Abstract
Introduction and Aims
The Rome III classification system treats functional constipation (FC) and irritable bowel syndrome with constipation (IBS-C) as distinct disorders, but this distinction appears artificial, and the same drugs are used to treat both. This study’s hypothesis is that FC and IBS-C defined by Rome III are not distinct entities.
Method
1,100 adults with a primary care visit for constipation and 1,700 age and gender matched controls from a health maintenance organization completed surveys 12 months apart; 66.2% returned the first questionnaire. Rome III criteria identified 231 with FC and 201 with IBS-C. The second survey was completed by 195 of the FC and 141 of the IBS-C cohorts. Both surveys assessed the severity of constipation and IBS, quality of life (QOL), and psychological distress.
Results
(1) Overlap: If the Rome III requirement that patients meeting criteria for IBS cannot be diagnosed FC is suspended, 89.5% of IBS-C cases meet criteria for FC and 43.8% of FC patients fulfill criteria for IBS-C. (2) No qualitative differences between FC and IBS-C: 44.8% of FC patients report abdominal pain, and paradoxically IBS-C patients have more constipation symptoms than FC. (3) Switching between diagnoses: by 12 months, 1/3 of FC transition to IBS-C and 1/3 of IBS-C change to FC.
Conclusions
Patients identified by Rome III criteria for FC and IBS-C are not distinct groups. Revisions to the Rome III criteria, possibly including incorporation of physiological tests of transit and pelvic floor function, are needed.
Keywords: Constipation, Irritable bowel, Diagnostic criteria, Symptom-based diagnosis
Introduction
The Rome III classification system for Functional Gastrointestinal Disorders (FGIDs) treats Functional Constipation (FC) and constipation predominant Irritable Bowel Syndrome (IBS-C) as distinct disorders, and implies that they have different pathophysiological mechanisms and therefore would require different treatment approaches1. The basis of FC is thought to involve delayed transit through the colon and/or failure to relax the pelvic floor muscles during attempted defecation whereas the pathophysiology of IBS-C is believed to involve a disorder of brain-gut interaction with a predominant symptom of pain or discomfort2.
When diagnoses of IBS-C or FC are made using the Rome III criteria there is no overlap because the Rome III criteria specifically exclude assigning a diagnosis of FC to a patient who fulfills the criteria for IBS-C (see Table 1)1. However, no rationale for this exclusion was provided by the authors of the Rome I3 or subsequent versions of the Rome criteria, and some clinicians regard this requirement as artificial. Moreover, it is noteworthy that both pro-kinetics such as Tegaserod4, Prucalopride5 and chloride channel agonists such as Lubiprostone6 have been found in clinical trials to be effective for the treatment of both IBS-C and FC7.
Table 1.
Rome III diagnostic criteria for FC and IBS-C
| Functional Constipation | Irritable Bowel Syndrome (constipation predominant subtype) |
|---|---|
|
|
Criteria for either FC or IBS-C must be fulfilled for the past 3 months, with onset of symptoms at least 6 months prior to diagnosis.
The purpose of this study was to test the hypothesis that FC and IBS-C as defined by the Rome III criteria are not distinct entities. To test this, we first examined the overlap between FC and IBS-C that would result if the requirement that no patient meeting criteria for IBS-C could also be diagnosed with FC were removed. Second, using the published Rome III criteria which exclude overlap, we classified patients into mutually exclusive categories and compared the symptom characteristics of FC and IBS-C. Third, we assessed the degree of switching between these diagnoses that occurs over time; if the disorders are distinct, subjects should either recover or retain their original diagnosis, whereas if they have substantially similar etiologies, one might expect subjects to alternate between these diagnoses. We also investigated patient characteristics that predict switching diagnoses over a 12-month period.
Methods
This is a secondary analysis of a larger prospective study on risk factors for chronic constipation and the impact of chronic constipation on quality of life and health care costs. The study was carried out at Group Health Cooperative of Puget Sound (GHC), which is a health management organization. Group Health members aged 18 years or older who made at least one clinic visit to a primary care provider between September 1, 2005 and December 31, 2005, and were enrolled at the GHC for all of the previous 5 years, were screened for inclusion in the study. Excluded were subjects with a history of gastrointestinal resection except appendectomy or cholecystectomy, and subjects with any ICD-9CM codes for gastrointestinal cancer.
