Abstract
Parkinson’s disease (PD) is a slowly progressive movement disorder that results from the loss of dopaminergic neurons in the substantia nigra, a small area of cells in the mid-brain. PD is a multifactorial disorder with unknown etiology, in which both genetic and environmental factors play important roles. Substantial evidence links α-synuclein, a small highly conserved presynaptic protein with unknown function, to both familial and sporadic PD. Rare familial cases of PD are associated with missense point mutations in α-synuclein, or with the hyper-expression of the wild type protein due to its gene duplication/triplication. Furthermore, α-synuclein was identified as the major component of amyloid fibrils found in Lewy body and Lewy neurites, the characteristic proteinaceous deposits that are the diagnostic hallmarks of PD. α-Synuclein is abundant in various regions of the brain and has two closely related homologs, β-synuclein and γ-synuclein. When isolated in solution, the protein is intrinsically disordered, but in the presence of lipid surfaces α-synuclein adopts a highly helical structure that is believed to mediate its normal function(s). A number of different conformational states of α-synuclein have been observed. Besides the membrane-bound form, other critical conformations include a partially-folded state that is a key intermediate in aggregation and fibrillation, various oligomeric species, and fibrillar and amorphous aggregates. A number of intrinsic and extrinsic factors that either accelerate or inhibit the rate of α-synuclein aggregation and fibrillation in vitro are known. There is a strong correlation between the conformation of α-synuclein (induced by various factors) and its rate of fibrillation. The aggregation process appears to be branched, with one pathway leading to fibrils and another to oligomeric intermediates that may ultimately form amorphous deposits. The molecular basis of Parkinson’s disease appears to be tightly coupled to the aggregation of α-synuclein and the factors that affect its conformation. This review focuses on the contributions of Prof. Anthony L. Fink to the field and presents some recent developments in this exciting area.
Keywords: α-Synuclein, synucleinopathies, aggregation, amyloid, fibril, neurodegeneration, intrinsically disordered protein, NMR, partially folded intermediate
1. PARKINSON’S DISEASE AS A TYPICAL SYNUC-LEINOPATHY
Parkinson’s disease, PD, is the most common aging-related movement disorder and the second most common neurodegenerative disorder after Alzheimer’s disease (AD). It is estimated that ~1.5 million Americans are affected by PD. The probability of sporadic PD development increases with age, with only a small percentage of patients diagnosed before the age of 50 [1]. The prevalence of PD is much greater among those who are at least 65 years old [2]. Approximately 1% of the population at 65–70 years of age is affected by PD, whereas the number of PD patients increases to 4–5% in 85-year-olds [3]. Based on these facts, PD is now considered an aging-related disease.
PD is a slowly progressive malady that affects the neurons in the substantia nigra, a small area of cells in the midbrain. Gradual degeneration of these cells causes a reduction in the dopamine content. This, in turn, can produce one or more of the classic signs of PD: resting tremor on one (or both) side(s) of the body; generalized slowness of movement or difficulty initiating movement (bradykinesia/akinesia); stiffness of limbs (rigidity); and gait or balance problems (postural instability). The substantia nigra consists of ~400,000 nerve cells, which begin to pigment after birth and are fully pigmented at age 18. The symptoms of PD become apparent after more than ~70% of the dopaminergic neurons die. The “normal” rate of nigral cell loss is ~2,400 per a year. Thus, if an unaffected person lives to be ~120 years old he (she) will probably develop PD. In clinical PD, the neuron loss is accelerated. Epidemiological studies and pathological analyses revealed a mean age of onset of 70 years in sporadic PD [4]. The detailed mechanism of the neuronal death is unknown. It is also unknown why the loss of the nerve cells is accelerated in PD. However, this acceleration seems to be determined by a combination of genetic susceptibility and environmental factors. Sporadic (or idiopathic) forms of this disease account for about 95% of PD patients [4, 5]. In addition to the sporadic form, there are multiple familial forms of the disease linked to mutations in a number of genes. These hereditary forms account for ~4% of PD patients, who develop early-onset disease before the age of 50 [6, 7].
In PD, some surviving nigral dopaminergic neurons contain cytosolic filamentous inclusions known as Lewy bodies (LBs) and Lewy neurites (LNs) [8, 9]. Besides the substantia nigra, LBs and LNs also are found in other brain regions, such as the dorsal motor nucleus of the vagus, the nucleus basalis of Meynert, and the locus coeruleus [9]. Abundant LBs and LNs in the cerebral cortex are also neuro-phathological hallmarks of dementia with LBs (DLB), a common late-life dementia that is clinically similar to AD [10], in LB variant of AD [11], diffuse LB disease [12], and multiple system atrophy (MSA) [11, 13]. Structurally, typical LBs appear as intracytoplasmic inclusions, 5–25 μm in diameter with a dense eosinophilic core and clearer surrounding halo. Ultra-structurally, they are composed of a dense core of filamentous and granular material that is surrounded by radially oriented filaments [14].
Several observations implicate α-synuclein in the pathogenesis of PD. A direct involvement of α-synuclein in the neurodegenerative processes in PD was demonstrated by genetic evidence. Autosomal dominant early-onset Parkinson’s disease is present in a small number of kindreds as a result of three different missense mutations in the α-synuclein gene, corresponding to A30P, E46K, and A53T substitutions in the α-synuclein protein sequence [15–17], or as a result of the hyper-expression of wild type α-synuclein due to the gene duplication/triplication [18–20]. Antibodies to α-synuclein detected this protein in LBs and LNs, the hallmark lesions of PD [21]. A substantial portion of fibrillar material in these specific inclusions was shown to be α-synuclein, and insoluble α-synuclein filaments were recovered from purified LBs [21, 22]. The production of wild type α-synuclein in transgenic mice [23] or of WT, A30P, and A53T in transgenic flies [24], led to motor deficits and neuronal inclusions reminiscent of PD. Cells transfected with α-synuclein develop LB-like inclusions under certain conditions. Other important observations correlating α-synuclein and PD pathogenesis, being reviewed in more detail elsewhere [25–32], are briefly outlined below. Numerous studies from different laboratories have established that recombinant α-synuclein easily assembles into amyloid-like fibrils in vitro and this process is modulated by familial point mutations. α-Synuclein is abnormally phosphorylated, ubiquitinated, and nitrated in pathology-related inclusions. Co-expression of chaperones or β-synuclein with α-synuclein in transgenic animals suppresses neurodegeneration. α-Synuclein-positive proteinaceous deposits were shown to accumulate in several animal models where Parkinsonism was induced by exposure to different neurotoxicants. Based on all these observations it is clear that PD is a typical synucleinopathy; i.e., a neurode-generative disorder whose pathogenesis is somehow linked to α-synuclein aggregation.
2. α-SYNUCLEIN AND SYNUCLEINOPATHIES
α-Synuclein is involved not only in pathogenesis of PD, but is also commonly associated with a diverse group of neurodegenerative diseases, known as synucleinopathies. These maladies share common pathologic inclusions composed of aggregated α-synuclein, which are deposited in selectively vulnerable neurons and glia [25, 33–35]. The term synucleinopathy was introduced in 1998 after filamentous α-synuclein deposits were found not only in PD, but also in MSA and DLB [36]. Since that discovery, α-synuclein-containing inclusions have been observed in increasing numbers of neurodegenerative diseases. Growing evidence associates the onset and progression of clinical symptoms, as well as the degeneration of affected brain regions, in all these neurodegenerative disorders with the formation of abnormal filamentous aggregates containing α-synuclein. Some of these maladies include: neurodegeneration with brain iron accumulation type 1 (NBIA1), pure autonomic failure, Down’s syndrome, complex of Guam, and several Lewy body disorders, such as diffuse Lewy body disease (DLBD), the Lewy body variant of Alzheimer’s disease (LBVAD), and PD dementia (PDD) [11–13, 21, 36–40]. All the aforementioned disorders are brain amyloidoses unified by pathological intracellular inclusions containing α-synuclein as a major component [22, 25, 33–36, 40, 41]. Some of the synucleinopathies are briefly outlined below.
2a. Dementia with Lewy Bodies
DLB is the second most frequent late-onset dementia after AD. It exists either in a pure form, or overlaps with the neuropathological features of AD. Neurophathological hallmarks of DLB are numerous α-synuclein-positive LBs and LNs in the substantia nigra [21], and in the cortical brain area [42]. In most DLB cases, LBs are frequently associated with typical AD-associated lesions: neurofibrillary tangles and amyloid plaques [43, 44].
2b. PD Dementia
The incidence of dementia in PD is higher than expected from aging alone [45]. In fact, dementia affects ~40% of PD patients [46], and the prevalence of dementia in PD patients is up to six times greater than observed in normal age matched controls [47]. It is believed that the LB diseases are characterized by a clinical continuum [48], from pure PD cases characterized solely by motor dysfunction, to numerous PD patients diagnosed clinically as having PD dementia (PDD), to DLB cases that combine movement dysfunction with dementia. DLB and PDD, being different in the temporal course of the disease, share most of the same clinical and neuropathologic features and are often considered as belonging to a spectrum of the same disease [49–51].
2c. Amyotrophic Lateral Sclerosis-Parkinsonsim-Dementia Complex of Guam
Guam disease (Guam ALS/PDC) frequently affects the Chamorro people of Guam [52–54] and has been proposed to be caused by seeds of the neurotoxic plant Cycas circinalis, commonly used by the Chamorro people as a traditional source of food and medicine [54], although more recent studies have questioned this conclusion [55, 56]. In general it appears that the three neurodegenerative diseases, ALS, dementia, and PD, co-occur within families more often than expected by chance, suggesting that there may be a shared genetic susceptibility to these maladies [57]. As might therefore be expected, α-synuclein pathology has been associated with Guam ALS/PDC [58].
2d. Other LB Diseases
LB deposition can affect several peripheral and central areas of the nervous system, such as the substantia nigra, hypothalamic nuclei, nucleus basalis of Meynert, dorsal raphe, locus ceruleus, dorsal vagus nucleus, and intermedio-lateral nucleus [59]. A ‘neuritic’ form of LB was also described in the dorsal vagus nucleus, sympathetic ganglia, and in intramural autonomic ganglia of the gastrointestinal tract, and cases are also known with extensive cortical and basal ganglia involvement [42, 60]. This broad spectrum of nervous system regions affected by LB deposition produces great variability in disease manifestation [27].
2e. Alzheimer’s Disease
AD is the most common aging-related neurological disorder. It is characterized by slow, progressive memory loss and dementia due to a gradual neurodegeneration in the cortex and hippocampus [61]. The pathological hallmarks of AD are neuronal loss, extracellular senile plaques containing the peptide Aβ, and neurofibrillary tangles composed of a hyperphosphorylated form of the microtubule associated protein tau [62]. α-Synuclein-positive inclusions resembling LBs and LNs were found in ~50% cases of sporadic AD [63]. LB-like intra-cytoplasmic inclusions were found in the amygdala, the temporal cortex, the parahippocampal gyrus, and in the parietal cortex, whereas LN-like inclusions were abundant in the amygdala, the CA2/3 region of hippocampus formation, parahippocampal gyrus, the temporal cortex, substantia nigra, locus ceruleus, the frontal cortex, and in the parietal cortex [63].
2f. Down’s Syndrome
Down’s syndrome is a genetic disorder characterized by an extra chromosome 21 (trisomy 21). Numerous LBs and LNs were found in the neurons of the limbic areas, predominantly of the amygdala, of Down’s syndrome patients with AD pathology [64, 65].
