novakovic G. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J Biomed Mater Res A. 2007 doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, Leor J, Cohen S. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci U S A. 2009;106(35):14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song H, Yoon C, Kattman SJ, Dengler J, Masse S, Thavaratnam T, Gewarges M, Nanthakumar K, Rubart M, Keller GM, Radisic M, Zandstra PW. Interrogating functional integration between injected pluripotent stem cell-derived cells and surrogate cardiac tissue. Proc Natl Acad Sci U S A. 2010;107(8):3329–3334. doi: 10.1073/pnas.0905729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reis LA, Chiu LL, Liang Y, Hyunh K, Momen A, Radisic M. A peptide-modified chitosan-collagen hydrogel for cardiac cell culture and delivery. Acta Biomater. 2011 doi: 10.1016/j.actbio.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Iyer RK, Chiu LL, Reis LA, Radisic M. Engineered cardiac tissues. Curr Opin Biotechnol. 2011 doi: 10.1016/j.copbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. Journal of the American College of Cardiology. 2006;48(5):907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7(12):1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H, Larson BL, Guillemette MD, Jain SR, Hua C, Engelmayr GC, Jr, Freed LE. The significance of pore microarchitecture in a multi-layered elastomeric scaffold for contractile cardiac muscle constructs. Biomaterials. 2011;32(7):1856–1864. doi: 10.1016/j.biomaterials.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32(35):9180–9187. doi: 10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nawroth JC, Lee H, Feinberg AW, Ripplinger CM, McCain ML, Grosberg A, Dabiri JO, Parker KK. A tissue-engineered jellyfish with biomimetic propulsion. Nat Biotechnol. 2012 doi: 10.1038/nbt.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagueh SF, Shah G, Wu Y, Torre-Amione G, King NM, Lahmers S, Witt CC, Becker K, Labeit S, Granzier HL. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 34.Omens JH. Stress and strain as regulators of myocardial growth. Prog Biophys Mol Biol. 1998;69(2-3):559–572. doi: 10.1016/s0079-6107(98)00025-x. [DOI] [PubMed] [Google Scholar]
- 35.Bouten CV, Dankers PY, Driessen-Mol A, Pedron S, Brizard AM, Baaijens FP. Substrates for cardiovascular tissue engineering. Advanced drug delivery reviews. 2011;63(4-5):221–241. doi: 10.1016/j.addr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Challenges in cardiac tissue engineering. Tissue engineering Part B, Reviews. 2010;16(2):169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen QZ, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: Ten years of research survey. Materials Science & Engineering R-Reports. 2008;59:1–37. [Google Scholar]
- 38.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95(7):3479–3487. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32(4):1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, Tiburcy M, Eschenhagen T. Heart muscle engineering: An update on cardiac muscle replacement therapy. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15(6):1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3- dimensional cell sheet manipulation technique and temperature- responsive cell culture surfaces. Circulation Research. 2002;90(3):e40–e48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. Faseb J. 2006;20(6):708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 44.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, Imanishi Y, Shimizu T, Okano T, Matsuda H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: Their integration with recipient myocardium. Transplantation. 2005;80:1586–1595. doi: 10.1097/01.tp.0000181163.69108.dd. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90(2):223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 46.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101(52):18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, Au HT, Radisic M, Vunjak-Novakovic G. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4(2):155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiu LL, Iyer RK, King JP, Radisic M. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tandon N, Marsano A, Maidhof R, Wan L, Park H, Vunjak-Novakovic G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med. 2011;5(6):e115–125. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu LL, Iyer RK, King JP, Radisic M. Biphasic electrical field stimulation aids in tissue engineering of multicell-type cardiac organoids. Tissue Eng Part A. 2011;17(11-12):1465–1477. doi: 10.1089/ten.tea.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nag AC. Study of non-muscle cells of the adult mammalian heart - a fine-structural analysis and distribution. Cytobios. 1980;28(109):41–61. [PubMed] [Google Scholar]
