Abstract
Objective
Epidemiological studies support an association of self-defined constipation with fiber and physical activity, but not liquid intake. The aims of this study were to assess the prevalence and associations of dietary fiber and liquid intake to constipation.
Methods
Analyses were based on data from 10,914 adults (≥20 years) from the 2005-2008 cycles of the National Health and Nutrition Examination Surveys (NHANES). Constipation was defined as hard or lumpy stools (Bristol Stool Scale types 1 or 2) as the “usual or most common stool type.” Dietary fiber and liquid intake from total moisture content were obtained from dietary recall. Co-variables included: age, race, education, poverty income ratio, body mass index, self-reported general health status, chronic illnesses, and physical activity. Prevalence estimates and prevalence odds ratios (POR) were analyzed in adjusted multivariable models using appropriate sampling weights.
Results
Overall, 9,373 (85.9%) adults (4,787 women and 4,586 men) had complete stool consistency and dietary data. Constipation rates were 10.2% (95% CI: 9.6,10.9) for women and 4.0 (95% CI: 3.2,5.0) for men (p<.001). After multivariable adjustment, low liquid consumption remained a predictor of constipation among women (POR: 1.3, 95% CI: 1.0,1.6) and men (POR: 2.4, 95% CI: 1.5,3.9); however, dietary fiber was not a predictor. Among women, African-American race/ethnicity (POR: 1.4, 95% CI: 1.0,1.9), being obese (POR: 0.7, 95% CI: 0.5,0.9), and having a higher education level (POR: 0.8, 95% CI: 0.7,0.9) were significantly associated with constipation.
Conclusions
The findings support clinical recommendations to treat constipation with increased liquid, but not fiber or exercise.
Keywords: constipation, functional bowel disorders, epidemiology
Introduction
Constipation is highly prevalent; estimated at 14% (95% Confidence Interval 12%-17%) in world-wide community-dwelling populations. It is higher among women, older adults, and those with a lower socio-economic status.1 Incidence rates of constipation over a 12-year period have been estimated at 17.5% (95% CI 14.5, 20.5) with higher rates reported among women than men and among adults over 70 years of age.2,3 Constipation is associated with impaired quality of life,4,5 increased health care costs estimated at $7422 in annual direct costs and $390 in annual out-of-pocket expenses (2005 US dollars),6 and with excess work absenteeism.4 About half of patients with constipation who consult physicians are not satisfied with their response to treatment.4
Epidemiological studies reporting the prevalence of CC have utilized differing definitions that include self-reported symptoms, stool frequency, and the Rome Foundation Criteria. Self-reported constipation rates (“frequent,” “usual” or “any” symptoms) and rates using the Rome Foundation Criteria vary widely in the literature and range from 2-27%.1,7-12 Lower prevalence rates, 5-9%, are found when using stool frequency (less than 3 bowel movements (BMs)/week).1,9,11 Stool consistency as defined by the validated Bristol Stool Form Scale (hard or lumpy stool consistency) has been advocated as a better measure for making a clinical diagnosis of constipation because it is more strongly correlated with objectively measured whole gut transit time13 and it is more frequently associated with clinical (i.e., physician) diagnosis of constipation than is a stool frequency of <3 BMs/week.14 Picture scales combined with standardized descriptors [the Bristol Stool Scale] were developed and validated for more reliable patient assessment of stool consistency.4 There are as yet no large epidemiological studies of the prevalence of constipation using stool consistency as a criterion, but clinicians are being encouraged to adopt the frequent occurrence of hard or lumpy stools as a way of identifying patients with constipation.15
Given that epidemiological and clinical studies support an association of constipation with fiber,9,12, 16, 17 and less is known about dietary liquid intake and physical activity, the primary aim of this study was to determine whether decreased intake of dietary fiber and liquid is associated with the presence of constipation, defined as hard or lumpy stools as the usual or most common stool type. Secondary aims were (1) to characterize the prevalence of constipation defined by stool consistency in a nationally representative sample of non-institutionalized U.S. adults, (2) to determine whether the prevalence of constipation varies by the type of definition used (stool frequency compared to stool consistency), and (3) to assess possible factors associated with constipation.
Materials and Methods
Study Population
The NHANES are cross-sectional surveys of a nationally representative sample of the non-institutionalized population sampled using a complex, stratified, multi-stage, probability cluster design. The National Center for Health Statistics (NCHS) Ethics Review Board approved the protocol, and all participants provided written informed consent.
The NHANES 2005-2006, and 2007-2008 cycles were combined for an overall description and characterization of the population. A sub-sample of 10,914 men and non-pregnant women aged 20 years or older who received a physical and laboratory examination in a mobile examination center (MEC) was identified. Questions about bowel symptoms were ascertained in the MEC Interview Room using a Computer-Assisted Personal Interview (CAPI) system.