The screening and recruitment algorithm is shown in Figure 1. We first identified 1,100 subjects for screening who had received a clinical diagnosis of constipation (ICD9CM code 569.0X) at a clinical visit between September 1, 2005 and December 31, 2005, and 1,700 age and sex matched subjects who had not received a clinical diagnosis of constipation at GHC for at least the last 5 years. Matching was accomplished by stratified sampling: Based on a previous study of the membership of Group Health Cooperative8, we were aware (a) that approximately 2/3 of patients with a clinical diagnosis of constipation would meet Rome III criteria for FC, (b) that a substantial number of people who had not consulted physicians for constipation would nevertheless meet symptom criteria for FC, and (c) that constipation is more prevalent in women than men and in older versus younger people. We therefore designed a quota sampling scheme in which we sampled approximately twice as many controls as we anticipated obtaining for constipated patients in each age stratum within each sex. There were 1,707 who completed the enrollment questionnaires (66.2% response rate) of whom 432 met the Rome III criteria for FC or IBS-C, and 336 (77.8%) of these completed the 12-month survey.
Figure 1.
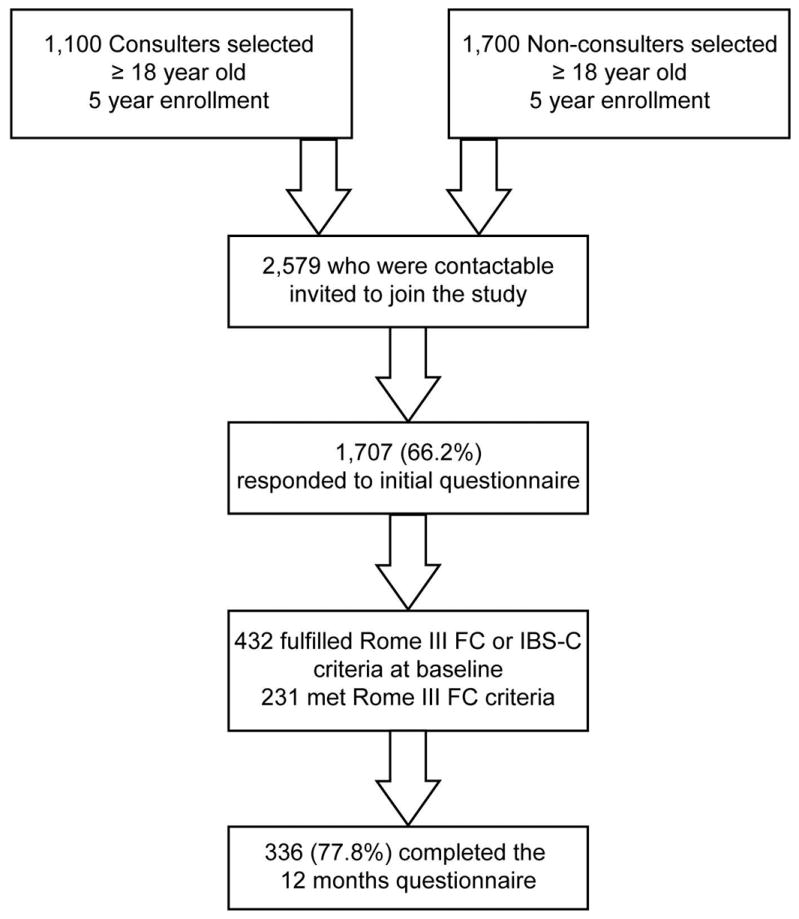
Study enrollment and follow-up flow chart.
Ethics Approval
The study was approved by the Institutional Review Boards for the Protection of Human Subjects at both the University of North Carolina at Chapel Hill and the GHC. As granted by both review boards, informed consent was inferred from completion and return of questionnaires, and subjects were not required to return a signed consent document. The cover letter sent with the survey contained all elements of informed consent.
