Abstract
Background
The use of direct-to-consumer genomewide profiling to assess disease risk is controversial, and little is known about the effect of this technology on consumers. We examined the psychological, behavioral, and clinical effects of risk scanning with the Navigenics Health Compass, a commercially available test of uncertain clinical validity and utility.
Methods
We recruited subjects from health and technology companies who elected to purchase the Health Compass at a discounted rate. Subjects reported any changes in symptoms of anxiety, intake of dietary fat, and exercise behavior at a mean (±SD) of 5.6±2.4 months after testing, as compared with baseline, along with any test-related distress and the use of health-screening tests.
Results
From a cohort of 3639 enrolled subjects, 2037 completed follow-up. Primary analyses showed no significant differences between baseline and follow-up in anxiety symptoms (P = 0.80), dietary fat intake (P = 0.89), or exercise behavior (P = 0.61). Secondary analyses revealed that test-related distress was positively correlated with the average estimated lifetime risk among all the assessed conditions (β = 0.117, P<0.001). However, 90.3% of subjects who completed follow-up had scores indicating no test-related distress. There was no significant increase in the rate of use of screening tests associated with genomewide profiling, most of which are not considered appropriate for screening asymptomatic persons in any case.
Conclusions
In a selected sample of subjects who completed follow-up after undergoing consumer genomewide testing, such testing did not result in any measurable short-term changes in psychological health, diet or exercise behavior, or use of screening tests. Potential effects of this type of genetic testing on the population at large are not known. (Funded by the National Institutes of Health and Scripps Health.)
Direct-to-consumer genomewide profiling to assess disease risk provides information about a person's genetic risk of 20 to 40 common polygenic diseases. The tests simultaneously genotype approximately 500,000 variant bases of a person's DNA. Consumers can purchase these tests, currently priced between $400 and $2,000, on the Internet.1-4 Consultation with a health care provider is not a prerequisite. Proponents argue that providing this type of information directly to consumers may result in improved compliance with health-screening practices and more healthful lifestyle choices. Skeptics assert that such testing has the potential to cause harm, including anxiety and increased use of unnecessary and expensive screening and medical procedures. The clinical validity and utility of these tests have not been demonstrated, and given their cost, many observers argue that their sale raises consumer-protection issues.
Studies of the psychological, behavioral, and clinical effects of genetic-risk disclosure for single diseases have generally been small and have yielded somewhat mixed findings.5-10 The Scripps Genomic Health Initiative was designed as a longitudinal cohort study to measure the effects of direct-to-consumer genomewide scans.11 Subjects purchased a commercially available genomewide risk scan at a subsidized rate and underwent Web-based standardized assessments at baseline and during follow-up, with the aim of gauging changes in anxiety level, diet, exercise, test-related distress, and use of screening tests.
Methods
Study Design and Instruments
The study was approved by the research ethics and institutional review boards at Scripps Health and Scripps Research Institute. Informed consent was obtained electronically from each study subject. Details regarding the study methods have been reported previously.11
All subjects underwent health assessments at baseline and during follow-up (after receipt of the risk-disclosure report) with the use of a Web-based survey tool, SurveyMonkey.12 The first follow-up assessment was administered 3 months after testing, and the second was scheduled for 12 months after testing. Here we report baseline and 3-month follow-up data.
Our baseline assessment included questions about demographic characteristics, family and individual health history, and attitudes toward genetic testing. Both the baseline and follow-up assessments included measures of anxiety, dietary fat intake, and exercise behavior. Situational anxiety was assessed with the 20-item “state anxiety” subscale of the Spielberger State–Trait Anxiety Inventory.13 A score of 39 or less on the anxiety subscale is thought to indicate a low-anxiety state. Using reliable-change-index methods,14 we estimated that a difference of 12 points or more on this measure between baseline and follow-up would indicate reliable and clinically meaningful change. Dietary fat intake was measured with the use of the 17-item Block Dietary Fat Screener,15 which includes the top sources of fat in the diets of Americans. A score of 23 or more is considered to indicate high fat intake. Exercise was measured with the use of the Godin Leisure-Time Exercise Questionnaire,16,17 which consists of three questions probing the frequency and duration of exercise of mild, moderate, and strenuous intensity in a typical week. A score of 7.5 or more is consistent with the minimum level of physical activity currently recommended by the Department of Health and Human Services; a score of 21 or more is consistent with the level recommended by the Institute of Medicine.
