Abstract
Common variable immune deficiency (CVID) is an assorted group of primary diseases that clinically manifest with antibody deficiency, infection susceptibility, and autoimmunity. Heterozygous mutations in the gene encoding the tumor necrosis factor receptor superfamily member TACI are associated with CVID and autoimmune manifestations, whereas two mutated alleles prevent autoimmunity. To assess how the number of TACI mutations affects B cell activation and tolerance checkpoints, we analyzed healthy individuals and CVID patients carrying one or two TACI mutations. We found that TACI interacts with the cleaved, mature forms of TLR7 and TLR9 and plays an important role during B cell activation and the central removal of autoreactive B cells in healthy donors and CVID patients. However, only subjects with a single TACI mutation displayed a breached immune tolerance and secreted antinuclear antibodies (ANAs). These antibodies were associated with the presence of circulating B cell lymphoma 6–expressing T follicular helper (Tfh) cells, likely stimulating autoreactive B cells. Thus, TACI mutations may favor CVID by altering B cell activation with coincident impairment of central B cell tolerance, whereas residual B cell responsiveness in patients with one, but not two, TACI mutations enables autoimmune complications.
Introduction
Common variable immune deficiency (CVID) is a heterogeneous group of primary diseases that manifest with antibody deficiency, susceptibility to infections, and autoimmunity (1). Since its identification as a clinical entity, CVID has been principally described as a B cell disorder (2). B cell receptor (BCR) and TLR7 and TLR9 responses are found altered in most CVID patients (3–5). However, CVID patients often have additional immunological abnormalities not apparently attributable to B cells, such as Treg deficiency, a finding associated with clinical autoimmunity (6, 7). Although the majority of CVID cases have no identifiable pathogenic mutation, 7%–10% of patients bear a mutation in the TNFRSF13B gene encoding TACI, a tumor necrosis factor receptor superfamily member expressed on B cells (8, 9). TACI can bind two ligands, a proliferation-inducing ligand (APRIL) and B cell activation factor (BAFF), both of which were found elevated in the serum of CVID patients (10–12). Interestingly, elevated serum BAFF concentrations in mice have been reported to interfere with the removal of autoreactive B cells (13, 14). TACI mutations in CVID patients are typically found in the heterozygous state, suggesting either that TACI mutations exert a dominant-negative effect on the unmutated allele, or that defects induced by TACI mutations result from haploinsufficiency (15–17). Yet, the lack of disease in the majority of carriers with TACI mutations and their puzzling relative commonness (approximately 1%) in the general population cast doubt on their role in the pathogenesis of immune deficiency (18). When associated with CVID, a single TACI mutation predicts the development of autoantibody-mediated autoimmune disease, whereas patients with two mutated alleles are mostly spared clinical autoimmune conditions, suggesting a complex role for TACI in maintaining B cell tolerance (19, 20).
In healthy controls, most autoreactivity is purged from the repertoire at two distinct B cell tolerance checkpoints (21). The first checkpoint occurs centrally in the bone marrow and is dependent upon B cell intrinsic factors including the BCR and TLR signaling pathways that mediate binding to self-antigens (22–25). In contrast, regulation of the peripheral B cell tolerance checkpoint involves Tregs and potentially plasma BAFF concentrations (26–28). To determine the impact of TACI mutations on the establishment of human B cell tolerance, we cloned and expressed in vitro recombinant antibodies from single new emigrant/translational and mature naive B cells from subjects with or without CVID carrying one or two TACI mutation(s). We found that TACI mutations impaired the removal of autoreactive B cells at the central B cell tolerance checkpoint by imposing BCR and TLR defects in a dose-dependent manner in all subjects, regardless of CVID status. In contrast, only healthy individuals, and not CVID patients, were capable of mitigating central B cell tolerance defects with an effective peripheral B cell tolerance checkpoint, which does not rely on functional TACI. Finally, we report that secreted antinuclear antibodies (ANAs) are common in CVID patients with one TACI mutation and correlate with the presence of circulating T follicular helper (Tfh) cells as well as a high incidence of autoimmunity, whereas subjects with two TACI mutations who are mostly protected from autoimmunity were completely devoid of ANAs and circulating Tfh cells.
Results
Central B cell tolerance is defective in all subjects with TACI mutations.
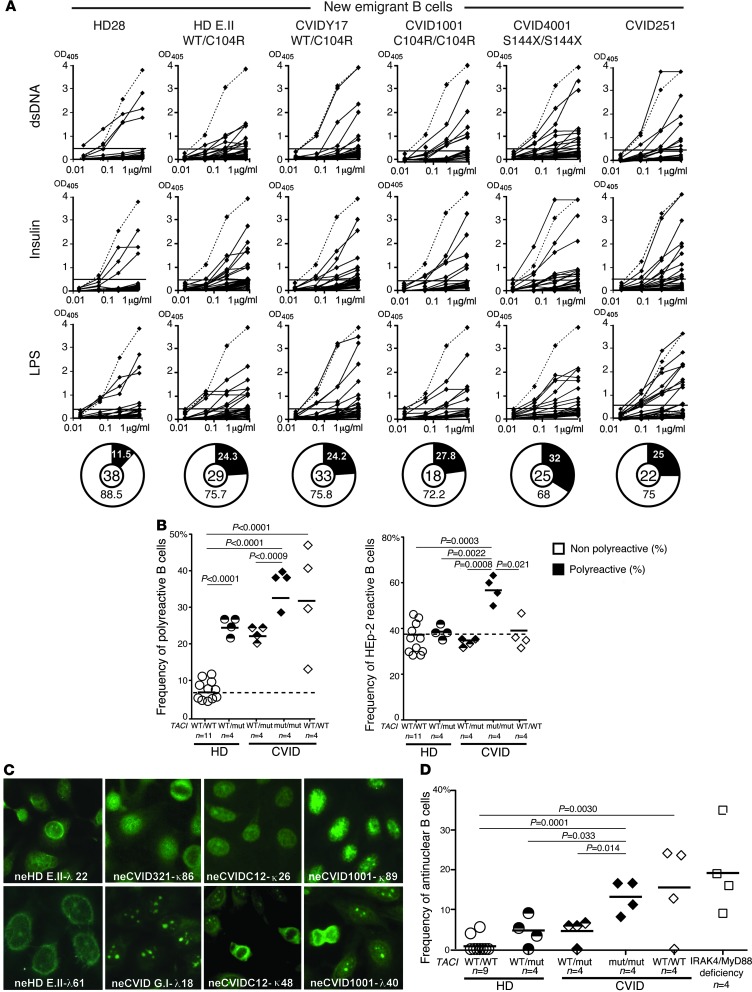
Central B cell tolerance is responsible for the removal of most polyreactive and antinuclear B cells (21). To determine whether this checkpoint is affected by TACI mutations, we cloned antibodies expressed by single CD10++CD21loIgMhiCD27–CD20+ new emigrant/transitional B cells from four representative individuals from the following three subject groups: healthy donors with one TACI mutation and CVID patients with one or two TACI mutations. We found a significant increase in the frequency of polyreactive clones in new emigrant/transitional B cells from all individuals with TACI mutations, which represented 20.0%–26.1% in either healthy individuals or CVID patients with a single mutation, compared with 5.3%–11.5% in healthy controls without mutations (Figure 1, A and B, and Supplemental Tables 1–18; supplemental material available online with this article; doi: 10.1172/JCI69854DS1). Polyreactive B cells were even more prevalent in patients with two TACI mutations comprising 28.5%–39.9% of their new emigrant/transitional B cells and were also frequent in CVID patients without TACI mutations as previously reported (Figure 1, A and B, and ref. 29). This increase in autoreactive clones in patients with two TACI mutations compared with subjects with a single TACI mutation was further evidenced by the significantly increased frequency of both HEp-2–reactive and nuclear-reactive new emigrant/transitional B cells in these subjects (Figure 1, B–D). Hence, TACI mutations interfere in a gene-dosage manner with the establishment of central B cell tolerance in all individuals regardless of their CVID status.