Survey questionnaires
The questionnaires completed by study subjects included the Rome III Diagnostic Questionnaire modules for functional constipation, IBS, and bloating. The development and validation of this questionnaire has been previously described9, 10. In addition to a Rome III diagnosis, these questionnaires were scored for two indices of symptom severity: (1) the number of constipation symptoms experienced, based on the 6 Rome III FC symptoms (see Table 1); and (2) the Constipation Severity Scale (CSS), a score calculated as the sum of the 5-point ordinal ratings (0=never, 1=sometimes, 2=often, 3=most of the time, 4=always) for the six Rome III functional constipation questions. The CSS was previously validated11. The frequency of abdominal pain or discomfort was assessed by the Rome III question, “In the last 3 months, how often did you have discomfort or pain anywhere in your abdomen for any reason?” Response choices were “never”, “less than one day a month”, “one day a month”, “2-3 days a month”, “one day a week”, “more than one day a week”, and “every day”. Demographic information such as age, gender, race, ethnicity, marital status, personal education as well as personal annual income were ascertained at the enrollment survey.
The baseline and follow-up surveys incorporated the SF-12 Health Survey, and the Patient Assessment of Constipation Quality of Life (PAC-QOL) questionnaire (which measures the degree of impairment in quality of life due to constipation). The SF-12 is scored for mental and physical component summary scores12. The PAC-QOL covers 4 dimensions pertaining to constipation (physical discomfort, worries and concerns, psychosocial discomfort, and satisfaction), and the results are presented as a total QOL impact score13. The survey also included the Brief Symptom Inventory (BSI), which provides an overall measure of psychological distress inferred from symptoms of anxiety, depression and somatization14. BSI scale scores were adjusted for sex differences by converting them to T-scores before statistical analysis.
Data analysis
Statistical analyses utilized SPSS software (version 15.0 for Windows, SPSS Inc., Chicago, IL). The Chi-square test was used for dichotomous variables, Student’s t-test was used for continuous variables, and the Mann-Whitney U test was used for ordinal scaled data. A two-tailed P-value of p< 0.05 was considered significant. Demographic factors that were significantly different between the groups at baseline were treated as potential confounders, and were controlled for using an analysis of covariance (ANCOVA).
Results
Overlap between IBS-C and FC at baseline
Two hundred and one patients met the Rome III criteria for IBS-C and 231 met criteria for FC at baseline. The Rome III diagnostic criteria for FC include the condition that if a subject fulfills criteria for IBS, they cannot be diagnosed as FC1, yet 180 of the 201 IBS-C patients would have met criteria for FC if this restriction were not present (Figure 2). Thus, without this restriction a total of 411 cases would fulfill criteria for FC of whom 43.8% would also fulfill criteria for IBS-C, and 89.5% of the 201 IBS-C cases would also fulfill the criteria for FC. The Venn diagram in Figure 2 illustrates this overlap at baseline.
Figure 2.

Venn diagram showing overlap between IBS-C and FC when the Rome III requirement that FC cannot be diagnosed in a patient who meets criteria for IBS is suspended.
Demographic Differences between FC and IBS-C
Table 2 compares the demographic characteristics of the 231 subjects who fulfilled the published Rome III criteria for FC (no overlap with IBS-C permitted) and the 201 who fulfilled the criteria for IBS-C at baseline. FC patients were older and had a higher educational level; therefore in the subsequent analyses, we adjusted for both of these factors as potential confounders (except for non-parametric tests of significance).
Table 2.
Demographic Profile of subjects
| Analysis Sample (n=1615) |
FC (n=231) |
IBS-C (n=201) |
p-value (FC vs. IBS-C) |
|
|---|---|---|---|---|
| Age in years | 66 | 76 | 61 | 0.001 |
| Female Gender | 68% | 70% | 78% | 0.06 |
| Caucasian Race | 86% | 88% | 89% | 0.83 |
| Education (college degree or higher) | 38% | 43% | 35% | 0.03 |
| Personal Income (≥ $30,000/year) | 54.4% | 51.5% | 53.7% | 0.92 |
Symptom and Psychosocial Differences between FC and IBS-C patients
Table 3 describes the baseline bowel symptoms and psychosocial indices of these two groups. There was a greater frequency of abdominal discomfort or pain among the IBS-C patients, and this was expected because the diagnostic criteria for IBS require pain and/or discomfort. However, 44.8% of FC patients also reported experiencing some abdominal pain or discomfort within the past 3 months (although they did not meet other symptom criteria for a diagnosis of IBS). Paradoxically, the IBS-C patients had significantly more constipation symptoms than their FC counterparts and a significantly worse CSS score, indicating a more severe degree of constipation at baseline. IBS-C subjects also had a greater impairment on the PAC-QOL, significantly worse psychological distress on the BSI, and greater impairment in the SF-12 Mental Composite Score.