The 3-month follow-up assessment included a measure of test-related distress and questions to gauge behavior associated with health surveillance or screening. Test-related distress was measured with the 22-item Impact of Events Scale–Revised.18,19 A score of more than 8 on the Avoidance and Intrusion subscales is thought to indicate “some impact,” and a score of more than 23 is thought to indicate clinically significant distress. We also asked whether subjects had undergone any of 13 health-screening tests since receiving their results, whether they intended to undergo any of these tests with greater frequency than before they had received their results, whether they had spoken with a genetic counselor about their results, and whether they had shared their results with their physician.
We designed our study to be consistent with the designs of previous single-gene, single-condition studies that have evaluated the psychological, behavioral, and clinical effects of genetic testing on conditions such as breast and colorectal cancer.6 The evaluation of clinical effects (e.g., on the use of screening tests) is particularly relevant, given the concern that direct-to-consumer testing may result in overutilization of medical testing and resources.20
Procedures
Study procedures pertaining to enrollment and administration of the baseline health assessment have been described in detail previously.11 Ninety days after subjects received their results, we sent an e-mail message to each of them, requesting that they complete the follow-up health assessment. Initially, we sent three e-mail requests within a 6-week time frame and classified subjects who did not complete follow-up within 6 weeks as lost to follow-up. Toward the end of the study, however, we streamlined the follow-up assessment so that the time-to-complete interval was cut by approximately 50%. We then sent a final set of e-mail reminders to subjects who had not completed follow-up, offering an incentive for their completion. Thus, what we call the “original” 3-month follow-up was closed to completion in February 2010, and the “short” follow-up was closed to completion in March 2010.
Personalized Genomic Test
We examined the effect of direct-to-consumer genomewide scanning with the Navigenics Health Compass,2 a commercially available genomewide risk test of uncertain clinical validity and utility (see the Methods section and Figure 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Table 1 provides an overview of the risk information provided by Navigenics to study subjects. In the context of this study, we focused our analyses on two risk-information formats provided to subjects: estimated lifetime risk (estimated risk of a particular condition, expressed as a percentage) and color-coded risk (with orange representing a condition for which the subject's estimated lifetime risk was more than 20% above average or for which the overall lifetime risk was more than 25%).
Table 1.
Risk Index Provided to Study Subjects by Navigenics and Composite Risk Analysis Variables.*
| Variable | Definition |
|---|---|
| Risk index | |
| Estimated lifetime risk | A subject's estimated lifetime risk of a particular condition, expressed as a percentage (i.e., the risk of the condition among persons of the same sex over an average life span); if a subject's estimated lifetime risk was more than 80%, “>80%” was the result provided to the subject rather than the actual estimate |
| Average lifetime risk | The average sex-specific lifetime risk in the population for a particular condition, expressed as a percentage |
| Percentile floor | Percent of HapMap CEU reference subjects with a lower genetic risk than the study subject |
| Percentile ceiling | Percent of HapMap CEU reference subjects with a higher genetic risk than the study subject |
| Dashboard color | Color-coded risk, with orange indicating either an overall lifetime risk of more than 25% or a risk that is more than 20% above average and gray indicating low risk |
| Composite risk analysis variables | |
| Total no. of conditions viewed | Total number of conditions the subject viewed out of 22 possible conditions (with breast and prostate cancer provided on a sex-specific basis) |
| Individual average estimated lifetime risk | Average estimated lifetime risk for all conditions that the subject viewed |
| Individual proportion of orange-coded conditions | Number of conditions for which the subject received a high-risk color code divided by the number of conditions viewed |
| Individual highest estimated lifetime risk | Highest estimated lifetime risk the subject viewed |
CEU denotes Utah residents with ancestry from northern and western Europe in the data set of the Centre d'Etude du Polymorphisme Humain.
Genetic Counseling and Safety Monitoring
Genetic counseling that is provided by the staff of board-certified genetic counselors at Navigenics was made available at no charge to study subjects. In addition, Navigenics provided proactive outreach to study subjects on the basis of their risk results (see the Methods section in the Supplementary Appendix).
Outcome Measures
The prespecified primary outcomes were changes in subjects' anxiety symptoms, dietary fat intake, and exercise behavior. The reduction of dietary fat intake is a common health recommendation aimed at reducing individual risk for several of the conditions on the Navigenics Health Compass panel. For this reason, total fat intake is a useful outcome measure for assessment of the behavioral effect of such genetic testing.