Constipation Definition
Stool consistency was assessed using the Bristol Stool Form Scale13 (color picture card with pictures and written descriptors of the 7 stool types) and the follow written question: “Please look at this card and tell me the number that corresponds with your usual or most common stool type.” Constipation was defined as a Type 1 (separate hard lumps, like nuts) or Type 2 rating (sausage like, but lumpy). Normal stool consistency was defined as Bristol Stool Scale Type 3, Type 4, and Type 5, as in other NHANES publications.18
In order to compare the constipation definitions using stool consistency to other definitions, we also assessed stool frequency with the following question, “How many times a week do you usually have a bowel movement?” Responses levels were not specified and the range varied from 1-70 bowel movements per week. Stool frequency results were dichotomized as less than 3 bowel movements per week (constipated) or ≥ 3 bowel movements per week (non-constipated).
Dietary Measures
For the 24-hour dietary data, a multiple-pass dietary recall method was used with a computer-assisted dietary interview, developed and validated by the US Department of Agriculture (USDA).19 Participants were asked to participate in two 24-hour dietary recall periods. The first 24-hour dietary recall was done during the MEC interview and the second 24-hour dietary recall was done 3 to 10 days later by telephone. From the USDA website, the dietary recall information for foods and beverages consumed by participants includes the name, USDA food code and description. The amounts of the foods/beverages consumed are in grams, with 64 food components/nutrients included. What We Eat in America (WWEIA) is conducted as a partnership between the USDA and the U.S. Department of Health and Human Services (DHHS). DHHS is responsible for the sample design and data collection, and USDA is responsible for the survey’s dietary data collection methodology, maintenance of the databases used to code and process the data, and data review and processing. USDA also funds the collection and processing of Day 2 dietary intake data, which are used to develop variance estimates and calculate usual nutrient intakes. Fiber consumption (gm/day) and liquid intake from total moisture consumption (gm/day including moisture from foods and beverages) were ascertained in the two 24-hour dietary recall periods and averaged. The distributions of fiber intake and liquid intake were divided into quartiles.
Other Measurements
Information on age (20-29, 30-39, 40-49, 50-59, 60-69, 70-79, ≥80 years), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), education (less than high school, high school, or more than high school), and poverty income ratio (≤2 times the poverty threshold, >2 times the poverty threshold) was self-reported. Participants’ weight and height were measured and BMI was calculated and binned as normal weight, over-weight, or obese (<25.0, 25.0-29.9, ≥30 kg/m2). Diabetes was defined by self-report of being told by a doctor or health professional and/or taking insulin and/or diabetic pills. Hypertension was defined by self-reported diagnosis and/or taking medication for hypertension. Participants with chronic disease were ascertained by self-report to questions about arthritis, chronic lung disease (emphysema, chronic bronchitis, asthma), chronic heart disease (congestive heart failure, coronary heart disease, angina, heart attack), stroke, any liver condition, or cancer. The numbers of chronic diseases were categorized from the individual diseases as 0, 1, 2, 3, or 4 or more. Self-reported general health status was categorized as “excellent/very good/good” and compared to “fair/poor.”
Vigorous physical activity was defined by different questions in the 2005-06 cycle and the 2007-08 cycle. The question used from 2005-06 inquired about “any” vigorous activities over the past 30 days for at least 10 minutes that “caused heavy sweating, or large increases in breathing or heart rate.” The questions from the 2007-08 cycle inquired about “vigorous-intensity activity that causes large increases in breathing or heart rate” either at work or during leisure time. Any positive responses to these questions defined vigorous physical activity. Negative responses to these questions defined not participating in any vigorous physical activity.
Statistical Methods
All estimates, standard errors, and association measures were derived using the sampling weights provided by the NCHS. These weights take into account unequal probabilities of selection resulting from the sample design, non-response, and planned over-sampling of the elderly, non-Hispanic Blacks, and Mexican Americans.
Appropriately weighted chi-square analysis and Pearson correlation coefficients for the entire sample and sub-group analysis according to gender were used to compare the two definitions for constipation according to stool frequency and stool form.
Separate analyses were conducted for men and women using the definition of constipation based on stool consistency. Estimates of the prevalence of constipation by survey waves were age-standardized by the direct method to the year 2000 Census population using the age group 20-29, 30-39, 40-49, 50-59, 60-69, 70-79, and 80 years or older. For estimates of population prevalence, the subjects who reported Bristol Stool Types 6 or 7 as their usual or most common stool type were included in the denominator, but for the associated factor analyses, these subjects with frequent loose or watery stools were excluded from analysis. Participants (n= 699 men and women) with loose or watery stools were excluded in the multivariable analyses due to potential differences in disease states that may contribute to loose stool and the dietary intake data that may impact stool consistency. We used appropriate sample weighting for 2-sample t tests and chi-square analysis for testing differences in means and proportions. Multivariable logistic regression analysis was used to calculate prevalence odds ratio (POR) estimates and corresponding 95% confidence intervals (CI) for constipation prevalence with adjustment for other variables associated with constipation. P values < 0.05 were considered statistically significant (no adjustment for multiple testing). Statistical analyses were performed using STATA statistical software version 8.2 (College Station, TX).
