Abstract
The total synthesis of amphidinolide C and a second-generation synthesis of amphidinolide F have been accomplished through the use of a common intermediate to access both the C1-C8 and the C18-C25 sections. The development of a Ag-catalyzed cyclization of a propargyl benzoate diol is described to access both trans-tetrahydrofuran rings. The evolution of a Felkin-controlled 2-lithio-1,3-dienyl addition strategy to incorporate C9-C11 diene as well as C8 stereocenter is detailed. Key controlling aspects in the sulfone alkylation / oxidative desulfurization to join the major subunits, including the exploration of the optimum masking group for the C18 carbonyl motif, are discussed. A Trost asymmetric alkynylation and a stereoselective cuprate addition to an alkynoate have been developed for the rapid construction of the C26-C34 subunit. A Tamura/Vedejs olefination to introduce the C26 sidearm of amphidnolides C and F is employed. The late-stage incorporation of the C15, C18 diketone motif proved critical to the successful competition of the total syntheses.
Introduction
Natural products continue to yield medicinally relevant leads for the treatment of human disease1 as well as an inspiration for the development of new synthetic strategy and chemical methodology for their construction.2 Macrolides such as epothilones,3 apoptolidins4 and bryostantins5 historically have provided a rich source of inspiration in both of these areas. The amphidinolide family of macrolides embodies another such collection of natural products that provides synthetic inspiration through their challenging architecture with equally intriguing biological function – particularly cytotoxic activity against multiple cancer cell lines. 6
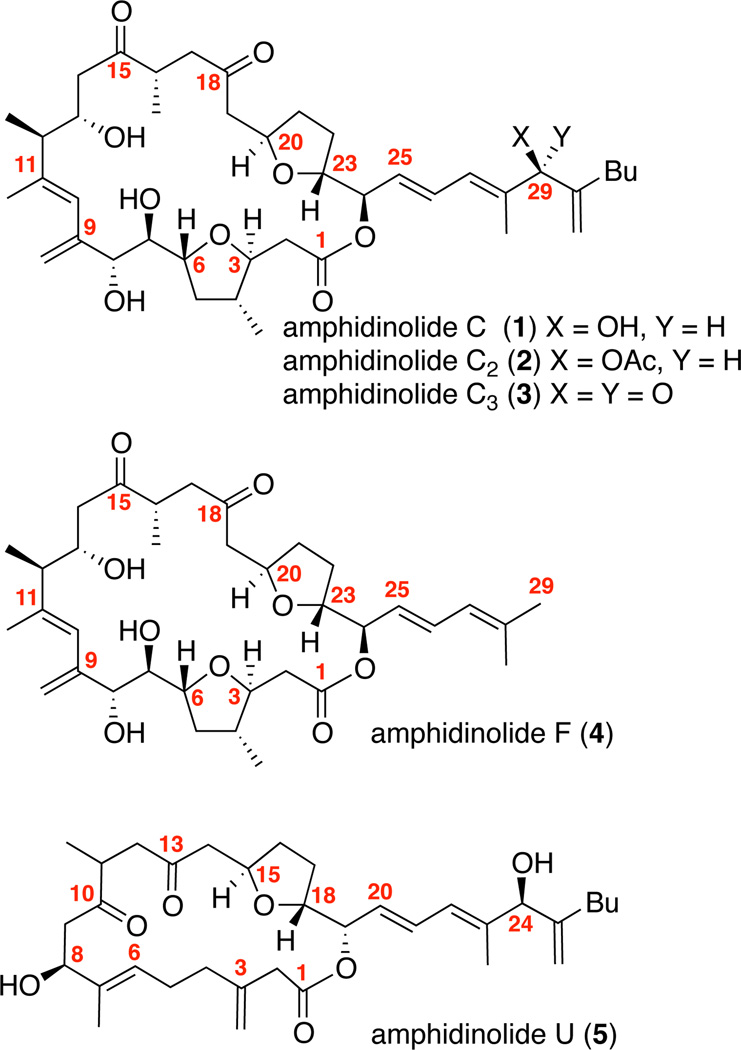
While multiple total syntheses of many members of this family have been reported,7 certain important subfamilies remain unaddressed. Of these unaddressed subfamilies, our laboratory became particularly interested in amphidinolides C and F, as they possess challenging (and identical) macrocyclic core and intriguing biological profile (Figure 1). Both amphidinolides C and F have attracted considerable synthetic attention8 including from our own laboratory;9 however, no total synthesis of either compound had been reported prior to our efforts.10,11 Amphidinolide C was isolated from the genus Amphidinium (Y-5, Y-56, Y-59 and Y-71 stains) in extremely small amounts (0.0015% yield) by Kobayashi and coworkers.12 The relative stereochemistry of 1 was determined by 1D and 2D NMR techniques and the absolute stereochemistry was established through degradation and Mosher ester analysis.121 exhibits impressive cytotoxic activity in multiple cancer cell lines (murine lymphoma L1210 cells: IC50 = 5.8 ng/mL and human epidermoid carcinoma KB cells: IC50 = 4.6 ng/mL).12 Subsequently, additional variants (amphidinolides C2 and C3) have been identified which bear esterification or oxidation at C29.13 Kobayashi has also reported the isolation of amphidinolide U (5). This compound contains the same side arm as amphidinolide C (1), but a simplified version of the macrocyclic core and has shown significantly reduced cytotoxicity data.14 Amphidinolide F (4) has also been isolated in limited qualities (0.00001% wet weigh yield) bearing an identical macrcocyclic core, but with a simplified sidearm.15 Interestingly, amphidinolide F shows greatly reduced cytotoxic activity as compared to 1.15 While the relative and absolute configurations of amphidinolide C had been established by Kobayashi, the definitive confirmation of the absolute stereochemistry of amphidinolide F and its relationship to amphidinolide C was not established until our laboratory completed its total synthesis in 2012.10 In this article, we provide a full account of our synthetic efforts towards amphidinolide F as well as the first reported total synthesis of amphidinolide C.
Figure 1.
Amphidinolides C, F and U.
Results and Discussion
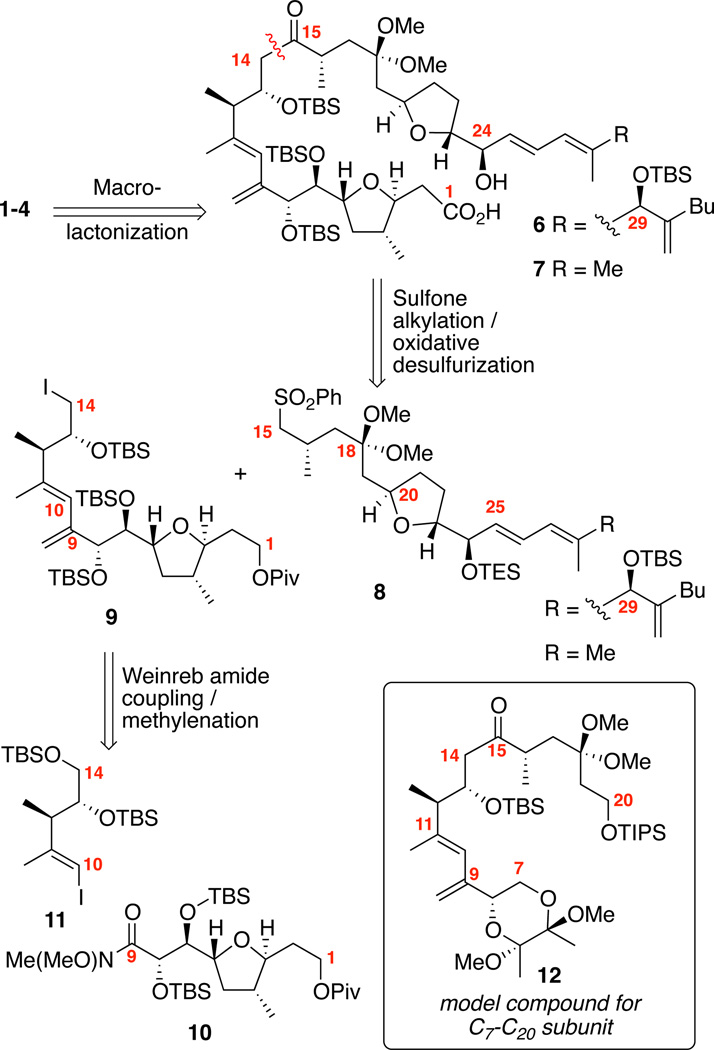
Our retrosynthetic strategy for accessing amphidinolides C and F is shown in Scheme 1. We envisioned formation of the 25-membered macrocycle through Yamaguchi macrolactonization. Next, we planned to join the two major subunits and incorporate the C15 carbonyl through a sulfone alkylation / oxidative desulfurization sequence.16 The nucleophilicity of sulfone carbanions is a powerful tool for the construction of sterically congested linkages such as the C14-C15 bond, in which branching at neighboring C13 and C16 would normally inhibit such strategies.17 Oxidative desulfurization is a chemical transformation that has been known for decades;18 however, it has received comparatively limited attention for the synthetic community.19 This umpolung approach also would allow us to regulate when the C15 carbonyl is incorporated while avoiding potential complications with dithiane chemistry.20 The iodide 9 could be accessed from the vinyl iodide 11 and Weinreb amide 10 by an organolithium coupling followed by methylenation. Prior to embarking on the total syntheses of compounds 1–4, we felt it would be prudent to study both our C9-C11 diene strategy and sulfone alkylation / oxidative desul-furization sequence on a C7-C20 model compound 12.
Scheme 1.
Retrosyntheses for Amphidinolides C-C3 and F.
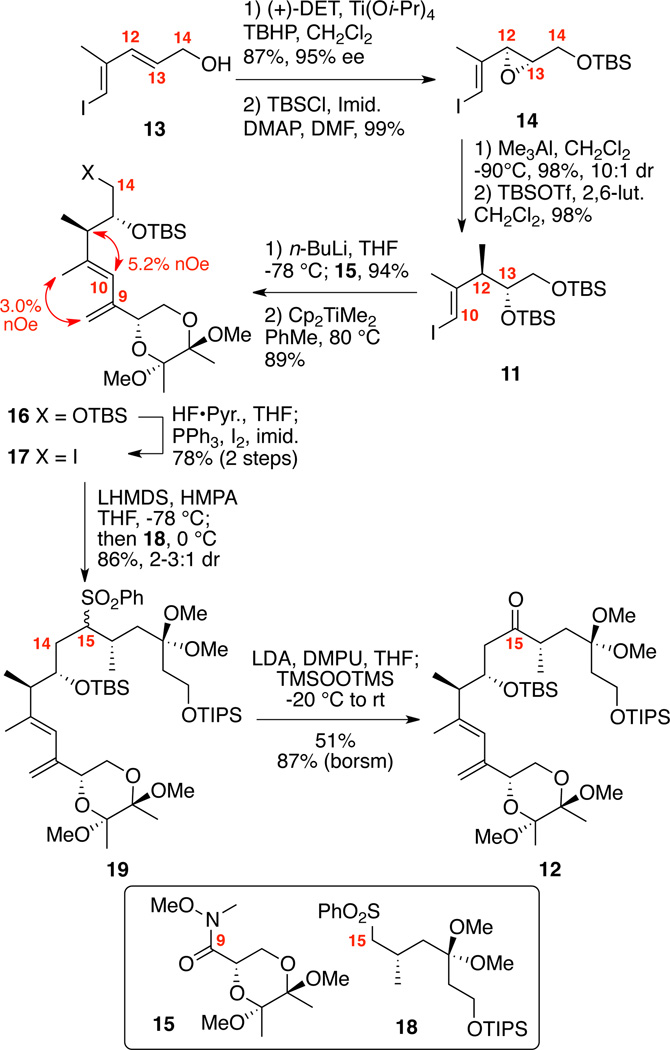
Our successful studies9a on the model compound 12 embarked from the readily available dienyl iodide 1321 (Scheme 2). Sharpless epoxidation22 followed by silyl protection produced 14. Me3Al-mediated opening of vinyl iodide / allyl epoxide 14 provided preferential SN2 opening at C12.23 Our originally published conditions proved somewhat scale de- pendent,9a but we found that modified conditions (portion-wise addition of reduced equivalents of AlMe3 and lowered reaction temperature to −90°C) gave reliable results on gram scale (>1.5 g scale, 98%, 10:1 dr). Subsequent silylation of C13 alcohol provided 11. Halogen / metal exchange and coupling with the Weinreb amide 159a followed by methenylation using Petasis conditions yielded the diene 16 with no observable E/Z isomerization. 1D nOe analysis confirmed that the desired olefin geometry was present after methylenation. Selective removal of the C14 TBS ether and conversion to iodide provided the requisite coupling partner 17. Next, our attention turned to the key sulfone alkylation / oxidative desulfurization sequence. The sulfone 18 (prepared in 9 steps from 3-hydrox-(2R)-methylpropionic acid methyl ester9a) was lithiated with LHMDS in the presence of HMPA and added to iodide 17 to cleanly provide the C14-C15 coupled material 19 in good yield as a mixture of diastereomers at C15. Given the sterically congested nature of both the nucleophile and electrophile, the high efficiency of this coupling was rewarding. Next, the oxidative desulfurization on sulfone 19 was examined. After some experimentation, we found that deprotonation with LDA in the presence of DMPU followed by the addition of TMSOOTMS produced the desired ketone 12 in 51% isolated yield (87% borsm).