Figure 1. Defective central B cell tolerance checkpoint in individuals carrying TACI mutation(s).
(A) Recombinant antibodies derived from new emigrant/transitional B cells from representative individuals were tested by ELISA for reactivity against dsDNA, insulin, and LPS (21). Antibodies were considered polyreactive when they reacted against all three antigens. Dashed lines show ED38 antibody–positive control, and solid lines show binding for each cloned recombinant antibody. Horizontal lines define the cutoff OD405 for positive reactivity. For each individual, the frequency of polyreactive and nonpolyreactive clones is summarized in pie charts, with the total number of antibodies tested indicated in the center. (B) The frequency of polyreactive new emigrant/transitional B cells increased in all individuals carrying TACI mutation(s) and most CVID patients without TACI mutations, whereas the frequency of HEp-2–reactive new emigrant/transitional B cells only increased in patients with two mutated TACI alleles. (C) Autoreactive antibodies expressed by new emigrant/transitional B cells from individuals carrying TACI mutation(s) included clones with various nuclear staining patterns on HEp-2 cells. Original magnification, ×40. (D) The frequency of antinuclear new emigrant/transitional B cells in individuals carrying TACI mutation(s) was elevated compared with healthy controls and is reminiscent of patients with MyD88 and IRAK4 deficiency (white squares) (23). Statistical significance by an unpaired Student’s t test is indicated.
B cell activation after BCR, TLR7, and TLR9 stimulation is TACI gene dosage dependent.
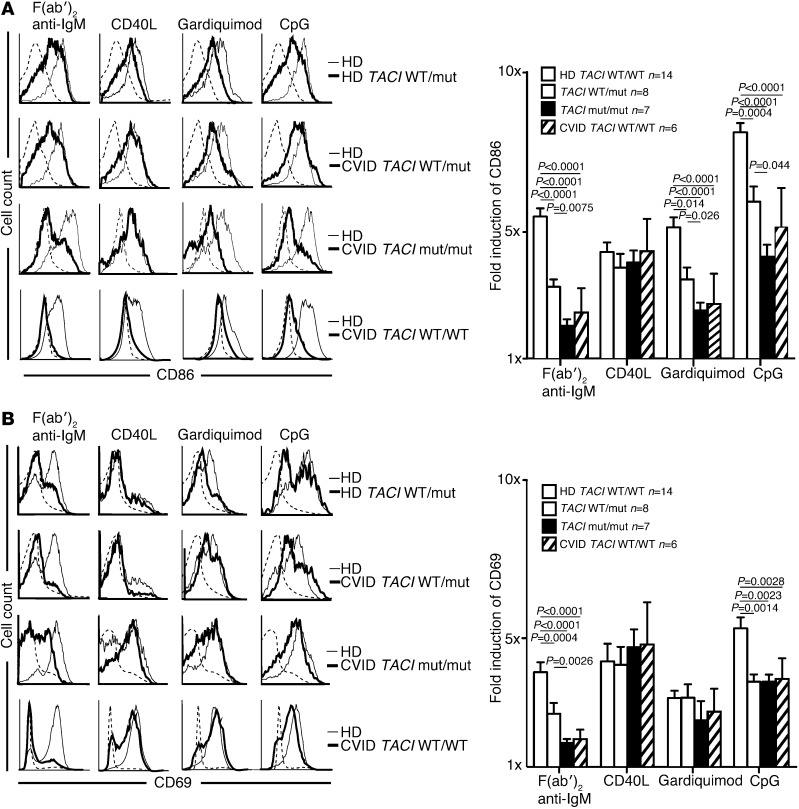
The high frequency of ANA-expressing new emigrant B cells in subjects with TACI mutations is reminiscent of IRAK4- and MYD88-deficient patients who display similar features, suggesting defective TLR functions in the presence of mutated TACI molecules (23). We therefore assessed the in vitro activation of naive B cells from individuals with and without TACI mutations; the cells were stimulated for 2 days through BCR, CD40, TLR7, or TLR9 (Figure 2). We found that independent of disease status, naive B cells carrying a single TACI mutation displayed a diminished induction of B cell activation markers CD86 and CD69 when stimulated with F(ab′)2 anti-human IgM, which triggers BCRs, guardiquimod (TLR7 agonist), or CpG (TLR9 agonist). By contrast, B cells with two TACI mutations were nearly unresponsive to such stimulation as also evidenced by the defective induction of the coactivation molecule ICOSL (Figure 2A and Supplemental Figure 1A). Similarly to adenosine deaminase (ADA) inhibition (25), TACI mutations imposed selective, not global, TLR7- and TLR9-dependent B cell activation defects because the induction of CD25 by TLR agonists was unaffected in B cells carrying TACI mutations (Figure 2B and Supplemental Figure 1B). In addition, B cells with TACI mutations did not suffer from an intrinsic inability to upregulate CD86 because this molecule was normally induced after CD40 triggering (Figure 2A). Moreover, we observed B cell activation defects in CVID patients without TACI mutations that were similar to the defects in B cells carrying TACI mutations but with overall greater variability (Figure 2, A and B, Supplemental Figure 1, A and B, and refs. 3, 4). Thus, TACI plays an important and selective role in mediating BCR, TLR7, and TLR9 functions during B cell activation.
Figure 2. TACI mutation(s) result in selective defects for in vitro naive B cell activation.
Purified naive B cells from representative individuals with and without TACI mutation(s) (thick lines) displayed decreased CD86 (A) but mostly normal CD69 (B) induction compared with healthy controls (thin lines) after 48 hours of stimulation with F(ab′)2 anti-IgM, CpG, and gardiquimod, but not CD40L. CVID patients without TACI mutation(s) (thick lines) displayed more variable CD86 and CD69 induction under activating conditions. Unstimulated healthy controls (dashed line) are shown for comparison. CVID patients (n = 3) and healthy subjects (n = 5) with a single TACI mutation were pooled because they displayed similar decreased B cell responses. Bar graphs (right) represent the combined data from multiple B cell activation experiments. Error bars represent the mean ± SEM. Statistical significance is indicated by an unpaired Student’s t test.
TACI interacts with TLR7 and TLR9 in B cells.