Results
From the 10,914 men and non-pregnant women aged 20 years or older from the NHANES 2005-06 and 2007-08 cycles (Figure 1), a total of 1,433 (13.1%) participants were missing questionnaire items for stool consistency/frequency and were excluded, leaving 4586 men and 4787 women in the final analytic sample (n = 9,373). Missing data on stool consistency and stool frequency from the 2 NHANES cycles were not statistically different (p>0.05). A total of 396 women (7.1%) and 303 men (5.7%) were excluded from the multivariable analysis who had loose stool consistency (Bristol Stool Form Types 6 and 7).
Figure 1. NHANES 2005-2006 and 2007-2008 Analytic and Multivariable Analysis Sample Size.
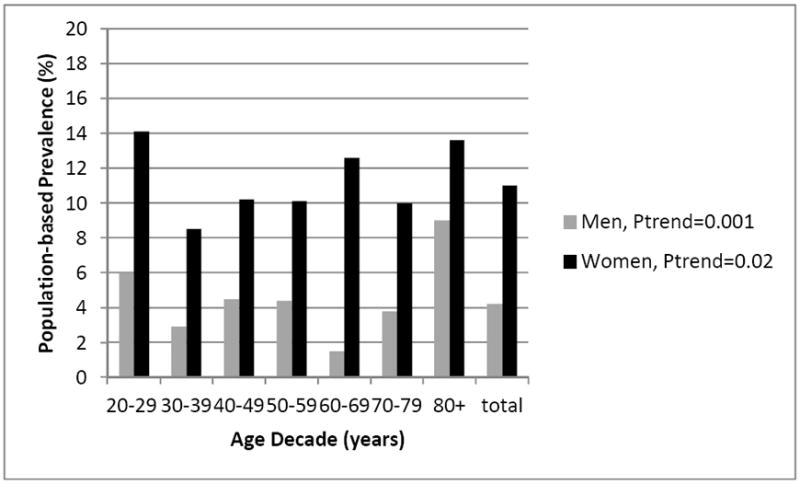
The population-based prevalence of constipation differed significantly by gender, with higher rates in women 10.2% (95% CI 9.6, 10.9) compared to men 4.0% (95% CI 3.2, 5.0), p<0.001. When comparing constipation prevalence among women and men, significant trends in prevalence existed among the age decades (Figure 2); however, no linear trends were evident: constipation prevalence was not associated with increasing age (per 10 years) among women (POR 1.0, 95% CI 0.9, 1.0) or men (POR 0.9, 95% CI 0.8, 1.0). When dichotomizing age as ≥60 years of age, women and men in the older age groups did not have higher prevalence rates of constipation than those <60 years of age, p>0.05.
Figure 2. Prevalence of Constipation by Age and Gender from NHANES, 2005-2006 and 2007-2008.
Prevalence estimates differed when constipation was defined by stool consistency (7.2%, 95% CI 6.7, 7.8) vs. stool frequency (3.1%, 95% CI 2.6, 3.8), Table 1. Only 0.8% (95% CI 0.6, 1.0) met both the stool consistency and stool frequency definitions for constipation; whereas 90.3% (95% CI 89.5, 91.2) of the NHANES participants did not have constipation by either definition. Stool consistency and stool frequency were weakly correlated in women (r2=0.12) and men (r2=0.06).
Table 1.
Comparison of Constipation Prevalence According to Stool Consistency and Stool Frequency Definitions in NHANES 2005-2006 and 2007-2008
| Stool Frequency (per week) | Stool Consistency | ||
|---|---|---|---|
| Bristol Types 1-2 | Bristol Types 3-7 | Total | |
| <3 | 0.8% (79) | 2.4% (241) | 3.1% (320) |
| >3 | 6.5% (659) | 90.3% (8377) | 96.8% (9036) |
| Total | 7.2% (738) | 92.3% (8618) | 100% (8674) |
All values are presented as percentage (n).
Univariate tests for risk factors
As shown in Table 2, higher constipation rates were seen among both men and women with lower education levels and fair/poor self-rated health (p<0.05). Among men, a significantly higher constipation prevalence was found among Mexican Americans and Non-Hispanic Black Americans (p=0.009) and those with lower poverty income ratios (p<0.001). Among women, a significantly higher constipation prevalence was seen among Non-Hispanic Black Americans when compared to all other racial/ethnic groups (p=0.03), without a significant trend seen among the individual racial/ethnic groups (p=0.34). No differences in constipation prevalence (p>0.05) were seen among women or men according to BMI, vigorous physical activity, or number of chronic diseases.