Scheme 2.
Synthesis of C7-C20 Segment: A Model Study.
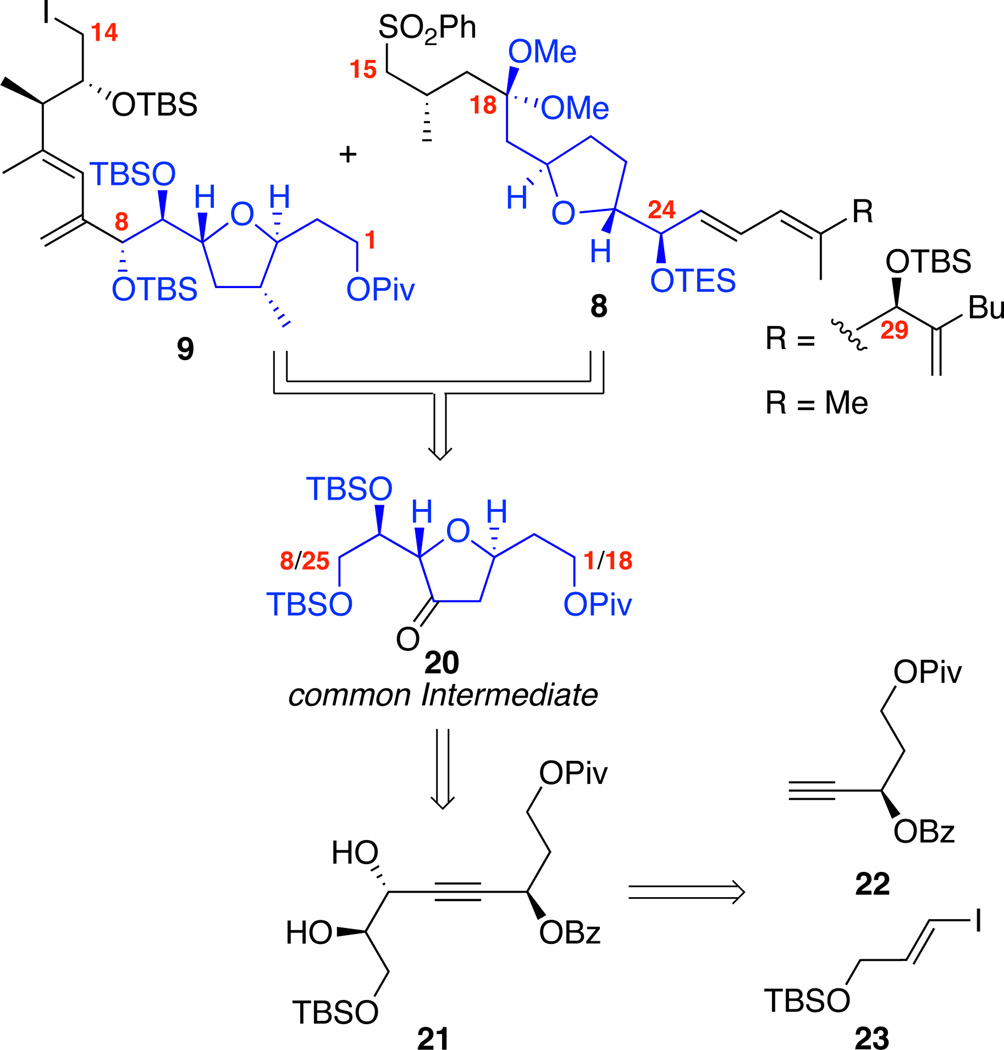
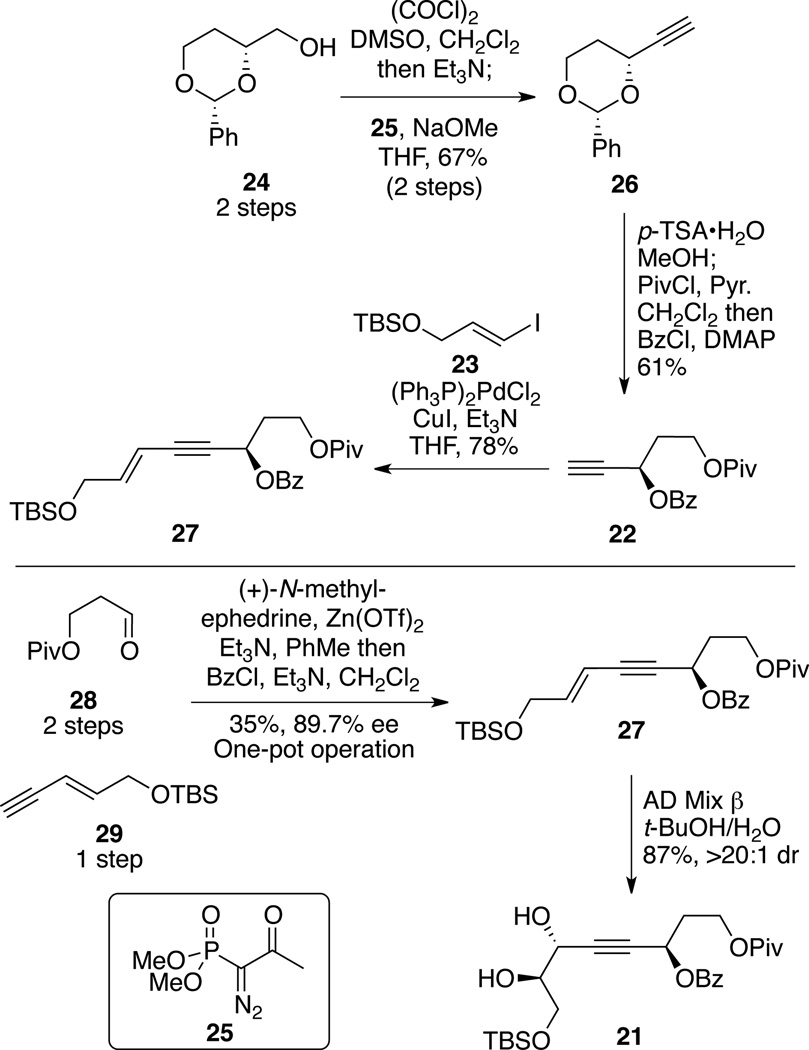
With an understanding that our strategy for the diene portion and coupling the two major subunits was likely to prove successful, we started our efforts towards the synthesis of the two THF segments (Scheme 3). Central to this strategy was the observation that a hidden symmetry element was present within the macrocyclic core. The functionality and stereochemistry of C1-C8 mapped nicely on the C18-C25 subunit. The lone exception to this correlation was the presence of the C4 methyl moiety. We identified that both the major fragments 9 and 8 could arise from a common subunit 20. This subunit in turn should be accessible from the propargyl benzoate / diol 21 through a metal-catalyzed cyclization. Pioneering work by Krause24 and Gagosz25 had demonstrated that Au- or Agcatalyzed processes were feasible; however, neither Krause nor Gagosz has tested the potential of this chemistry on diol systems such as 21 or in the presence of considerable additional functionality.
Scheme 3.
Common Intermediate Approach to Major Subunits.
Synthesis of the cyclization precursor 21 is shown in Scheme 4. Starting from the known alcohol 24 (available in two steps from D-malic acid),26 Swern oxidation followed by alkyne formation using the Ohira-Bestmann reagent 25 provided 26. The alkyne 26 could also be accessed from the aldehyde via the two-step Corey-Fuchs protocol (Ph3P, CBr4, CH2Cl2; n-BuLi, THF, 69% over 2 steps). Diol deprotection and subsequent esterifications provided the propargyl benzoate 22. Sonogashira coupling27 with known vinyl iodide 2328 generated the enyne 27. While this seven-step route provided access to the enyne 27 in multigram quantities, a more expedient route was feasible through the known aldehyde 2829 and enyne 2930 using Carreira’s asymmetric alkynylation31 with in situ benzoate ester formation to provide 27 in four fewer steps (LLS). Sharpless dihydroxylation with AD Mix β32,33 provided the cyclization precursor 21 in high yield and excellent dr.
Scheme 4.
Synthesis of Cyclization Precursor.
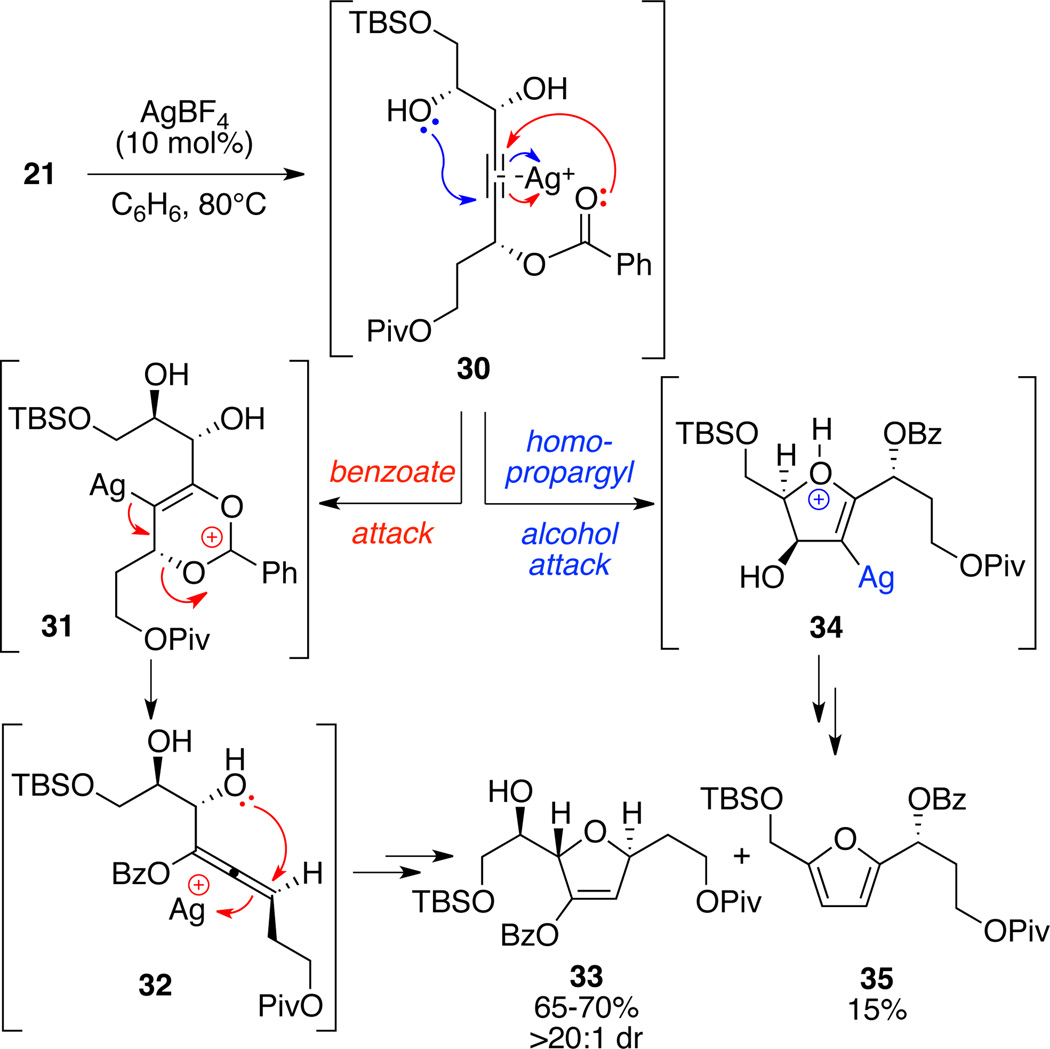
We were pleased to find that the desired metal-catalyzed cyclization could be cleanly effected by treatment of 21 with AgBF4 (10 mol%) in degassed benzene at 80°C to produce the trans-DHF 33 in 65–70% yield on 5-gram scale (Scheme 5). Interestingly, Au-catalyzed versions of this cyclization proved unsuccessful in our hands. Key to this transformation was the absence of light; performing this transformation in a lighted room led to greatly diminished yield (≈ 25%). Selection of the pivaloyl protecting group was also key as use of electron rich moieties (e.g. PMB, DMB) led to reduced yield (0–30%). In addition to the desired DHF 33, a small amount (15%) of the furan byproduct 35 was also produced. We hypothesize that nucleophilic attack by the benzoate oxygen (marked in red) on activated alkyne 30 might produce the allene species 32 via stabilized carbocationic intermediate 31. Another silvermediated activation of allene 32 could promote the nucleophilic attack by proximal alcohol moiety to deliver the DHF 33 after protodemetallation. Alternately, byproduct 35 might arise from a competitive attack by the distal hydroxyl nucleophile on activated alkyne 30 (marked in blue) to generate the vinyl silver intermediate 34. Protodemetallation followed by Lewis (or Bronsted) acid-activated aromatization would produce the furan 35.