Prespecified secondary outcomes were test-related distress and the use of screening tests, as measured by self-reported completion of screening or medical testing and intention to undergo screening or medical testing with greater frequency after the genetic testing.
Study Oversight
The study was funded by the National Institutes of Health and the Scripps Genomic Medicine Division of Scripps Health, which provided subsidies for the subjects' purchase of the Navigenics test and receipt of genetic-counseling services. Navigenics did not provide any financial support for the study and was not involved in the study design, the accrual or analysis of data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Statistical Analysis
All statistical analyses were conducted with the use of statistical software packages SPSS and OriginPro 8.1 and the Dimension Research resources for computing z-tests and confidence intervals for proportions.21 Two-sided t-tests, Mann–Whitney U tests, or chi-square tests were used to compare baseline variables for subjects who completed follow-up with those for subjects who were lost to follow-up. Baseline and follow-up scores on assessments of anxiety level, dietary fat intake, and exercise activity were adjusted for age, sex, education, ancestry (white or nonwhite), income, health-related occupation, follow-up interval in days, and original versus short follow-up. Adjusted scores were then tested by means of the Wilcoxon signed-rank test for related samples. We totaled the number of screening tests that each subject reported they would complete with greater frequency after genetic testing, with adjustment for covariates, and used a one-sample Wilcoxon signed-rank test to determine whether the number was significantly increased from zero. Percentages were used to qualitatively describe scores on the Impact of Events Scale–Revised.
We used linear regression, with adjustment for the eight covariates and baseline scores on each measure, to assess the relationship between follow-up assessments (scores for anxiety level, dietary fat intake, and exercise activity) and the average estimated lifetime risk of all conditions for which results were viewed, the proportion of conditions that were color-coded orange, and the estimated lifetime risk and color-coded risk for each of the 23 individual conditions. Each subject received results for 22 conditions, with sex-specific calculated risks of breast and prostate cancer. We carried out a parallel analysis of scores on the Impact of Events Scale–Revised. We tested for a correlation between the use of screening tests and the two composite risk estimates (as above), using Spearman's rank correlation coefficients. However, to test associations with risk estimates for specific conditions, each screening test was matched with corresponding conditions of varying degrees of relevance and tested with the use of logistic regression, with the screening test as the dependent variable and the eight covariates plus the condition-specific risk estimate as the independent variables (Table 1 in the Supplementary Appendix).
We also determined the proportion of subjects who reported speaking with a genetic counselor about their results or sharing their results with their physician. We tested the extent to which either of these factors was associated with changes in follow-up scores for anxiety, diet, exercise, or test-related distress, using linear regression. A P value of less than 0.05 was considered to indicate statistical significance. All reported P values are uncorrected for multiple testing.
Results
Characteristics of the Subjects
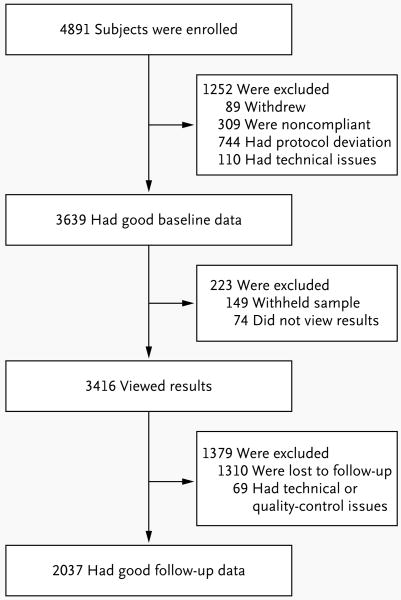
Figure 1 depicts enrollment numbers and outcomes. Descriptive statistics for demographic and outcome variables are shown in Table 2, as are baseline comparisons of these variables for the 2037 subjects (56.0%) who completed follow-up versus the 55 subjects who completed a baseline assessment but eventually withdrew, the 223 subjects who withheld their sample or did not view their results, and the 1310 subjects who viewed their results but were lost to follow-up. Median baseline scores for anxiety, dietary fat intake, and exercise suggest that the general health of the subjects was good. The same baseline comparisons were also performed between the 1720 subjects who completed the originally planned 3-month assessment (84.4%) and the 317 subjects who completed the short follow-up assessment (15.6%) (Table 2 in the Supplementary Appendix). The mean (±SD) follow-up interval was 5.6±2.4 months.