Table 3.
Symptoms, quality of life and psychosocial distress in patients with functional constipation compared to IBS-C at baseline
| FC (n=231) |
IBS-C (n=201) |
p-value* | |
|---|---|---|---|
| Frequency of abdominal discomfort or pain (median of ordinal scale ratings 0-6) | 0 “never” | 4 “1 day/wk” | <0.001 |
| Number of chronic constipation symptoms (Range 0-6) | 3.21 | 3.76 | <0.001 |
| Constipation Severity Scale (Range 0-24) | 7.1 | 9.7 | <0.001 |
| PAC-QOL total score (Range 0-4; higher scores =more impact on QOL) | 1.07 | 1.70 | <0.001 |
| SF-12 Physical Composite Score (Mean T-score for normal population=50: higher scores = less impact on QOL) | 42.9 | 41.1 | 0.17 |
| SF-12 Mental Composite Score (Mean T-score for normal population=50: higher scores = less impact on QOL) | 52.4 | 48.9 | <0.001 |
| BSI General Severity Index T-score (Mean T-score for normal population=50: higher scores = greater psychological distress) | 48.7 | 52.3 | <0.001 |
p-values were computed after adjusting for age and education
Switching of diagnoses
Twelve months after they enrolled in the study, 79/195 (40.5%) of those with FC had recovered and were no longer constipated (i.e. no longer met the FC or IBS-C criteria), and 30/141 (25.5%) of those who had IBS-C at enrollment were no longer constipated (i.e., no longer met criteria for IBS-C or FC). The proportion who recovered was nearly twice as great for FC compared to IBS-C (Table 4).
Table 4.
Diagnoses at baseline and 12 months
| FC at 12 mo %(n) |
IBS-C at 12 mo %(n) |
IBS-M at 12mo %(n) |
Well at 12 mo %(n) |
|
|---|---|---|---|---|
| FC at baseline (n=195) | 38.5% (75) | 13.3% (26) | 5.1% (10) | 40.5% (79) |
| IBS-C at baseline (n=141) | 25.5% (36) | 35.5% (50) | 16.3% (23) | 21.3% (30) |
Abbreviations: mo=months, FC=functional constipation; IBS-C=IBS with constipation
To investigate the tendency to switch diagnoses over time, we excluded patients who recovered and examined the diagnoses of those who retained symptoms of constipation at 12 months: Approximately one third (36/111) of FC patients switched and met criteria for IBS-C or IBS-M at 12 months, which was matched by a third (36/109) of IBS-C patients who switched and met criteria for FC at 12 months follow-up.
Differences in baseline characteristics of patients who switched diagnoses
To explore the possible reasons why IBS-C and FC patients switch diagnoses, we compared the constipation and pain characteristics at baseline of subjects who switched versus those who retained their original diagnostic labels. Amongst patients with FC at baseline, those who switched to IBS-C at 12 months reported more frequent abdominal pain or discomfort at baseline (median of less than 1 day/mo vs. never, p=0.001 by Mann-Whitney U test) as well as at 12 months (median of 2-3 days/mo vs. never, p<0.001) compared to those who remained FC patients. Conversely, IBS-C patients who switched to FC had less frequent abdominal pain or discomfort at baseline (median of 2-3 days/mo vs. 1 day/week, p<0.001), and at 12 months (median of less than 1 day/mo vs. 1 day/week, p<0.001) as compared with those who remained IBS-C.
When those who retained their diagnoses for 12 months were compared to those who switched (Table 5), there was no difference in the number of constipation symptoms at baseline or in their baseline CSS scores. This was true for both the group who had FC at baseline and the group with IBS-C at baseline. The PAC-QOL, SF-12, and BSI scores also failed to predict whether subjects would switch or remain in the same diagnostic category. Age and education were treated as covariates in these analyses.