Scheme 5.
Silver-Catalyzed Cyclization to Dihydrofuran.
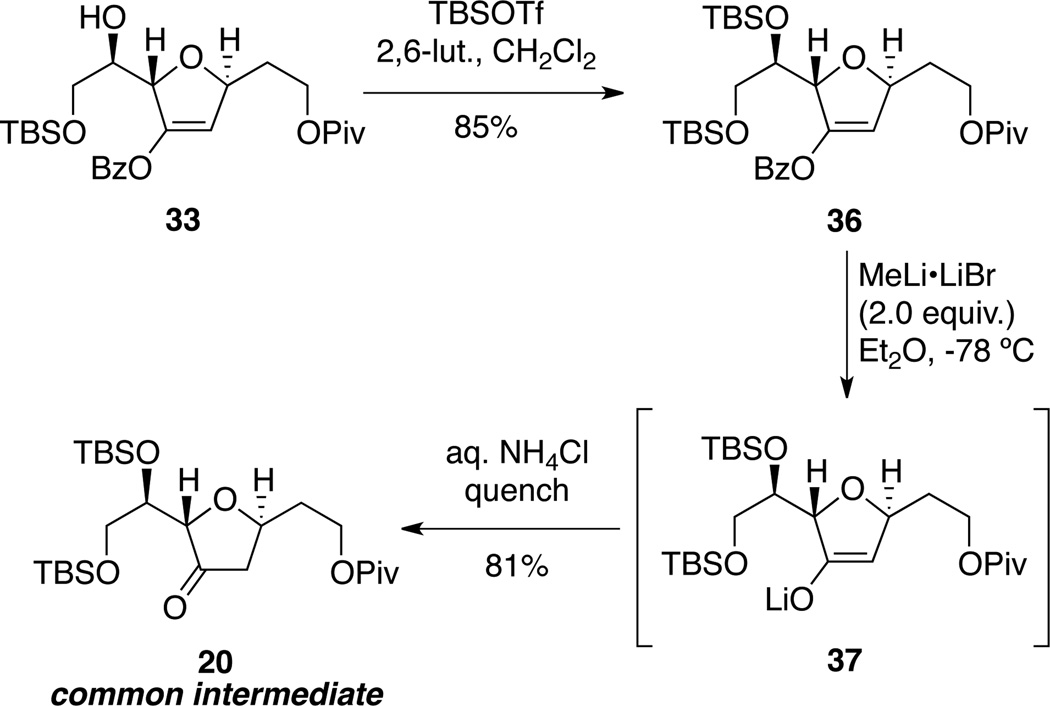
Synthesis of the common intermediate 20 is shown in Scheme 6. While alcohol 33 proved to be unstable to prolonged storage, protection as its TBS ether 36 quickly addressed that shortcoming. Removal of the benzoate ester in presence of the pivaloate (Piv) moiety was problematic. Fortunately, we found that treatment with modulated methyl lithium (MeLi•LiBr)34 provided conditions that selectively cleaved the benzoate moiety to reveal the in situ enolate 37 which was protonated with aqueous ammonium chloride to provide the common intermediate 20. A small amount of the pivaloate deprotected product (≈10%) was observed under these conditions; however, use of MeLi instead of MeLi•LiBr led to significantly larger amount of depivaloated product.
Scheme 6.
Synthesis of the Common Intermediate.
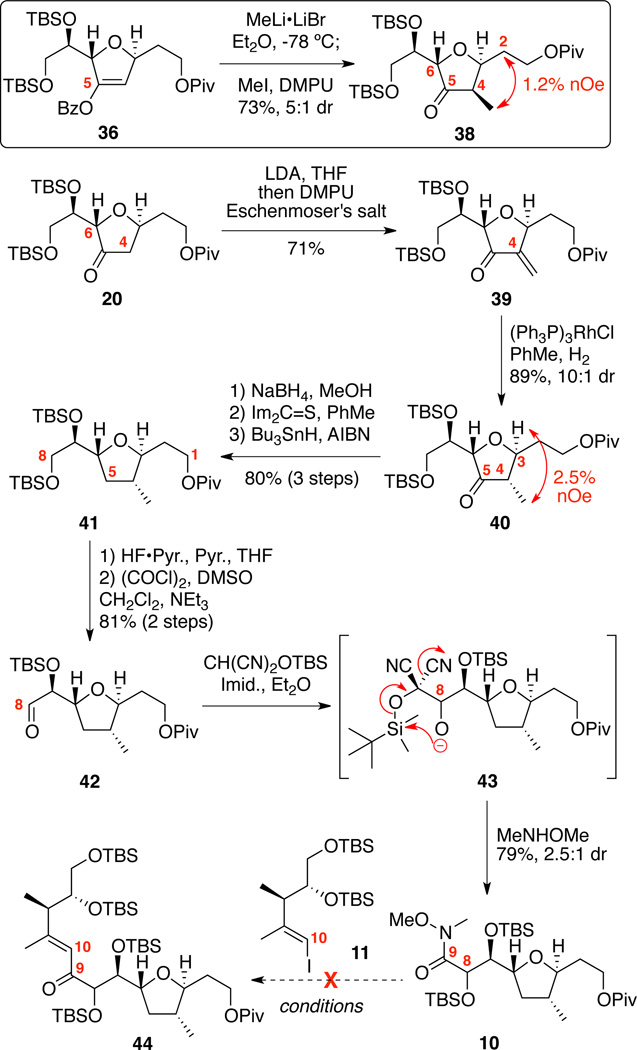
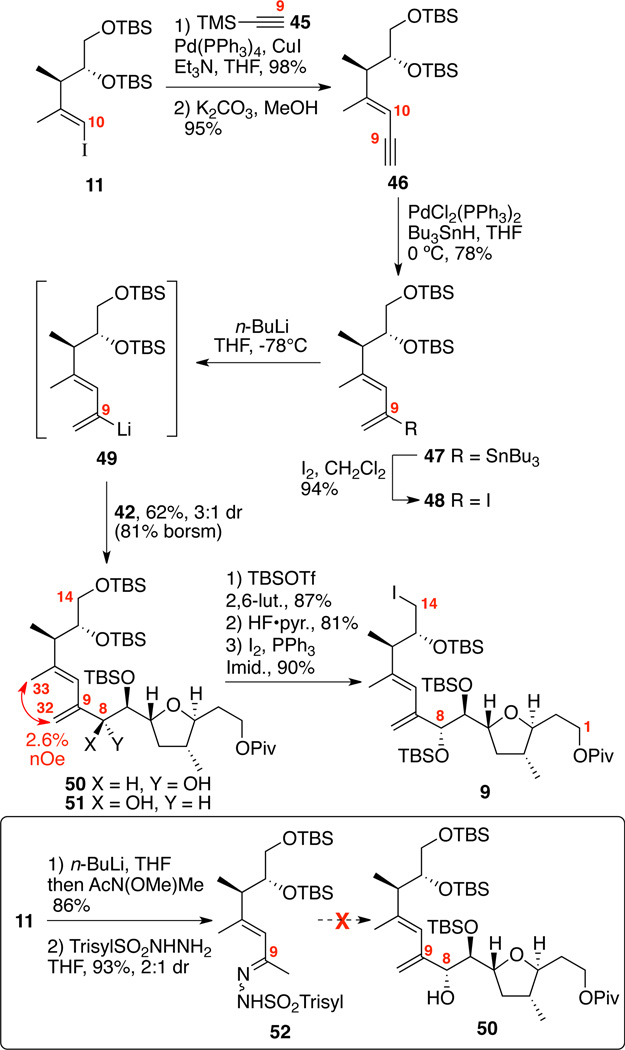
With the common intermediate 20 in hand, we first set out to develop a general approach to the C1-C14 portion of both amphidinolides C and F (Scheme 7). While we had hoped that simple alkylation of the enolate 37 (e.g. generated in situ from MeLi•LiBr treatment of benzoate 36) would provide the desired stereochemical outcome, we experimentally observed the undesired C4 stereochemistry in 5:1 dr. Interestingly, the C6 stereocenter overrode the more proximate C3 position to control the stereochemistry of this transformation. This stereochemical bias was successfully harnessed by first methylenation of the ketone 20 using Eschenmoser’s salt followed by hydrogenation with Wilkinson’s catalyst to give the desired stereochemical combination in 10:1 dr. In both cases 38 and 40, the C4 stereochemistry was determined by nOe analysis. Next, deoxygenation of ketone 40 was first explored using a Wolff-Kishner strategy. Myers had recently reported an elegant improvement35 to the traditional harsh conditions for this transformation that appeared well-suited to our substrate. While we were able to form the TBS-hydrazone intermediate, we were unable to effect the necessary reduction – leading only to decomposition or no reaction under a variety of conditions. We next turned to a Barton-McCombie strategy.36 Reduction of the ketone to alcohol followed by conversion to the thioate and Bu3SnH-mediated reduction cleanly provided the deoxygenated product 41 in excellent overall yield.37 It was important that the Bu3SnH reduction be conducted in deoxygenated solvent. Next, removal of the C8 TBS ether was cleanly effected using HF•pyr. conditions followed by Swern oxidation to yield the aldehyde 42. In order to access the presumed coupling partner (e.g. 10), it was required to incorporate the C9 Weinreb amide and to establish the C8 stereocenter. Nemoto has reported an elegant potential solution for this challenge, which utilized a silyoxy malononitrile nucleo-phile.38 We were pleased to see that these conditions nicely proceeded via the presumed intermediate 4339 to provide the Weinreb amide 10 in good yield and modest diastereoselectivity. While stereochemical outcome of this experiment was expected to be the Felkin (syn) product, we did not rigorously determine the C8 stereochemistry. Unfortunately, despite considerable efforts using either the major or minor C8 diastereomers, we were unable to facilitate the subsequent coupling experiment between the organolithium species derived from iodide 11 and the Weinreb amide 10 using a variety of halogen / metal exchange conditions (e.g. n-BuLi, t-BuLi) and solvents (THF, Et2O, THF / hexanes). Based on these unexpected results, a revised approach was needed to circumvent the iterative formation of the C8-C9 and the C9-C10 bonds.
Scheme 7.
Initial Route for C1-C14 Subunit.
The successful synthesis of the C1-C14 subunit is shown in Scheme 8. In order to circumvent the problematic addition chemistry with Weinreb amide 10, we chose to utilize a nucleophilic 1,3-diene motif (e.g. organolithium 49) for diastereoselective addition to aldehyde 42. We were unaware of any prior example of exploiting similar strategy with 2-lithio-1,3-dienes. While a related vinyl iodide have been employed in cross coupling strategy to form the diene motif present in amphidinolide B,40 the organolithium strategy brought with its potential for metallotropic rearrangement41 of 49. We initially explored accessing this lithio species via a Shapiro process from the corresponding hydrazone 52; however, this approach led to rapid decomposition. We hypothesized that proportionately milder halogen-metal exchange process at lower temperature might circumvent this decomposition process. Thus, we targeted 2-iodo-1,3-diene 48 as a suitable precursor for accessing the lithiated species. In preparation for this strategy, Sonogashira coupling27 between iodide 11 and TMS-acetylene (45) cleanly furnished the enyne 46 in excellent chemical yield. Use of a Pd(II) salts [e.g. (Ph3P)2PdCl2] gave reduced chemical yields as compared to (Ph3P)4Pd. Next, Pd(0)-catalyzed hydrostannylation42 followed by iodination produced the dienyl iodide 48. To our delight, halogen-metal exchange followed by addition of aldehyde 42 cleanly provided the targeted allylic alcohol 50 in 62% yield and 3:1 dr (50:51). This strategy allowed us to produce the 1,3-diene and secure the C8 stereochemistry in a single operation. The C8 stereochemistry was confirmed by advanced Mosher ester analysis.43 After TBS protection at C8, selective desilylation at C14 and conversion to correspondng iodide provided the C1-C14 subunit 9.