Figure 1. Enrollment and Outcomes.
Subjects who withdrew from the study cited financial reasons, insurance concerns, and change of mind. Subjects who did not complete the baseline health assessment after three e-mail requests were considered to not be in compliance. The unintentional release of a subject's genetic results before the completion of the baseline health assessment was considered a protocol deviation. A subject's submission of duplicate or triplicate surveys was considered a technical issue. Of the 89 subjects who withdrew, only 55 completed a baseline assessment.
Table 2.
Baseline Comparisons between Subjects Who Completed Follow-up and Those Who Withdrew, Withheld DNA Sample or Did Not View Results, or Were Lost to Follow-up.*
| Variable | Completed Follow-up | Withdrew† | P Value | Withheld Sample or Did Not View Results | P Value | Viewed Results but Lost to Follow-up | P Value |
|---|---|---|---|---|---|---|---|
| No. of subjects | 2037 | 55 | NA | 223 | NA | 1310 | NA |
| Female sex (%) | 55.3 | 67.3 | 0.08 | 53.4 | 0.58 | 49.3 | 0.001 |
| Age (yr) | 0.49 | 0.54 | 0.008 | ||||
| Mean | 46.7±12.0 | 45.6±13.6 | 46.2±11.2 | 45.6±11.6 | |||
| Range | 19–85 | 21–71 | 22–82 | 19–85 | |||
| Income ($) | 0.02 | 0.02 | 0.009 | ||||
| Median | 100,000–149,999 | 100,000–149,999 | 100,000–149,999 | 150,000–199,999 | |||
| Modal | 100,000–149,999 | 50,000–99,999 | 100,000–149,999 | 100,000–149,999 | |||
| Median educational level | Some postgraduate education | Graduate of 4-yr college | 0.04 | Graduate of 4-yr college | <0.001 | Graduate of 4-yr college | 0.001 |
| White race (%)‡ | 84.2 | 63.6 | <0.001 | 69.1 | <0.001 | 76.6 | <0.001 |
| Scripps Health employee (%) | 23.6 | 41.8 | 0.002 | 19.7 | 0.20 | 16.5 | <0.001 |
| Anxiety score§ | 0.04 | 0.58 | 0.01 | ||||
| Mean | 35.2±9.6 | 37.8±9.5 | 35.8±10.2 | 36.2±10.0 | |||
| Median | 34.0 | 37.0 | 35.0 | 35.0 | |||
| Range | 20–72 | 22–57 | 20–65 | 20–74 | |||
| Dietary fat score¶ | 0.02 | 0.97 | 0.34 | ||||
| Mean | 16.0±7.9 | 13.6±8.6 | 16.0±7.8 | 15.8±8.2 | |||
| Median | 15.0 | 12.0 | 15.0 | 15.0 | |||
| Range | 0–51 | 0–34 | 1–41 | 0–54 | |||
| Exercise score | 0.67 | 0.10 | 0.005 | ||||
| Mean | 28.6±23.0** | 27.5±22.3 | 26.7±22.2†† | 27.7±25.3‡‡ | |||
| Median | 24.0** | 23.8 | 21.0†† | 22.1‡‡ | |||
| Range | 0–246** | 0–112 | 0–110†† | 0–238 ‡‡ |
Plus–minus values are means ±SD. P values are for comparisons with the group of subjects who completed follow-up. P values for comparisons of sex, race, and employment by Scripps Health were calculated by means of Pearson's chi-square test; those for age by means of independent samples t-test; and those for income, education, and scores on assessments of anxiety, dietary fat intake, and exercise by means of the Mann–Whitney U test. NA denotes not applicable because no statistical test was performed.
Of the 89 subjects who withdrew, only 55 completed a baseline assessment.
Race was self-reported.
Anxiety was assessed with the 20-item subscale of the Spielberger State–Trait Anxiety Inventory; scores range from 20 to 80, with higher scores indicating higher anxiety. A score of 39 or less is considered to indicate low anxiety. A difference of 12 points or more on this measure is considered to indicate a reliable and clinically meaningful change.
Dietary fat intake was measured with the 17-item Block Dietary Fat Screener; scores range from 0 to 68, with higher scores indicating higher fat intake. A difference of 11 points or more on this measure is considered to indicate a reliable and clinically meaningful change.