2g. Multiple System Atrophy
MSA is an adult-onset progressive neurodegenerative disorder of unknown etiology. It is characterized by any combination of parkinsonian, autonomic, cerebellar or pyramidal symptoms, and pathologically by the variable neuron loss in the striatum, substantia nigra pars compacta, cerebellum, pons, inferior olives and intermediolateral column of the spinal cord [66]. The histological hallmarks of MSA include argyrophilic fibrillary inclusions in the oligodendrocytes and glial cytoplasmic inclusions (GCIs), which are also known as Papp-Lantos bodies [67]. Fibrillar inclusions are also found in the neuronal somata, axons, and nucleus and neuronal cytoplasmic inclusions are frequently seen in the pontine and inferior olivary nuclei [68]. A major component of these MSA-specific glial and neuronal inclusions is α-synuclein [36, 68].
2h. Neurodegeneration with Brain Iron Accumulation Type 1
NBIA1, formerly know as Hallervorden-Spatz disease (HSD) or adult neuroaxonal dystrophy, is a rare neurodegenerative malady that occurs in both sporadic and familial forms. Clinically, NBIA 1 is characterized by rigidity, dystonia, dyskinesia, and choreoathetosis [69–72], together with dysarthria, dysphagia, ataxia, and dementia [72–74]. Histopathologically, NBIA1 is characterized by neuronal loss, neuraxonal spheroids, and iron deposition in the globus pallidus and substantia nigra pars compacta, as well as by the presence of LB-like and GCI-like inclusions and dystrophic neuritis [73]. These LB-like inclusions throughout the cortex and brainstem, axonal swellings, and the rare GCI-like inclusions of the midbrain clearly possess α-synuclein immunoreactivity [75–77]. Importantly, axonal spheroids were also shown to contain α-synuclein [77, 78].
3. STRUCTURAL PROPERTIES AND CONFORMATIONAL BEHAVIOR OF α-SYNUCLEIN
α-Synuclein is an abundant brain protein of 140 residues, lacking both cysteine and tryptophan residues. This protein is present in high concentration at presynaptic terminals and is found in both soluble and membrane-associated fractions of the brain. α-Synuclein was estimated to account for as much as 1% of the total protein in soluble cytosolic brain fractions [79]. Several possible functions have been suggested, including synaptic vesicle release and trafficking, fatty acid binding and physiological regulation of certain enzymes, transporters, and neurotransmitter vesicles, as well as roles in neuronal survival [29]. α-Synuclein was shown to physically interact with at least 50 ligands including >30 proteins [29, 31, 80]. Recently, a proteomic analysis identified 324 α-synuclein-interacting proteins in the dopaminergic cells of the MES cell line. Almost half of these interactors (141 proteins) experienced a significant change in their relative abundance (increase or decrease) after the MES cell were treated with rotenone [81], a pesticide that produces parkinsonism in animals and induces LB-like inclusions in the remaining dopaminergic neurons [82]. The involvement of α-synuclein in the control of the neuronal apoptotic response and in the protection of neurons from various apoptotic stimuli was demonstrated [83].
At the level of amino acid sequence, the structure of α-synuclein can be divided into three regions: residues 1–60, which contain four 11-amino acid imperfect repeats (coding for amphipathic helices) with a conserved motif (KTKEGV); residues 61–95, which contain the highly amyloidogenic NAC region and two additional repeats; and the highly charged C-terminal region, residues 96–140. The first two regions comprise a lipid-binding domain, whereas the C-terminal tail is thought to be a protein-protein interaction motif.
In 1996, Weinreb et al. [84] showed that when isolated in solution, α-synuclein (known at that time as NACP for the “non-Aβ component of Alzheimer’s disease amyloid plaque” (NAC) precursor protein) had a much larger Stokes radius (34 Å) but sedimented more slowly (s20,w = 1.7S) than globular proteins of similar molecular weight suggesting that it is elongated. According to circular dichroism (CD) in the far-UV region and Fourier-transform infrared spectroscopy (FTIR), NACP/α-synuclein was shown to be devoid of significant amounts of secondary structure. The structure of this protein was practically unaffected by boiling, pH, salt, and chemical denaturants and was independent of protein concentration. All this indicated that NACP/α-synuclein likely belongs to a class of proteins referred to as natively unfolded proteins [84].
3a. Basic Structural Properties of α-Synuclein
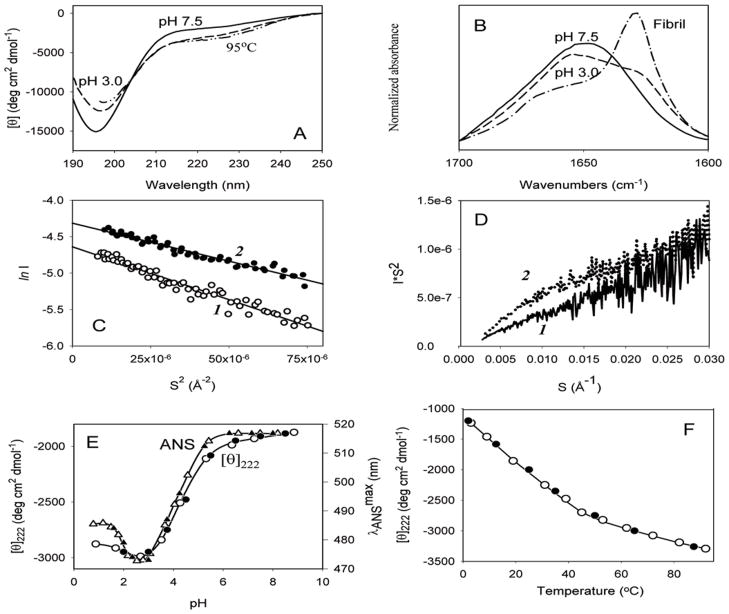
Subsequent studies supported this conclusion and added some new features to the structural description of this protein. Fig. (1) presents some of the data obtained in the laboratory of Prof. Anthony L. Fink (Tony) by the middle of 2000 [85]. At neutral pH, α-synuclein possessed far-UV CD (Fig. (1A)) and FTIR (amide I region, Fig. (1B)) spectra typical of an unfolded polypeptide chain. In fact, the far-UV CD spectrum was characterized by a minimum in the vicinity of 196 nm and the absence of bands in the 210–230 nm region, whereas a broad peak at 1650 cm−1 in FTIR spectrum suggested that the majority of the molecule (~70%) was disordered [85]. The hydrodynamic properties of α-synuclein analyzed by size exclusion chromatography and small-angle X-ray scattering (SAXS) were in agreement with the results of far-UV CD and FTIR studies. α-Synuclein was shown to be slightly more compact than expected for a random coil [85, 86], as the Stokes radius measured for α-synuclein by size-exclusion chromatography was notably lower than that calculated for a completely unfolded polypeptide chain of the appropriate molecular mass. NMR diffusion measurements confirmed these results as well [87] and Rg values calculated for α-synuclein at neutral pH from SAXS data using the Guinier approximation (40±1 Å, Fig. (1C)) were also significantly smaller than those estimated for a random coil polypeptide of the same length (52 Å) [85]. The analysis of SAXS data in the form of Kratky plots provides information on the globularity (packing density) and the conformation of a polymer molecule, being able to distinguish between a compact globular conformation and a chain-like extended conformation [88–98]. The scattering curve for a globular conformation follows Porod’s law — I(Q) ∞ Q−4 — at large values of Q and the scattering intensity from the expanded chain molecule is proportional to Q−2 in the moderate Q region and to Q−1 in the high Q region. Thus, the Kratky plot for a globular conformation shows a clear peak, whereas the plot for a chain-like conformation has a plateau and then rises monotonically [95]. Fig. (1D) shows that the profile of the Kratky plot of α-synuclein at neutral pH was typical for a random coil conformation. Thus, at neutral pH α-synuclein was shown to be essentially disordered, but slightly more compact than a random coil [85]. The compactness of α-synuclein was also later confirmed by NMR studies, which posited [99] and subsequently demonstrated [100–102] the presence of transient long-range contacts within the protein. Thus, α-synuclein definitely belongs to the family of intrinsically disordered proteins, and more specifically to the subfamily of its most disordered members, known as natively unfolded proteins, which are characterized by a unique combination of low overall hydrophobicity and high net charge [103–107].
Fig. 1.
Structural properties and conformational behavior of human α-synuclein. (A). Far-UV CD spectra measured under different conditions. (B). FTIR spectra measured for natively unfolded, partially folded and fibrillar forms of α-synuclein. Guinier (C) and Kratky plot (D) representation of the results of SAXS analysis of human α-synuclein at different experimental conditions: 1 – pH 7.5; (E). pH-Induced partial folding of α-synuclein. (F). Temperature-induced folding of the natively unfolded α-synuclein.
3b. Structure-Forming Effects of pH and Temperature
At the logical next step, the effects of basic environmental factors on structural properties of α-synuclein were evaluated in Tony’s lab. This was a fundamental question at the time, as very little was known about the conformational behavior of structureless proteins. An educated guess, based on the assumption that the unfolded nature of α-synuclein is determined by low overall hydrophobicity and high net charge, posited that any alterations in the protein environment leading to an increase in hydrophobicity and/or decrease in net charge should be accompanied by at least partial folding of the intrinsically disordered protein. At a basic level, these two parameters, charge and hydrophobicity, can be modulated via changes in the protein environment: the excess negative charge of α-synuclein at neutral pH (pI = 4.7) would be neutralized at low pH values, and the overall hydrophobicity of a protein will increase with increasing temperature. Therefore, the protein was expected to partially fold under conditions of low pH or high temperature [85].
Data presented in Fig. (1) support this hypothesis. According to Fig. (1A–1D), α-synuclein became more ordered at pH 3.0 or at high temperature. The protein clearly gained some ordered secondary structure (Fig. (1A) and (1B)), became a bit more compact (Fig. (1C), and developed the beginnings of a tightly packed core (Fig. (1D)). This meant that at acidic pH natively unfolded α-synuclein was transformed into a partially folded conformation with a significant amount of β-structure [85]. Furthermore, Fig. (1E) shows that a decrease in pH led to a noticeable blue shift of the ANS fluorescence maximum (from ~515 to ~475 nm, open triangles in Fig. (1C)) and that the pH-induced structural transitions monitored by ANS fluorescence and CD occur simultaneously in a co-operative manner and were completely reversible, excluding aggregation. This meant that protonation of α-synuclein resulted in the transformation of this natively unfolded protein into a partially folded conformation with a significant amount of ordered secondary structure, some compactness, the beginnings of a tightly packed core, and a high affinity for ANS [85].
Comparable results were obtained for the protein at high temperatures (Fig. (1)). The temperature-dependence of [θ]222 shown in Fig. (1F) revealed that an increase in temperature was also sufficient to induce the reversible formation of some ordered secondary structure in α-synuclein [85]. The major spectral changes occurred over the range of 3 to 50°C, with subsequent heating leading to a less pronounced effect. Analyses of these results indicated that high temperatures induced a reversible transition of α-synuclein into a partially folded intermediate with spectral properties similar to those of the species found at low pH [85].
Taken together, the results of this study indicated that α-synuclein, while being highly unstructured at neutral pH, is nevertheless not a random coil and has some residual structure [85]. Either a decrease in pH, or an increase in temperature is sufficient to transform α-synuclein into a partially folded pre-molten globule-like conformation. The structure-forming effects of low pH were attributed to the effective minimization of the high net charge of this protein. This net charge minimization decreased intramolecular charge-charge repulsion and therefore promoted the hydrophobic-driven collapse to a partially folded intermediate. The effect of elevated temperatures was attributed to the increased strength of the hydrophobic effect at higher temperatures, leading to a stronger hydrophobic driving force for folding [85].
