Abstract
The distal nephron is composed of two main cell types: principal cells and intercalated cells. These cells have distinct morphologic features that allow them to be readily distinguished by light microscopy, as well as distinct suites of proteins that facilitate cell-specific transport properties. In this issue of the JCI, Gueutin and colleagues describe a new mechanism by which β-intercalated cells, via release of ATP and prostaglandin E2 (PGE2), influence the activity of transporters in principal cells.
Challenging tradition
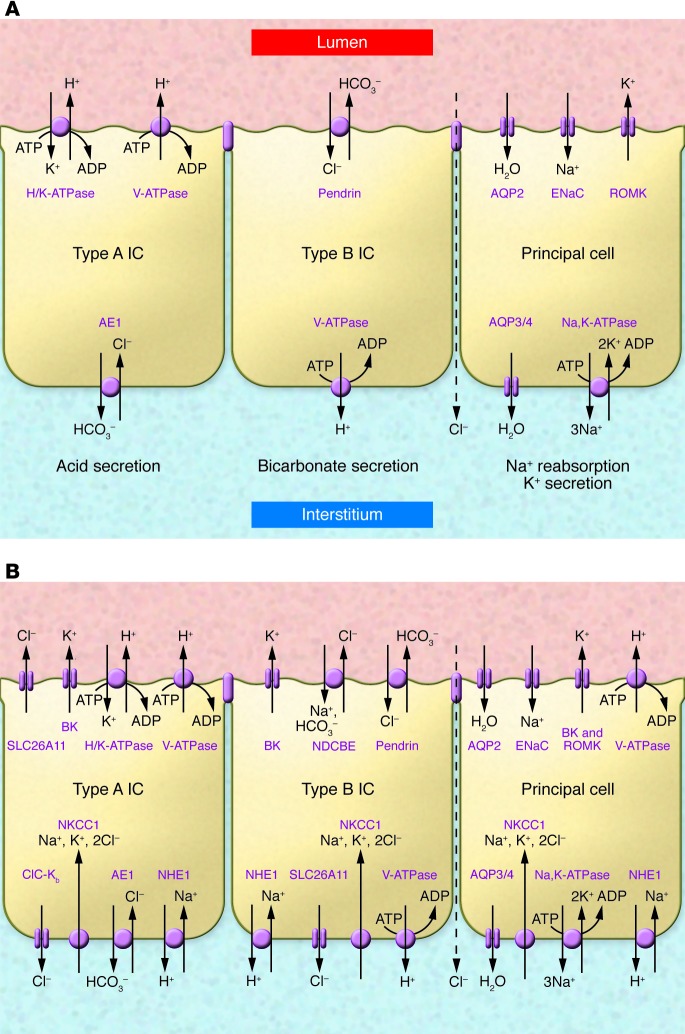
The traditional view of the distal nephron considers principal cells to be primarily responsible for reabsorption of filtered Na+ and for K+ secretion into the ultrafiltrate, whereas intercalated cells account for urinary acidification. Sodium reabsorption by principal cells occurs via the Na+/Cl– cotransporter (NCC) in the early distal nephron and the epithelial Na+ channel (ENaC) in later nephron segments. Potassium is secreted via the renal outer medullary K+ channel (ROMK; Figure 1A). Intercalated cells are primarily responsible for urinary acidification, through H+ secretion by the vacuolar H+-ATPase or the H+/K+-ATPase found in α-intercalated cells. When required, HCO3– secretion into the ultrafiltrate occurs via the Cl–/HCO3– exchanger pendrin (also known as SLC4A4) in β-intercalated cells (1, 2). This view presumes limited crosstalk between principal and intercalated cells, as a lack of gap junctions between these cell types limits their communication (3, 4).
Figure 1. Evolving understanding of the distal nephron.
(A) Traditional view, highlighting key transport proteins involved with acid/base, salt, and water balance in kidney collecting duct α (type A) and β (type B) intercalated cells (ICs) and principal cells. (B) Updated view, based on studies performed in mice, rats, or rabbits. Although SLC26A11 is represented as a Cl– channel, there is evidence that it may also function as a Cl–/HCO3– exchanger. AE1, anion exchanger 1; AQP, aquaporin; ClC-Kb, Cl– channel, kidney-specific (type B); NDCBE, Na+-dependent Cl–/HCO3– exchanger; NHE1, Na+/H+ exchanger 1; NKCC1, Na+/K+/2Cl– cotransporter 1; V-ATPase, vacuolar H+-ATPase.