Table 5.
Comparison of baseline characteristics of patients who switched vs. those who retained their original diagnoses
| Patients diagnosed as FC at Baseline | Patients diagnosed as IBS-C at Baseline | |||||
|---|---|---|---|---|---|---|
| FC at 12 mths (75) | IBS-C at 12 mths (26) | p-value | IBS-C at 12 mths (50) | FC at 12 mths (36) | p-value | |
| Age/yrs | 75.1 | 65.7 | 0.008 | 57.3 | 67.1 | 0.01 |
| Frequency of abdominal pain or discomfort (median)# | 0 “never” | 1 “<1 day/mo” | 0.001* | 4 “1 day/week” | 3 “2-3 days/mo” | <0.001* |
| No of Constipation symptoms | 3.59 | 3.62 | 0.92 | 4.02 | 4.14 | 0.70 |
| PAC-QOL total score | 1.10 | 1.24 | 0.32* | 1.74 | 1.56 | 0.14 |
| SF-12 Physical Composite Score | 41.2 | 43.5 | 0.44 | 43.3 | 42.1 | 0.66 |
| SF-12 Mental Composite Score | 53.7 | 50.7 | 0.25 | 47.1 | 48.8 | 0.43 |
| BSI General Severity Index (T-score) | 47.7 | 49.5 | 0.37 | 52.3 | 52.7 | 0.85 |
Significant after controlling for age using an ANCOVA.
Frequency of abdominal discomfort was described using an ordinal scale : 0 = Never; 1 = <1 day a month, 2 = one day a month, 3 = 2 to 3 days a month, 4 = one day a week. 5 = >1 day a week, 6 = Everyday
Discussion
The Rome criteria were designed as a tool to categorize patients with functional gastrointestinal diseases into distinct diagnoses based on their presenting symptoms1. Our study is the first to evaluate in a large population of subjects with constipation, the ability of the Rome III criteria to achieve this distinction. The data show that the Rome III criteria for FC and IBS-C does not separate patients into distinct groups. This is shown by the following observations:
Diagnostic overlap – If the artificial requirement that FC and IBS-C cannot be diagnosed in the same person is suspended, 89.5% of those with IBS-C also meet criteria for FC, and conversely 43.8% of patients fulfilling criteria for FC also meet criteria for IBS-C (Figure 2). This implies that IBS-C is a subset of FC.
Lack of specificity in symptom profiles – While IBS-C patients report more frequent pain or discomfort than FC, 44% of patients meeting only FC criteria also report some pain or discomfort. Moreover, IBS-C patients paradoxically report more severe constipation than FC patients.
Switching diagnostic labels – When patients who recover from constipation are excluded, approximately 1/3 of patients with each diagnostic label at study enrollment switch to the other diagnostic label by 12 months follow-up.
Previous investigators have reported a similar overlap in symptoms in population based samples and have recognized that the “splitting” of functional gastrointestinal disorders into discrete diagnoses may not be practical. Locke et al15 demonstrated in a population survey that there is a significant overlap between functional GI symptoms that have been used to define symptom complexes. Of relevance, they suggested that up to 6.5% of the population surveyed had an IBS/constipation overlap. In an early study that alluded to the ambiguity in the definition of constipation, Probert et al showed that the likelihood of having IBS was significantly increased in those who met the Rome definition of constipation16. However, none have directly addressed the issue of the precision of the Rome criteria for distinguishing between the different diagnostic labels assigned to constipated patients, namely between functional constipation and IBS-C. In our study we showed that at baseline there was a large overlap between the diagnostic labels of IBS-C and FC, with approximately 90% of the IBS-C patients fulfilling the diagnostic criteria for FC, and nearly half of the FC patients meeting the criteria for IBS-C. This speaks to the fact that while the Rome III criteria for these two conditions require them to be mutually exclusive, in reality these criteria are unable to clearly separate IBS-C from FC patients based on the presenting symptoms.