3c. Effect of Other Factors on α-Synuclein Structure
Detailed analysis using a combination of low resolution techniques, such as CD, FTIR, fluorescence, and several hydrodynamic approaches [108–117], revealed that the PD-related point mutations A30P, E46K, and A53T do not affect the overall structure of human α-synuclein, which remains natively unfolded [108, 116, 117]. However, high resolution solution NMR spectroscopy revealed that the A30P mutation strongly attenuates the helical propensity found in the N-terminal region of wild type α-synuclein [99, 126]. The A53T mutation was found to exert a more modest influence on local structural propensity, resulting in a slightly enhanced preference for extended conformations in a small region around the site of mutation [99]. The E46K mutation results in subtle changes in the conformation of the monomeric protein [118]. These mutations were also proposed to modify long-range transient structure in α-synuclein [119] although this conclusion remains controversial [102]
Human α-synuclein was shown to bind specifically to synthetic vesicles containing acidic phospholipids [120, 121]. This binding was accompanied by a dramatic increase in α-helical content [120, 121] and was attributed to the formation of two curved antiparallel but non-contacting α-helices (Val3-Val37 and Lys45-Thr92) connected by a well ordered, extended linker [122-125], whereas the acidic, glutamate-rich C-terminal region (Asp98-Ala140) was shown to behave as a highly mobile tail; i.e., it remained unstructured even in the presence of membranes [125–127]. More recently, the helical structure of α-synuclein was shown to be dependent on the size of the lipid particle it is bound to [128] with the two helices able to fuse into a single long helix [129], and this transition has been proposed to be functionally important [130].
In general, the conformation of α-synuclein is highly pliable and sensitive to modification by numerous environmental factors. Although in the majority of cases such factors were shown to induce partial folding, the non-compact natively unfolded conformation was effectively stabilized via methionine oxidation [131–133]. In addition to low pH and high temperature [85], a pre-molten globule-like partially folded conformation was stabilized in α-synuclein by low concentrations of organic solvents [134] and TMAO [135], by different metal ions [136], salts [137], pesticides/herbicides [138–140], heparin and other glycosoaminoglycans [132], some polycations [141], or by spontaneous oligomerization both in vitro and in vivo [142]. The addition of high concentrations of different alcohols dramatically increased the degree of folding of α-synuclein [134], with simple alcohols inducing a β-sheet-enriched conformation and fluorinated alcohols promoting α-helix-rich species [134]. Alcohol-induced α-helical and β-structural species were initially monomeric but readily underwent association over longer time scales [134]. High concentrations of TMAO induced α-synuclein to form oligomeric α-helical globular species with rigid tertiary structure [135].
Prolonged incubation of α-synuclein at elevated temperatures results in a progressive aggregation, with pre-molten globule-like dimers formed first [142]. Under conditions of oxidative stress, the formation of oxidative dimers with dityrosine cross-links was also reported [143]. Besides covalent and non-covalent dimers, α-synuclein is able to form morphologically different soluble oligomers. For example, nitrated α-synuclein assembles into oligomeric spheroids [144]. The incubation of α-synuclein with different metals for 24 hours at 4° gave rise to three different classes of oligomers: Cu2+, Fe3+ and Ni2+ yielded 0.8–4 nm spherical particles, similar to α-synuclein incubated without metal ions; Mg2+, Cd2+ and Zn2+ gave larger, 5–8 nm spherical oligomers; Co2+ and Co2+ produced annular (doughnut-like) oligomers, 70–90 nm in diameter with Ca2+ and 22–30 nm in diameter with Co2+ [145]. Finally, under appropriate conditions, α-synuclein was shown to form amorphous aggregates and fibrils (see Fig. (2)), with the appearance of each type of insoluble aggregate being determined by the environmental conditions. Based on this astonishing conformational behavior, the concept of a protein-chameleon was proposed, according to which the structure of α-synuclein is mainly determined by the peculiarities of its environment [146].
Fig. 2.
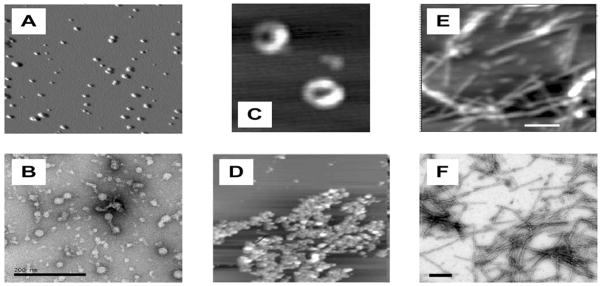
Various aggregated forms of human α-synuclein. Spherical oligomers as seen by AFM (A) and EM (B); annular oligomers (C) and amorphous aggregates (D) visualized by AFM; amyloid fibrils as seen by AFM (E) and EM (F).
4. AGGREGATION OF α-SYNUCLEIN: MOLECULAR MECHANISMS OF FATAL ATTRACTION
4a. Critical Amyloidogenic Partially Folded Intermediate
The formation of amyloid fibrils, amorphous aggregates or various oligomers induces different degrees of α-synuclein folding. Early stages of fibril formation by α-synuclein involve the formation of a pre-molten globule-like partially folded intermediate that is critical for α-synuclein fibrillation [85]. Fig. (1) shows that a decrease in pH or an increase in temperature forced α-synuclein to gain some ordered structure. As these same conditions led to the dramatic acceleration of α-synuclein fibrillation, it has been concluded that the pre-molten globule-like partially structured conformation is a key intermediate in the fibril-forming pathway [85]. This suggests a simple kinetic model of fibrillation involving conversion of monomeric α-synuclein into the critical partially folded intermediate, which leads to formation of the amyloidogenic nucleus and subsequent propogation into fibrils. Such intermediates typically have sizable nonpolar patches (i.e., spatial clusters of hydrophobic side chains) on their surface, which lead to hydrophobic interactions between molecules, resulting in intermolecular interactions and aggregation. These hydrophobic patches are absent in the natively unfolded monomeric α-synuclein. Factors that increase the concentration of such intermediates will favor aggregation [147]. This model has strong predictive power, as all intrinsic or extrinsic factors shifting the equilibrium in favor of this monomeric partially folded conformation are expected to facilitate the fibrillation process. As α-synuclein is a natively unfolded protein whose structure is extremely sensitive to its environment, such folding-promoting factors seem to be wide-ranging. Obvious instances include point mutations, non-polar molecules including some pesticides (that might preferentially bind to the partially-folded intermediate), cations (that might mimic the effect of low pH/high proton concentration), as well as factors that result in an increase in the concentration of α-synuclein itself or cause modifications of the protein (e.g., via oxidative damage, etc.) (reviewed in [31, 32, 146, 147]). Consistent with this model, the rate of α-synuclein fibril formation in vitro was shown to be highly dependent on the conditions used. In vitro α-synuclein fibril formation kinetics are described by a typical sigmoid curve that shows an initial lag phase followed by an exponential growth phase and a final plateau, usually attributed to a nucleation-dependent polymerization. Agitation is a strong accelerator of the fibril formation process: typically fibrillation of 70 FM (1 mg/mL) α-synuclein is completed within 3 days with agitation, whereas without agitation it takes months [147]. Many endogenous and exogenous factors were shown to increase the fibrillation rates. This acceleration can be attributed to the increased concentration of the amyloidogenic intermediate caused by the various conditions. Certain environmental factors are also known to inhibit fibril formation by α-synuclein. This inhibition of fibrillation occurs when either the monomer or non-fibrillogenic oligomers are stabilized [31, 32, 146, 147]. Some of these rate-modulating conditions are considered below.
4b. Genetic Factors
There are two major genetic abnormalities that directly link α-synuclein with neurodegeneration in familial early onset PD: overexpression of α-synuclein due to duplication or triplication of the α-synuclein gene locus [18–20] and missense mutations in the α-synuclein gene, corresponding to A30P, E46K, and A53T substitutions in α-synuclein protein [15–17]. Even in the absence of additional factors, α-synuclein exists in an equilibrium between the natively unfolded and the partially folded conformations. Although the overall probability for intermediate formation is normally low, increased protein concentrations will necessarily raise the total concentration of amyloidogenic intermediates, thus increasing accordingly the rate of fibril formation. In agreement with this argument, increases in α-synuclein concentration have been shown to result in decreased lag times and increased fibril elongation rates in vitro [85]. This observation is consistent with and may underlie the fact that autosomal-dominant PD (average age of onset, 34 years), ranging clinically from DLB to typical PD, is associated with the duplication/triplication of α-synuclein locus, which potentially enhances the level of α-synuclein production [18–20].
Studies of several kindreds with familial PD clearly show that a single mutation in the human α-synuclein gene is sufficient to cause PD. Importantly, all three PD-related point mutations, A30P, E49K, and A53T, were shown to accelerate α-synuclein aggregation (but not necessarily fibrillation) in vitro [108, 109, 111, 112, 114–117, 148]. The absence of major structural differences (at least those detectable by low resolution techniques, such as CD, FTIR, fluorescence, and hydrodynamics) between groups of natively unfolded and partially folded conformations of all these α-synuclein variants raised the question of how the mutations affect the aggregation propensity of the protein. Analysis of hydrophobicity and propensities to form β-sheet or α-helix for WT, A30P and A53T revealed that both mutations reduced hydrophobicity in the vicinity of the substitution, but the propensity to form α-helical structure was somewhat diminished in the N-terminal region of both mutants, whereas the predisposition to form β-structure was predicted to be slightly enhanced [116]. In agreement with these observations, high-resolution solution NMR analysis established that the A30P mutation disrupts a region of residual helical structure that exists in the wild type protein [126], whereas the A53T mutation results in a slight enhancement of a preference for extended conformation in a small region around the mutation site [99]. All this suggests that the increased internal susceptibility of A30P and A53T to form β-sheets may not be strong enough to alter the structure of the monomeric proteins, but may affect the aggregation behavior of the α-synuclein mutants through specific stabilization of an intermolecular β-structure [116]. As a result, the mutants show a faster rate of fibrillation (A53T) or amorphous aggregation (A30P).
As far as the E46K mutation is concerned, it is located in the fourth KTKEGV-type repeat in the amino-terminal region of α-synuclein. It has been emphasized that a Glu residue similar to E46 is present in five of the seven degenerative repeats in α-synuclein, and the only repeat that does not have such a residue (repeat 2) has Glu residues adjacent to each side of the repeat [117]. Based on these observations it has been suggested that the N-terminal region of α-synuclein and, more specifically, Glu residues in the repeats may be important in regulating the ability of α-synuclein to polymerize into amyloid fibrils [117].
4c. Molecular Crowding
The natural environment of any protein inside a living cell is extremely crowded, as the concentration of macromolecules, including proteins, nucleic acids, carbohydrates, and small solutes within a living cell is estimated to be as high as 400 g/L [149]. The volume occupied by solutes is unavailable to other molecules, giving rise to a phenomenon known as “excluded volume effects” [149, 150]. It has been suggested that volume exclusion in physiological media could modulate the rate and the extent of the amyloid formation in vivo [151]. The validity of this hypothesis was confirmed for the in vitro fibrillation of human α-synuclein [152, 153]. Pesticides and metals, which are linked to increased risk of PD via the epidemiological studies (see below), were also shown to further accelerate α-synuclein fibrillation when present under conditions of molecular crowding [154–156].
4d. Anions and Salts
Partial folding of α-synuclein at neutral pH to the potentially amyloidogenic intermediate can be induced by various anions. However, the magnitude of the fibrillation accelerating effect varied significantly between anions and generally followed the position of the anions in the Hofmeister series, suggesting that the major role of anions in fibrillation is their modulation of protein-water interactions [154]. Based on these observations it has been concluded that the enhanced fibrillation of α-synuclein in the presence of anions is the result of partial folding induced by the loss of uncompensated charge and an increase in preferential hydration, which promotes partial folding and aggregation by strengthening hydrophobic interactions [154].
4e. Polyanions and Polycations
In several human diseases, glycosaminoglycans (GAGs) and proteoglycans (PGs) are involved in the formation of proteinaceous deposits [157, 158]. Different GAGs (heparin, heparan sulfate) and other highly sulfated polymers (dextran sulfate) as well as agrin (an extracellular matrix and trans-membrane heparan sulfate proteoglycan in the central neuronal system [159–161]) were shown to bind to α-synuclein and stimulate its fibrillation in vitro [132, 162]. Agrin and α-synuclein were shown to be co-localized in LBs and LNs found in the substantia nigra of PD patients, indicating that this PG may contribute to the etiology of PD by modulating the aggregation state of α-synuclein in dopaminergic neurons [162]. Similarly, several unstructured polycations such as polyethyleneimine, spermine, spermidine, polyLys, and polyArg were shown to interact with α-synuclein, to induce partial folding of this protein and to accelerate its oligomerization and fibrillation [141, 163].