Scheme 8.
Synthesis of the C1-C14 Subunit.
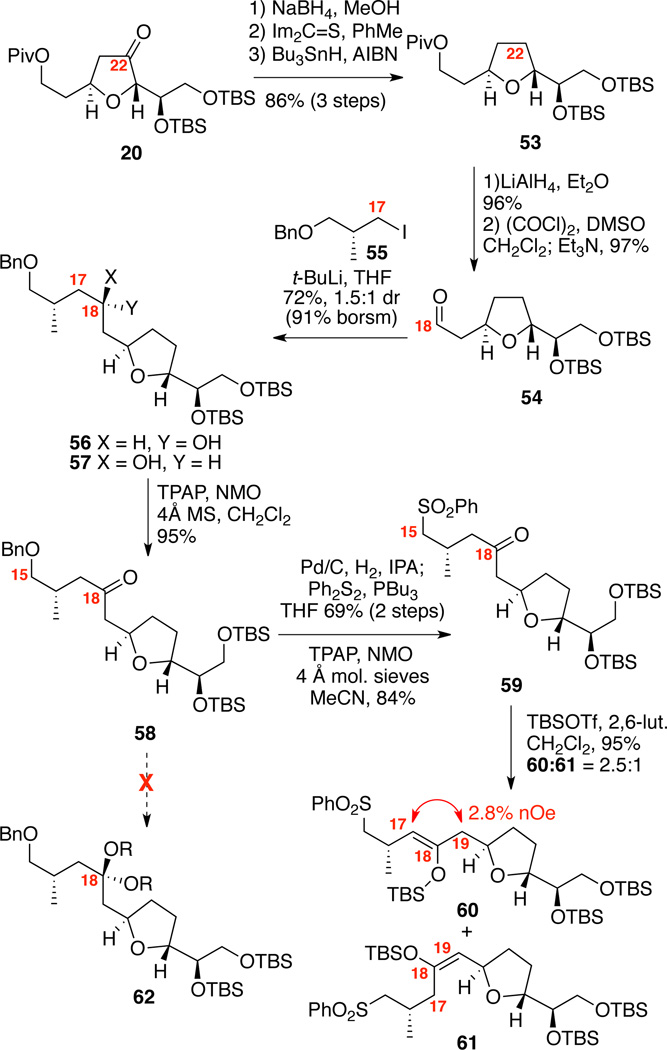
With a viable, unified route to the C1-C14 domain, our attention shifted to construction of the remaining sulfone subunit (Scheme 9). Starting from the common ketone intermediate 20, borohydride reduction provided corresponding alcohol as a 1.7:1 mixture at C22. Thiolate formation under basic conditions led to silyl migration, but use of thermolysis in presence of thiocarbonyldiimidazole cleanly yielded the thiolate. Barton-McCombie deoxygenation proceeded smoothly to provide THF 53. After, pivaloate deprotection and Swern oxidation to yield aldehyde 54, coupling with the organolithium species derived from iodide 5544 produced the alcohols 56/57 as a inseparable mixture of diastereomers. Attempted coupling the C15 thiophenyl version45 of 55 proved problematic in our hands. Oxidation generated the C18 ketone 58. As we had done previously,9a we planned to mask the C18 ketone as ketal 62. Despite our considerable efforts, we were unable to effect this process. Consequently, it was necessary to develop an alternate method for masking the C18 carbonyl moiety. One option was to construct a silyl enol-ether that should be readily cleavable under mild fluoride conditions; however, its utility was potentially complicated due to the possibility for formation of four different isomers. Fortunately, after conversion to the sulfone 59, treatment with TBSOTf under mildly basic conditions cleanly produced just two of the four possible isomers in excellent yield.
Scheme 9.
Synthesis of the Silyl Enol-ethers.
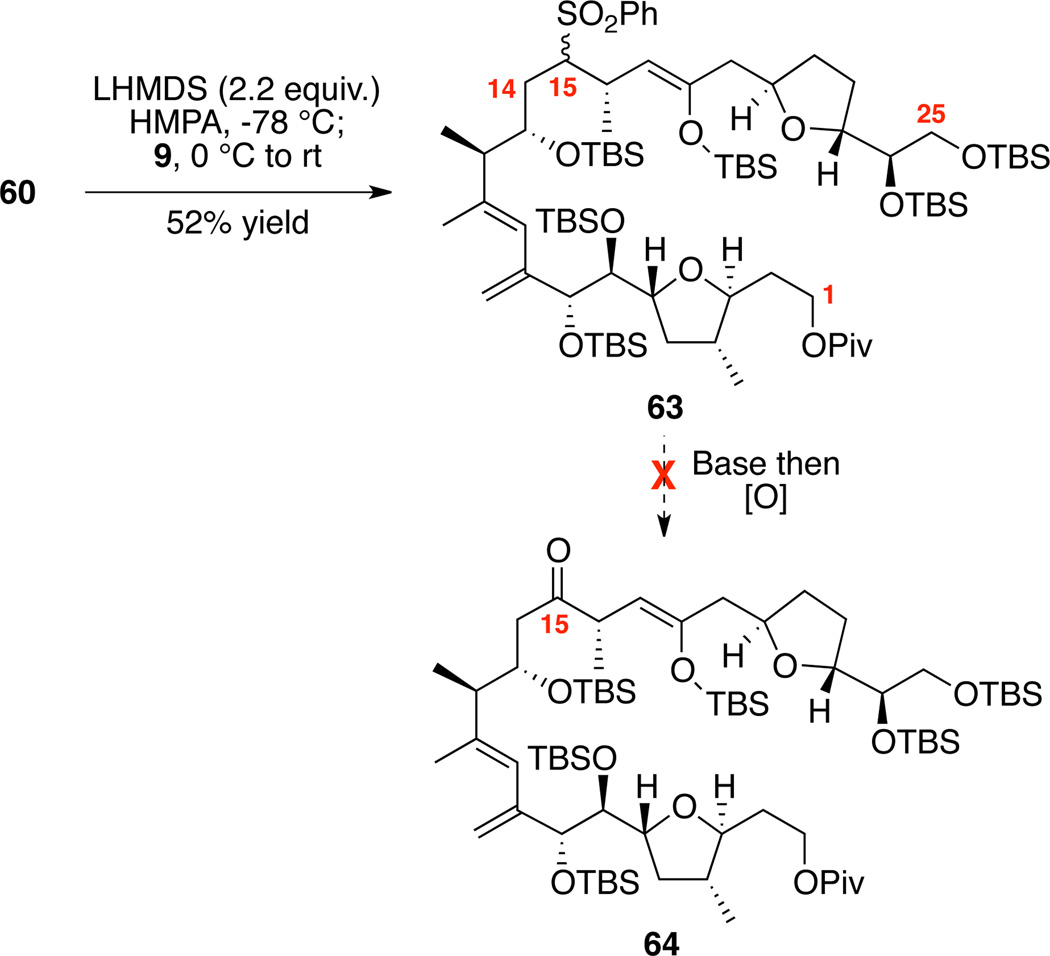
We next set out to test the viability of our coupling strategy on the enol-ethers 60 and 61 (Scheme 10). After modification of the stoichiometry of base as compared to previously developed conditions, we were able to once again facilitate the key C-C bond-forming event. While the yields were modest in the coupling process [52% yield for 60 and 45% yield for 61 (not shown)46], we were more focused on the critical oxidative desulfurization. We were disappointed to observe only decomposition under a range of conditions for this critical step using 63 as well as its silyl enol-ether isomer (not shown). One possible explanation for the divergence in reactivity between our model system 19 and the silyl enol-ether series was the absence of a chelatable group at C18 to help direct lithiation at C15 and stabilize any resultant anion.
Scheme 10.
Exploration of Silyl Enol-ether Series in Sulfone Alkylation / Oxidative Desulfurization Sequence.
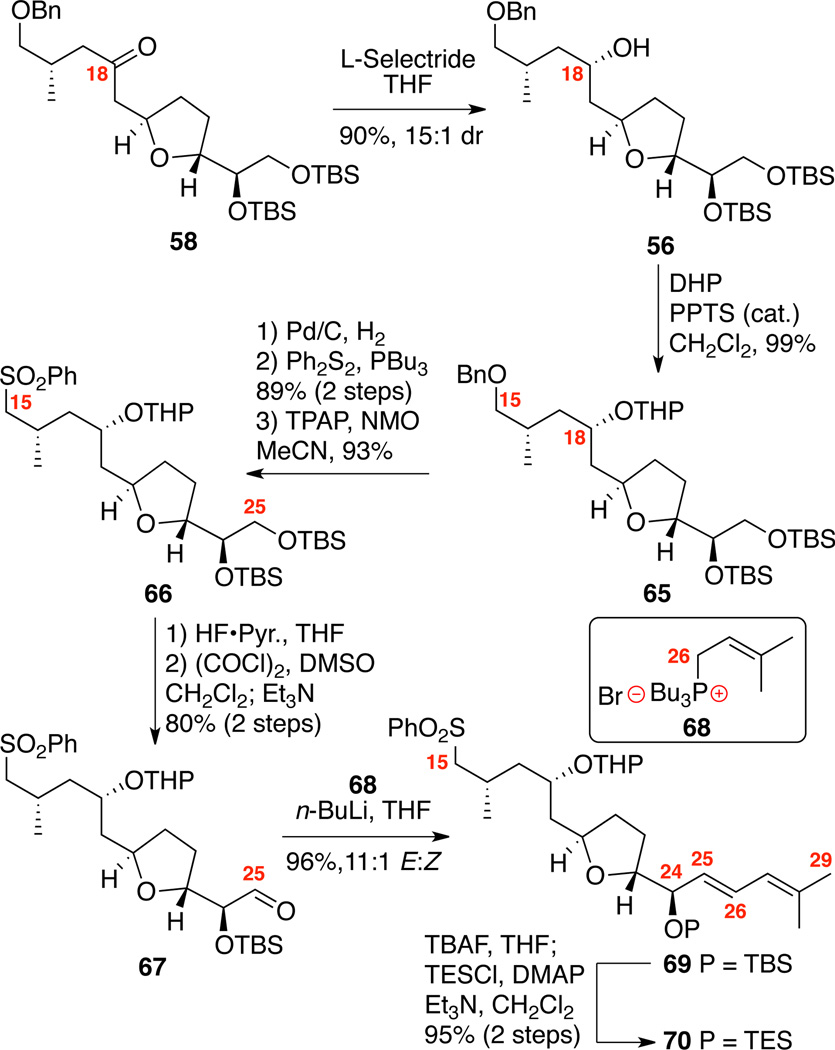
Based on this speculative C18-chelation hypothesis, we embarked on the synthesis of a fully functionalized C15-C29 system containing an appropriately selected protecting group at C18 (Scheme 11). We strategically targeted amphidinolide F (4) first due to the C25 simplified sidearm with the expectation that lessons learned could be applied to amphidinolide C (1). Given the presumed acid sensitivity of the macrolactone, our choices were likely limited to protecting groups readily removable under mild conditions. We initially selected a THP protecting group at C18 as it is well known to be labile under mildly acidic conditions.47 Starting from ketone 58, L-Selectride reduction cleanly provided the 18S isomer 56 as determined by advanced Mosher ester analysis.43 Protection at C18 using DHP generated the mixed acetal 65 in excellent yield. Subsequent removal of the benzyl ether under hydrogenative conditions followed by sulfide incorporation and oxidation using TPAP, NMO in acetonitrile yielded the C15 sulfone 66. Selective removal of the C25 TBS ether followed by Swern oxidation yielded the α-oxy aldehyde 67. Olefination using the Tamura/Vedejs-type tributylphosphonium salt 6848 cleanly produced the desired diene 69 with high E/Z selectivity and chemical yield (96%, 11:1 E:Z). C24 protecting group exchange produced the necessary coupling partner 70 in excellent yield.