Table 2.
Univariate Analysis of Factors Associated with Constipation among US Women and Men from NHANES, 2005-2006 and 2007-2008
| Men, n=4586 | Women, n=4787 | |||||
|---|---|---|---|---|---|---|
| Variable | No. of Mena | % (95% CI)b | P valuec | No. of Womena | % (95% CI)b | P valuec |
| Overall constipation prevalence | 221 | 4.0 (3.2, 5.0) | 518 | 10.2 (9.6, 10.9) | ||
| Age (years) | 4586 | 0.001 | 4787 | 0.02 | ||
| 20-29 | 48 | 5.9 (4.0, 8.7) | 135 | 13.6 (11.0, 16.6) | ||
| 30-39 | 32 | 2.7 (1.8, 4.1) | 77 | 7.7 (6.0, 10.0.) | ||
| 40-49 | 41 | 4.3 (2.7, 6.6) | 83 | 9.4 (7.5, 11.8) | ||
| 50-59 | 31 | 4.0 (2.8, 5.8) | 64 | 9.5 (7.3, 12.2) | ||
| 60-69 | 19 | 1.4 (0.7, 2.9) | 79 | 11.6 (9.4, 14.1) | ||
| 70-79 | 24 | 3.5 (2.1, 5.8) | 46 | 9.0 (7.0, 11.7) | ||
| 80+ | 26 | 8.4 (5.5, 12.8) | 34 | 12.5 (9.0, 17.0) | ||
| Race/ethnicity | 4586 | 0.009 | 4787 | 0.42 | ||
| Hispanic-Mexican American | 50 | 6.3 (4.9, 8.0) | 89 | 9.4 (7.4, 12.1) | ||
| Hispanic – Other | 21 | 5.4 (3.0, 9.7) | 43 | 10.3 (7.3, 14.3) | ||
| Non-Hispanic white | 88 | 3.3 (2.3, 4.7) | 243 | 9.9 (9.1, 10.9) | ||
| Non-Hispanic black | 55 | 6.2 (4.7, 8.1) | 126 | 12.6 (10.4, 15.2) | ||
| Other – Including multi-racial | 7 | 4.7 (2.1, 8.1) | 17 | 9.9 (5.4,17.5) | ||
| Education | 4584 | 0.004 | 4785 | <0.001 | ||
| <High school | 94 | 5.4 (4.0, 7.4) | 152 | 12.2 (10.4, 14.2) | ||
| High school or GED | 59 | 5.4 (3.7, 7.7) | 151 | 12.1 (10.4, 13.9) | ||
| >High school | 68 | 2.8 (2.0, 4.0) | 214 | 8.8 (7.9, 9.7) | ||
| Family poverty income ratio | 4586 | <0.001 | 4787 | 0.09 | ||
| < 2 | 121 | 5.9 (4.6, 7.4) | 11.4 (10.1, 13.0) | |||
| ≥ 2 | 100 | 3.2 (2.4, 4.3) | 10.3 (9.3, 11.5) | |||
| Body mass index | 4556 | 0.14 | 4756 | 0.05 | ||
| < 25.0 | 68 | 5.2 (3.7, 7.5) | 188 | 11.4 (9.6, 13.4) | ||
| 25.0 – 29.9 | 85 | 3.7 (2.7, 4.9) | 161 | 11.1 (9.3, 13.4) | ||
| > 30.0 | 67 | 3.5 (2.4, 4.9) | 166 | 8.3 (6.9, 9.9) | ||
| Self-rated health | 4586 | 0.02 | 4787 | 0.03 | ||
| Excellent/very good/good | 151 | 3.7 (2.8, 4.9) | 377 | 9.7 (9.1, 10.4) | ||
| Fair/poor | 70 | 5.9 (4.4, 7.7) | 141 | 12.7 (10.3, 15.5) | ||
| Chronic diseases | 4502 | 0.30 | 4723 | 0.49 | ||
| None | 116 | 4.6 (3.6, 5.9) | 223 | 10.3 (8.8, 11.9) | ||
| 1 | 41 | 3.1 (1.8, 5.1) | 124 | 9.4 (7.5,11.7) | ||
| 2 | 32 | 3.6 (2.2, 5.7) | 88 | 11.5 (9.1, 14.5) | ||