4f. Environmental PD Risk Factors
PD is now considered as an “environmental” disease, since several lines of evidence point to environmental exposures as potential contributing factors in the pathogenesis of this malady [164–170]
4f.i. Pesticides and Herbicides
In a search for a potential explanation of the increased risk of PD associated with exposure to pesticides, the in vitro effect of several commonly used pesticides and herbicides on the structure and aggregation of human α-synuclein was analyzed. These studies revealed that several pesticides and herbicides induced a conformational change in α-synuclein and significantly accelerated its fibrillation [138–140]. These structure-forming and fibrillation-accelerating effects of hydrophobic pesticides were ascribed to their ability to bind and stabilize the amyloidogenic partially folded conformation [138, 139]. Importantly, a noticeable up-regulation of α-synuclein production was induced by the administration of paraquat to mice, which also led to the accumulation of α-synuclein containing fibrilar aggregates within the neurons of the substantia nigra [140]. These observations provide a rationale for using both in vitro and in vivo approaches for the screening of putative neurotoxicants, which, by affecting α-synuclein conformation and aggregation, may play a role in the pathogenesis of synucleinopathies [140].
4f.ii. Heavy Metals
Exposure to heavy metals is another potential PD risk factor [171–178]. Motivated by the results of epidemiological studies and postmortem analyses of the brain tissues of PD patients, Tony’s in vitro analysis established that a number of mono-, di-, and trivalent metal ions are able to accelerate the α-synuclein fibrillation process [136]. The effectiveness of metal cations in inducing fibrillation was shown to correlate with increasing ion charge density and with their ability to induce amyloidogenic partially folded species due to the masking of intramolecular Coulombic charge-charge repulsion in the natively unfolded α-synuclein molecule [136, 179].
4f.iii. Organic Solvents
Because of the increased incidence of PD resulting from the exposure to organic solvents [180–184], the structural properties and aggregation/fibrillation propensities of α-synuclein in water-organic solvent mixtures were also analyzed [134]. At low concentrations, all organic solvents studied effectively induced the amyloidogenic partially folded conformation and favored very rapid α-synuclein fibrillation [134].
4f.iv. Oxidative Stress: Aggregation Enhancers
Oxidative injury has been implicated in the pathogenesis of several disorders, such as AD [185, 186], PD [187], DLB [188], amyotrophic lateral sclerosis [189], Huntington’s disease [190], atherosclerosis and inflammatory diseases [191], chronic renal failure [192], cataractogenesis [193], and brain ischemia and carcinogenesis [194], and oxidatively modified proteins are known to accumulate during normal aging [195–197]. As α-synuclein does not have cysteines and tryptophanes, the primary targets for oxidative modifications are its methionine and tyrosine residues. Under mild oxidative conditions (1–2% H2O2), all four methionines of α-synuclein can be oxidized to the methionine sulfoxide, MetO [198]. Methionine oxidized α-synuclein was found to be more highly unfolded than the non-oxidized protein [131, 133, 198], less prone to oligomerize and aggregate, and even able to inhibit the fibrillation of non-modified α-synuclein [198]. The inhibition α-synuclein fibrillation by methionine oxidation was shown to be proportional to the number of oxidized methionines [199]. The presence of certain metals (Ti3+, Zn2+, Al3+ and Pb2+) could overcome this inhibition [131]. This suggests that the balance between the protective anti-oxidant role of the methionine residues and the protective anti-fibrillation effect of oxidized methionine residues in α-synuclein may fail under conditions of industrial pollution due to exposure to lead, aluminum, zinc, titanium, and other metals. In the presence of enhanced concentrations of such industrial pollutants, toxic insult-induced up-regulation of α-synuclein no longer plays a protective role; rather, it may represent a new risk factor, leading to the effective metal-triggered fibrillation of the methionine-oxidized protein [131].
4f.v. Oxidative Stress: Aggregation Inhibitors
All PD risk factors considered thus far are strong accelerators of α-synuclein fibrillation. Although for a long time it was believed that amyloid fibrils themselves were harmful, a novel emerging paradigm favors the idea that the deposited proteinaceous inclusions (such as senile plaques in AD or LBs and LNs in PD, etc.) are not cytotoxic [200] and even may be protective [201] favoring cell survival. As a result, an alternate amyloid hypothesis, the oligomer hypothesis, was proposed which claims that although mature amyloid fibrils may not be toxic, some species formed during the fibril assembly process may be responsible for cell damage. As fibrillation is known to involve oligomeric species, the formation of some small oligomers, known as protofibrils, was proposed to be responsible for neurotoxicity [202–209]. One of the potential mechanisms of this cytotoxicity is the ability of oligomeric intermediates populated during the conversion of proteins from a monomeric state into amyloid fibrils to permeabilize lipid bilayers and cell membranes [204–206, 208, 209]. Paragraphs below consider several PD risk factors potentially linked to the formation of such toxic oligomers.
One of the most frequent oxidative modifications of tyrosines is their nitration [210–212]. The selective and specific nitration of α-synuclein in PD and other synucleinopaties has been proposed to directly link oxidative and nitrative damage to the onset and progression of these maladies [213]. Nitration of α-synuclein in vitro was shown to be accompanied by partial folding and oligomerization of this protein [144, 214]. The formation of these soluble oligomers completely inhibited the fibrillation of nitrotyrosinated α-synuclein at neutral pH, and the addition of nitrated α-synuclein substantially inhibited the fibrillation of the non-modified protein in a concentration-dependent manner [144, 214].
In addition to various protein oxidative modifications, lipid oxidation is frequently induced as a result of the oxidative stress. One of the most crucial products of lipid oxidation is 4-hydroxy-2-nonenal (HNE), which has been implicated in PD pathogenesis. Recently, it has been shown that the co-incubation of HNE with α-synuclein led to covalent modification of the protein, with up to six HNE molecules incorporated as Michael addition products [215]. Highly increased β-sheet content was found by CD and FTIR in the HNE-modified α-synuclein, whereas fibrillation of this protein was inhibited in an HNE concentration-dependent manner. This inhibition was caused by the formation of soluble oligomers, which were compact and tightly packed according to SAXS analysis. The HNE-induced oligomers were very stable and their addition to primary mesencephalic cultures caused marked neurotoxicity [215].
4g. Protein-Protein Interactions
α-Synuclein is known to interact with various proteins (see above). Such interactions might have a significant influence on the aggregation and fibrillation of this protein. Therefore, several illustrative examples were studied in Tony’s lab. For example, the addition of either β- or γ-synuclein (two natively unfolded members of the synuclein family [86, 102]) inhibited α-synuclein fibrillation in a concentration-dependent manner [86]. As α-synuclein effectively inhibited α-synuclein aggregation in animal models [216], it has been suggested that β- and γ-synucleins may act as regulators of α-synuclein fibrillation in vivo, potentially acting as chaperones [86].
Later, core histones were shown to physically interact with α-synuclein and to accelerate α-synuclein fibrillation [163]. It also has been shown that paraquat administration triggers an upregulation of α-synuclein in the mouse brain [140] and is accompanied by α-synuclein translocation into the nucleus [163]. Nuclear translocation of α-synuclein and the formation of histone-α-synuclein complexes was suggested to provide a mechanism by which α-synuclein-related neuronal response may be activated or sustained. In this model, α-synuclein-histone complexes have a regulatory role by decreasing the pool of free histones available for DNA binding. The subsequent destabilization of nucleosomes and enhanced manifestation of the DNA matrix activity could lead to increased transcription, and ultimately to the production of proteins in response to a variety of stimuli, including toxic insults [163].
Another α-synuclein-binding protein analyzed in Tony’s lab was DJ-1 [217], a multifunctional protein known to prevent protein aggregation and to serve as a molecular chaperone [218]. The fibrillation inhibition efficiency of DJ-1 was shown to be directly related to its oxidation state: native (unoxidized) DJ-1 did not interact with α-synuclein and did not inhibit its fibrillation, whereas being oxidized via the sulfinic acid of Cys106 formation, DJ-1 very effectively prevented α-synuclein fibrillation in vitro [217].
4h. Interaction with Membranes
Several studies have demonstrated α-synuclein’s association with lipids [79, 121, 219–225]. In fact, this protein is found both in the cytosol and in association with membranes in the presynaptic region of neurons. The membrane-bound state of α-synuclein has also been suggested to play an important role in fibril formation [226]. The N-terminal region (approximately residues 1–95) of α-synuclein contains six 11-amino acid imperfect repeats with a highly conservative hexamer motif (KTKEGV), resulting in a variation in hydrophobicity with a strictly conserved periodicity of 11 residues [122, 124, 127]. Such a periodicity is characteristic of the amphipathic lipid-binding α-helical domains of apolipoproteins [223, 227]. In 2003, the interaction of α-synuclein with lipid vesicles of different composition and sizes and the effects of these interactions on the protein conformation and the fibrillation kinetics were analyzed [228]. Different factors, such as the phospholipid composition, the size of the vesicles, and the ratio of α-synuclein to phospholipid, were shown to play a role in modulation of α-synuclein-vesicle interaction. High lipid concentrations induced α-helical structure in α-synuclein and inhibited its fibrillation. At relatively low lipid concentrations, acidic phospholipids were shown to induce the partial folding of α-synuclein and to accelerate its fibrillation. A strong correlation was observed between the induction of α-helix in α-synuclein and the inhibition of fibril formation. This suggested that α-helical, membrane-bound α-synuclein is unlikely to contribute to aggregation and fibrillation [228].
Of great importance was Tony’s related finding that α-synuclein-membrane interactions affect both protein and membrane [229]. The association of the protein with the bi-layer led to disruption of the membrane, and the kinetics of α-synuclein fibril formation were significantly affected by the protein association and subsequent membrane disruption. The ability of α-synuclein to disrupt membranes was shown to be correlated with the protein’s binding affinity and the protein’s α-helicity. In these experiments, protofibrillar α-synuclein caused a much more rapid destruction of the membrane than soluble monomeric protein, clearly showing that protofibrils (oligomers) are likely to be significantly neurotoxic [229].
It has recently been reported that α-synuclein can be associated with lipid rafts and caveolae [230–232]. Caveolae are specialized neuronal membrane domains that are enriched in the caveolin family of proteins and sphingolipids, mostly gangliosides and sphingomyelin. Lipid rafts are specialized plasma membrane microdomains that are enriched in cholesterol and sphingolipids, with similar lipid composition to caveolae. GM1 ganglioside is a molecular marker for these regions and is specifically enriched in the caveolae. Interaction of α-synuclein with ganglioside GM1-containing small unilamellar vesicles (SUVs,) induced substantial α-helical structure in this protein and inhibited or completely eliminated α-synuclein fibril formation, depending on the amount of GM1 present [233]. The interaction of α-synuclein with GM1-containing SUVs was accompanied by α-synuclein oligomer formation. The familial A53T mutation had no effect on the interaction between α-synuclein and GM1-containing SUVs, whereas the A30P mutation dramatically inhibited this interaction [233]. This GM1-modulated recruitment of α-synuclein to caveolae and lipid rafts could explain the preferential localization of this protein to presynaptic membranes [233].
4i. Small Molecules
α-Synuclein aggregation and dopamine are obviously brought together in vivo, as the common pathway for both idiopathic and familial PD is the damage and subsequent loss of dopaminergic neurons accompanied by the formation of α-synuclein containing LBs and LNs in the substantia nigra pars compacta [234]. Several catecholamines, including L-dopa and dopamine, were shown to inhibit α-synuclein fibrillation and even to dissolve the preformed fibrils in vitro [235]. The oxidation products derived from these catecholamines were more potent inhibitors of the α-synuclein fibrillation than the non-oxidized catecholamines [235, 236].