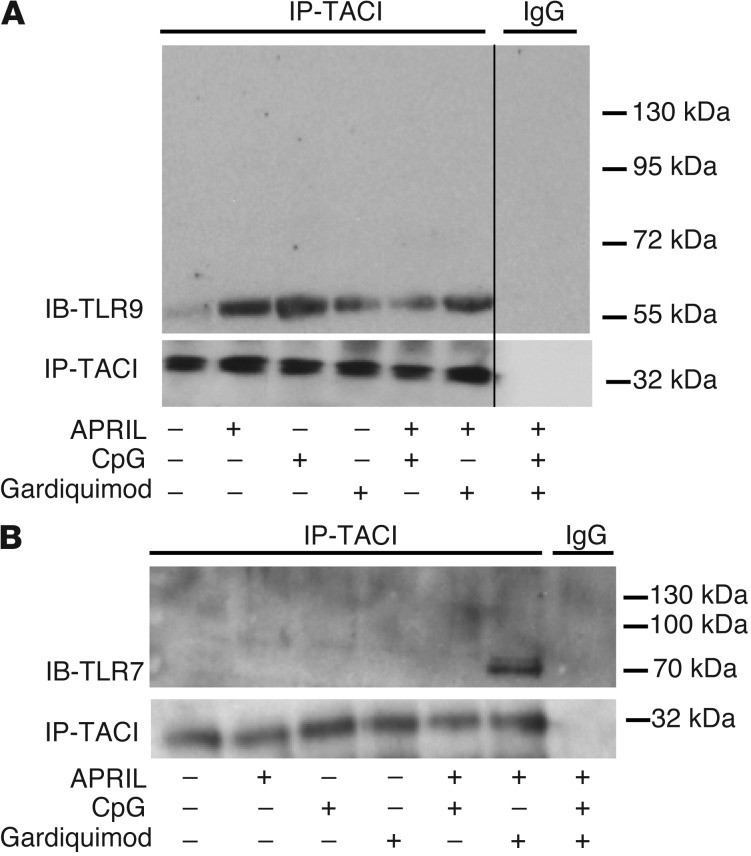
The identification of TACI-dependent TLR7 and TLR9 functions in B cells and the rapid induction of TACI on the B cell surface after activation by these TLRs suggest an interplay between TACI, TLR7, and TLR9, since each uses MyD88 for intracellular signaling (25, 30, 31). To explore the potential interaction between TACI and these TLRs, we performed coprecipitation experiments using healthy donor splenic B cells under different stimulating conditions (Figure 3). We found that TACI coprecipitated with TLR9 and that the interaction was enhanced upon activation of these receptors by their respective ligands APRIL and CpG, but less so by the TLR7 ligand gardiquimod (Figure 3A). TACI did not associate with the 120-kDa unactivated full-length TLR9, but associated only with its cleaved 65-kDa mature form created after the binding of its ligand, DNA (32). Hence, TACI only interacts with TLR9 after activation (Figure 3A). Coprecipitation of TACI and mature TLR9 was also observed in the human IgD+ E2E B cell line under similar activating conditions (Supplemental Figure 2). In contrast to TLR9, the interaction between TACI and TLR7 was only detected upon simultaneous activation by APRIL and gardiquimod, respectively (Figure 3B). Once more, TACI did not bind to the 120-kDa unactivated full-length TLR7, but was found to bind only to its cleaved 75-kDa mature form, thereby demonstrating that the association of TACI with TLR7 follows TLR7 activation by its ligand (Figure 3B and ref. 33).
Figure 3. TACI interacts with activated/cleaved TLR7 and TLR9.
TACI associates with activated/cleaved 65-kDa TLR9 (A) or 75-kDa TLR7 (B) after the receptors triggered or cotriggered with their respective ligands. Immunoprecipitations were performed using human splenic B cells stimulated or not by the TACI ligand APRIL, TLR9 ligand CpG, and/or TLR7 ligand gardiquimod, as indicated below the blots. The thin black vertical line in A signifies the absence of a single lane from an otherwise contiguous blot that was removed, as it did not further clarify the TACI-TLR9 interaction.
A role for TACI in potentiating TLR7 and TLR9 functions in B cells was further revealed by B cell activation experiments using TACI and TLR costimulations. Indeed, TACI triggering by its ligand APRIL enhanced TLR7- or TLR9-induced B cell proliferation and IgM secretion in vitro, whereas APRIL alone did not elicit any responses (Supplemental Figure 3). The specificity of the synergy between TACI and TLR7 or TLR9 was demonstrated by the addition of TACI-Ig, a decoy receptor for soluble APRIL and BAFF that blocked the enhanced B cell responses induced by APRIL and TLR agonists (Supplemental Figure 3 and ref. 33). The in vivo activation of TACI likely relies upon the production of its ligands in trans by non-B cells, including dendritic cells, since B cells did not seem to secrete substantial amounts of BAFF or APRIL (Supplemental Figure 4 and refs. 34, 35). Hence, we identified ligand-dependent interactions between TACI and endosomal receptors TLR7 and TLR9, which further reveal that TACI plays an important role in mediating human B cell activation by these TLRs.
Peripheral B cell tolerance is defective in CVID patients.
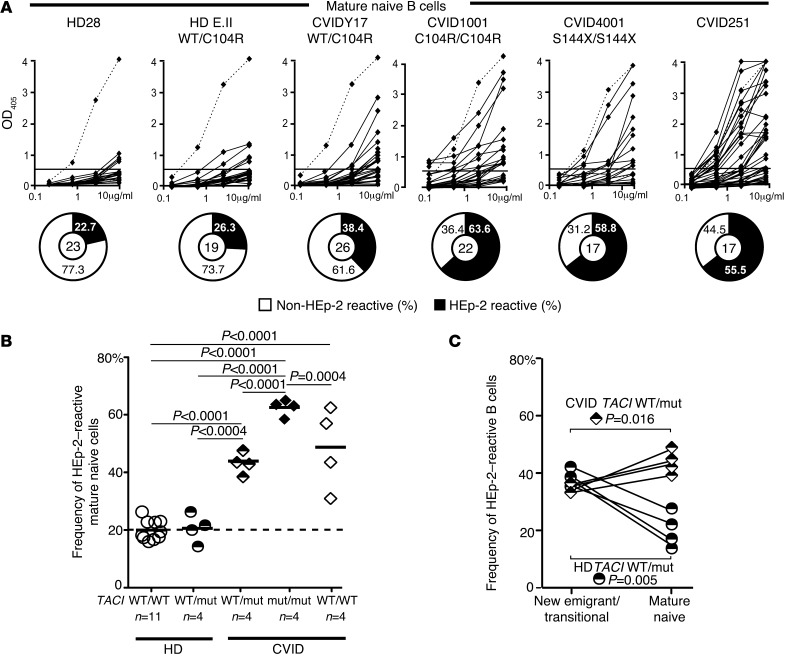
A second B cell tolerance checkpoint is responsible for the elimination of additional autoreactive B cells before they enter the CD10–CD21+IgM+CD27–CD20+ mature naive B cell compartment (21). We assessed the impact of TACI mutations on peripheral B cell tolerance by testing HEp-2 cell lysates for the reactivity of recombinant antibodies cloned from mature naive B cells from healthy subjects and CVID patients with or without TACI mutations (Supplemental Tables 19–35). We observed a significantly elevated frequency of HEp-2 reactive mature naive B cells in all tested CVID patients with one TACI mutation (38.5%–47.6%; P < 0.001), those with two TACI mutations (58.5%–65%; P < 0.001), and those without TACI mutations (28.6%–56%; P < 0.001) compared with healthy controls (16.0%–26.3%), demonstrating an impaired peripheral B cell tolerance checkpoint in these patients (Figure 4, A and B). Peripheral B cell tolerance defects were further evidenced by higher frequencies of polyreactive mature naive clones in CVID patients compared with healthy controls, with the highest frequency being observed in B cells from subjects with two TACI mutations that frequently displayed antinuclear reactivity (Supplemental Figure 5 and Supplemental Figure 6, A and B). In contrast, between the new emigrant/transitional and mature naive B cell developmental stages, healthy donors with one TACI mutation showed a significant reduction in both HEp-2–reactive (37.7% to 19.3%; P = 0.005) and polyreactive (24.9% to 18.2%; P = 0.046) clones (Figure 4 and Supplemental Figure 6B). We conclude that a single TACI mutation does not affect the peripheral B cell tolerance checkpoint, which is functional in all healthy subjects but defective in CVID patients.