As we learn more about properties of the distal nephron, distinctions between principal and intercalated cells are beginning to fade. Recent studies have shown that intercalated cells are capable of reabsorbing filtered Na+ and Cl– via a luminal Na+-dependent Cl–/HCO3– exchanger, SLC4A8, which operates in parallel with pendrin (Figure 1B and refs. 2, 5). Reabsorption is not blocked by amiloride, a prototypic ENaC inhibitor, but is blocked by thiazide diuretics, which are also inhibitors of NCC. The finding that mice overexpressing pendrin develop salt-sensitive hypertension suggests that this NaCl transport system has an important role in the reabsorption of filtered NaCl and regulation of extracellular fluid volume and blood pressure (6).
Evidence also suggests that intercalated cells, along with principal cells, participate in K+ secretion that is activated by increased tubular flow rates. In this context, K+ secretion is mediated by the luminal large-conductance Ca2+-activated K+ channel (referred to as the BK channel) and the basolateral Na+/K+/2Cl– cotransporter (7, 8). The findings that both individuals with Bartter syndrome as a result of ROMK loss-of-function mutations and mice lacking ROMK expression have robust renal K+ secretion highlight the importance of this BK channel–mediated K+ secretory pathway (9). Although neonates with loss of function ROMK mutations may exhibit early hyperkalemia (10), this likely reflects a delay in BK channel expression in the immediate postnatal period. Finally, identification of a luminal H+-ATPase in principal cells, although modest compared with intercalated cell H+-ATPase, raises the possibility that these cells may contribute to renal H+ secretion (11).
Working together
Given the recent findings that principal and intercalated cells transport similar cations, it is likely that mechanisms have evolved to coordinate the transport of specific ions across these distinct cell types. For example, pendrin-dependent transport of HCO3– into the tubular lumen by intercalated cells enhances ENaC activity in principal cells (12). High tubular flow activates autocrine/paracrine prostaglandin E2 (PGE2) release in the distal nephron, presumably via activation of cytosolic phospholipase 2 (cPLA2), which in turn regulates flow-stimulated Na+ and K+ transport in cortical collecting ducts (13). Gueutin and colleagues address the issue of functional crosstalk between intercalated and principal cells of the distal nephron in this issue of the JCI (14). Distal renal tubular acidosis is a clinical disorder associated with reduction or loss of distal nephron acid secretion. In humans, this disorder is accompanied by increased urinary Ca2+ losses, nephrocalcinosis, and chronic kidney disease. It is not surprising that humans with this disorder also exhibit enhanced renal Na+ and K+ losses, as this could simply reflect damaged cells within the distal nephron (15). The authors studied a mouse model of distal renal tubular acidosis in which the gene encoding the B1 subunit of the vacuolar H+-ATPase was disrupted (16). Previous characterization determined that these animals have a blunted response to increased acid load and do not have enhanced urinary Ca2+ losses or nephrocalcinosis.
Surprisingly, the authors found that these animals have an impaired ability to adapt to a low-NaCl diet. Normally, transition to a low-NaCl diet is accompanied by enhanced Na+ reabsorption in the nephron and reduced urinary Na+ excretion. Studies of cortical collecting ducts isolated from mice lacking the vacuolar H+-ATPase B1 subunit revealed that both Na+ and Cl– absorption were suppressed, as were transporters responsible for Na+ and Cl– absorption (ENaC α and γ subunits and pendrin) in this nephron segment. The impaired Na+ reabsorption was not due to reductions in the renin-angiotensin-aldosterone system, which is known to activate Na+ transporters in the distal nephron. These findings raised the possibility that there are other factors responsible for blunting NaCl absorption in the distal nephron.
The authors found increased urinary excretion of PGE2 and ATP in mice lacking the vacuolar H+-ATPase B1 subunit. β-intercalated cells have a key role in the release of PGE2, as blocking vacuolar H+-ATPase in β-intercalated cells within isolated cortical collecting ducts was associated with enhanced PGE2 release. This prostanoid is a known inhibitor of ENaC (17). Extracellular ATP has a role in this process, as PGE2 release was dependent on ATP-dependent signaling via purinergic receptors. Extracellular ATP, released by connexin hemichannels and signaling through purinergic receptors, is also a known ENaC inhibitor that reduces channel open probability (18, 19).
In addition to the changes in renal Na+ handling, mice lacking expression of the vacuolar H+-ATPase B1 subunit had a urinary concentrating defect, reflecting reduced aquaporin 2 expression. When fed a low-Na+ diet, these mice also exhibited enhanced renal K+ loss, which appeared to be due to increased BK channel expression and increased urinary flow.