According to epidemiological studies, leprosy patients have a significantly lower probability of developing AD if they had been undergoing antileprosy treatment with rifampicin and closely related drugs for the preceding several years [237–239]. Therefore, it has been suggested that these antileprosy drugs might inhibit Aβ aggregation and prevent amyloid deposition [240]. In fact, rifampicin and its analogs were shown to inhibit Aβ aggregation and neurotoxicity in vitro [240–242]. Similarly, rifampicin eliminated α-synuclein fibrillation in vitro [243]. Furthermore, it is was able to disaggregate preformed α-synuclein fibrils in a concentration-dependent manner and led to the formation of soluble oligomers comprised of partially folded α-synuclein [243].
The flavonoid baicalein is the main component of the traditional Chinese herbal medicine Scutellaria baicalensis, also known as Chinese skullcap or Huang Qin, which has multiple biological activities including antiallergic, anticarcinogenic, antiviral, antibacterial, anti-inflammatory, and anti-HIV properties [114, 244–247]. Micromolar concentrations of baicalein or its oxidized forms were shown to inhibit the formation of α-synuclein fibrils, and to disaggregate pre-formed fibrils in vitro giving rise to soluble oligomers [248]. Recently, the structural properties of the baicalein-stabilized α-synuclein oligomers, purified by SEC-HPLC, and their conformational stability was analyzed using a set of biophysical techniques such as CD, FTIR, SEC-HPLC, SAXS, EM, and AFM [249]. This analysis revealed that the baicalein-stabilized oligomers possess very specific structural features, being spherical in shape (according to the AFM and EM data), having relatively globular structure with packing density intermediate between that of pre-molten globules and typical globular proteins (according to the Kratky plot analysis of the SAXS data), and having relatively well-developed secondary structure (according to the FTIR and far-UV CD analysis). These oligomers were characterized by high thermodynamic stability (as evidenced by the results of the unfolding analyses and by the fact that their shape did not change after the prolonged incubation) and were able to inhibit fibrillation of non-baicalein-treated α-synuclein. The most important finding was the fact that these highly stable and fibrillation-inhibiting oligomers did not disrupt the integrity of biological membrane (at least they are not more disruptive than the monomeric protein) [249]. This important observation demonstrated for the first time that formation of soluble oligomers is not always harmful and can be beneficial. These findings have paved the road for the development of novel therapies for PD, whose molecular mechanism of action could be similar to that of baicalein – specific stabilization of the thermodynamically stable, non-fibrillating soluble oligomers that can inhibit α-synuclein fibrillation and do not cause the inappropriate membrane permealization [249].
4j. Smoking and PD
Interestingly, there is an apparent negative association between smoking or caffeine intake and PD [250, 251] (the risk of PD in nonsmokers is about twice that of smokers, cigarette smokers are about 50% less likely to have PD and patients with PD are about 50% less likely to have smoked cigarettes during their lifetime [252, 253]). This association is again correlated with in vitro studies, where the presence of nicotine and caffeine was shown to have no accelerating effect on the kinetics of α-synuclein fibrillation [139]. Recently, more detailed analysis of the fibrillation of α-synuclein in the presence of five different compounds found in cigarette smoke: anabasine, cotinine, hydroquinone, nicotine and nornicotine, revealed that nicotine and hydroquinone effectively inhibited α-synuclein fibrillation and stabilized soluble oligomeric forms [254]. In fact, instead of insoluble amyloid-like fibrils, three stable soluble oligomeric forms were formed, which according to AFM imaging were mostly spherical species with heights of ~16 nm, ~10 nm, and ~4 nm. Furthermore, it was shown that nicotine and hydroquinone successfully inhibited A53T fibrillation as well [254]. The fact that nicotine and hydroquinone effectively inhibited α-synuclein fibrillation and stabilized soluble oligomeric forms, combined with the results of epidemiological studies showing that smoking and PD incidence are negatively correlated, suggested that the nicotine- or hydroquinone-stabilized α-synuclein oligomers might be similar to those stabilized by baicalein (see above). These studies supported the hypothesis that protein aggregates (including oligomers) are highly heterogeneous. This heterogeneity might be caused either by heterogeneous starting materials or by multiple pathways of assembly, or by both these factors. Therefore, it is unlikely that all soluble oligomers, considering their astonishing morphological variability, will be cytotoxic to the same degree [254].
V. CONCLUDING REMARKS
The contributions of Tony Fink to understanding the structure of α-synuclein and the molecular mechanisms of its aggregation are truly remarkable. His research in this area was extremely productive: out of 1216 ISI Web of Knowledge entries dealing with α-synuclein aggregation, 44 papers were from Tony’s lab. On average, each of his 44 papers was cited 56 times, which is almost twice as high as the average citation rate of the remaining 1172 papers. As of January 7, 2009, his first paper on the structure and fibrillation of human α-synuclein [85], being cited 230 time, occupies position 18 among the 1216 papers on α-synuclein aggregation. Out of 189 highly cited α-synuclein-related papers (i.e., papers that are cited 50 or more times), 18 are from his lab. In other words, 40.9% of Tony’s papers are highly cited, whereas this parameter is equal to 14.6% for all other papers in this field. These numbers provide a formal basis for our opinion that Tony’s research on α-synuclein structure and aggregation was very productive and his work exerted a significant impact on this field. This review includes an overview of some of his papers, covering a very broad range of topics, from meticulous descriptions of α-synuclein structure and conformational behavior, to elucidation of the molecular mechanisms of α-synuclein aggregation and fibrillation in vitro, to detailed evaluation of the various PD risk factors in the simplest in vitro model of the highly complex syndrome known as PD, i.e., in physiological solutions containing purified α-synuclein.
Acknowledgments
This work was supported in part by NIH R01 grants LM007688 and GM071714 (VU) and AG019391 and AG025440 (DE), by the Program of the Russian Academy of Sciences for the “Molecular and cellular biology” (VU), by the Irma T. Hirschl Foundation (DE), and by a gift from Herbert and Ann Siegel (DE). The support of the IUPUI Signature Centers Initiative is gratefully acknowledged (VU).
ABBREVIATIONS USED
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- DLB
Dementia with Lewy bodies
- MSA
Multiple system atrophy
- DLBD
Diffuse Lewy body disease
- LBVAD
Lewy body variant of Alzheimer’s disease
- HSD
Hallervorden-Spatz disease
- NBIA1
Neurodegeneration with brain iron accumulation type 1
- PDD
PD dementia
- LB
Lewy body
- LN
Lewy neurite
- NAC
Non-Aβ component of AD plagues
- Aβ
Amyloid beta peptide
- MetO
Methionine sulfoxide
- GAG
Glycosaminoglycan
- PG
Proteoglycan
- HNE
4-Hydroxy-2-nonenal
References
- 1.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 2.Moghal S, Rajput AH, D’Arcy C, Rajput R. Prevalence of movement disorders in elderly community residents. Neuroepidemiology. 1994;13:175–178. doi: 10.1159/000110376. [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann NY Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanner CM. Is the cause of Parkinson’s disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–142. [PubMed] [Google Scholar]
- 5.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 6.Mizuno Y, Hattori N, Kitada T, Matsumine H, Mori H, Shimura H, Kubo S, Kobayashi H, Asakawa S, Minoshima S, Shimizu N. Familial Parkinson’s disease: alpha-synuclein and parkin. Adv Neurol. 2001;86:13–21. [PubMed] [Google Scholar]
- 7.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 8.Lewy FH. In: Paralysis Agitans Pathologische Anatomie. Lewandowski M, editor. Handbuch der Neurologie, Springer; Berlin: 1912. pp. 920–933. [Google Scholar]
- 9.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki H, Lipkin LE, Aronson SM. Diffuse intracytoplasmic ganglionic inclusions (Lewy type) associated with progressive dementia and quadriparesis in flexion. J Neuropathol Exp Neurol. 1961;20:237–244. doi: 10.1097/00005072-196104000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Trojanowski JQ, Goedert M, Iwatsubo T, Lee VM. Fatal attractions: abnormal protein aggregation and neuron death in Parkinson’s disease and Lewy body dementia. Cell Death Differ. 1998;5:832–837. doi: 10.1038/sj.cdd.4400432. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Abnormal accumulation of NACP/alpha-synuclein in neurodegenerative disorders. Am J Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 13.Lucking CB, Brice A. Alpha-synuclein and Parkinson’s disease. Cell Mol Life Sci. 2000;57:1894–1908. doi: 10.1007/PL00000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tennyson VM. Electron microscopic study of the developing neuroblast of the dorsal root ganglion of the rabbit embryo. J Comp Neurol. 1965;124:267–317. doi: 10.1002/cne.901240302. [DOI] [PubMed] [Google Scholar]
- 15.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 16.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 17.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 18.Singleton A, Gwinn-Hardy K, Sharabi Y, Li ST, Holmes C, Dendi R, Hardy J, Crawley A, Goldstein DS. Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain. 2004;127:768–772. doi: 10.1093/brain/awh081. [DOI] [PubMed] [Google Scholar]
- 19.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 20.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 21.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 22.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 24.Feany MB, Bender WW. A Drosophila model of Parkinson’s disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 25.Trojanowski JQ, Lee VM. Parkinson’s disease and related alpha-synucleinopathies are brain amyloidoses. Ann NY Acad Sci. 2003;991:107–110. doi: 10.1111/j.1749-6632.2003.tb07468.x. [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW. Alpha-synuclein and the Lewy body disorders. Curr Opin Neurol. 2001;14:423–432. doi: 10.1097/00019052-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 28.Goedert M. Parkinson’s disease and other alpha-synucleinopathies. Clin Chem Lab Med. 2001;39:308–312. doi: 10.1515/CCLM.2001.047. [DOI] [PubMed] [Google Scholar]
- 29.Dev KK, Hofele K, Barbieri S, Buchman VL, van der Putten H. Part II: alpha-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/s0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 30.Uversky VN, Fink AL. Amino acid determinants of alpha-synuclein aggregation: putting together pieces of the puzzle. FEBS Lett. 2002;522:9–13. doi: 10.1016/s0014-5793(02)02883-1. [DOI] [PubMed] [Google Scholar]
- 31.Uversky VN. Alpha-synuclein misfolding and neurodegenerative diseases. Curr Protein Pept Sci. 2008;9:507–540. doi: 10.2174/138920308785915218. [DOI] [PubMed] [Google Scholar]
- 32.Uversky VN. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 33.Goedert M. Filamentous nerve cell inclusions in neurodegenerative diseases: tauopathies and alpha-synucleinopathies. Philos Trans R Soc Lond B Biol Sci. 1999;354:1101–1118. doi: 10.1098/rstb.1999.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spillantini MG, Goedert M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann NY Acad Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 35.Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 36.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. Alpha-synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 38.Gai WP, Power JH, Blumbergs PC, Blessing WW. Multiple-system atrophy: a new alpha-synuclein disease? Lancet. 1998;352:547–548. doi: 10.1016/s0140-6736(05)79256-4. [DOI] [PubMed] [Google Scholar]