Figure 4. A defective peripheral B cell tolerance checkpoint in CVID patients.
(A) Recombinant antibodies derived from mature naive B cells from subjects or CVID patients with or without TACI mutation(s) were tested by ELISA for anti–HEp-2 cell reactivity. Dashed lines show the ED38-positive control (21). Horizontal lines define the cutoff OD405 for positive reactivity. For each individual, the frequencies of HEp-2–reactive and non–HEp-2–reactive clones are summarized in pie charts, with the number of antibodies tested indicated in the center. (B) The frequency of HEp-2–reactive mature naive B cells was higher in all CVID patients than was observed in healthy donors with or without one TACI mutation. (C) The frequency of HEp-2–reactive clones rose between the new emigrant/transitional and mature naive B cell stages in CVID patients with a TACI mutation, whereas it decreased in healthy carriers with one TACI mutation. Statistical significance is indicated by an unpaired or paired (for C) Student’s t test.
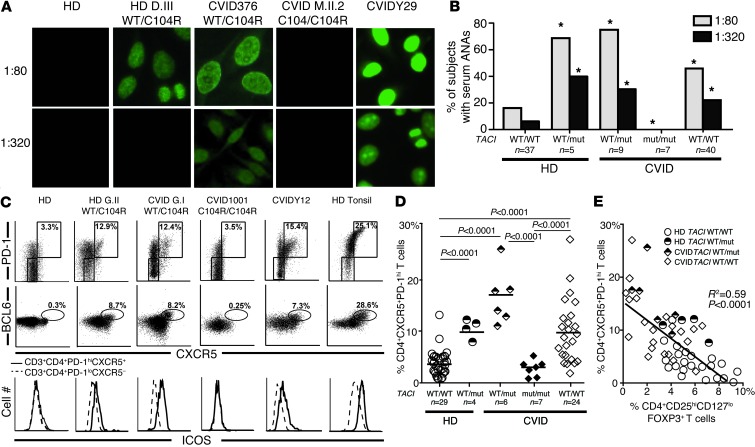
Figure 6. Heterozygous TACI mutations correlate with secreted ANAs and circulating Tfh cells.
(A and B) ANAs with diverse nuclear staining patterns at low (1:80) and high (1:320) dilutions were common in individuals with one TACI mutation, but were absent in patients with two mutated alleles. *P < 0.05 (statistical differences compared with healthy controls by χ2 testing). (C and D) Expanded CD4+PD-1hiCXCR5+ Tfh cells in the peripheral blood of individuals with one, but not two, TACI mutations and some CVID patients without TACI mutations. In addition to PD-1 and CXCR5 expression, Tfh cells in carriers of a single TACI mutation were further evidenced by increased intracellular BCL6 expression in and increased cell surface ICOS expression on CD4+PD-1hiCXCR5+ T cells (solid line in lower panel of C) compared with CD4+PD-1loCXCR5– T cells (dashed line in lower panel of C). (E) The frequency of circulating Tfh cells was inversely correlated with the frequency of circulating Tregs in enrolled subjects with or without single TACI mutations. Statistical significance is indicated using χ2 testing (B), an unpaired Student’s t test (D), or linear regression analysis (E).
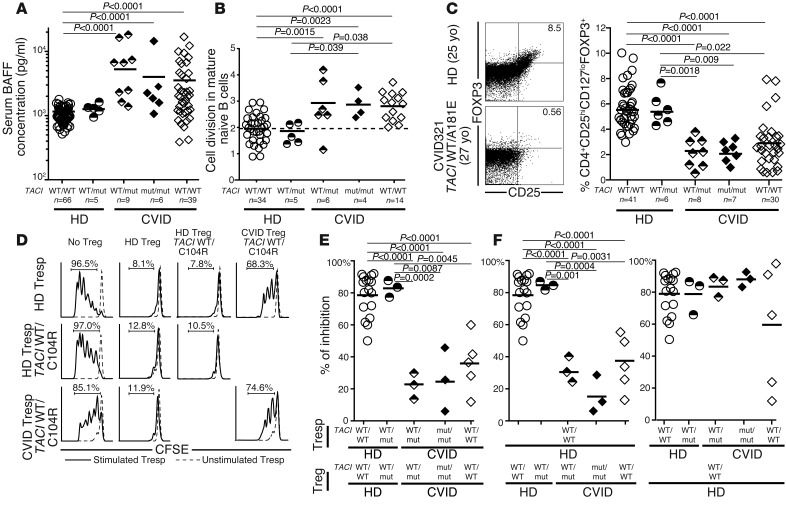
Peripheral B cell tolerance defects in CVID patients correlate with elevated plasma BAFF concentrations and decreased Treg frequencies.
Defects in peripheral B cell tolerance have been correlated with elevated plasma BAFF concentrations and lower Treg frequencies, both features that have already been reported in CVID (7, 11, 12, 26–28). We found 4- to 5-fold increased elevations in plasma BAFF concentrations in CVID patients, contrasting with normal BAFF concentrations in healthy donors with a single TACI mutation (Figure 5A). Using a quantitative PCR–based assay for enumerating κ-deletion recombination excision circles (KRECs) that estimates the number of divisions undergone by a B cell population (36), we found that mature naive B cells from CVID patients with and without TACI mutations displayed significantly increased homeostatic proliferation, with an average of 3.0 divisions in vivo compared with 1.9 divisions in B cells from healthy donors both with and without TACI mutations (Figure 5B). Both the defective peripheral B cell tolerance checkpoint and the dysregulated homeostatic B cell proliferation observed in CVID patients were characteristic of FOXP3-deficient patients who do not have functional Tregs, suggesting a similarly altered Treg compartment in CVID (28). Indeed, we found that the frequency of CD4+CD25hiCD127loFOXP3+ Tregs was decreased in most CVID patients, independent of the presence and/or number of TACI mutations, but not in healthy donors with a single TACI mutation (Figure 5C). Impaired Treg function in CVID patients was further suggested by their altered Ki67+CD45RO+CD25lo phenotype, also reminiscent of nonfunctional Tregs from FOXP3-deficient IPEX patients (Supplemental Figure 7, A and B, and ref. 28). Our direct assessment of Treg suppressive function in vitro demonstrated the impaired ability of Tregs from CVID patients with and without TACI mutations to inhibit the proliferation of CFSE-labeled CD3+CD4+CD25– autologous and heterologous T responder (Tresp) cells (Figure 5, D and E). In contrast, Tregs from healthy donors with a single TACI mutation were as suppressive as Tregs from controls with no TACI mutation, further demonstrating that TACI mutations are not inducing Treg defects per se (Figure 5, D–F). Thus, TACI mutations are not responsible for the elevated plasma BAFF concentrations, the low Treg frequencies, or the poor Treg suppressive function associated with the majority of CVID patients displaying a defective peripheral B cell tolerance checkpoint.