Scheme 11.
Synthesis of the Tetrahydropyranyl Series.
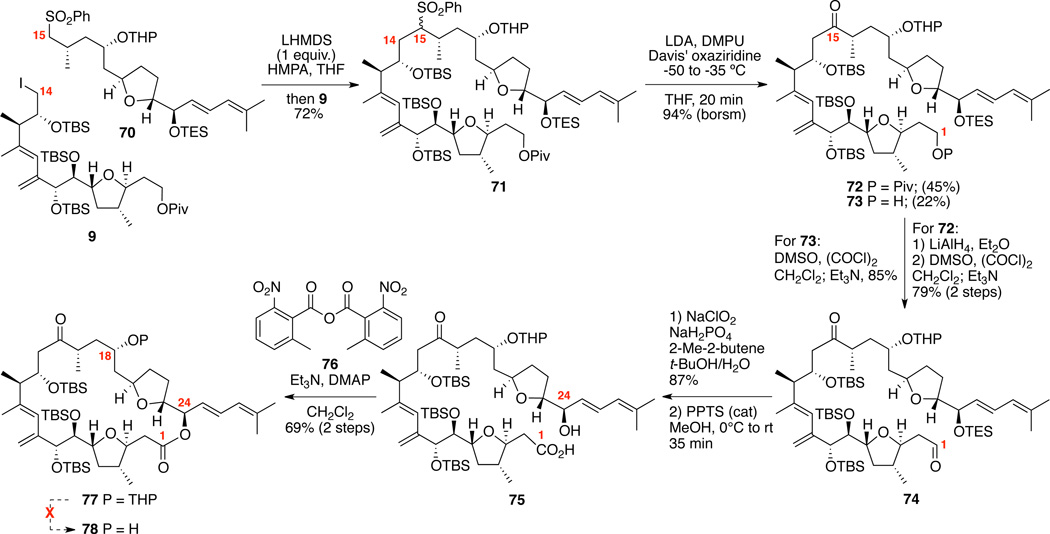
With both the major subunits in hand, we set out to explore the critical sulfone alkylation / oxidative desulfurization sequence (Scheme 12). To our delight, treatment of sulfone 70 with LHMDS in presence of HMPA followed by addition of the iodide 9 yielded the C14-C15 coupled material 71 in a gratifying 72% yield. Only one equivalent of base with respect to sulfone 70 was necessary to effect the transformation. For the oxidative desulfurization, a modification of our original conditions provided the desired ketone in excellent overall yield. Davis’ oxaziridine appeared to be key to this transformation as use of alternate oxidants (e.g. MoOPH, TMSOOTMS etc.) gave inferior results. Presence of the C18 chelating protecting group is likely key to the success of both the alkylation and the oxidative desulfurization. Both the C1 Piv-protected and deprotected products (72 and 73 respectively) were obtained from this transformation (likely due to adventitious water facilitating its saponification); however, both compounds were productive contributors to the synthetic sequence. For 73, Swern oxidation directly produced the aldehyde 74. For 72, LiAlH4 reduction removed the pivaloate with concomitant reduction of the C15 carbonyl and subsequent oxidation under Swern conditions generated the same aldehyde 74. Pinnick oxidation provided the carboxylic acid. Next, we required the selective deprotection of the C24 TES ether in presence of multiple 2° TBS ethers and a OTHP moiety. Fortunately, mild acidic conditions (PPTS, MeOH) selectively removed the C24 TES ether to provide seco acid 75. We speculated that the sterically congested nature of the C18 OTHP group inhibited its deprotection under these conditions. Little did we know that this positive short-term accomplishment was foreboding of future events. Next, macrolactonization of seco-acid 75 under Shina conditions49 provided the 25-membered macrolactone 77 in good yield (69% over 2 steps). Yamaguchi macrolactonization conditions50 were also effective in this transformation – albeit in a slightly lower chemical yield (65%). Despite the THP moiety’s well-known lability under Brønsted and Lewis acidic conditions, we were unable to successfully facilitate its removal under a range of conditions 5:1:1 AcOH/THF/H2O, MgBr2,51 Me2AlCl,52 BF3•OEt2/1,2-ethanedithiol53) – ultimately leading to decomposition in each case. We speculated that the acid sensitivity of the macrocycle 77 was due to preferential ionization at C24, which would generate a highly stabilized dienyl cation.
Scheme 12.
Initial Construction of Amphidinolide F Macrocycle.
Despite this significant setback to our campaign towards amphidinolide F, two negative results provided a possible pathway to circumvent this reactivity. Unlike other conditions screened, treatment of 77 with either 4:2:1 AcOH/THF/H2O or PPTS/MeOH54 did not decompose the macrocycle (nor was any appreciable deprotection of the C18 OTHP observed). We hypothesized if we could identify a more acid labile protecting group at C18 that could be cleaved with these mildly acidic conditions, we could access the needed alcohol at that position. We cautiously turned to the underutilized ethoxyethyl ether (OEE) protecting group as a possible candidate. The OEE moiety is known to be significantly more labile than an OTHP group (circa 250 times in one study)47 while maintaining the necessary chelating ability for the sulfone alkylation / oxidative desulfurization sequence.
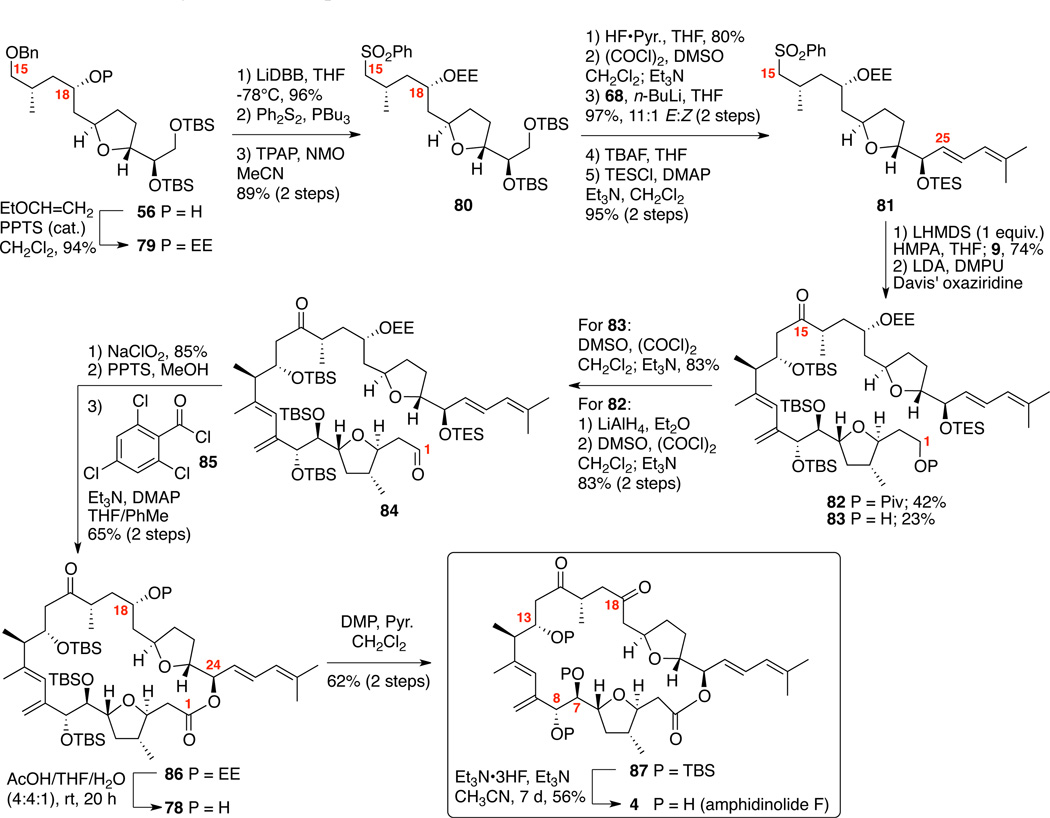
The successful execution of this C18 OEE strategy for the total synthesis of amphidinolide F is shown in Scheme 13. Acetalization was best accomplished with PPTS and ethoxyvinyl ether in high yield. We quickly became concerned with the viability of this route, as the next required transformation (C15 debenzylation) proved problematic under our prior Pd/C, H2 conditions (see Supporting Information). Use of the Freeman reagent55 nicely circumvented the problem. Fortunately, the subsequent sequence principally followed our prior OTHP route. After formation of the required sulfone 81, sulfone alkylation / oxidation proceeded in near identical yields to our OTHP route. For the macrocyclization, it was found the Yamaguchi conditions50 to be optimum for accessing 86 in 65% yield over 2 steps. With the key macrocycle 86 in hand, we returned to the previously problematic C18 deprotection. We were thrilled to find that our OEE hypothesis proved valid as aqueous acetic acid conditions smoothly provided the corresponding C18 alcohol 78. This alcohol 78 existed as a mixture of the hydroxyl ketone and C15 hemiketal; however, the equilibrium could be driven to the C15, C18 diketone 87 by oxidation using Dess-Martin’s periodinane (DMP). It is important to note that while macrolactonization, EE deprotection and DMP oxidation proceeded smoothly, NMR analysis of the corresponding macrolactones often generated broaden spectral patterns – indicating a conformational equilibrium likely existed on the NMR time scale. We explored multiple deprotection conditions for the three remaining TBS ethers (e.g. HF•pyr., TASF56); however, prolonged exposure to Et3N•3HF57 ultimately proved to be effective – yielding am-phidinolide F (4) in 56% isolated yield. Spectral comparison of synthetic amphidinolide F was in good agreement with the spectral data (1H, 13C, [α]D) reported by Kobayashi and coworkers. It should be noted that both Kobayashi58 and our own laboratory observed some concentration dependent shifts to the NMR spectra; however, comparison at 0.0036 M concentration (0.4 mg 4 in 0.18 mL CDCl3) proved optimum. Thus, the total synthesis of 4 was achieved starting from 1,3- propanediol in 29 steps longest linear sequence (LLS) based on our second-generation route employing the Carreira asymmetric alkynylation sequence (Scheme 4).
Scheme 13.
Total Synthesis of Amphidinolide F.
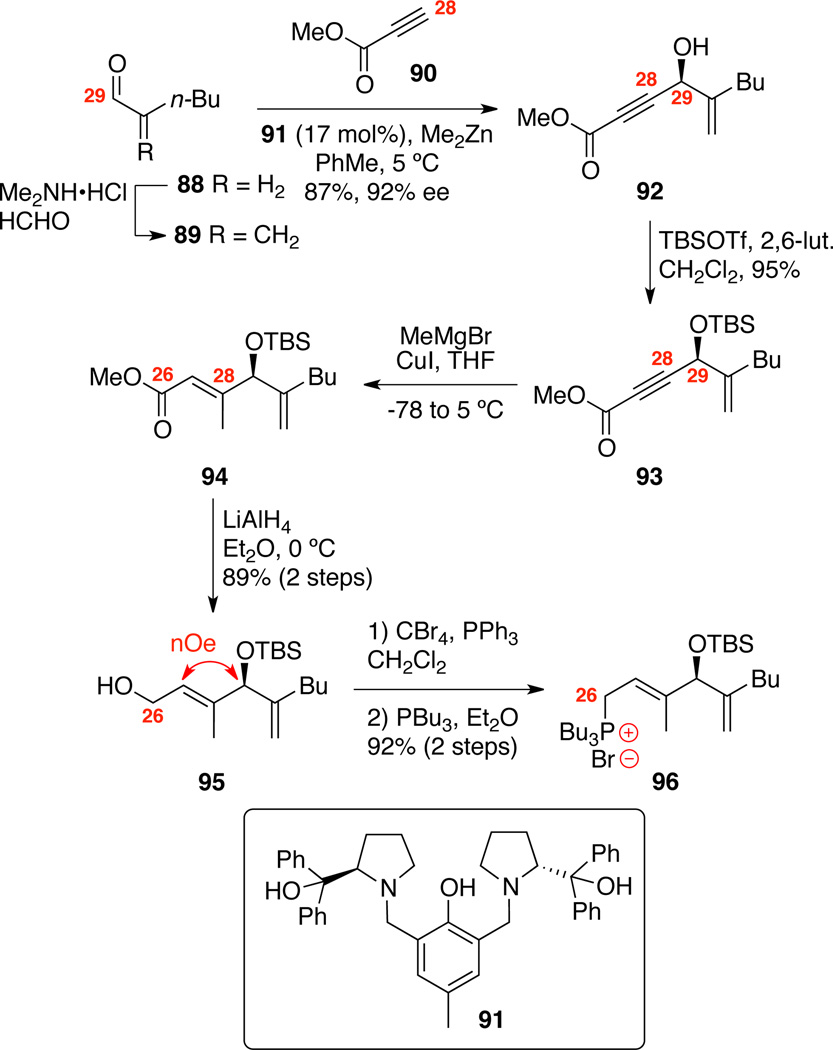
We next set out to apply this overall strategy to the synthesis of the most bioactive member of this subfamily 1–4, am-phidinolide C (1). In fact, macrolide 1 is one of the most biologically potent members of the entire amphidinolide family of >35 macrolides. Our approach toward this compound employs the identical C1-C14 subunit 9, but a more complicated sulfone coupling partner 99. Starting from known aldehyde 89 [available in one-step from hexanal (88)59], Trost asymmetric alkynylation60 with commercially available alkyne 90 gave the desired propargyl alcohol 92 in high yield and enantioselectivity (Scheme 14). After silyl protection at C29, cuprate addition to the alkynoate 93 generated the desired E-alkene 94 in complete stereoselectivity. LiAlH4 reduction produced the allyl alcohol 95 in 89% yield over two steps. This compound was employed to determine the absolute configuration at C29 by desilylation (TBAF) and advanced Mosher ester analysis.43 Conversion of alcohol 95 to the corresponding tributylphosphonium salt 96 was accomplished by treatment with CBr4, Ph3P followed by displacement with PBu3 in high overall yield. The overall route proved highly efficient (6 steps, 68% overall yield) yielding the salt 96 in multigram quantity.