- 39.Arawaka S, Saito Y, Murayama S, Mori H. Lewy body in neurodegeneration with brain iron accumulation type 1 is immunoreactive for alpha-synuclein. Neurology. 1998;51:887–889. doi: 10.1212/wnl.51.3.887. [DOI] [PubMed] [Google Scholar]
- 40.Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson’s disease. Neurosci Lett. 1997;239:45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- 41.Lundvig D, Lindersson E, Jensen PH. Pathogenic effects of alpha-synuclein aggregation. Brain Res Mol Brain Res. 2005;134:3–17. doi: 10.1016/j.molbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta Neuropathol (Berl) 1978;42:127–134. doi: 10.1007/BF00690978. [DOI] [PubMed] [Google Scholar]
- 43.Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clin Neuropathol. 1984;3:185–192. [PubMed] [Google Scholar]
- 44.Deramecourt V, Bombois S, Maurage CA, Ghestem A, Drobecq H, Vanmechelen E, Lebert F, Pasquier F, Delacourte A. Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2006;65:278–288. doi: 10.1097/01.jnen.0000205145.54457.ea. [DOI] [PubMed] [Google Scholar]
- 45.Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- 46.Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, Stern Y. A population-based investigation of Parkinson’s disease with and without dementia. Relationship to age and gender. Arch Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- 47.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 48.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 49.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 50.McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O’Brien J, Playfer J, Reid W. Dementia with Lewy bodies. Lancet Neurol. 2004;3:19–28. doi: 10.1016/s1474-4422(03)00619-7. [DOI] [PubMed] [Google Scholar]
- 51.Emre M. Dementia in Parkinson’s disease: cause and treatment. Curr Opin Neurol. 2004;17:399–404. doi: 10.1097/01.wco.0000137529.30750.ab. [DOI] [PubMed] [Google Scholar]
- 52.Plato CC, Cruz MT, Kurland LT. Amyotrophic lateral sclerosis-Parkinsonism dementia complex of Guam: further genetic investigations. Am J Hum Genet. 1969;21:133–141. [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt HP, Emser W, Heimes C. Familial occurrence of amyotrophic lateral sclerosis, parkinsonism, and dementia. Ann Neurol. 1984;16:642–648. doi: 10.1002/ana.410160604. [DOI] [PubMed] [Google Scholar]
- 54.Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 55.Stone R. Guam: deadly disease dying out. Science. 1993;261:424–426. doi: 10.1126/science.8332906. [DOI] [PubMed] [Google Scholar]
- 56.Steele JC, McGeer PL. The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology. 2008;70:1984–1990. doi: 10.1212/01.wnl.0000312571.81091.26. [DOI] [PubMed] [Google Scholar]
- 57.Majoor-Krakauer D, Ottman R, Johnson WG, Rowland LP. Familial aggregation of amyotrophic lateral sclerosis, dementia, and Parkinson’s disease: evidence of shared genetic susceptibility. Neurology. 1994;44:1872–1877. doi: 10.1212/wnl.44.10.1872. [DOI] [PubMed] [Google Scholar]
- 58.Sebeo J, Hof PR, Perl DP. Occurrence of alpha-synuclein pathology in the cerebellum of Guamanian patients with parkinsonism-dementia complex. Acta Neuropathol. 2004;107:497–503. doi: 10.1007/s00401-004-0840-4. [DOI] [PubMed] [Google Scholar]
- 59.den Hartog Jager WA, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kosaka K, Mehraein P. Dementia-Parkinsonism syndrome with numerous Lewy bodies and senile plaques in cerebral cortex. Arch Psychiatr Nervenkr. 1979;226:241–250. doi: 10.1007/BF00342237. [DOI] [PubMed] [Google Scholar]
- 61.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 62.Clark CM, Ewbank D, Lee VM-Y, Trojanowski JQ. Molecular Pathology of Alzheimer’s Disease: Neuronal Cytoskeletal Abnormalities. In: Growdon JH, Rossor MN, editors. The Dementias Vol 19 of Blue Books of Practical Neurology. Butterworth–Heinemann; Boston: 1998. pp. 285–304. [Google Scholar]
- 63.Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y. Alpha-synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res. 2001;888:287–296. doi: 10.1016/s0006-8993(00)03082-1. [DOI] [PubMed] [Google Scholar]
- 64.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol. 1999;45:353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Marui W, Iseki E, Ueda K, Kosaka K. Occurrence of human alpha-synuclein immunoreactive neurons with neurofibrillary tangle formation in the limbic areas of patients with Alzheimer’s disease. J Neurol Sci. 2000;174:81–84. doi: 10.1016/s0022-510x(99)00327-5. [DOI] [PubMed] [Google Scholar]
- 66.Daniel S. The Neuropathology and Neurochemistry of Multiple System Atrophy. In: Mathias CJ, Bannister R, editors. Autonomic Failure. Oxford University Press; Oxford: 1999. pp. 321–328. [Google Scholar]
- 67.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 68.Wakabayashi K, Takahashi H. Cellular pathology in multiple system atrophy. Neuropathology. 2006;26:338–345. doi: 10.1111/j.1440-1789.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 69.Taylor TD, Litt M, Kramer P, Pandolfo M, Angelini L, Nardocci N, Davis S, Pineda M, Hattori H, Flett PJ, Cilio MR, Bertini E, Hayflick SJ. Homozygosity mapping of Hallervorden-Spatz syndrome to chromosome 20p12.3-p13. Nat Genet. 1996;14:479–481. doi: 10.1038/ng1296-479. [DOI] [PubMed] [Google Scholar]
- 70.Malandrini A, Cesaretti S, Mulinari M, Palmeri S, Fabrizi GM, Villanova M, Parrotta E, Montagnani A, Montagnani M, Anichini M, Guazzi GC. Acanthocytosis, retinitis pigmentosa, pallidal degeneration. Report of two cases without serum lipid abnormalities. J Neurol Sci. 1996;140:129–131. doi: 10.1016/0022-510x(96)00155-4. [DOI] [PubMed] [Google Scholar]
- 71.Sugiyama H, Hainfellner JA, Schmid-Siegel B, Budka H. Neuroaxonal dystrophy combined with diffuse Lewy body disease in a young adult. Clin Neuropathol. 1993;12:147–152. [PubMed] [Google Scholar]
- 72.Swaiman KF. Hallervorden-Spatz syndrome and brain iron metabolism. Arch Neurol. 1991;48:1285–1293. doi: 10.1001/archneur.1991.00530240091029. [DOI] [PubMed] [Google Scholar]
- 73.Dooling EC, Schoene WC, Richardson EP., Jr Hallervorden-Spatz syndrome. Arch Neurol. 1974;30:70–83. doi: 10.1001/archneur.1974.00490310072012. [DOI] [PubMed] [Google Scholar]
- 74.Jankovic J, Kirkpatrick JB, Blomquist KA, Langlais PJ, Bird ED. Late-onset Hallervorden-Spatz disease presenting as familial parkinsonism. Neurology. 1985;35:227–234. doi: 10.1212/wnl.35.2.227. [DOI] [PubMed] [Google Scholar]
- 75.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 76.Wakabayashi K, Yoshimoto M, Fukushima T, Koide R, Horikawa Y, Morita T, Takahashi H. Widespread occurrence of alpha-synuclein/NACP-immunoreactive neuronal inclusions in juvenile and adult-onset Hallervorden-Spatz disease with Lewy bodies. Neuropathol Appl Neurobiol. 1999;25:363–368. doi: 10.1046/j.1365-2990.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 77.Galvin JE, Giasson B, Hurtig HI, Lee VM, Trojanowski JQ. Neurodegeneration with brain iron accumulation, type 1 is characterized by alpha-, beta-, and gamma-synuclein neuropathology. Am J Pathol. 2000;157:361–368. doi: 10.1016/s0002-9440(10)64548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neumann M, Adler S, Schluter O, Kremmer E, Benecke R, Kretzschmar HA. Alpha-synuclein accumulation in a case of neurodegeneration with brain iron accumulation type 1 (NBIA-1, formerly Hallervorden-Spatz syndrome) with widespread cortical and brainstem-type Lewy bodies. Acta Neuropathol (Berl) 2000;100:568–574. doi: 10.1007/s004010000224. [DOI] [PubMed] [Google Scholar]
- 79.Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. The precursor protein of non-A beta component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 80.Payton JE, Perrin RJ, Clayton DF, George JM. Protein-protein interactions of alpha-synuclein in brain homogenates and transfected cells. Brain Res Mol Brain Res. 2001;95:138–145. doi: 10.1016/s0169-328x(01)00257-1. [DOI] [PubMed] [Google Scholar]
- 81.Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J. Identification of novel proteins interacting with both a-synuclein and DJ-1. Mol Cell Proteomics. 2006 doi: 10.1074/mcp.M600182-MCP200. [DOI] [PubMed] [Google Scholar]
- 82.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 83.da Costa CA, Ancolio K, Checler F. Wild-type but not Parkinson’s disease-related ala-53 --> Thr mutant alpha-synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 84.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP a protein implicated in Alzheimer’s disease and learning is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 85.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 86.Uversky VN, Li J, Souillac P, Millett IS, Doniach S, Jakes R, Goedert M, Fink AL. Biophysical properties of the synucleins and their propensities to fibrillate: inhibition of alpha-synuclein assembly by beta- and gamma-synucleins. J Biol Chem. 2002;277:11970–11978. doi: 10.1074/jbc.M109541200. [DOI] [PubMed] [Google Scholar]
- 87.Morar AS, Olteanu A, Young GB, Pielak GJ. Solvent-induced collapse of alpha-synuclein and acid-denatured cytochrome c. Protein Sci. 2001;10:2195–2199. doi: 10.1110/ps.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lattman EE. Small angle X-ray scattering studies of protein folding. Curr Opin Struct Biol. 1994;4:87–92. [Google Scholar]
- 89.Kataoka M, Goto Y. X-ray solution scattering studies of protein folding. Fold Des. 1996;1:R107–114. doi: 10.1016/S1359-0278(96)00047-8. [DOI] [PubMed] [Google Scholar]
- 90.Kataoka M, Hagihara Y, Mihara K, Goto Y. Molten globule of cytochrome c studied by small angle X-ray scattering. J Mol Biol. 1993;229:591–596. doi: 10.1006/jmbi.1993.1064. [DOI] [PubMed] [Google Scholar]
- 91.Kataoka M, Nishii I, Fujisawa T, Ueki T, Tokunaga F, Goto Y. Structural characterization of the molten globule and native states of apomyoglobin by solution X-ray scattering. J Mol Biol. 1995;249:215–228. doi: 10.1006/jmbi.1995.0290. [DOI] [PubMed] [Google Scholar]
- 92.Semisotnov GV, Kihara H, Kotova NV, Kimura K, Amemiya Y, Wakabayashi K, Serdyuk IN, Timchenko AA, Chiba K, Nikaido K, Ikura T, Kuwajima K. Protein globularization during folding: a study by synchrotron small-angle X-ray scattering. J Mol Biol. 1996;262:559–574. doi: 10.1006/jmbi.1996.0535. [DOI] [PubMed] [Google Scholar]
- 93.Kataoka M, Kuwajima K, Tokunaga F, Goto Y. Structural characterization of the molten globule of alpha-lactalbumin by solution X-ray scattering. Protein Sci. 1997;6:422–430. doi: 10.1002/pro.5560060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uversky VN, Karnoup AS, Segel DJ, Seshadri S, Doniach S, Fink AL. Anion-induced folding of Staphylococcal nuclease: characterization of multiple equilibrium partially folded intermediates. J Mol Biol. 1998;278:879–894. doi: 10.1006/jmbi.1998.1741. [DOI] [PubMed] [Google Scholar]
- 95.Hagihara Y, Hoshino M, Hamada D, Kataoka M, Goto Y. Chain-like conformation of heat-denatured ribonuclease A and cytochrome c as evidenced by solution X-ray scattering. Fold Des. 1998;3:195–201. doi: 10.1016/S1359-0278(98)00027-3. [DOI] [PubMed] [Google Scholar]
- 96.Uversky VN, Gillespie JR, Millett IS, Khodyakova AV, Vasiliev AM, Chernovskaya TV, Vasilenko RN, Kozlovskaya GD, Dolgikh DA, Fink AL, Doniach S, Abramov VM. Natively unfolded human prothymosin alpha adopts partially folded collapsed conformation at acidic pH. Biochemistry. 1999;38:15009–15016. doi: 10.1021/bi990752+. [DOI] [PubMed] [Google Scholar]
- 97.Uversky VN, Gillespie JR, Millett IS, Khodyakova AV, Vasilenko RN, Vasiliev AM, Rodionov IL, Kozlovskaya GD, Dolgikh DA, Fink AL, Doniach S, Permyakov EA, Abramov VM. Zn(2+)-mediated structure formation and compaction of the “natively unfolded” human prothymosin alpha. Biochem Biophys Res Commun. 2000;267:663–668. doi: 10.1006/bbrc.1999.2013. [DOI] [PubMed] [Google Scholar]
- 98.Doniach S. Changes in biomolecular conformation seen by small angle X-ray scattering. Chem Rev. 2001;101:1763–1778. doi: 10.1021/cr990071k. [DOI] [PubMed] [Google Scholar]