| 3 | 13 | 3.6 (1.4,6.5) | 54 | 11.1 (9.1, 14.5) | ||
| 4 or more | 11 | 5.5 (2.5,11.8) | 22 | 7.4 (4.3, 12.6) | ||
| Vigorous physical activity | 4585 | 0.12 | 4787 | 0.53 | ||
| No | 143 | 4.6 (3.5, 5.9) | 220 | 10.5 (9.6, 11.5) | ||
| Yes | 78 | 3.4 (2.4, 4.7) | 117 | 9.6 (7.8, 11.9) | ||
| Dietary fiber intake | 3918 | 0.008 | 4257 | 0.08 | ||
| Lowest quartile (<10.1 gm/day) | 49 | 6.2 (4.0,9.5) | 149 | 12.0 (10.5,13.7) | ||
| Middle lower quartile (≥10.1 to 14.5 gm/day) | 34 | 3.3 (2.2,4.9) | 134 | 10.8 (9.2,12.5) | ||
| Middle upper quartile (≥14.5 to 20.1 gm/day) | 47 | 3.7 (2.2,6.1) | 108 | 9.6 (7.8 12.0) | ||
| Highest quartile (>20.1 gm/day) | 42 | 2.6 (2.0,3.6) | 76 | 8.1 (6.2, 10.5) | ||
| Total dietary liquid intake | 3918 | <0.001 | 4257 | 0.008 | ||
| Lowest quartile (<1881.9 mg/day) | 54 | 8.6 (5.5, 13.1) | 172 | 13.3 (11.7,15.2) | ||
| Middle lower quartile (≥1881.9 to 2498.5 mg/day) | 40 | 3.5 (2.5,4.8) | 123 | 11.6 (9.7, 13.9) | ||
| Middle upper quartile (≥2498.5 to 3319.0 mg/day) | 45 | 3.6 (2.3, 5.5) | 95 | 8.2 (6.5, 10.3) | ||
| Highest quartile (>3319.0 mg/day) | 33 | 2.5 (1.7, 3.7) | 77 | 8.1 (5.8,11.2) | ||
Total number of men and women responding for the category or condition under study (denominator) excluding those with missing data
Weighted prevalence reported
p-value: Pearson’s design-based Chi-square test with appropriate sampling weights
When evaluating the dietary intake of fiber and liquid from the total moisture content in food and liquid sources (Table 2 and Figures 3A and 3B), significantly higher rates of constipation were seen within both genders at the lowest quartile of intake of dietary fiber (<10.1 grams/day) and dietary liquid (<1882 milligrams/day). Men and women who reported constipation did not have higher levels of dietary fiber intake within the upper quartiles than those without constipation (Table 3). From Table 3, 30.5% of the men and 32.8% of the women with constipation reported fiber intake within the lowest quartile (<10.1 grams/day) compared to 17.3% of the men and 27.1% of the women without constipation.
Figure 3. A and B. Prevalence of Constipation According to Intake of Dietary Fiber and Total Dietary Moisture from NHANES, 2005-06 and 2007-2008.
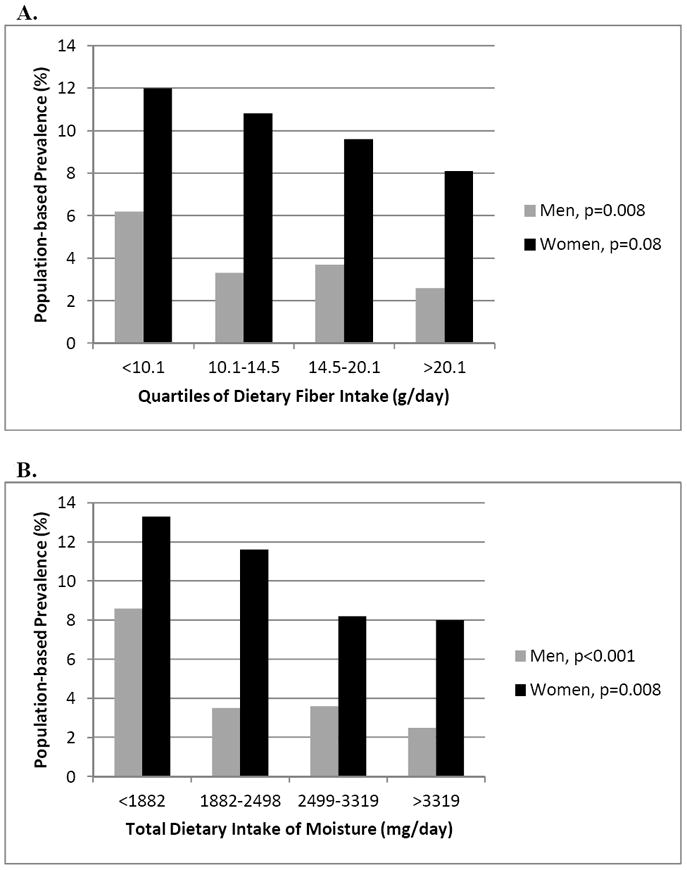
Table 3.