A cooperative future
In summary, the work presented by Gueutin and colleagues (14) introduces a new paradigm of crosstalk between principal and intercalated cells and provides further evidence that both cell types are important in maintaining Na+ balance and thus blood pressure. This work also raises a number of questions that we hope will be addressed in future studies. While inhibition of basolateral vacuolar H+-ATPase in β-intercalated cells was necessary to see the crosstalk between intercalated and principal cells, we do not know whether this regulatory interaction is also seen when β-intercalated cell vacuolar H+-ATPase activity is reduced by endogenous regulatory factors, such as increased acid load associated with a “typical” Western diet. Do inhibitors of prostaglandin synthesis (e.g., indomethacin and other nonsteroidal antiinflammatory drugs) have a role in preventing urinary loss of Na+ in individuals with congenital or acquired distal renal tubular acidosis, with the caveat that long-term use of the drugs may damage the kidney? What are the cellular mechanisms that lead to increased ATP release when vacuolar H+-ATPase in β-intercalated cells is inhibited? Are impairments in different components of this paracrine signaling pathway involved in the pathogenesis of salt-sensitive hypertension? The answers to these questions should provide useful information by which to understand the interaction between principal and intercalated cells, and also direct development of therapeutics for renal disease. On a final note, the authors’ observations raise the possibility that other mechanisms of crosstalk exist between these cells to facilitate the coordinated regulation of transporters between intercalated and principal cells.
Acknowledgments
This work was supported by NIH grants DK038470 (to L.M. Satlin), DK051391 (to T.R. Kleyman), DK065161 (to T.R. Kleyman), and DK075048 (to K.R. Hallows).
Footnotes
Conflict of interest: Kenneth R. Hallows is the principal investigator of a grant to the University of Pittsburgh from Dialysis Clinics Inc. to study the role of kinases in kidney epithelial transport regulation.
Citation for this article: J Clin Invest. 2013;123(10):4139–4141. doi:10.1172/JCI71944.
See the related article beginning on page 4219.
References
- 1.Madsen KM, Tisher CC. Structural-functional relationship along the distal nephron. Am J Physiol. 1986;250(6 pt 3):F1–F15. doi: 10.1152/ajprenal.1986.250.1.F1. [DOI] [PubMed] [Google Scholar]
- 2.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol. 2012;74:325–349. doi: 10.1146/annurev-physiol-020911-153225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biemesderfer D, Stanton B, Wade JB, Kashgarian M, Giebisch G. Ultrastructure of Amphiuma distal nephron: evidence for cellular heterogeneity. Am J Physiol. 1989;256(4 pt 1):C849–C857. doi: 10.1152/ajpcell.1989.256.4.C849. [DOI] [PubMed] [Google Scholar]
- 4.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285(5):F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 5.Leviel F, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120(5):1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacques T, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24(7):1104–1113. doi: 10.1681/ASN.2012080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006;291(5):F923–F931. doi: 10.1152/ajprenal.00192.2006. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, et al. Role of NKCC in BK channel-mediated net K(+) secretion in the CCD. Am J Physiol Renal Physiol. 2011;301(5):F1088–F1097. doi: 10.1152/ajprenal.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bailey MA, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70(1):51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 10.Finer G, et al. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr. 2003;142(3):318–323. doi: 10.1067/mpd.2003.100. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Pastor-Soler NM, Schreck C, Zavilowitz B, Kleyman TR, Satlin LM. Luminal flow modulates H+-ATPase activity in the cortical collecting duct (CCD). Am J Physiol Renal Physiol. 2012;302(1):F205–F215. doi: 10.1152/ajprenal.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pech V, et al. Pendrin modulates ENaC function by changing luminal HCO3. J Am Soc Nephrol. 2010;21(11):1928–1941. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores D, Liu Y, Liu W, Satlin LM, Rohatgi R. Flow-induced prostaglandin E2 release regulates Na and K transport in the collecting duct. Am J Physiol Renal Physiol. 2012;303(5):F632–F638. doi: 10.1152/ajprenal.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gueutin V, et al. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123(10):4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sebastian A, McSherry E, Morris RC. Impaired renal conservation of sodium and chloride during sustained correction of systemic acidosis in patients with type 1, classic renal tubular acidosis. J Clin Invest. 1976;58(2):454–469. doi: 10.1172/JCI108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finberg KE, et al. The B1-subunit of the H(+) ATPase is required for maximal urinary acidification. Proc Natl Acad Sci U S A. 2005;102(38):13616–13621. doi: 10.1073/pnas.0506769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stokes JB, Kokko JP. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pochynyuk O, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283(52):36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem. 2011;286(2):1054–1060. doi: 10.1074/jbc.M110.176552. [DOI] [PMC free article] [PubMed] [Google Scholar]