- 99.Bussell R, Jr, Eliezer D. Residual structure and dynamics in Parkinson’s disease-associated mutants of alpha-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 100.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in alpha-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 101.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured alpha-synuclein. Proc Natl Acad Sci USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sung YH, Eliezer D. Residual structure, backbone dynamics, and interactions within the synuclein family. J Mol Biol. 2007;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uversky VN, Gillespie JR, Fink AL. Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins. 2000;41:415–427. doi: 10.1002/1097-0134(20001115)41:3<415::aid-prot130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 104.Uversky VN. Natively unfolded proteins: a point where biology waits for physics. Protein Sci. 2002;11:739–756. doi: 10.1110/ps.4210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uversky VN. What does it mean to be natively unfolded? Eur J Biochem. 2002;269:2–12. doi: 10.1046/j.0014-2956.2001.02649.x. [DOI] [PubMed] [Google Scholar]
- 106.Uversky VN. Protein folding revisited: a polypeptide chain at the folding-misfolding-nonfolding cross-roads: which way to go? Cell Mol Life Sci. 2003;60:1852–1871. doi: 10.1007/s00018-003-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 108.Li J, Uversky VN, Fink AL. Conformational behavior of human alpha-synuclein is modulated by familial Parkinson’s disease point mutations A30P and A53T. Neurotoxicology. 2002;23:553–567. doi: 10.1016/s0161-813x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 109.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 110.El-Agnaf OM, Jakes R, Curran MD, Wallace A. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson’s disease. FEBS Lett. 1998;440:67–70. doi: 10.1016/s0014-5793(98)01419-7. [DOI] [PubMed] [Google Scholar]
- 111.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 112.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 113.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 114.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr Accelerated oligomerization by Parkinson’s disease linked alpha-synuclein mutants. Ann NY Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 115.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization not fibrillization is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 117.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 118.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT., Jr The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 119.Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of alpha-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem. 2005;280:30649–30652. doi: 10.1074/jbc.C500288200. [DOI] [PubMed] [Google Scholar]
- 120.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson’s disease variants with phospholipids. Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 121.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 122.Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 123.Chandra S, Chen X, Rizo J, Jahn R, Sudhof TC. A broken alpha-helix in folded alpha-Synuclein. J Biol Chem. 2003;278:15313–15318. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- 124.Bussell R, Jr, Ramlall TF, Eliezer D. Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein. Protein Sci. 2005;14:862–872. doi: 10.1110/ps.041255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 126.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 127.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci USA. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Borbat P, Ramlall TF, Freed JH, Eliezer D. Inter-helix distances in lysophospholipid micelle-bound alpha-synuclein from pulsed ESR measurements. J Am Chem Soc. 2006;128:10004–10005. doi: 10.1021/ja063122l. [DOI] [PubMed] [Google Scholar]
- 129.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound alpha-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eliezer D. Protein Folding and Aggregation in In Vitro Models of Parkinson’s Disease: Structure and Function of Alpha-Synuclein. In: Nash R, Przedborski S, editors. Parkinson’s Disease: Pathogenic and Therapeutic Insights from Toxin and Genetic Models. Academic Press; New York: 2008. pp. 575–595. [Google Scholar]
- 131.Yamin G, Glaser CB, Uversky VN, Fink AL. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem. 2003;278:27630–27635. doi: 10.1074/jbc.M303302200. [DOI] [PubMed] [Google Scholar]
- 132.Cohlberg JA, Li J, Uversky VN, Fink AL. Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from alpha-synuclein in vitro. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]
- 133.Glaser CB, Yamin G, Uversky VN, Fink AL. Methionine oxidation, alpha-synuclein and Parkinson’s disease. Biochim Biophys Acta. 2005;1703:157–169. doi: 10.1016/j.bbapap.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 134.Munishkina LA, Phelan C, Uversky VN, Fink AL. Conformational behavior and aggregation of alpha-synuclein in organic solvents: modeling the effects of membranes. Biochemistry. 2003;42:2720–2730. doi: 10.1021/bi027166s. [DOI] [PubMed] [Google Scholar]
- 135.Uversky VN, Li J, Fink AL. Trimethylamine-N-oxide-induced folding of alpha-synuclein. FEBS Lett. 2001;509:31–35. doi: 10.1016/s0014-5793(01)03121-0. [DOI] [PubMed] [Google Scholar]
- 136.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein: a possible molecular NK between Parkinson’s disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 137.Munishkina LA, Henriques J, Uversky VN, Fink AL. Role of protein-water interactions and electrostatics in alpha-synuclein fibril formation. Biochemistry. 2004;43:3289–3300. doi: 10.1021/bi034938r. [DOI] [PubMed] [Google Scholar]
- 138.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of alpha-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 139.Uversky VN, Li J, Bower K, Fink AL. Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology. 2002;23:527–536. doi: 10.1016/s0161-813x(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 140.Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 141.Goers J, Uversky VN, Fink AL. Polycation-induced oligomerization and accelerated fibrillation of human alpha-synuclein in vitro. Protein Sci. 2003;12:702–707. doi: 10.1110/ps.0230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J Biol Chem. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- 143.Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000;275:18344–18349. doi: 10.1074/jbc.M000206200. [DOI] [PubMed] [Google Scholar]
- 144.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 145.Lowe R, Pountney DL, Jensen PH, Gai WP, Voelcker NH. Calcium(II) selectively induces alpha-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci. 2004;13:3245–3252. doi: 10.1110/ps.04879704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Uversky VN. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. J Biomol Struct Dyn. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 147.Fink AL. The aggregation and fibrillation of alpha-synuclein. Acc Chem Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 148.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human alpha-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 149.Zimmerman SB, Trach SO. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]
- 150.Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem. 2001;276:10577–10580. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]
- 151.Minton AP. Implications of macromolecular crowding for protein assembly. Curr Opin Struct Biol. 2000;10:34–39. doi: 10.1016/s0959-440x(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 152.Uversky VN, Cooper EM, Bower KS, Li J, Fink AL. Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett. 2002;515:99–103. doi: 10.1016/s0014-5793(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 153.Shtilerman MD, Ding TT, Lansbury PT., Jr Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry. 2002;41:3855–3860. doi: 10.1021/bi0120906. [DOI] [PubMed] [Google Scholar]
- 154.Munishkina LA, Cooper EM, Uversky VN, Fink AL. The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit. 2004;17:456–464. doi: 10.1002/jmr.699. [DOI] [PubMed] [Google Scholar]
- 155.Munishkina LA, Fink AL, Uversky VN. Concerted action of metals and macromolecular crowding on the fibrillation of alpha-synuclein. Protein Pept Lett. 2008;15:1079–1085. doi: 10.2174/092986608786071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Munishkina LA, Ahmad A, Fink AL, Uversky VN. Guiding protein aggregation with macromolecular crowding. Biochemistry. 2008;47:8993–9006. doi: 10.1021/bi8008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kisilevsky R. Review: amyloidogenesis-unquestioned answers and unanswered questions. J Struct Biol. 2000;130:99–108. doi: 10.1006/jsbi.2000.4222. [DOI] [PubMed] [Google Scholar]
- 158.McLaurin J, Yang D, Yip CM, Fraser PE. Review: modulating factors in amyloid-beta fibril formation. J Struct Biol. 2000;130:259–270. doi: 10.1006/jsbi.2000.4289. [DOI] [PubMed] [Google Scholar]
- 159.Tsen G, Halfter W, Kroger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995;270:3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- 160.Halfter W, Schurer B, Yip J, Yip L, Tsen G, Lee JA, Cole GJ. Distribution and substrate properties of agrin, a heparan sulfate proteoglycan of developing axonal pathways. J Comp Neurol. 1997;383:1–17. [PubMed] [Google Scholar]
- 161.Cohen NA, Kaufmann WE, Worley PF, Rupp F. Expression of agrin in the developing and adult rat brain. Neuroscience. 1997;76:581–596. doi: 10.1016/s0306-4522(96)00345-4. [DOI] [PubMed] [Google Scholar]
- 162.Liu IH, Uversky VN, Munishkina LA, Fink AL, Halfter W, Cole GJ. Agrin binds alpha-synuclein and modulates alpha-synuclein fibrillation. Glycobiology. 2005;15:1320–1331. doi: 10.1093/glycob/cwj014. [DOI] [PubMed] [Google Scholar]
- 163.Goers J, Manning-Bog AB, McCormack AL, Millett IS, Doniach S, Di Monte DA, Uversky VN, Fink AL. Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry. 2003;42:8465–8471. doi: 10.1021/bi0341152. [DOI] [PubMed] [Google Scholar]
- 164.Tanner CM. The role of environmental toxins in the etiology of Parkinson’s disease. Trends Neurosci. 1989;12:49–54. doi: 10.1016/0166-2236(89)90135-5. [DOI] [PubMed] [Google Scholar]
- 165.Tanner CM, Chen B, Wang W, Peng M, Liu Z, Liang X, Kao LC, Gilley DW, Goetz CG, Schoenberg BS. Environmental factors and Parkinson’s disease: a case-control study in China. Neurology. 1989;39:660–664. doi: 10.1212/wnl.39.5.660. [DOI] [PubMed] [Google Scholar]
- 166.Di Monte DA. The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol. 2003;2:531–538. doi: 10.1016/s1474-4422(03)00501-5. [DOI] [PubMed] [Google Scholar]
- 167.McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 168.Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson’s disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 169.Di Monte DA. The role of environmental agents in Parkinson’s disease. Clin Neurosci Res. 2001;1:419–426. [Google Scholar]
- 170.Uversky VN. Neurotoxicant-induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- 171.Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, Richardson RJ. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20:239–247. [PubMed] [Google Scholar]
- 172.Altschuler E. Aluminum-containing antacids as a cause of idiopathic Parkinson’s disease. Med Hypotheses. 1999;53:22–23. doi: 10.1054/mehy.1997.0701. [DOI] [PubMed] [Google Scholar]
- 173.Zayed J, Ducic S, Campanella G, Panisset JC, Andre P, Masson H, Roy M. Environmental factors in the etiology of Parkinson’s disease. Can J Neurol Sci. 1990;17:286–291. [PubMed] [Google Scholar]
- 174.Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Occupational metal exposures and the risk of Parkinson’s disease. Neuroepidemiology. 1999;18:303–308. doi: 10.1159/000026225. [DOI] [PubMed] [Google Scholar]
- 175.Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson’s disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8:87–92. doi: 10.1002/mds.870080116. [DOI] [PubMed] [Google Scholar]
- 176.Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: an X-ray microanalysis. J Neurochem. 1991;56:446–451. doi: 10.1111/j.1471-4159.1991.tb08170.x. [DOI] [PubMed] [Google Scholar]