Comparison of Dietary Fiber Intake Among Men and Women with and without Constipation
| Men, n = 3918 | Women, n = 4257 | |||||
|---|---|---|---|---|---|---|
| Quartile of fiber intake | No constipation | Constipation | p value* | No constipation | Constipation | p value* |
| Lowest quartile (<10.1 gm/day) | 17.3% | 30.5% | <0.001 | 27.1% | 32.8% | 0.009 |
| Middle lower quartile (≥10.1 to 14.5 gm/day) | 22.3% | 19.4% | 0.37 | 26.4% | 28.2% | 0.40 |
| Middle upper quartile (≥14.5 to 20.1 gm/day) | 27.1% | 26.7% | 0.92 | 27.2% | 24.3% | 0.19 |
| Highest quartile (>20.1 gm/day) | 33.3% | 23.4% | 0.007 | 19.4% | 14.7% | 0.02 |
Two-sample proportiontests among individual quartiles
Multivariable analyses of risk factors for constipation
After multivariable adjustment (Table 4), factors associated with constipation differed among the women and men. In women, after controlling for age and other factors, African-American race/ethnicity, having more education (protective), being obese (protective), and consuming less total liquid in the diet was marginally associated with constipation; whereas low dietary fiber intake and more vigorous physical activity were not significantly associated with constipation. In men, after controlling for age, race/ethnicity, education, poverty status, self-rated health status, BMI, and chronic diseases, only low liquid intake remained a significant predictor of constipation; whereas low fiber intake and vigorous physical activity did not predict constipation.
Table 4.
Weighted Multivariable Models for Factors Associated with Constipation among US Men and Women from NHANES, 2005-06 and 2007-08
| WOMEN* POR (95% CI) N=3841 | MEN* POR (95% CI) N=3561 | |
|---|---|---|
| African American race/ethnicity | 1.39 (1.00, 1.93) | 1.40 (0.82, 2.41) |
| Living above poverty income | 0.93 (0.72, 1.20) | 0.71 (0.48, 1.04) |
| Higher education | 0.82 (0.71, 0.94) | 0.92 (0.69, 1.21) |
| Comorbidity | 1.00 (0.87, 1.15) | 0.97 (0.79, 1.19) |
| BMI (obese) | 0.65 (0.49, 0.88) | 0.91 (0.55, 1.52) |
| Poor/fair self-rated health | 1.24 (0.86, 1.78) | 1.31 (0.83, 2.05) |
| Vigorous physical activity | 0.96 (0.68, 1.36) | 0.74 (0.45, 1.20) |
| Low fiber intake (lowest quartile) | 1.07 (0.84, 1.36) | 1.40 (0.88, 2.20) |
| Low dietary liquid intake (lowest quartile) | 1.29 (1.02, 1.64) | 2.42 (1.51, 3.88) |
All multivariable models controlled for age (in decades) and included appropriate sampling weights.
Discussion
From a US population-based survey that involved private in-person interviews with a computer-assisted questionnaire, constipation symptoms using a definition based on stool consistency revealed a higher overall prevalence (10.2%, 95% CI 9.6, 10.9, in women vs. 4.0%, 95% CI 3.2, 5.0 in men). Constipation symptoms defined by stool consistency were higher than a stool frequency-based definition (7.2%, 95% CI 6.7, 7.8 vs. 3.1%, 95% CI 2.6, 3.8, respectively). After controlling for other known factors for chronic constipation in adults, modifiable factors that may improve constipation included increasing the dietary intake of liquids.
Our prevalence estimate using a definition based on stool consistency was overall lower, 7.2%, 95% CI 6.7, 7.8, than a recent systematic review and meta-analysis of the world-wide prevalence of chronic constipation, 14%, (95% CI 12, 17)1 and the cumulative incidence rate of constipation over a 12-year period, 17.5% (95% CI 14.5, 20.5) in one community-based US study.3 In this same community-based US study, the authors reported significantly different rates of persistent (3%) and non-persistent constipation symptoms (21%) over a 20-year period.2
The wide variations in published prevalence estimates for constipation are dependent on the type of survey and the questions used to estimate the prevalence.1 Often, higher prevalence rates are seen with self-reported constipation symptoms compared to the use of validated symptom-based questionnaires or stool frequency for measuring constipation. Our definition was based on stool consistency and we employed a validated questionnaire, the Bristol Stool Form Scale. This yielded a higher prevalence than did surveys based on a definition using stool frequency (7.2%, 95% CI 6.7, 7.8 for stool consistency compared to 3.1%, 95% CI 2.6, 3.8 for stool frequency). The lower prevalence rate we estimated using stool frequency is slightly lower than other reported prevalence rates, 5-9%, when using the same stool frequency threshold (less than 3 BMs/week) to define constipation.1,9,11 We support using a validated measure of stool consistency to define constipation given the wide variation in prevalence estimates from the literature. However, using a combination definition which requires abnormal stool consistency as well as abnormal stool frequency may underestimate the prevalence of constipation, as reported in Table 1.