Scheme 14.
Synthesis of the Phosphonium Salt.
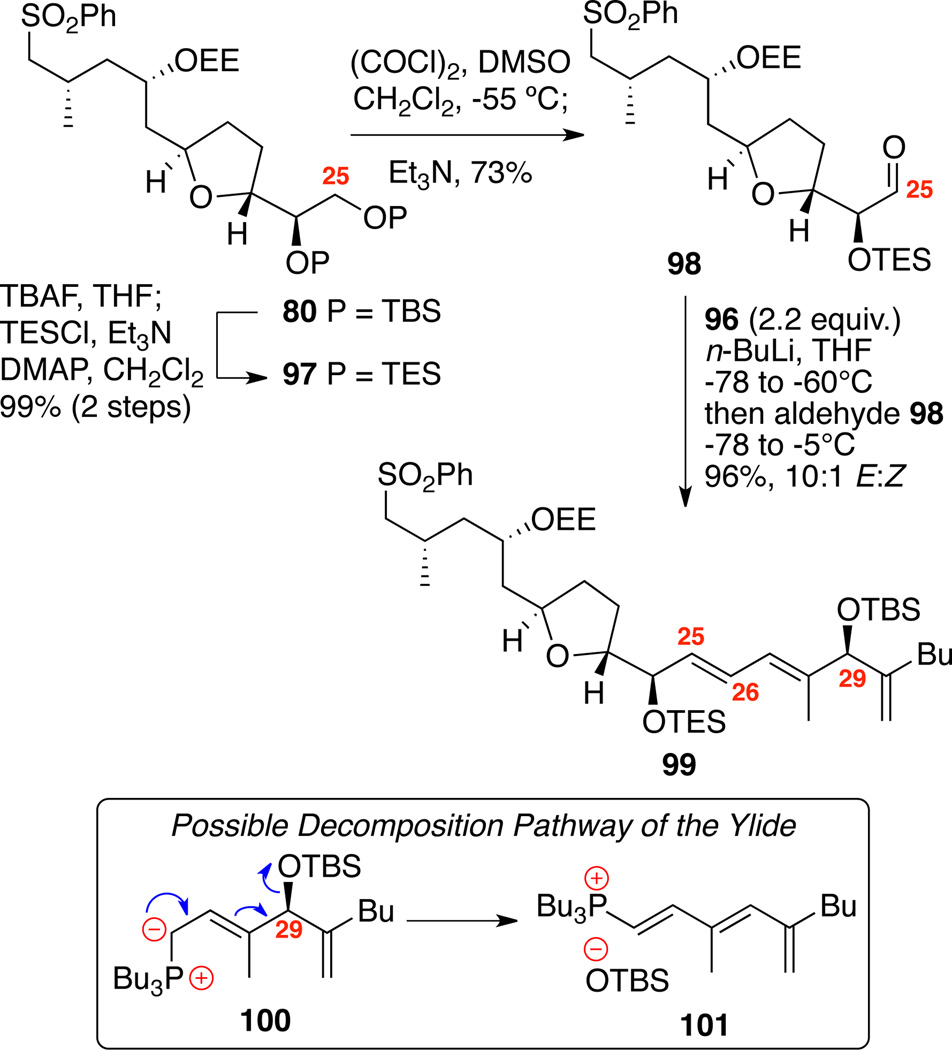
Synthesis of the C15-C34 sulfone 99 is shown in Scheme 15. Starting from previously made bis-TBS ether 80, TBAF mediated desilylation followed by bis-TES protection produced 97. Next, tandem C25 deprotection and oxidation using Swern conditions produced the α-oxy aldehyde 98. We initially screened our previously optimized Tamura / Vedejs olefination conditions for attaching the necessary side arm; however, only decomposition was observed. This outcome was not entirely unexpected as base-induced elimination of ylide 100 would generate a conjugated triene 101. Fortunately, reduction of the reaction temperature and an increase in the equivalence of the salt 96 (1.5 to 2.2 equiv.) led to excellent conversion to the desired triene 99 (96% yield, 10:1 E:Z).
Scheme 15.
Synthesis of the Sulfone Subunit.
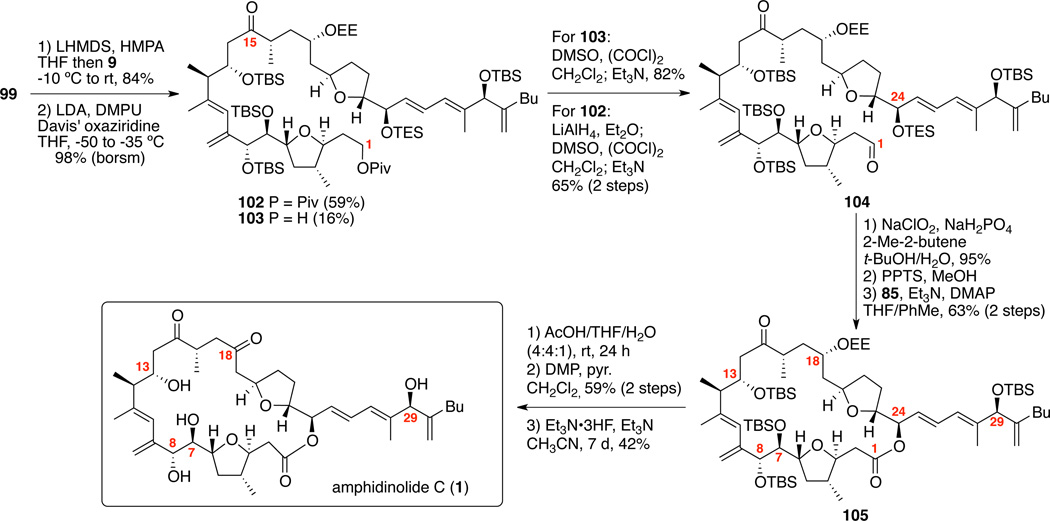
The completion of the total synthesis of amphidinolide C is shown in Scheme 16. Lithiation of sulfone 99 followed by addition of the iodide 9 generated the C14-C15 coupled material in excellent yield (84%). Oxidative desulfurization proceeded smoothly using LDA, DMPU and Davis’ oxaziridine to produce 59% of 102 and 16% of 103. As before, both compounds were useful for accessing the aldehyde 104. Pinnick oxidation of aldehyde 104 followed by careful removal of the C24 TES ether generated the seco acid. Yamaguchi macrolactonization produced the 25-membered macrolactone 105 in 63% yield over two steps. Aqueous acetic acid conditions again proved effective for selective removal of the C18 OEE moiety. Subsequent oxidation using DMP yielded tetra-TBS protected amphidinolide C. Gratifyingly, global deprotection using Et3N•3HF produced the natural product 1, which was matched nicely with the observed spectra (1H, 13C NMR in C6D6).12b–c Additionally, the optical rotation data was in agreement with the literature value [Synthetic: [α]D23 = −98.5° (c = 0.21, CHCl3); Natural:12 [α]D26 = −106° (c = 1.0, CHCl3)]. This approach constitutes a 28-step synthesis (LLS) of amphidinolide C (1).
Scheme 16.
Total Synthesis of Amphidinolide C.
Conclusion
The total syntheses of amphidinolides C and F have been accomplished (28 and 29 LLS respectively). Central to these syntheses is the use of a common intermediate strategy to access approximately 65% of the macrocyclic core and the THF rings present in the two natural products. A stereoselective silver-catalyzed cyclization of a propargyl benzoate/diol was employed to construct the needed trans-stereochemistry of the THF rings. A Felkin-controlled, 2-lithio-1,3-dienyl addition to an α-silyloxy aldehyde incorporated the C9-C11 diene and established the C8 stereocenter in single operation. An efficient 6-step sequence provided access to the C26-C34 aphidinolide C subunit and the Tamura-Vedejs olefination incorporated the C25-C29 sidearm of amphidinolide F and the C25-C34 sidearm of amphidinolide C. A sterically congested sulfone alkylation / oxidative desulfurization sequence was utilized to couple the major subunits and incorporate the C15 ketone. The presence of chelating moiety at C18 was critical to the success of the oxidative desulfurization step. A carefully orchestrated sequence for stepwise revealing of the C24 alcohol followed by macrolactonization, C18 deprotection and oxidation provided access to the protected amphidinolide natural products. The final global deprotection was uniquely feasible utilizing Et3N•3HF as desilylating agent. With a viable route to accessing the amphidinolide C/F subfamily, this work opens the door to exploring the pronounced influence of the C25 sidearm on biological activity. These studies will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
Prof. Takaaki Kubota and Jun’ichi Kobayashi (Hokkaido University) are acknowledged for assistance with the NMR spectra for compounds 1 and 4, and Prof. Claudia Maier and Jeff Morré (OSU) for mass spectra data. Finally, the authors are grateful to Prof. James D. White (OSU) and Dr. Roger Hanselmann (Rib-X Pharmaceuticals) for their helpful discussions.