- 52.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: Toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277(2 Pt 2):H433–444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 53.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114(1 Suppl):I72–78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 54.Sussman MA, McCulloch A, Borg TK. Dance band on the titanic - biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;91(10):888–898. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- 55.Parratt JR, Vegh A, Zeitlin IJ, Ahmad M, Oldroyd K, Kaszala K, Papp JG. Bradykinin and endothelial-cardiac myocyte interactions in ischemic preconditioning. Am J Cardiol. 1997;80(3A):A124–A131. doi: 10.1016/s0002-9149(97)00467-0. [DOI] [PubMed] [Google Scholar]
- 56.Shah AM, Mebazaa A, Yang ZK, Cuda G, Lankford EB, Pepper CB, Sollott SJ, Sellers JR, Robotham JL, Lakatta EG. Inhibition of myocardial crossbridge cycling by hypoxic endothelial cells - a potential mechanism for matching oxygen supply and demand? Circ Res. 1997;80(5):688–698. doi: 10.1161/01.res.80.5.688. [DOI] [PubMed] [Google Scholar]
- 57.Asakawa N, Shimizu T, Tsuda Y, Sekiya S, Sasagawa T, Yamato M, Fukai F, Okano T. Pre-vascularization of in vitro three-dimensional tissues created by cell sheet engineering. Biomaterials. 2010;31(14):3903–3909. doi: 10.1016/j.biomaterials.2010.01.105. [DOI] [PubMed] [Google Scholar]
- 58.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 59.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S. Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci U S A. 2011;108(36):14789–14794. doi: 10.1073/pnas.1017825108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J Physiol Cell Physiol. 2003;285(1):C171–182. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 61.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105(6):1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 62.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109(1):47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose RA, Jiang H, Wang X, Helke S, Tsoporis JN, Gong N, Keating SC, Parker TG, Backx PH, Keating A. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Guo X, Palmer AF, Das H, Guan J. High-efficiency matrix modulus-induced cardiac differentiation of human mesenchymal stem cells inside a thermosensitive hydrogel. Acta Biomater. 2012;8(10):3586–3595. doi: 10.1016/j.actbio.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 65.Lin X, Li HY, Chen LF, Liu BJ, Yao Y, Zhu WL. Enhanced differentiation potential of human amniotic mesenchymal stromal cells by using three-dimensional culturing. Cell and Tissue Research. 2013 doi: 10.1007/s00441-013-1576-z. in press. [DOI] [PubMed] [Google Scholar]
- 66.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378(9806):1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104(4):e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11(5):723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a kdr+ embryonic-stem-cell-derived population. Nature. 2008;453(7194):524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 72.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci U S A. 2005;102(37):13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical wnt signaling. Proc Natl Acad Sci U S A. 2012;109(27):E1848–1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Didie M, Christalla P, Rubart M, Muppala V, Doker S, Unsold B, El-Armouche A, Rau T, Eschenhagen T, Schwoerer AP, Ehmke H, Schumacher U, Fuchs S, Lange C, Becker A, Tao W, Scherschel JA, Soonpaa MH, Yang T, Lin Q, Zenke M, Han DW, Scholer HR, Rudolph C, Steinemann D, Schlegelberger B, Kattman S, Witty A, Keller G, Field LJ, Zimmermann WH. Parthenogenetic stem cells for tissue-engineered heart repair. J Clin Invest. 2013 doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013 doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Keller G, Kong CW, Li RA. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation Arrhythmia and electrophysiology. 2013;6(1):191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6(10):e26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5(9):e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, Davis ME. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8(12):4357–4364. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22(10):1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 81.Gepstein L, Ding C, Rahmutula D, Wilson EE, Yankelson L, Caspi O, Gepstein A, Huber I, Olgin JE. In vivo assessment of the electrophysiological integration and arrhythmogenic risk of myocardial cell transplantation strategies. Stem Cells. 2010;28(12):2151–2161. doi: 10.1002/stem.545. [DOI] [PubMed] [Google Scholar]