Similar to other cross-sectional studies of chronic constipation, we found several associated factors that have been consistently reported in the literature.1,8,12,20 Overall, we confirmed risk factors commonly reported for constipation including: female gender,7,9,11,12,20,21 African-American race/ethnicity (only among women),9,12,20 lower socioeconomic status,12,20 and low educational status.9,12,20 Interestingly, we did not find that age decade was a significant factor associated with constipation among women or men in this study. Many other cross-sectional and longitudinal studies report age as a significant risk factor for constipation.1,9,10,12,20 Even when using stool frequency as the definition for constipation, no trends for increased prevalence of constipation by age decade were observed. One other population-based study using stool frequency as a definition also failed to find an increased trend in constipation prevalence by age.7
In addition to using stool consistency with a validated scale to define constipation in this population-based study, the use of validated dietary data to quantify total daily intake of fiber and liquids was a unique component to this study. Previous epidemiologic studies did not use validated methodology for the assessment of dietary intake or did not include a measure of fluid intake from beverages and food.8,12 When tested separately, higher rates of constipation were seen for men and women in the lower quartiles of dietary intake for fiber and total liquids (from foods and beverages). However, when controlling for other factors in this analysis (including vigorous physical activity), women and men had an increased odds of having constipation with low amounts of total dietary sources of liquid (OR 1.29, 95% CI 1.02, 1.64 for women and OR 2.42, 95% CI 1.51, 3.88 for men) but these associations disappeared for low fiber intake after multivariate adjustment. In women the odds of having constipation with low total dietary liquid intake were marginal despite women consuming more dietary liquids than men (Figure 3B). Women also had higher rates of dietary fiber intake (Figure 3A) and more vigorous physical activity than the men (Table 2) without a significant association seen with constipation in women or men when controlling for other associated factors. These observational findings show that more evidence is needed in clinical trials for the nonpharmacologic approaches (i.e. dietary changes and differing levels of exercise intensity) for constipation.16,22 Given our findings regarding fluid intake, more studies are needed to define optimal amounts of dietary fluid which may have a synergistic effect with dietary fiber intake.17,23
Study limitations include the cross-sectional nature of NHANES data. Causation cannot be determined nor the temporal relationship between onset of constipation and dietary intake. Therefore, we cannot comment on whether increasing fiber and fluid intake will improve constipation symptoms. We were unable to ascertain the impact of increasing levels of physical activity on constipation and only evaluated the impact of self-reported “vigorous” exercise on constipation. Other limitations include the lack of data on the duration of self-reported constipation symptoms or the severity of constipation symptoms for comparison with stool frequency and stool consistency. A recall bias may also exist with the terminology used to ascertain “usual” stool consistency in this cross-section study design. However, in previous reports, constipation diagnosed by stool consistency agreed with a physician diagnosis of constipation better than did stool frequency.14 Given that very few men or women reported taking supplemental fiber in the 2007-08 cycle (n=25), further statistical analysis could not be done to compare the intake of supplemental fiber to the amount of dietary fiber intake. Additionally, we were not able to evaluate the usage of laxatives (prescription or non-prescription) on constipation in this study. The possibility exists that a participant on laxatives may have been misclassified by our definition using stool consistency.
In conclusion, our study used a validated measure of stool consistency to define constipation prevalence in a nationally representative sample of non-institutionalized U.S. adults. Factors associated with constipation among women were similar to other population-based studies when defined by stool consistency. Our finding that low dietary intake of liquids increased the odds of having constipation supports clinical guidelines for the treatment of constipation with increased fluids, but not increasing dietary fiber intake. In general, more evidence is needed to support increased fiber and fluid intake for the treatment of constipation.
Study Highlights.
What is known?
Constipation has a world-wide prevalence of 14% and varies based on type of definition used
Constipation is associated with female gender, increasing age, and lower socioeconomic status
Low fiber intake is associated with constipation in epidemiologic studies
What is new?
Prevalence rate of constipation defined by hard stool consistency is 7% in the US
African-American women, but not men, have higher rates of constipation
Low liquid intake, but not low fiber intake, was associated with constipation
Acknowledgments
Funding/Support:
This study was supported in part from a Veterans Health Administration Career Development Award (CDA-2) to Dr. A. Markland and supported in part by R01 DK31369 to Dr. W.E. Whitehead.