One of the few longitudinal studies that attempted to determine the stability of FGID symptoms was a natural history population survey by Halder et al, which describes a high rate of turnover in the symptom status of patients17. Their data showed that nearly equal proportions of IBS-C subjects became FC and vice-versa over follow-up. As compared to Halder’s study, we focused on patients with a clinical diagnosis of constipation, with the exclusion of any concurrent gastrointestinal organic pathology, rather than relying on a population incidence. As such, we had much larger subject numbers and data that we believe can be extrapolated to patients in the clinical setting.
What is also unique about our study is that we investigated patients who had symptoms of constipation that were severe enough to result in a medical clinic visit, and who received a clinical diagnosis of constipation from their physician. This contrasts with the studies of largely healthy subjects in previous publications. We focused on the constipation characteristics of these patients, and so had detailed data relating to symptom characteristics, quality of life and other measures which allowed us to test for differences between the FC and IBS-C patients and the factors that predicted the stability of their diagnoses.
Abdominal pain or discomfort is the hallmark symptom of IBS18, raising the question of whether this symptom uniquely distinguishes IBS-C from FC. Our data confirm that IBS-C patients have more frequent pain or discomfort than FC patients and that FC patients who had more pain at baseline or who developed more abdominal pain or discomfort during the 12 month follow-up period were more likely to switch from a diagnosis of FC to a diagnosis of IBS-C. However, the symptom of abdominal pain or discomfort lacked specificity for IBS-C diagnosis: 44% of patients meeting only criteria for FC at baseline had some abdominal pain or discomfort, and a reduction in the level of abdominal pain or discomfort was about as likely to occur and to result in a change of diagnosis as compared to an increase in abdominal pain or discomfort. These observations are consistent with the hypothesis that the differences between IBS-C and FC in abdominal pain are quantitative but not qualitative differences, i.e., they only reflect differences in the frequency of pain or discomfort. Further studies investigating the pathophysiological mechanisms for pain and discomfort in these two groups are needed.
Psychological distress is another symptom that has been thought to be more closely linked to IBS than to FC. Our data confirm greater psychological distress in IBS-C compared to FC, but neither baseline levels of psychological distress nor changes in psychological distress from baseline to the end of follow-up were significant predictors of changes in diagnosis from baseline to 12 months follow-up.
A limitation of this study is that we did not measure relevant physiological parameters such as whole gut transit time, pelvic floor function during defecation, or pain thresholds. It is possible that these physiological variables could identify pathophysiologically distinct groups of patients with constipation. The patients in this study were also older than average for IBS patients in other series although ages were typical for functional constipation. It is difficult to see how these limitations could affect our conclusion that the Rome III criteria in their current form do not identify distinct groups of patients with FC and IBS-C.
There are implications on a number of fronts – First, the Rome symptom based diagnoses are based on the premise that they “breed true” across clinical and population groups and provide a framework for identifying patients for research and in collecting accurate epidemiologic data1. Our findings suggest that a re-definition of the Rome criteria, as they pertain to patients with constipation, may be needed to avoid confusion in treatment trials and disease prevalence reporting. Second, there are clinical ramifications. Physicians seeking to assign a concrete disease label to their patients or base their treatment algorithms upon making a definitive diagnosis will likely be frustrated as they find their patients switching between disorders, and will wonder if their diagnosis was accurate in the first place.
Conclusions
In summary, the overlap in symptoms between IBS-C and FC and the tendency to alternate between diagnoses suggest that the Rome III criteria for IBS-C and FC may not identify etiologically distinct groups of patients. FC and IBS-C appear to be conditions that are quantitatively different, with IBS-C being more severe than FC, but they appear to be qualitatively similar. Therefore, rather than being separate diagnoses, we proposed that they are actually part of the same condition, anchored on different ends of the spectrum of severity.
In trying to define the true differences between FC and IBS-C, we propose that the current Rome criteria based on symptom definition are inadequate, but we acknowledge that physiological tests of motility or transit might identify qualitative differences between FC and IBS-C.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Functional Constipation and Irritable Bowel Syndrome are classified as separate entities by the Rome III criteria.
In reality, clinicians have found it difficult to differentiate these two conditions
Several drugs (Lubiprostone, Tegaserod, Linaclotide) have been found to be effective for both.
WHAT IS NEW HERE
There is a large overlap between FC and IBS-C, and patients frequently transition between these diagnoses over time.