- 82.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: Insights into the development of cell-based pacemakers. Circulation. 2005;111(1):11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 83.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human es-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012 doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kraehenbuehl TP, Ferreira LS, Hayward AM, Nahrendorf M, van der Vlies AJ, Vasile E, Weissleder R, Langer R, Hubbell JA. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32(4):1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chiu LL, Radisic M. Scaffolds with covalently immobilized vegf and angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31(2):226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 86.Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M, Li RK. Biodegradable collagen patch with covalently immobilized vegf for myocardial repair. Biomaterials. 2011;32(5):1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 87.Chiu LL, Weisel RD, Li RK, Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med. 2011;5(1):69–84. doi: 10.1002/term.292. [DOI] [PubMed] [Google Scholar]
- 88.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107(34):15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leung BM, Sefton MV. A modular approach to cardiac tissue engineering. Tissue Eng Part A. 2010;16(10):3207–3218. doi: 10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iyer RK, Chiu LL, Vunjak-Novakovic G, Radisic M. Biofabrication enables efficient interrogation and optimization of sequential culture of endothelial cells, fibroblasts and cardiomyocytes for formation of vascular cords in cardiac tissue engineering. Biofabrication. 2012;4(3) doi: 10.1088/1758-5082/4/3/035002. 035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiu LL, Montgomery M, Liang Y, Liu H, Radisic M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc Natl Acad Sci U S A. 2012;109(50):E3414–3423. doi: 10.1073/pnas.1210580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sekine H, Shimizu T, Sakaguchi K, Dobashi I, Wada M, Yamato M, Kobayashi E, Umezu M, Okano T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat Commun. 2013;4:1399. doi: 10.1038/ncomms2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction a 5-year update. Journal of the American College of Cardiology. 2011;58(25):2615–2629. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. Journal of the American College of Cardiology. 2004;44(3):654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 95.Nakamuta JS, Danoviz ME, Marques FLN, dos Santos L, Becker C, Goncalves GA, Vassallo PF, Schettert IT, Tucci PJF, Krieger JE. Cell therapy attenuates cardiac dysfunction post myocardial infarction: Effect of timing, routes of injection and a fibrin scaffold. PLoS ONE. 2009;4:e6005. doi: 10.1371/journal.pone.0006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo HD, Wang HJ, Tan YZ, Wu JH. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Pt A. 2011;17(1-2):45–58. doi: 10.1089/ten.TEA.2010.0124. [DOI] [PubMed] [Google Scholar]
- 97.Danoviz ME, Nakamuta JS, Marques FLN, dos Santos L, Alvarenga EC, dos Santos AA, Antonio EL, Schettert IT, Tucci PJ, Krieger JE. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS ONE. 2010;5:e12077. doi: 10.1371/journal.pone.0012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang X, Wang H, Ma X, Adila A, Wang B, Liu F, Chen B, Wang C, Ma Y. Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med (Maywood) 2010;235(12):1505–1515. doi: 10.1258/ebm.2010.010175. [DOI] [PubMed] [Google Scholar]
- 99.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112(9 Suppl):I173–177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 100.Wang T, Jiang XJ, Tang QZ, Li XY, Lin T, Wu DQ, Zhang XZ, Okello E. Bone marrow stem cells implantation with alpha-cyclodextrin/mpeg-pcl-mpeg hydrogel improves cardiac function after myocardial infarction. Acta Biomater. 2009;5(8):2939–2944. doi: 10.1016/j.actbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 101.Wall ST, Yeh CC, Tu RY, Mann MJ, Healy KE. Biomimetic matrices for myocardial stabilization and stem cell transplantation. J Biomed Mater Res A. 2010;95(4):1055–1066. doi: 10.1002/jbm.a.32904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin Y-D, Yeh M-L, Yang Y-J, Tsai D-C, Chu T-Y, Shih Y-Y, Chang M-Y, Liu Y-W, Tang ACL, Chen T-Y, Luo C-Y, Chang K-C, Chen J-H, Wu H-L, Hung T-K, Hsieh PCH. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010:S132–S141. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 103.Lu WN, Lu SH, Wang HB, Li DX, Duan CM, Liu ZQ, Hao T, He WJ, Xu B, Fu Q, Song YC, Xie XH, Wang CY. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 104.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10(3-4):403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 105.Wang H, Zhou J, Liu Z, Wang C. Injectable cardiac tissue engineering for the treatment of myocardial infarction. J Cell Mol Med. 2010;14(5):1044–1055. doi: 10.1111/j.1582-4934.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7(1):1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Trans Res. 2011;4(5):528–542. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 108.Johnson TD, Christman KL. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin Drug Deliv. 2012;10(1):59–72. doi: 10.1517/17425247.2013.739156. [DOI] [PubMed] [Google Scholar]
- 109.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117(11):1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 110.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in swine. Journal of the American College of Cardiology. 2009;54(11):1014–1023. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, Demaria AN, Dib N, Christman KL. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59(8):751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laham RJ, Rezaee M, Post M, Sellke FW, Braeckman RA, Hung D, Simons M. Intracoronary and intravenous administration of basic fibroblast growth factor: Myocardial and tissue distribution. Drug Metab Dispos. 1999;27(7):821–826. [PubMed] [Google Scholar]
- 113.Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD, Pettigrew RI, Whitehouse MJ, Yoshizawa C, Simons M. Intracoronary basic fibroblast growth factor (fgf-2) in patients with severe ischemic heart disease: Results of a phase i open-label dose escalation study. J Am Coll Cardiol. 2000;36(7):2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- 114.Krause K, Jaquet K, Schneider C, Haupt S, Lioznov MV, Otte KM, Kuck KH. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: First-in-man study. Heart. 2009;95(14):1145–1152. doi: 10.1136/hrt.2008.155077. [DOI] [PubMed] [Google Scholar]
- 115.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30(29):5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 117.McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: Data from the heart and soul study. J Am Soc Echocardiogr. 2009;22(2):190–197. doi: 10.1016/j.echo.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010;107(25):11507–11512. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, Gorman JH, 3rd, Gorman RC, Burdick JA. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011;12(11):4127–4135. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: A finite element model simulation. Circulation. 2006;114(24):2627–2635. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 121.Tous E, Purcell B, Ifkovits JL, Burdick JA. Injectable acellular hydrogels for cardiac repair. J Cardiovasc Transl Res. 2011;4(5):528–542. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 122.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. Journal of cardiac failure. 2009;15(7):629–636. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25(9):1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 124.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11 Suppl 1):S29–37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 125.Sawa Y, Miyagawa S, Sakaguchi T, Fujita T, Matsuyama A, Saito A, Shimizu T, Okano T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue lvas in a patient with dcm: Report of a case. Surg Today. 2012;42(2):181–184. doi: 10.1007/s00595-011-0106-4. [DOI] [PubMed] [Google Scholar]
- 126.Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18(9-10):910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T. Development of a drug screening platform based on engineered heart tissue. Circ Res. 2010;107(1):35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 128.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in timothy syndrome. Nature. 2011;471(7337):230–234. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-qt syndrome. N Engl J Med. 2010;363(15):1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 130.Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of leopard syndrome. Nature. 2010;465(7299):808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4(130):130ra147. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stamm C, Nasseri B, Choi YH, Hetzer R. Cell therapy for heart disease: Great expectations, as yet unmet. Heart, lung & circulation. 2009;18(4):245–256. doi: 10.1016/j.hlc.2008.10.014. [DOI] [PubMed] [Google Scholar]