Footnotes
Alayne D. Markland, DO, MSc – conception, initiation, and writing
Olafur Palsson, PsyD - conception, initiation, and writing
Patricia S. Goode, MD, MSN - conception and writing
Kathryn L. Burgio, PhD – conception and writing
Jan Busby-Whitehead, MD – conception and writing
William E. Whitehead, PhD - conception, initiation, and writing
Disclosure/Conflict of Interest:
The author acting as the submission’s guarantor must be identified. The submission’s guarantor is Alayne D. Markland, DO, MSc.
The authors must certify the role that each author had in conceiving, initiating and writing up the research project.
Declaration of all financial and, if relevant, any editorial assistance received to support the research project and/or preparation of the article.
Identification of any relationships that any author or other entity that provided financial or editorial support may have in potential competing interests to those referenced in the submission.
References
- 1.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011 Sep;106(9):1582–1591. doi: 10.1038/ajg.2011.164. quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 2.Choung RS, Locke GR, Rey E, et al. Factors Associated with Persistant and Non-Persistant Chronic Constipation, Over 20 Years. Clin Gastroenterol Hepatol. 2012 Jan; doi: 10.1016/j.cgh.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Cumulative incidence of chronic constipation: a population-based study 1988-2003. Aliment Pharmacol Ther. 2007 Dec;26(11-12):1521–1528. doi: 10.1111/j.1365-2036.2007.03540.x. [DOI] [PubMed] [Google Scholar]
- 4.Johanson JK. Chronic constipation: a survey of the patient perspective. Alimentary Pharmacology & Therapeutics. 2007;25(5):599–608. doi: 10.1111/j.1365-2036.2006.03238.x. [DOI] [PubMed] [Google Scholar]
- 5.Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002 Aug;97(8):1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 6.Nyrop KA, Palsson OS, Levy RL, et al. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007 Jul;26(2):237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 7.Harari D, Gurwitz JH, Avorn J, Bohn R, Minaker KL. Bowel habit in relation to age and gender. Findings from the National Health Interview Survey and clinical implications. Arch Intern Med. 1996 Feb 12;156(3):315–320. [PubMed] [Google Scholar]
- 8.Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum. 1989 Jan;32(1):1–8. doi: 10.1007/BF02554713. [DOI] [PubMed] [Google Scholar]
- 9.Everhart JE, Go VL, Johannes RS, Fitzsimmons SC, Roth HP, White LR. A longitudinal survey of self-reported bowel habits in the United States. Dig Dis Sci. 1989 Aug;34(8):1153–1162. doi: 10.1007/BF01537261. [DOI] [PubMed] [Google Scholar]
- 10.Talley NJ, Weaver AL, Zinsmeister AR, Melton LJ. Functional constipation and outlet delay: a population-based study. Gastroenterology. 1993 Sep;105(3):781–790. doi: 10.1016/0016-5085(93)90896-k. [DOI] [PubMed] [Google Scholar]
- 11.Pare P, Ferrazzi S, Thompson WG, Irvine EJ, Rance L. An epidemiological survey of constipation in canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001 Nov;96(11):3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 12.Sandler RS, Jordan MC, Shelton BJ. Demographic and dietary determinants of constipation in the US population. Am J Public Health. 1990 Feb;80(2):185–189. doi: 10.2105/ajph.80.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992 Jun;33(6):818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrazzi S, Thompson GW, Irvine EJ, Pare P, Rance L. Diagnosis of constipation in family practice. Can J Gastroenterol. 2002 Mar;16(3):159–164. doi: 10.1155/2002/740413. [DOI] [PubMed] [Google Scholar]
- 15.Lembo A, Camilleri M. Chronic Constipation. N Engl J Med. 2003 Oct 2;349(14):1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 16.Wald A. Constipation in the primary care setting: current concepts and misconceptions. Am J Med. 2006 Sep;119(9):736–739. doi: 10.1016/j.amjmed.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33(8):895–901. doi: 10.1111/j.1365-2036.2011.04602.x. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Borrud L, Goode PS, et al. Fecal Incontinence in US Adults: Epidemiology and Risk Factors. Gastroenterology. 2009;137(2):512. doi: 10.1053/j.gastro.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD) What We Eat In America, NHANES 2005-2006. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=18354 Retrieved January 21, 2013.
- 20.Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989 Oct;11(5):525–536. doi: 10.1097/00004836-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Talley NJ, Jones M, Nuyts G, Dubois D. Risk factors for chronic constipation based on a general practice sample. The American Journal of Gastroenterology. 2003;98(5):1107–1111. doi: 10.1111/j.1572-0241.2003.07465.x. [DOI] [PubMed] [Google Scholar]
- 22.Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005 Apr;100(4):936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 23.Gallegos-Orozco JF, Foxx-Orenstein AE, Sterler SM, Stoa JM. Chronic constipation in the elderly. Am J Gastroenterol. 2012;107(1):18–25. doi: 10.1038/ajg.2011.349. [DOI] [PubMed] [Google Scholar]