Funding Sources
Financial support was provided by the National Institutes of Health (NIH) (GM63723) and Oregon State University (Harris and Shoemaker Fellowships for SM).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Complete experimental procedures are provided, including 1H and 13C spectra, of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Brachmachari G. In: Bioactive Natural Products. Brachmachari G, editor. Hackensack, NJ: World Scientific Publishing; 2011. pp. 1–199. [Google Scholar]
- 2.(a) Nicolaou KC, Sorensen JE, editors. Classics in Total Synthesis: Targets, Strategies, Methods. New York: Wiley-VCH; 1996. [Google Scholar]; (b) Wilson RM, Danishefsky SJ. J. Org. Chem. 2006;71:8329–8351. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]; (c) Cossy J, Arseniyadis S, editors. Modern Tools for the Synthesis of Complex Bioactive Molecules. John Wiley and Sons; 2012. [Google Scholar]
- 3.Kinghorn AD, Falk H, Kobayashi J, editors. The Epothilones: An Outstanding Family of Anti-Tumor Agents. From Soil to the Clinic. NewYork, NY, USA: SpringerWien; 2009. [Google Scholar]
- 4.Daniel PT, Koert U, Schuppan J. Angew. Chem. Int. Ed. 2006;45:872–893. doi: 10.1002/anie.200502698. [DOI] [PubMed] [Google Scholar]
- 5.Hale KJ, Manaviazar S. Chem. Asian. J. 2010;5:704–754. doi: 10.1002/asia.200900634. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ishibashi M, Kobayashi J. Heterocycles. 1997;44:543–572. [Google Scholar]; (b) Chakraborty TK, Das S. Curr. Med. Chem. Anti-cancer Agents. 2001;1:131–149. doi: 10.2174/1568011013354660. [DOI] [PubMed] [Google Scholar]; (c) Kobayashi J, Shimbo K, Kubota T, Tsuda M. Pur. App. Chem. 2003;75:337–342. [Google Scholar]; (d) Kobayashi J, Tsuda M. Nat. Prod. Rep. 2004;21:77–93. doi: 10.1039/b310427n. [DOI] [PubMed] [Google Scholar]; (e) Colby EA, Jamison TF. Org. Biomol. Chem. 2005;3:2675–2684. doi: 10.1039/b507315b. [DOI] [PubMed] [Google Scholar]; (f) Kobayashi J, Kubota T. J. Nat. Prod. 2007;70:451–460. doi: 10.1021/np0605844. [DOI] [PubMed] [Google Scholar]; (g) Hiersemann N, Kobayashi J. J. Antibiot. 2008;61:271–284. doi: 10.1038/ja.2008.39. [DOI] [PubMed] [Google Scholar]; (h) Fürstner A. Israel J. Chem. 2011;51:329–345. [Google Scholar]
- 7.(a) Williams D, Kissel WS. J. Am. Chem. Soc. 1988;120:11198–11199. [Google Scholar]; (b) Williams DR, Meyer BJ, Mi L. Org. Lett. 2000;2:945–948. doi: 10.1021/ol0000197. [DOI] [PubMed] [Google Scholar]; (c) Williams DR, Meyer KG. J. Am. Chem. Soc. 2001;123:765–766. doi: 10.1021/ja005644l. [DOI] [PubMed] [Google Scholar]; (d) Lam HW, Pattenden G. Angew. Chem. Int. Ed. 2002;41:508–511. doi: 10.1002/1521-3773(20020201)41:3<508::aid-anie508>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]; (e) Maleczka RE, Terrell LR, Jr, Geng F, Ward JS., III Org. Lett. 2002;4:2841–2844. doi: 10.1021/ol0262284. [DOI] [PubMed] [Google Scholar]; (f) Fürstner A, Aissa C, Riveiros R, Ragot J. Angew. Chem. Int. Ed. 2002;41:4763–4766. doi: 10.1002/anie.200290042. [DOI] [PubMed] [Google Scholar]; (g) Trost BM, Chisholm JD, Wrobleski SJ, Jung M. J. Am. Chem. Soc. 2002;124:12420–12421. doi: 10.1021/ja027883+. [DOI] [PubMed] [Google Scholar]; (h) Aiessa C, Riveiros R, Ragot J, Fürstner A. J. Am. Chem. Soc. 2003;125:15512–15520. doi: 10.1021/ja038216z. [DOI] [PubMed] [Google Scholar]; (i) Ghosh AK, Liu C. J. Am. Chem. Soc. 2003;125:2374–2375. doi: 10.1021/ja021385j. [DOI] [PubMed] [Google Scholar]; (j) Lepage O, Kattnig E, Fürstner A. J. Am. Chem. Soc. 2004;126:15970–15971. doi: 10.1021/ja044130+. [DOI] [PubMed] [Google Scholar]; (k) Ghosh AK, Gong G. J. Am. Chem. Soc. 2004;126:3704–3705. doi: 10.1021/ja049754u. [DOI] [PubMed] [Google Scholar]; (l) Trost BM, Harrington PE. J. Am. Chem. Soc. 2004;126:5028–5029. doi: 10.1021/ja049292k. [DOI] [PubMed] [Google Scholar]; (m) Trost BM, Papillion JPN. J. Am. Chem. Soc. 2004;126:13618–13619. doi: 10.1021/ja045449x. [DOI] [PubMed] [Google Scholar]; (n) Trost BM, Wrobleski ST, Chisholm JD, Harrington PE, Jung M. J. Am. Chem. Soc. 2005;127:13589–13597. doi: 10.1021/ja0533646. [DOI] [PubMed] [Google Scholar]; (o) Harrington PE, Chisholm JD, Wrobleski ST. J. Am. Chem. Soc. 2005;127:13598–13610. doi: 10.1021/ja053365y. [DOI] [PubMed] [Google Scholar]; (p) Colby EA, O’Brien KC, Jamison TF. J. Am. Chem. Soc. 2005;127:4297–4307. doi: 10.1021/ja042733f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Trost BM, Papillon JPN, Nussbaumer T. J. Am. Chem. Soc. 2005;127:17921–17937. doi: 10.1021/ja055967n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Va P, Roush WR. J. Am. Chem. Soc. 2006;128:15960–15961. doi: 10.1021/ja066663j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Kim CH, An HJ, Shin WK, Yu W, Woo SK, Jung SK, Lee E. Angew. Chem. Int. Ed. 2006;45:8019–8021. doi: 10.1002/anie.200603363. [DOI] [PubMed] [Google Scholar]; (t) Ghosh AK, Gong G. J. Org. Chem. 2006;71:1085–1093. doi: 10.1021/jo052181z. [DOI] [PubMed] [Google Scholar]; (u) Fürstner A, Kattnig E, Lepage O. J. Am. Chem. Soc. 2006;128:9194–9204. doi: 10.1021/ja061918e. [DOI] [PubMed] [Google Scholar]; (v) Nicoloau KC, Brenzovich WE, Bulger PG, Francis TM. Org. Biomol. Chem. 2006;4:2119–2157. doi: 10.1039/b602020h. [DOI] [PubMed] [Google Scholar]; (w) Nicolaou KC, Bulger PG, Brenzovich WE. Org. Biomol. Chem. 2006;4:2158–2183. doi: 10.1039/b602021f. [DOI] [PubMed] [Google Scholar]; (x) Deng L-S, Huang X-P, Zhao G. J. Org. Chem. 2006;71:4625–4635. doi: 10.1021/jo0605086. [DOI] [PubMed] [Google Scholar]; (y) Jin J, Chen Y, Li Y, Wu J, Dai W-M. Org. Lett. 2007;9:2585–2588. doi: 10.1021/ol0710360. [DOI] [PubMed] [Google Scholar]; (z) Va P, Roush WR. Tetrahedron. 2007;63:5768–5796. doi: 10.1016/j.tet.2007.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (aa) Fürstner A, Bouchez LC, Funel J-A, Liepins V, Poree F-H, Gilmour R, Beaufils F, Laurich D, Tamiya M. Angew. Chem. Int. Ed. 2007;46:9265–9270. doi: 10.1002/anie.200704024. [DOI] [PubMed] [Google Scholar]; (bb) Dai W-M, Chen Y, Jin J, Wu J, Lou J, He Q. Synlett. 2008:1737–1741. [Google Scholar]; (cc) Barbazanges M, Meyer C, Cossy J. Org. Lett. 2008;10:4489–4492. doi: 10.1021/ol801708x. [DOI] [PubMed] [Google Scholar]; (dd) Kim CH, An HJ, Shin WK, Yu W, Woo SK, Jung SK, Lee E. Chem. Asian. J. 2008;3:1523–1534. doi: 10.1002/asia.200800062. [DOI] [PubMed] [Google Scholar]; (ee) Rodriquez-Escrich C, Urpi F, Vilarrasa J. Org. Lett. 2008;10:5191–5194. doi: 10.1021/ol8021676. [DOI] [PubMed] [Google Scholar]; (ff) Lu L, Zhang W, Carter RG. J. Am. Chem. Soc. 2008;130:7253–7255. doi: 10.1021/ja803012n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (gg) Lu L, Zhang W, Carter RG. J. Am. Chem. Soc. 2008;130:11834. doi: 10.1021/ja803012n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (hh) Fürstner A, Bouchez LC, Morency L, Funel J-A, Liepins V, Poree F-H, Gilmour R, Lau-rich D, Beaufils F, Tamiya M. Chem. Eur. J. 2009;15:3983–4010. doi: 10.1002/chem.200802067. [DOI] [PubMed] [Google Scholar]; (ii) Hangyou M, Ishiyama H, Takahashi Y, Kobayashi J. Org. Lett. 2009;11:5046–5049. doi: 10.1021/ol902026f. [DOI] [PubMed] [Google Scholar]; (jj) Ko HM, Lee CW, Kwon HK, Chung HS, Choi SY, Chung YK, Lee E. Angew. Chem. Int. Ed. 2009;47:2364–2366. doi: 10.1002/anie.200805266. [DOI] [PubMed] [Google Scholar]; (kk) Fürstner A, Flügge S, Larionov O, Takahashi Y, Kubota T, Kobayashi J. Chem. Eur. J. 2009;15:4011–4029. doi: 10.1002/chem.200802068. [DOI] [PubMed] [Google Scholar]; (ll) Yadav JS, Reddy CS. Org. Lett. 2009;11:1705–1708. doi: 10.1021/ol9002724. [DOI] [PubMed] [Google Scholar]; (jj) Li H, Wu J, Luo J, Dai W-M. Chem. Eur. J. 2010;16:11530–11534. doi: 10.1002/chem.201001794. [DOI] [PubMed] [Google Scholar]; (ll) Wu D, Li H, Jin J, Wu J, Dai W-M. Synlett. 2011:895–898. [Google Scholar]; (mm) Sun L, Wu D, Wu J, Dai W-M. Synlett. 2011:3036–3040. [Google Scholar]; (nn) Hara A, Morimoto R, Iwasaki Y, Saitoh T, Ishikara Y, Nishiyama S. Angew. Chem. Int. Ed. 2012;51:9877–9880. doi: 10.1002/anie.201204992. [DOI] [PubMed] [Google Scholar]; (oo) Lu L, Zhang W, Nam S, Horne DA, Jove R, Carter RG. J. Org. Chem. 2013;78:2213–2247. doi: 10.1021/jo3026077. [DOI] [PMC free article] [PubMed] [Google Scholar]; (pp) Williams DR, Myers BJ, Mi L. Org. Lett. 2013;15:2070. doi: 10.1021/ol0000197. [DOI] [PubMed] [Google Scholar]; (qq) Williams DR, Myers BJ, Mi L, Binder RJ. J. Org. Chem. 2013;78:4762–4778. doi: 10.1021/jo4002382. [DOI] [PMC free article] [PubMed] [Google Scholar]; (rr) Volchkov I, Lee D. J. Am. Chem. Soc. 2013;135:5324–5427. doi: 10.1021/ja401717b. [DOI] [PubMed] [Google Scholar]
- 8.(a) Ishiyama H, Ishibashi M, Kobayashi J. Chem. Pharm. Bull. 1996;44:1819–1822. [Google Scholar]; (b) Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2003;59:1613–1625. [Google Scholar]; (c) Shotwell JB, Roush WR. Org. Lett. 2004;12:3865–3868. doi: 10.1021/ol048381z. [DOI] [PubMed] [Google Scholar]; (d) Mohapatra DK, Rahaman H, Chorghade MS, Gurjar MK. Synlett. 2007:567–570. [Google Scholar]; (e) Bates RH, Shotwell JB, Roush WR. Org. Lett. 2008;9:4343–4346. doi: 10.1021/ol801852j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Armstrong A, Pyrkotis C. Tetrahedron Lett. 2009;50:3325–3328. [Google Scholar]; (g) Paudyal MP, Rath NP, Spilling CD. Org. Lett. 2010;12:2954–2957. doi: 10.1021/ol100959a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ferri L, Figadre B. Org. Lett. 2010;12:4976–4979. doi: 10.1021/ol1021228. [DOI] [PubMed] [Google Scholar]; (i) Roy S, Spilling CD. Org. Lett. 2010;12:5326–5329. doi: 10.1021/ol102345v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Morra NA, Pagenkopf BL. Org. Lett. 2011;13:572–575. doi: 10.1021/ol1030074. [DOI] [PubMed] [Google Scholar]; (k) Fischer DA, Williams DR, De R, Fultz M, Morales-Ramos A, Rodriguez-Reyes D. San Diego, CA. 243st National American Chemical Society Meeting; March 25–29 2012; ORGN-756. [Google Scholar]; (l) Wu D, Forsyth CJ. Org. Lett. 2013;15:1178–1181. doi: 10.1021/ol303515h. [DOI] [PubMed] [Google Scholar]; (m) Clark JS, Yang G, Osnowski AP. Org. Lett. 2013;15:1460–1463. doi: 10.1021/ol4004838. [DOI] [PubMed] [Google Scholar]; (n) Clark JS, Yang G, Osnowski AP. Org. Lett. 2013;15:1464–1467. doi: 10.1021/ol400482j. [DOI] [PubMed] [Google Scholar]
- 9.(a) Mahapatra S, Carter RG. Org. Biomol. Chem. 2009;7:4582–4585. doi: 10.1039/b916744g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mahapatra S, Carter RG. Anaheim, CA. 241st National American Chemical Society Meeting; March 27–31, 2011; 2011. ORGN-295. [Google Scholar]; (c) Mahapatra S, Carter RG. Portland, OR. Northwest Regional American Chemical Society Meeting; June 26–30, 2011; NORM-261. [Google Scholar]
- 10.Mahapatra S, Carter RG. Angew. Chem., Int. Ed. 2012;51:7948–7951. doi: 10.1002/anie.201203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fürstner and co-orkers have recently completed an elegant total synthesis of amphidinolide F:Valot G, Regens CS, O’Malley DP, Godineau E, Takikawa H, Fürstner A. Angew. Chem. Int. Ed. doi: 10.1002/anie.201301700. Early View.