- 177.Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 178.Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 179.Sung YH, Rospigliosi C, Eliezer D. NMR mapping of copper binding sites in alpha-synuclein. Biochim Biophys Acta. 2006;1764:5–12. doi: 10.1016/j.bbapap.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 180.Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, Oertel WH, Ulm G, Schneider E. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: a case-control study in Germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 181.Davis LE, Adair JC. Parkinsonism from methanol poisoning: benefit from treatment with anti-Parkinson drugs. Mov Disord. 1999;14:520–522. doi: 10.1002/1531-8257(199905)14:3<520::aid-mds1026>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 182.Hageman G, van der Hoek J, van Hout M, van der Laan G, Steur EJ, de Bruin W, Herholz K. Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. J Neurol. 1999;246:198–206. doi: 10.1007/s004150050334. [DOI] [PubMed] [Google Scholar]
- 183.Pezzoli G, Strada O, Silani V, Zecchinelli A, Perbellini L, Javoy-Agid F, Ghidoni P, Motti ED, Masini T, Scarlato G, Agid Y, Hirsch EC. Clinical and pathological features in hydrocarbon-induced parkinsonism. Ann Neurol. 1996;40:922–925. doi: 10.1002/ana.410400616. [DOI] [PubMed] [Google Scholar]
- 184.Uitti RJ, Snow BJ, Shinotoh H, Vingerhoets FJ, Hayward M, Hashimoto S, Richmond J, Markey SP, Markey CJ, Calne DB. Parkinsonism induced by solvent abuse. Ann Neurol. 1994;35:616–619. doi: 10.1002/ana.410350516. [DOI] [PubMed] [Google Scholar]
- 185.Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch Neurol. 1999;56:1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 186.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurode-generative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lyras L, Perry RH, Perry EK, Ince PG, Jenner A, Jenner P, Halliwell B. Oxidative damage to proteins, lipids, and DNA in cortical brain regions from patients with dementia with Lewy bodies. J Neurochem. 1998;71:302–312. doi: 10.1046/j.1471-4159.1998.71010302.x. [DOI] [PubMed] [Google Scholar]
- 189.Cookson MR, Shaw PJ. Oxidative stress and motor neurone disease. Brain Pathol. 1999;9:165–186. doi: 10.1111/j.1750-3639.1999.tb00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington’s disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Witko-Sarsat V, Khoa TN, Jungers P, Drueke T, Descamps-Latscha B. Advanced oxidation protein products: oxidative stress markers and mediators of inflammation in uremia. Adv Nephrol Necker Hosp. 1998;28:321–341. [PubMed] [Google Scholar]
- 192.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillere-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drueke T, Descamps-Latscha B. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol. 1998;161:2524–2532. [PubMed] [Google Scholar]
- 193.Fu S, Dean R, Southan M, Truscott R. The hydroxyl radical in lens nuclear cataractogenesis. J Biol Chem. 1998;273:28603–28609. doi: 10.1074/jbc.273.44.28603. [DOI] [PubMed] [Google Scholar]
- 194.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- 195.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stadtman ER, Oliver CN, Starke-Reed PE, Rhee SG. Age-related oxidation reaction in proteins. Toxicol Ind Health. 1993;9:187–196. doi: 10.1177/0748233793009001-213. [DOI] [PubMed] [Google Scholar]
- 197.Leeuwenburgh C, Hansen P, Shaish A, Holloszy JO, Heinecke JW. Markers of protein oxidation by hydroxyl radical and reactive nitrogen species in tissues of aging rats. Am J Physiol. 1998;274:R453–461. doi: 10.1152/ajpregu.1998.274.2.R453. [DOI] [PubMed] [Google Scholar]
- 198.Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS Lett. 2002;517:239–244. doi: 10.1016/s0014-5793(02)02638-8. [DOI] [PubMed] [Google Scholar]
- 199.Hokenson MJ, Uversky VN, Goers J, Yamin G, Munishkina LA, Fink AL. Role of individual methionines in the fibrillation of methionine-oxidized alpha-synuclein. Biochemistry. 2004;43:4621–4633. doi: 10.1021/bi049979h. [DOI] [PubMed] [Google Scholar]
- 200.Tompkins MM, Hill WD. Contribution of somal Lewy bodies to neuronal death. Brain Res. 1997;775:24–29. doi: 10.1016/s0006-8993(97)00874-3. [DOI] [PubMed] [Google Scholar]
- 201.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 202.Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer’s disease. Proc Natl Acad Sci USA. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein especially the Parkinson’s disease-associated mutants forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 205.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 206.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 207.Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- 208.Eliezer D. Amyloid ion channels: a porous argument or a thin excuse? J Gen Physiol. 2006;128:631–633. doi: 10.1085/jgp.200609689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sokolov Y, Kozak JA, Kayed R, Chanturiya A, Glabe C, Hall JE. Soluble amyloid oligomers increase bilayer conductance by altering dielectric structure. J Gen Physiol. 2006;128:637–647. doi: 10.1085/jgp.200609533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ischiropoulos H. Biological significance and clinical relevance of nitric oxide-mediated protein modifications. Free Radic Res. 2003;37:15. [Google Scholar]
- 211.Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem Biophys Res Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. [DOI] [PubMed] [Google Scholar]
- 212.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 213.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 214.Uversky VN, Yamin G, Munishkina LA, Karymov MA, Millett IS, Doniach S, Lyubchenko YL, Fink AL. Effects of nitration on the structure and aggregation of alpha-synuclein. Brain Res Mol Brain Res. 2005;134:84–102. doi: 10.1016/j.molbrainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 215.Qin Z, Hu D, Han S, Reaney SH, Di Monte DA, Fink AL. Effect of 4-hydroxy-2-nonenal modification on alpha-synuclein aggregation. J Biol Chem. 2007;282:5862–5870. doi: 10.1074/jbc.M608126200. [DOI] [PubMed] [Google Scholar]
- 216.Hashimoto M, Rockenstein E, Mante M, Mallory M, Masliah E. beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron. 2001;32:213–223. doi: 10.1016/s0896-6273(01)00462-7. [DOI] [PubMed] [Google Scholar]
- 217.Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J Mol Biol. 2006;356:1036–1048. doi: 10.1016/j.jmb.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 218.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 221.Jenco JM, Rawlingson A, Daniels B, Morris AJ. Regulation of phospholipase D2: selective inhibition of mammalian phospholipase D isoenzymes by alpha- and beta-synucleins. Biochemistry. 1998;37:4901–4909. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- 222.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 223.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 224.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein alpha-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 225.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of alpha-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 227.George JM. The synucleins. Genome Biol. 2002;3:REVIEWS 3002. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu M, Fink AL. Lipid binding inhibits alpha-synuclein fibril formation. J Biol Chem. 2003;278:16873–16877. doi: 10.1074/jbc.M210136200. [DOI] [PubMed] [Google Scholar]
- 229.Zhu M, Li J, Fink AL. The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 230.Hashimoto M, Takenouchi T, Rockenstein E, Masliah E. Alpha-synuclein up-regulates expression of caveolin-1 and down-regulates extracellular signal-regulated kinase activity in B103 neuroblastoma cells: role in the pathogenesis of Parkinson’s disease. J Neurochem. 2003;85:1468–1479. doi: 10.1046/j.1471-4159.2003.01791.x. [DOI] [PubMed] [Google Scholar]
- 231.Spencer B, Crews L, Masliah E. Climbing the scaffolds of Parkinson’s disease pathogenesis. Neuron. 2007;53:469–470. doi: 10.1016/j.neuron.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 232.Benarroch EE. Lipid rafts, protein scaffolds, and neurologic disease. Neurology. 2007;69:1635–1639. doi: 10.1212/01.wnl.0000279590.22544.c3. [DOI] [PubMed] [Google Scholar]
- 233.Martinez Z, Zhu M, Han S, Fink AL. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 234.Maguire-Zeiss KA, Federoff HJ. Convergent pathobiologic model of Parkinson’s disease. Ann NY Acad Sci. 2003;991:152–166. doi: 10.1111/j.1749-6632.2003.tb07473.x. [DOI] [PubMed] [Google Scholar]
- 235.Li J, Zhu M, Manning-Bog AB, Di Monte DA, Fink AL. Dopamine and L-dopa disaggregate amyloid fibrils: implications for Parkinson’s and Alzheimer’s disease. FASEB J. 2004;18:962–964. doi: 10.1096/fj.03-0770fje. [DOI] [PubMed] [Google Scholar]
- 236.Conway KA, Rochet JC, Bieganski RM, Lansbury PT., Jr Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294:1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 237.Namba Y, Kawatsu K, Izumi S, Ueki A, Ikeda K. Neurofibrillary tangles and senile plaques in brain of elderly leprosy patients. Lancet. 1992;340:978. doi: 10.1016/0140-6736(92)92870-l. [DOI] [PubMed] [Google Scholar]
- 238.Chui DH, Tabira T, Izumi S, Koya G, Ogata J. Decreased beta-amyloid and increased abnormal Tau deposition in the brain of aged patients with leprosy. Am J Pathol. 1994;145:771–775. [PMC free article] [PubMed] [Google Scholar]
- 239.Mcgeer PL, Harada N, Kimura H, Mcgeer EG, Schulzer M. Prevalence of dementia amongst elderly japanese with leprosy - apparent effect of chronic drug-therapy. Dementia. 1992;3:146–149. [Google Scholar]
- 240.Tomiyama T, Shoji A, Kataoka K, Suwa Y, Asano S, Kaneko H, Endo N. Inhibition of amyloid beta protein aggregation and neurotoxicity by rifampicin. Its possible function as a hydroxyl radical scavenger. J Biol Chem. 1996;271:6839–6844. doi: 10.1074/jbc.271.12.6839. [DOI] [PubMed] [Google Scholar]
- 241.Tomiyama T, Kaneko H, Kataoka K, Asano S, Endo N. Rifampicin inhibits the toxicity of pre-aggregated amyloid peptides by binding to peptide fibrils and preventing amyloid-cell interaction. Biochem J. 1997;322(Pt 3):859–865. doi: 10.1042/bj3220859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tomiyama T, Asano S, Suwa Y, Morita T, Kataoka K, Mori H, Endo N. Rifampicin prevents the aggregation and neurotoxicity of amyloid beta proteinin vitro. Biochem Biophys Res Commun. 1994;204:76–83. doi: 10.1006/bbrc.1994.2428. [DOI] [PubMed] [Google Scholar]
- 243.Li J, Zhu M, Rajamani S, Uversky VN, Fink AL. Rifampicin inhibits alpha-synuclein fibrillation and disaggregates fibrils. Chem Biol. 2004;11:1513–1521. doi: 10.1016/j.chembiol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 244.Wu JA, Attele AS, Zhang L, Yuan CS. Anti-HIV activity of medicinal herbs: usage and potential development. Am J Chin Med. 2001;29:69–81. doi: 10.1142/S0192415X01000083. [DOI] [PubMed] [Google Scholar]
- 245.Ikezoe T, Chen SS, Heber D, Taguchi H, Koeffler HP. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate. 2001;49:285–292. doi: 10.1002/pros.10024. [DOI] [PubMed] [Google Scholar]
- 246.Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001;43:173–178. doi: 10.1006/phrs.2000.0761. [DOI] [PubMed] [Google Scholar]
- 247.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 248.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 249.Hong DP, Fink AL, Uversky VN. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008;383:214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 251.Litvan I, Halliday G, Hallett M, Goetz CG, Rocca W, Duyckaerts C, Ben-Shlomo Y, Dickson DW, Lang AE, Chesselet MF, Langston WJ, Di Monte DA, Gasser T, Hagg T, Hardy J, Jenner P, Melamed E, Myers RH, Parker D, Jr, Price DL. The etiopathogenesis of Parkinson disease and suggestions for future research. Part I. J Neuropathol Exp Neurol. 2007;66:251–257. doi: 10.1097/nen.0b013e3180415e42. [DOI] [PubMed] [Google Scholar]
- 252.Fratiglioni L, Wang HX. Smoking and Parkinson’s and Alzheimer’s disease: review of the epidemiological studies. Behav Brain Res. 2000;113:117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 253.Quik M. Smoking, nicotine and Parkinson’s disease. Trends Neurosci. 2004;27:561–568. doi: 10.1016/j.tins.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 254.Hong DP, Fink AL, Uversky VN. Smoking and Parkinson’s disease: does nicotine affect alpha-synuclein fibrillation? Biochim Biophys Acta. 2009;1794:282–290. doi: 10.1016/j.bbapap.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]