Figure 5. Increased plasma BAFF concentrations, homeostatic naive B cell proliferation, and an impaired Treg compartment are features common to CVID patients with and without TACI mutations.
TACI mutations did not affect plasma BAFF concentrations (pg/ml) measured by ELISA (A) or mature naive B cell expansion detected by KREC analysis (B). Both were greater in CVID patients compared with healthy controls. (C–F) CVID status, but not TACI mutations, affected Treg frequency and function. (C) Dot plots represent CD4+ gated CD25hiFOXP3+ T cells of a healthy donor control and an age-matched CVID patient with one TACI mutation. Scatter plots reveal that decreased CD4+CD25hiCD127loFOXP3+ Treg frequency only correlated with CVID. yo, year-old. (D) Representative histograms of Treg-mediated suppression of autologous and heterologous CFSE-labeled Tresp cells on day 4.5 from a CVID patient and a healthy relative both carrying a TACI mutation were compared with a healthy control. Dashed lines display nonstimulated Tresp cells. The autologous and heterologous suppressive activities of Tregs from healthy donor controls, healthy carriers with a single TACI mutation, CVID patients with one or two mutated TACI alleles, as well as CVID patients without TACI mutations are summarized in E and F, respectively. Statistical significance is indicated by an unpaired Student’s t test.
Circulating ANAs and BCL6+ Tfh cells are associated with a single TACI mutation.
CVID patients with a single TACI mutation are prone to developing autoimmune syndromes, whereas carrying two mutations seems to prevent autoimmunity (19, 20). To further investigate this apparent discrepancy, we assessed the plasma of healthy donors and CVID patients with or without TACI mutation(s) for IgG ANAs. In agreement with previous reports, 16.2% of healthy donors were found positive for low-titer (1:80 dilution) plasma ANAs, with very few displaying high ANA titers (5.4% at a dilution of 1:320) (37). In contrast, most CVID patients with one TACI mutation displayed low-titer plasma ANAs (1:80 dilution), and 30% of them had detectable high-titer ANAs (1:320) (Figure 6, A and B). We found that 40% of healthy donors carrying a single TACI mutation also frequently harbored significant high-titer ANAs, thereby suggesting a role for TACI in preventing autoantibody secretion (Figure 6, A and B). Remarkably, ANAs were undetectable even at a low 1:80 titer in all seven CVID patients with two TACI mutations, revealing that the absence of functional TACI blocks autoreactive B cell activation (Figure 6, A and B).
Since the generation of IgG ANAs is considered T cell dependent, we analyzed effector T cell subsets potentially contributing to autoantibody production, such as CXCR5+PD-1hiCD4+ circulating Tfh cells (38, 39). These T cells are rare in the peripheral blood of control healthy donors without a TACI mutation and in CVID patients with two TACI mutations, comprising 3.7% and 2.1% of their CD4+ T cells, respectively (Figure 6, C and D). In contrast, we found a high average frequency of CXCR5+PD-1hiCD4+ T cells in healthy donors and CVID patients with a single TACI mutation as well as in some CVID patients without TACI mutations (healthy donor [HD] TACI WT/mut: 10.1%; CVID TACI WT/mut: 17.1%; and CVID: 9.9% of CD4+ cells) (Figure 6, C and D). These cells resembled tonsilar Tfh cells, which are normally localized in GCs, since they expressed intracellular BCL6, the master transcriptional regulator responsible for Tfh differentiation, as well as the coactivation molecules ICOS and CD40L (Figure 6C and data not shown) (40). Tfh cells were generally most prevalent in the peripheral blood of subjects who had the lowest frequencies of circulating Tregs, whereas in subjects with two TACI mutations, Tfh cells were nearly absent (Figure 6E). In addition, several cytokines secreted by Tfh cells, including IL-4 and IL-10, but not IL-21, were found at elevated concentrations in the plasma of CVID patients but not in that of healthy donors with one TACI mutation (Supplemental Figure 8). Hence, a single TACI mutation correlates with an increased frequency of circulating CD4+CXCR5+PD-1hiICOShiBCL6+ Tfh cells and the production of high-titer IgG ANAs, thereby revealing an additional, more distal B cell tolerance breach often associated with autoimmune syndromes. In contrast, a second TACI mutation resulting in a lack of functional TACI counteracts the effects of a single TACI mutation, creating a scenario of scant circulating Tfh cells, absent ANAs, and a reduced susceptibility to autoantibody-mediated disease.
Discussion
We report herein several roles played by TACI mutations in the development of CVID. Previously, the association between TACI mutation(s) and CVID was unclear because many people carrying TACI mutations are healthy and without infectious susceptibility or decreased serum immunoglobulins (18, 41). However, we found that all individuals with TACI mutations failed to remove developing autoreactive B cells in the marrow. This defective central B cell tolerance checkpoint is the first evidence of the full penetrance of TACI mutations in B cell development. This effect is not influenced by the nature of the mutation itself or the CVID status of the carrier. Indeed, healthy carriers with a single C104R TACI mutation abrogating ligand binding displayed abnormalities similar to those in CVID patients carrying the A181E mutation affecting the TACI transmembrane domain. Likewise, patients homozygous for the allele encoding the C104R mutation showed profound B cell defects and elevated autoreactive B cell frequencies similar to those in the S144X/S144X individual in whom no TACI protein is expressed (8). Hence, various mutated TACI alleles encode similarly nonfunctional molecules. It remains to be determined whether these mutations result in the expression of interfering dominant-negative products or whether their impact on B cell activation and central selection is solely due to haploinsufficiency.
How do TACI mutations affect the removal of developing autoreactive B cells? Previous studies have suggested that the regulation of central B cell tolerance is intrinsic to B cells and involves the sensing of self-antigens through binding with BCRs and potentially TLRs (23). Our data from subjects with TACI mutations further support such a model, as TACI is expressed primarily by B cells and regulates BCR, TLR7, and TLR9 functions. Indeed, B cells carrying a single TACI mutated allele display decreased CD86 upregulation in vitro when BCR, TLR7, or TLR9 are triggered, whereas B cells with two mutated TACI alleles fail to upregulate this costimulatory molecule. Interestingly, defective CD86 induction after BCR and TLR9 stimulation as well as impaired TLR responses are features already reported for B cells from CVID patients and may represent one mechanism by which TACI mutations favor the development of CVID (5, 42). TACI-deficient patients as well as subjects with a single TACI mutation share a similar defective central B cell tolerance checkpoint that is also characterized by a failure to remove antinuclear clones. The presence of antinuclear clones in the new emigrant/transitional B cell compartment is reminiscent of IRAK4-deficient, MYD88-deficient, and ADA-deficient severe combined immune deficiency patients who have revealed the IRAK4/MYD88 and ADA pathways to be essential for the counterselection of such reactivity (23, 25). Since TLR7 and TLR9 bind nucleic acids and require IRAK4, MYD88, ADA, and TACI to function in B cells, these receptors may therefore play an important role in establishing central tolerance against RNA- and DNA-containing antigens. In addition, TACI’s intracellular domain binds MYD88 (30), and our data show that TACI can interact with both TLR7 and TLR9 upon coengagement of these receptors. Hence, polyreactive and antinuclear BCRs that bind self-antigens in the bone marrow may internalize and deliver them to endosomal compartments. TLR7 and TLR9 may then recognize RNA- and DNA-containing complexes, leading to TLR proteolytic activation and interaction with TACI, which in turn may allow the amplification ofIRAK4/MYD88–dependent signals to mediate central tolerance mechanisms. We conclude that TACI plays an essential role in the establishment of central B cell tolerance in humans.