- 12.(a) Kobayashi J, Ishibashi M, Wälchli NR, Nakamura H, Yamasu T, Hirata Y, Sasaki T, Ohizumi Y. J. Am. Chem. Soc. 1988;110:490–494. [Google Scholar]; (b) Kubota T, Tsuda M, Kobayashi J. Org. Lett. 2001;3:1363–1366. doi: 10.1021/ol015741z. [DOI] [PubMed] [Google Scholar]; (c) Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2001;57:5975–5977. [Google Scholar]; (d) Kubota T, Tsuda M, Kobayashi J. Tetrahedron. 2003;59:1613–1625. [Google Scholar]
- 13.(a) Kubota T, Sakuma Y, Tsuda M, Kobayashi J. Mar. Drugs. 2004;2:83–87. [Google Scholar]; (b) Kubota T, Suzuki A, Yamada M, Baba S, Kobayashi J. Heterocycles. 2010;82:333–338. [Google Scholar]
- 14.Tsuda M, Endo T, Kobayashi J. Tetrahedron. 1999;55:14565–14570. [Google Scholar]
- 15.Kobayashi J, Tsuda M, Ishibashi M, Shigemori H, Yamasu T, Hirota H, Sasaki T. J. Antibiot. 1991;44:1259–1261. doi: 10.7164/antibiotics.44.1259. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X-T, Carter RG. Angew. Chem. Int. Ed. 2006;45:1787–1790. doi: 10.1002/anie.200503733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuwa H, Okamura Y, Natsugari H. Tetrahedron. 2004;60:5341–5532. [Google Scholar]
- 18.(a) Little RD, Myong SO. Tetrahedron Lett. 1980;21:3339–3342. [Google Scholar]; (b) Hwu JR. J. Org. Chem. 1983;48:4432–4433. [Google Scholar]; (c) Yamada S, Nakayama K, Takayama H. Tetrahedron Lett. 1984;25:3239–3242. [Google Scholar]; (d) Bonaparte AC, Betush MP, Panseri BM, Mastarone DJ, Murphy RK, Murphree SS. Org. Lett. 2011;13:1447–1449. doi: 10.1021/ol200135m. [DOI] [PubMed] [Google Scholar]
- 19.(a) Paquette LA, Barriault L, Pissarnitski D. J. Am. Chem. Soc. 1999;121:4542–4543. [Google Scholar]; (b) Arjona O, Menchaca R, Plumet J. Org. Lett. 2001;3:107–109. doi: 10.1021/ol000334t. [DOI] [PubMed] [Google Scholar]
- 20.Smith AB, Adams CM., III Acc. Chem. Res. 2004;37:365–377. doi: 10.1021/ar030245r. [DOI] [PubMed] [Google Scholar]
- 21. Menche D, Hassfeld J, Li J, Rudolph S. J. Am. Chem. Soc. 1996;129:6100–6101. doi: 10.1021/ja071461o.See also: Harris H, Jarowicki K, Kocienski P, Bell R. Synlett. 1996:903–905. Hanisch I, Bruckner R. Synlett. 2006:374–378. Yin N, Wang G, Qian M, Negishi E. Angew. Chem., Int. Ed. 2006;45:2916–2920. doi: 10.1002/anie.200600012.
- 22.(a) Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974–5976. [Google Scholar]; (b) Gao Y, Klunder JM, Hanson RM, Masamune H, Ko SY, Sharpless KB. J. Am. Chem. Soc. 1987;109:5765–5780. [Google Scholar]; (C) Schomaker JM, Pul-gam VR, Borhan B. J. Am. Chem. Soc. 2004;126:13600–13601. doi: 10.1021/ja0469075. [DOI] [PubMed] [Google Scholar]
- 23.Shanmugam P, Miyashita M. Org. Lett. 2003;5:3265–3268. doi: 10.1021/ol035075x. [DOI] [PubMed] [Google Scholar]
- 24.(a) Hoffmann-Röder A, Krause N. Org. Lett. 2001;3:2537–2538. doi: 10.1021/ol016205+. [DOI] [PubMed] [Google Scholar]; (b) Volz F, Krause N. Org. Biomol. Chem. 2007;5:1519–1521. doi: 10.1039/b703995f. [DOI] [PubMed] [Google Scholar]
- 25.Buzas A, Istrate F, Gagosz F. Org. Lett. 2006;8:1957–1759. doi: 10.1021/ol0606839. [DOI] [PubMed] [Google Scholar]
- 26.(a) Flögel O, Amombo MGO, Reiβig H-U, Zahn G, Brüdgam I, Hartl H. Chem. Eur. J. 2003;9:1405–1415. doi: 10.1002/chem.200390160. [DOI] [PubMed] [Google Scholar]; (b) Herradon B. Tetrahedron: Asymm. 1991;2:191–194. [Google Scholar]
- 27.Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467–4470. [Google Scholar]
- 28.Gagnon D, Lauzon S, Godbout C, Spino C. Org. Lett. 2005;7:4769–4771. doi: 10.1021/ol052034n. [DOI] [PubMed] [Google Scholar]
- 29.Judd WR, Ban S, Aubé J. J. Am. Chem. Soc. 2006;128:13736–13741. doi: 10.1021/ja063411+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mapp AK, Heathcock CH. J. Org. Chem. 1999;64:23–27. doi: 10.1021/jo9813742. [DOI] [PubMed] [Google Scholar]
- 31.Boyall D, López F, Sasaki H, Frantz D, Carreira EM. Org. Lett. 2001;2:4233–4236. doi: 10.1021/ol006791r. [DOI] [PubMed] [Google Scholar]
- 32.Kolb HC, Van Nieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 33.Carter RG, Weldon DJ. Org. Lett. 2000;2:3913–3916. doi: 10.1021/ol006674w. [DOI] [PubMed] [Google Scholar]
- 34.(a) Turks M, Fairweather KA, Scopelliti R, Vogel P. Eur. J. Org. Chem. 2011:3317–3328. [Google Scholar]; (b) Exner CJ, Turks M, Fonquerne F, Vogel P. Chem. Eur. J. 2011;17:4246–4253. doi: 10.1002/chem.201003264. [DOI] [PubMed] [Google Scholar]; (c) Exner CJ, Lalcef S, Poli F, Turks M, Vogel P. J. Org. Chem. 2011;76:840–845. doi: 10.1021/jo102035d. [DOI] [PubMed] [Google Scholar]
- 35.Furrow ME, Myers AG. J. Am. Chem. Soc. 2004;126:5436–5445. doi: 10.1021/ja049694s. [DOI] [PubMed] [Google Scholar]
- 36.Barton DHR, McCombie SW. J. Chem. Soc., Perkin Trans. 1975;1:1574–1585. [Google Scholar]
- 37.Please note that 41 was converted to a known degradation intermediate of amphidinolide C.12d See supporting information for full details.
- 38.Nemoto H, Ma R, Moriguchi H, Kawamura T, Kamiya M, Shibuya M. J. Org. Chem. 2007;72:9850–9853. doi: 10.1021/jo701859a. [DOI] [PubMed] [Google Scholar]
- 39.Nemoto H, Kawamura T, Miyoshi N. J. Am. Chem. Soc. 2005;127:14546–14547. doi: 10.1021/ja054010h. [DOI] [PubMed] [Google Scholar]
- 40.Mandal AK, Schneekloth JS, Jr, Kuramochi K, Crews CM. Org. Lett. 2006;8:427–430. doi: 10.1021/ol052620g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann RW, Polachowski A. Chem. Eur. J. 1998;4:1724–1730. [Google Scholar]
- 42.This reactivity was initially noted by Smith and coworkers as an unwanted side reaction in their synthesis of rapamycin. Smith AB, III, Condon SM, McCauley JA, Leazer JL, Jr, Leahy JW, Maleczka RE., Jr J. Am. Chem. Soc. 1990;119:962–973. For alternative routes to similar stannane, see: Oehlschager AC, Hutzinger MW, Aksela R, Sharma S, Singh SM. Tetrahedron Lett. 1990;31:165–168. Suzenet F, Blart E, Quintard J-P. Synlett. 1998:879–881.
- 43.Ohtani I, Kusumi T, Kashman Y, Kakisawa H. J. Am. Chem. Soc. 1991;113:4092–4096. [Google Scholar]
- 44.(a) White JD, Kawaski M. J. Org. Chem. 1992;57:5292–5300. [Google Scholar]; (b) Vong BG, Abraham S, Xiang AX, Theodorakis EA. Org. Lett. 2003;5:1617–1620. doi: 10.1021/ol034243i. [DOI] [PubMed] [Google Scholar]; (c) Kopecky DJ, Rychnovsky SD. J. Am. Chem. Soc. 2001;123:8420–8421. doi: 10.1021/ja011377n. [DOI] [PubMed] [Google Scholar]
- 45.Kabalka GW, Gooch EE, Sastry KAR. J. Nuc. Med. 1981;22:908–912. [PubMed] [Google Scholar]
- 46.See supporting information for details on this coupling reaction.
- 47.Greene TW, Wuts PGM., 3rd . Protective Groups in Organic Synthesis. New York: John Wiley & Sons, Inc.; 1999. chapter 2. [Google Scholar]
- 48.(a) Tamura R, Saegusa K, Kakihana M, Oda D. J. Org. Chem. 1998;53:2723–2728. [Google Scholar]; (b) Wang Y, Panagabko C, Atkinson J. Bioorg. Med. Chem. 2010;18:777–786. doi: 10.1016/j.bmc.2009.11.051. [DOI] [PubMed] [Google Scholar]; (c) Vedejs E, Marth CF, Ruggeri R. J. Am. Chem. Soc. 1998;110:3940–3948. [Google Scholar]
- 49.Shiina I, Kubota M, Oshiumi H, Hashizume M. J. Org. Chem. 2004;69:1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- 50.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
- 51.Kim S, Park JH. Tetrahedron Lett. 1987;28:439–440. [Google Scholar]
- 52.Ogawa Y, Shibasaki M. Tetrahedron Lett. 1984;25:663–664. [Google Scholar]
- 53.Nambiar KP, Mitra A. Tetrahedron Lett. 1994;35:3033–3036. [Google Scholar]
- 54.Miyashita M, Yoshikoshi A, Grieco PA. J. Org. Chem. 1977;42:3772–3774. [Google Scholar]
- 55.Freeman PK, Hutchinson LL. J. Org. Chem. 1980;45:1924–1930. [Google Scholar]
- 56.For TASF mediated desilylation in amphidinolide B synthesis, see Reference 7ff and 7hh.
- 57.(a) Pirrung MC, Shuey SW, Lever DC, Fallon L. Bioorg. Med. Chem. Lett. 1994;4:1345–1346. [Google Scholar]; (b) Dunetz JR, Julian LD, Newcom JS, Roush WR. J. Am. Chem. Soc. 2008;130:16407–16416. doi: 10.1021/ja8063205. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hanessian S, Schroeder BR, Giacometti RB, Merner BL, Østergaard M, Swayze EE, Seth PP. Angew. Chem. Int. Ed. 2012;51:11242–11245. doi: 10.1002/anie.201203680. [DOI] [PubMed] [Google Scholar]
- 58.Kubota T, Kobayashi J. Personal Communication [Google Scholar]
- 59.Ragoussis V, Giannikopoulos A, Skoka E, Grivas P. J. Agric. Food. Chem. 2007;55:5050–5052. doi: 10.1021/jf0704662. [DOI] [PubMed] [Google Scholar]
- 60.Trost BM, Weiss AH, Wangelin AK-V. J. Am. Chem. Soc. 2006;128:8–9. doi: 10.1021/ja054871q. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.