The Rome III criteria for FC and IBS-C do not identify pathophysiologically distinct groups and may require revision.
Acknowledgments
Supported by a generous grant from Novartis Pharmaceuticals and by grants R24 DK067674 and R01 DK031369 from the National Institute of Diabetes, Digestive, and Kidney Diseases.
Footnotes
Roles of authors:
Reuben K Wong – data analysis, writing manuscript
Olafur S Palsson – data management and statistical analysis
Marsha J Turner – contributions to project administration and data collection
Rona L Levy – contributions to design and data collection
Andrew D Feld – contributions to design and data collection
Michael Von Korff – contributions to design and data collection
William E Whitehead – primary responsibility for study design and funding, contributions to writing manuscript
Potential competing interests:
William E. Whitehead has served on the board of the Rome Foundation since 1990. He has also served on advisory boards for Novartis Pharmaceuticals, Takeda Pharmaceuticals, and Ironwoods, each of which has developed and/or marketed drugs for the treatment of functional constipation and irritable bowel syndrome with constipation. He has been the recipient of research grants from Novartis and Takeda pharmaceutical companies. None of the other authors have competing interests.
Reference List
- 1.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III: The Functional Gastrointestinal Disorders. McLean, Virginia: Degnon Associates; 2006. [Google Scholar]
- 2.Locke GR, III, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterol. 2000;119:1766–1778. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WG . the Working Team for Functional Bowel Disorders. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Richter JE, Talley NJ, Thompson WG, Corazziari E, Whitehead WE, editors. The functional gastrointestinal disorders. Boston: Little, Brown and Company; 1994. pp. 115–173. [Google Scholar]
- 4.Kamm MA, Muller-Lissner S, Talley NJ, Tack J, Boeckxstaens G, Minushkin ON, Kalinin A, Dzieniszewski J, Haeck P, Fordham F, Hugot-Cournez S, Nault B. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 6.Drossman DA, Chey W, Panas R, Wahle A, Scott C, Ueno R. Lubiprostone Significantly Improves Symptom Relief Rates in Adults With Irritable Bowel Syndrome and Constipation (IBS-C): Data from Two Twelve-Week, Randomized, Placebo-Controlled, Double-Blind Trials. 132. 2007. pp. 2586–2587. [Google Scholar]
- 7.Pohl D, Tutuian R, Fried M. Pharmacologic treatment of constipation: what is new? Curr Opin Pharmacol. 2008;8:724–728. doi: 10.1016/j.coph.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Nyrop KA, Palsson OS, Levy RL, Korff MV, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson WG, Drossman DA, Talley NJ, Walker L, Whitehead WE. Rome III diagnostic questionnaire for the adult functional GI disorders (including alarm questions) and scoring algorithm. In: Drossman DA, Cprazzoaro E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. III. McLean, VA: Degnon Associates, Inc.; 2006. pp. 917–951. [Google Scholar]
- 10.Whitehead WE. Development and validation of the Rome III diagnostic questionnaire. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. 3. McLean, Virginia: Degnon Associates; 2006. pp. 835–853. [Google Scholar]
- 11.Palsson OS, Turner MJ, Levy RL, Feld AD, Von Korff M, Drossman DA, Whitehead WE. The Rome III questionnaire functional constipation module as a constipation severity scale. 2008:A-421. [Google Scholar]
- 12.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Frank L, Kleinman L, Farup C, Taylor L, Miner P., Jr Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. doi: 10.1080/003655299750025327. [DOI] [PubMed] [Google Scholar]
- 14.Derogatis LR. BSI 18 Brief Symptom Inventory 18: Administration, scoring, and procedures manual. NCS Pearson, Inc; 2000. [Google Scholar]
- 15.Locke GR, III, Zinsmeister AR, Fett SL, Melton LJ, III, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 16.Probert CS, Emmett PM, Cripps HA, Heaton KW. Evidence for the ambiguity of the term constipation: the role of irritable bowel syndrome. GUT. 1994;35:1455–1458. doi: 10.1136/gut.35.10.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halder SL, Locke GR, III, Schleck CD, Zinsmeister AR, Melton LJ, III, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterol. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterol. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]