TACI mutations potentially favor CVID development by impairing B cell activation. However, many individuals carrying a TACI mutation do not develop CVID (18, 41). By comparing such subjects with CVID patients with TACI mutation(s), we found that disease correlated with an abnormal peripheral B cell tolerance checkpoint independently of TACI mutations. Indeed, all asymptomatic individuals with a single TACI mutation managed to prevent the accumulation of autoreactive clones in their mature naive B cell compartment. This demonstrates that peripheral B cell tolerance can be established despite a defective central B cell tolerance checkpoint. The increased frequency of autoreactive mature naive B cells in patients with autoimmune diseases such as rheumatoid arthritis, lupus erythematosus, and type 1 diabetes — all of whom display defective central B cell tolerance — is therefore likely due to a defective peripheral B cell tolerance checkpoint and not just an accumulation in the periphery of autoreactive clones produced by the marrow (24, 43, 44). The defect in peripheral tolerance of CVID patients is associated with an impaired Treg compartment, which plays an important role in regulating this checkpoint (28). In addition, decreased Treg functions may also lead to increased proliferative events in the mature naive B cell compartment of CVID patients compared with healthy donors with or without TACI mutations (28). The elevated BAFF concentration in the plasma of CVID patients may also favor B cell homeostatic expansion and provide survival signals to autoreactive clones, thereby preventing their elimination in the periphery (14, 26–28, 45). Hence, one TACI mutation is not sufficient to induce the development of CVID and is not responsible for the defective peripheral B cell tolerance checkpoint observed in CVID patients. It is unclear at this point whether another susceptibility gene is responsible for CVID development, potentially affecting Treg function, or whether nongenetic environmental factors are more important in the development of this disease.
It was previously reported that CVID patients with a single TACI mutation are prone to autoimmune cytopenias, whereas patients devoid of functional TACI are protected from autoimmunity (20). This apparent discrepancy was initially puzzling, as we found that subjects with two mutated TACI alleles had increased frequencies of autoreactive clones in all naive B cell compartments compared with CVID patients with a single TACI mutation. However, in the absence of functional TACI, this increase in autoreactive B cells is accompanied by profound impairments of B cell activation after BCR, TLR7, and TLR9 triggering that are significantly worse than in subjects with a single TACI mutation. Moreover, TLRs play an essential role in the development of autoimmunity (46). Hence, severe TLR function impairment in TACI-deficient individuals is likely to be protective against autoimmunity despite profoundly defective autoreactive B cell counterselection, leaving these individuals with an immunodeficient phenotype. In agreement with this hypothesis, we could detect ANAs in the plasma of CVID patients and asymptomatic individuals with a single TACI mutation, but not in subjects with two mutated TACI alleles.
High-titer plasma ANAs in individuals with a single TACI mutation correlated with the presence of circulating CD4+CXCR5+PD-1hiICOShiBCL6+ Tfh cells, which normally reside in GCs and provide B cell help. B cells in GCs also produce reciprocal signals for Tfh differentiation. The absence of functional TACI resulting from two mutated TACI alleles impairs costimulatory ICOSL and CD86 expression and thereby precludes the development of Tfh cells and limits ANA secretion. Indeed, another group of CVID patients who are spared autoimmune disease and devoid of circulating Tfh cells are those with ICOS deficiency, whose lymph node GCs display a disturbed architecture (47–49). Thus, the absence of circulating Tfh cells in patients with two TACI mutations may illustrate dysfunctional GC reactions and decreased isotype-switched antibody secretion. In agreement with this hypothesis, murine models have demonstrated that either CD86 or ICOSL deficiency abolish GC formation and impair the generation of Tfh cells (50–52). However, expanded Tfh cells driven by overexpression of ICOSL on B cells have been reported in Taci knockout mice (53). Such differences between mouse and man may reconcile the development of an SLE-like autoimmune condition in TACI-deficient mice with the absence of autoimmune manifestations in CVID patients with two TACI mutations (54). On the other hand, as in Taci–/– mice, Tfh cells are enriched in CVID patients with a single TACI mutation and likely contribute to the activation of autoreactive B cells through cell-cell interactions and the production of cytokines such as IL-4 and IL-10, leading to ANA secretion. It is unknown at this point why Tfh cell frequency is increased in CVID patients with a single TACI mutation or without TACI mutations, but their low Treg numbers and poor Treg suppressive function may fail to downregulate Tfh production in GCs (55, 56). Nevertheless, increased Tfh cells may favor autoreactive B cell activation in CVID patients and contribute to the development of autoimmune conditions characteristic of these patients.
In summary, TACI mutations favor CVID development by altering B cell activation after BCR, TLR7, and TLR9 stimulation. However, additional genetic or environmental factors are required for the development of CVID. Since TACI regulates the function of BCR, TLR7, and TLR9, which are likely involved in sensing self-antigens in the marrow, subjects with TACI mutation(s) not only suffer from B cell activation defects but also from an impaired removal of developing autoreactive B cells. B cells from CVID patients with a single TACI mutation may respond to self-antigen stimulation and receive additional T cell help due to the presence of Tfh cells, leading to autoantibody secretion and an increased risk for autoimmune syndromes. In contrast, autoreactive B cells from subjects with two mutated TACI alleles lack the functional TACI necessary for B cell activation, resulting in diminished immune and autoimmune humoral responses.
Methods
Patients.
We obtained peripheral blood from 21 individuals with TACI mutations including 9 CVID patients with one TACI mutation and 7 immune-deficient patients with two TACI mutations, 6 with CVID, and 1 with hypogammaglobulinemia (Supplemental Table 1). Patient A.II.1, a TACI S144X homozygote who has a complete absence of measurable TACI-specific mRNA and protein, has no increased susceptibility to infections and does not require Ig replacement therapy, yet is nonetheless hypogammaglobulinemic (serum IgG 410 mg/dl) (8). Although not a classical CVID phenotype, this patient was grouped here with other CVID patients carrying two TACI mutations to simplify subject categorization. Healthy relatives of these patients made up the remaining 5 subjects; each carried one TACI mutation. TACI alleles present in our study cohort included common missense variants associated with CVID (C104R and A181E) as well as rare mutations such as insertions (204insA, 571insG) and truncations (S144X and S194X). For purposes of comparison, we obtained peripheral blood samples from 50 healthy controls and 50 CVID patients without TACI mutations. The study groups were controlled for age and sex. In addition, healthy controls analyzed for their autoreactive B cell frequencies were previously reported and included a 50-year-old male of mixed European descent previously splenectomized after accidental blunt-force trauma (HD28) (25). We also included HD29, the 29-year-old healthy brother of CVID patient CVID332. All subjects were genotyped at the TACI gene loci by Sanger sequencing using published primer sets (exons 1–4) (8). For exon 5, the following primer pair was used: 5′-CCTAGTGCAGGGCCAGGCCTG-3′ and 5′-CCGACCTCCTGCTCTATCT-3′. All samples were collected in accordance with Yale University’s IRB-approved protocol HIC0906005336.
Cell staining and sorting, cDNA, RT-PCR, antibody production, ELISAs, and indirect fluorescence assays.
Peripheral B cells were purified from the peripheral blood of research subjects by positive selection using CD20 magnetic beads (Miltenyi Biotec). Single CD19+CD21loCD10+IgMhiCD27– new emigrant/transitional and CD19+CD21+CD10–IgM+CD27– peripheral mature naive B cells from patients and control donors were sorted on a FACSVantage cell sorter (BD) into 96-well PCR plates. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, recombinant antibody expression, recombinant antibody purification, and recombinant antibody reactivity determination were as previously described (21). For indirect immunofluorescence assays, HEp-2 cell–coated slides (Bion Enterprises) were incubated in a moist chamber at room temperature with patient plasma samples at 1:80 and 1:320 dilutions in PBS according to the manufacturer’s instructions. FITC-conjugated goat anti-human IgG was used as a detection reagent. The following antibodies were used for flow cytometric stainings: CD19 APC-Cy7, CD27 PerCP-Cy5.5, CD10 PE-Cy7, IgM FITC, CD21 APC, CD25 FITC, CD69 PE, CD80 FITC, CD86 PE, CD4 APC-Cy7, CD25 PE-Cy7, CD127 PerCP-Cy5.5, CD45RO Pacific Blue, TACI PE, CD303 FITC, CD14 Pacific Blue, CD4 APC-Cy7, CXCR5 PerCP-Cy5.5, PD-1 PE-Cy7, ICOSL PE, CD25 PE, and CD40 APC (all from Biolegend), CD3 eFluor 605NC, BCL6 PE (both from eBioscience), and CD21 BD Horizon V450 (BD). Intracellular staining for FOXP3 Alexa Fluor 488 (clone PCH101; eBioscience) was performed using the FOXP3/Transcription Factor Staining Buffer Set (eBioscience) in accordance with the manufacturer’s instructions.
B cell activation and proliferation.
Naive B cells were enriched from the blood of healthy donors by negative selection using the Naive B Cell Isolation Kit II (Miltenyi Biotec) and plated for 48 hours at 150,000–200,000 cells per well in a 96-well plate in RPMI 10% FBS and either 2.5 μg/ml polyclonal F(ab′)2 rabbit anti-human IgM (Jackson ImmunoResearch), multimeric soluble recombinant human CD40L 1.0 μg/ml (Alexis Biochemicals), 0.5 μg/ml CpG (TLR9 agonist; Invitrogen), or 1.0 μg/ml gardiquimod (TLR7 agonist; InvivoGen). In proliferation and antibody secretion experiments, total human splenic B cells isolated by negative selection using EasySep were stimulated for 5 days with CpG and gardiquimod in the presence or absence of 500 ng/ml APRIL (R&D Systems). APRIL was blocked with 30 μg/ml of TACI-Ig (Ancell). Mouse IgG1 with irrelevant binding activity was used as a control for TACI-Ig. Cell proliferation was assessed by CFSE staining using the CellTrace CFSE Cell Proliferation Kit (Invitrogen) according to the manufacturer’s instructions. Supernatants were collected and analyzed to test the presence of BAFF and APRIL using a soluble human BAFF ELISA Kit (Adipogen) and a LEGEND MAX Human APRIL/TNFSF13 ELISA Kit (BioLegend). IgM secretion was detected by ELISA. In brief, M96-Nunc ELISA plates were coated with anti-human IgM (SouthernBiotech). After blocking with 1% BSA in PBS, samples were added to the plate overnight. Purified anti IgM-HRP antibody (Cappel) and TMB substrate solution (BD) were used according to the manufacturer’s instructions.
Coimmunoprecipitation experiments.
FACS-sorted human CD19+ splenic B cells were treated with 0.2 μg/ml recombinant human APRIL (R&D Systems), 0.5 μg/ml CpG (Invitrogen), and/or 1.0 μg/ml gardiquimod (InvivoGen) for 2 hours. For each condition, 107 cells were used. Cells were homogenized and solubilized in lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% Triton, 0.2 mg/ml BSA, and protease inhibitors) using a Dounce homogenizer. An equal amount of each protein lysate was incubated with anti-TACI biotinylated antibody (Abcam) overnight at 4°C, followed by incubation with 50 μl of streptavidin-sepharose beads (GE Healthcare) for 3 hours. Different buffers were used to wash streptavidin-antigen-antibody complexes (wash buffer 1: 50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 0.1% Triton; wash buffer 2: 10 mM Tris-HCl, pH 7.5, 0.1% Triton). Denaturated proteins were analyzed by SDS-polyacrylamide gel electrophoresis. The membranes were immunoblotted with anti-TLR9 (Pierce), anti-TLR7 (Abcam), and anti-TACI (Abcam) antibodies.
KREC assay.
The ratio of KREC joints (signal joint) to the Jκ-Cκ recombination genomic joints (coding joint) was determined as previously described (36). In brief, genomic DNA was isolated from sorted B cell fractions by lysing cell pellets in 10 mM Tris-HCl, pH 8.0, containing 100 μg/ml proteinase K (Roche), incubating for 1 hour at 56°C, and heat inactivating the enzyme at 95°C for 10 minutes. Two separate RT-PCR reactions were performed, one reaction to amplify the signal joint and another to amplify the coding joint, as previously detailed. The number of cell divisions was calculated by subtracting the cycle threshold of the PCR detecting the coding joint from that detecting the signal joint.
In vitro Treg suppression assay.
CD4+ T cells were enriched using the EasySep Human CD4+T cell enrichment kit (STEMCELL Technologies). CD4+CD25hiCD127lo/– Tregs were sorted by flow cytometry, whereas CD3+CD4+CD25– Tresp cells were obtained after the depletion of CD25+ cells with anti-human CD25 microbeads (Miltenyi Biotec) and then labeled with CellTrace CFSE (InvivoGen) at 5 μM. Treg and Tresp cells were cocultured at a 1:1 ratio in the presence of beads loaded with anti-CD2, anti-CD3, and anti-CD28 (Treg suppression inspector human; Miltenyi Biotec). On days 3.5 to 4.5, cocultures were stained for viability with the LIVE/DEAD kit (Invitrogen), and proliferation of the viable Tresp was analyzed by CFSE dilution.
Cytokine detection.
Plasma BAFF concentrations were determined by ELISA according to the manufacturer’s instructions (R&D Systems). Cytokines (IL-4, IL-10) in plasma were measured with the High Sensitivity Human Cytokine Magnetic Bead Kit (Millipore) using a Luminex 200. IL-21 was measured by ELISA with the Human IL-21 ELISA Ready-Set-Go Kit (eBioscience).
Statistics.
Statistical analysis was performed using GraphPad Prism software, version 5.0 (GraphPad Software). Differences between groups of research subjects were analyzed for statistical significance with unpaired or paired (when within groups), two-tailed Student’s t tests except for plasma indirect immunofluorescence assay comparisons that used χ2 testing. For these statistical tests, α was set to α = 0.05; accordingly, a P value less than or equal to 0.05 was considered significant. The relationship between Tfh cells and Tregs was statistically analyzed using linear regression analysis.
Supplementary Material
Acknowledgments
We thank J. Bussel, F. Kantor, P. Askenase, C. Randolf, J. Sproviero, F. Hsu, C. Price, and O. Pagovich for providing blood samples from CVID patients, and S. Rudchenko, L. Devine, and C. Wang for cell sorting. This work was supported by grants from the NIH-NIAID (AI071087, AI082713, and AI095848, to E. Meffre, and AI061093, to E. Meffre, A. Cerutti, and C. Cunningham-Rundles); and the NIH-NICHD (K12HD0141401-10, to N. Romberg); and by a CIS/Talecris Fellowship Award (to N. Romberg). T. Kinnunen was supported by the Sigrid Juselius Foundation, the Finnish Medical Foundation, and the Saastamoinen Foundation. L. Menard was supported by NIH-NIAID grant T32 AI089704. T. Cantaert received support from the Rubicon program, Netherlands Organization for Scientific Research, and H. Morbach received support from the German Research Foundation (DFG, MO 2160/2-1).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(10):4283–4293. doi:10.1172/JCI69854.
See the related Commentary beginning on page 4142.
References
- 1.Al-Herz W, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanford JP, Favour CB, Tribeman MS. Absence of serum gamma globulins in an adult. N Engl J Med. 1954;250(24):1027–1029. doi: 10.1056/NEJM195406172502403. [DOI] [PubMed] [Google Scholar]
- 3.Bryant A, Calver NC, Toubi E, Webster AD, Farrant J. Classification of patients with common variable immunodeficiency by B cell secretion of IgM and IgG in response to anti-IgM and interleukin-2. Clin Immunol Immunopathol. 1990;56(2):239–248. doi: 10.1016/0090-1229(90)90145-G. [DOI] [PubMed] [Google Scholar]
- 4.Yu JE, et al. Toll-like receptor 7 and 9 defects in common variable immunodeficiency. J Allergy Clin Immunol. 2009;124(2):349–356. doi: 10.1016/j.jaci.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denz A, Eibel H, Illges H, Kienzle G, Schlesier M, Peter HH. Impaired up-regulation of CD86 in B cells of “type A” common variable immunodeficiency patients. Eur J Immunol. 2000;30(4):1069–1077. doi: 10.1002/(SICI)1521-4141(200004)30:4<1069::AID-IMMU1069>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Horn J, et al. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156(3):446–454. doi: 10.1111/j.1365-2249.2009.03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arumugakani G, Wood PM, Carter CR. Frequency of Treg cells is reduced in CVID patients with autoimmunity and splenomegaly and is associated with expanded CD21lo B lymphocytes. J Clin Immunol. 2010;30(2):292–300. doi: 10.1007/s10875-009-9351-3. [DOI] [PubMed] [Google Scholar]
- 8.Salzer U, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 9.Castigli E, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275(45):35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 11.Knight AK, Radigan L, Marron T, Langs A, Zhang L, Cunningham-Rundles C. High serum levels of BAFF, APRIL, and TACI in common variable immunodeficiency. Clin Immunol. 2007;124(2):182–189. doi: 10.1016/j.clim.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreuzaler M, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188(1):497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 13.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20(4):441–453. doi: 10.1016/S1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 14.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20(6):785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Garibyan L, Lobito AA, Siegel RM, Call ME, Wucherpfennig KW, Geha RS. Dominant-negative effect of the heterozygous C104R TACI mutation in common variable immunodeficiency (CVID). J Clin Invest. 2007;117(6):1550–1557. doi: 10.1172/JCI31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chinen J, et al. Transmembrane activator and CAML interactor (TACI) haploinsufficiency results in B-cell dysfunction in patients with Smith-Magenis syndrome. J Allergy Clin Immunol. 2011;127(6):1579–1586. doi: 10.1016/j.jaci.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JJ, et al. The C104R mutant impairs the function of transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) through haploinsufficiency. J Allergy Clin Immunol. 2010;126(6):e1234–e1232. doi: 10.1016/j.jaci.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan-Hammarstrom Q, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39(4):429–430. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120(5):1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salzer U, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113(9):1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 22.Ng Y-S, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase (Btk) is essential for human B cell tolerance. J Exp Med. 2004;200(7):927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isnardi I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29(5):746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menard L, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer AV, Morbach H, Brigida I, Ng YS, Aiuti A, Meffre E. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. J Clin Invest. 2012;122(6):2141–2152. doi: 10.1172/JCI61788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herve M, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204(7):1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyers G, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011;108(28):11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kinnunen T, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595–1603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romberg N, Ng YS, Cunningham-Rundles C, Meffre E. Common variable immunodeficiency patients with increased CD21-/lo B cells suffer from altered receptor editing and defective central B cell tolerance. Blood. 2011;118(22):5977–5978. doi: 10.1182/blood-2011-09-373969. [DOI] [Google Scholar]
- 30.He B, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11(9):836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/S1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 32.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208(4):643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 35.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 36.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. 2007;204(3):645–655. doi: 10.1084/jem.20060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan EM, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40(9):1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 38.Mietzner B, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita R, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. . Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Gallo M, Radigan L, Almejún MB, Martínez-Pomar N, Matamoros N, Cunningham-Rundles C. TACI mutations and impaired B-cell function in subjects with CVID and healthy heterozygotes. J Allergy Clin Immunol. 2013;131(2):468–476. doi: 10.1016/j.jaci.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham-Rundles C, Radigan L, Knight AK, Zhang L, Bauer L, Nakazawa A. TLR9 activation is defective in common variable immune deficiency. J Immunol. 2006;176(3):1978–1987. doi: 10.4049/jimmunol.176.3.1978. [DOI] [PubMed] [Google Scholar]
- 43.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Human B cell tolerance and its failure in rheumatoid arthritis. Ann N Y Acad Sci. 2005;1062:116–126. doi: 10.1196/annals.1358.014. [DOI] [PubMed] [Google Scholar]
- 44.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadanaga A, et al. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56(5):1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 47.Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4(3):261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 48.Bossaller L, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177(7):4927–4932. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 49.Warnatz K, et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood. 2006;107(8):3045–3052. doi: 10.1182/blood-2005-07-2955. [DOI] [PubMed] [Google Scholar]
- 50.Borriello F, et al. B7-1 B7-2 have overlapping, critical roles in immunoglobulin class switching germinal center formation. Immunity. 1997;6(3):303–313. doi: 10.1016/S1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 51.Mak TW, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4(8):765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 52.Salek-Ardakani S, et al. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J Immunol. 2011;186(9):5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ou X, Xu S, Lam KP. Deficiency in TNFRSF13B (TACI) expands T-follicular helper germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A. 2012;109(38):15401–15406. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18(2):279–288. doi: 10.1016/S1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 55.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.