Exercise and physical activity were measured with the Godin Leisure-Time Exercise Questionnaire; scores range from 0 to 246, with higher scores indicating greater physical activity (i.e., a greater number of metabolic-equivalent hours per week). A difference of 29 points or more on this measure is considered to indicate a reliable and clinically meaningful change.
A total of 1943 subjects were included in this category.
A total of 215 subjects were included in this category.
A total of 1272 subjects were included in this category.
We further compared selected demographic characteristics of our sample with those of a representative sample of Navigenics customers who did not participate in the study, and observed no significant differences (Table 3 in the Supplementary Appendix). In addition, there was no significant difference in the composite genetic risk estimates between subjects who completed follow-up and those who were lost to follow-up or between those who completed the original follow-up assessment and those who completed the short follow-up assessment (Tables 4 and 5 in the Supplementary Appendix).
Overall Effect of Testing
We observed no significant differences in the level of anxiety, dietary fat intake, or exercise behavior between baseline and follow-up for the sample as a whole (Table 3). On the Impact of Events Scale–Revised, the mean score for the sample overall was 3.2±7.1, indicating no distress. On the Avoidance and Intrusion subscales, 90.3% of subjects had a total score of 8 or less, indicating no test-related distress, and 97.2% had a total score of 23 or less, indicating no clinically significant test-related distress.
Table 3.
Primary Outcome Measures before and after Receipt of Results of Genetic Testing for 2037 Subjects Who Completed Follow-up.*
| Outcome Measure | Baseline Score | Follow-up Score | P Value† |
|---|---|---|---|
| Anxiety | 35.2±9.6 | 34.6±10.0 | 0.80 |
| Dietary fat intake | 16.0±7.9 | 15.2±7.5 | 0.89 |
| Exercise‡ | 28.6±23.0 | 28.6±22.9 | 0.61 |
Plus–minus values are means ±SD. The assessment tools and ranges of scores for each category are listed in Table 2.
All P values were calculated with the use of the Wilcoxon signed-rank test after adjustment for covariates.
A total of 1943 subjects were included in this analysis.
Table 4 shows actual and intended use of screening tests after genetic testing. Overall, the subjects were nearly evenly divided on whether they intended to undergo additional screening or medical testing on the basis of their results (Fig. 2 in the Supplementary Appendix). However, the number of screening tests that subjects intended to complete with greater frequency after genetic testing was found to be significantly increased from zero (mean, 1.8±2.6; P<0.001).
Table 4.
Association between Condition-Specific Risk Estimates from Genomewide Profiling and Intended or Actual Completion of Screening Tests.*
| Screening Test and Condition | Assessment of Clinical Benefit of Screening Test | Completion of Screening Test† | Intention to Increase Frequency of Screening Test‡ | Completion of Screening Test | Intention to Increase Frequency of Screening Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Lifetime Risk | Orange Color Code§ | Estimated Lifetime Risk | Orange Color Code§ | ||||||||
| percent | odds ratio (95%CI) | P value | odds ratio (95%CI) | P value | odds ratio (95%CI) | P value | odds ratio (95%CI) | P value | |||
| Thyroid test for Graves' disease | No benefit | 24.8 | 9.5 | 1.04 (0.93–1.16) | 0.47 | 1.18 (0.91–1.52) | 0.22 | 1.01 (0.86–1.18) | 0.92 | 1.34 (0.93–1.94) | 0.11 |
|
| |||||||||||
| Skin examination for psoriasis | No benefit | 23.2 | 15.9 | 0.99 (0.92–1.09) | 0.97 | 1.03 (0.82–1.29) | 0.82 | 1.05 (0.96–1.15) | 0.30 | 1.24 (0.96–1.60) | 0.09 |
|
| |||||||||||
| Ophthalmic examination | 29.2 | 14.4 | |||||||||
|
| |||||||||||
| Glaucoma | Possible benefit | 1.02 (0.98–1.07) | 0.32 | 1.22 (0.96–1.56) | 0.10 | 1.12 (1.06–1.18) | <0.001 | 1.63 (1.22–2.17) | 0.001 | ||
|
| |||||||||||
| Macular degeneration | Possible benefit | 1.01 (1.00–1.03) | 0.04 | 1.14 (0.91–1.42) | 0.25 | 1.05 (1.04–1.06) | <0.001 | 2.02 (1.56–2.63) | <0.001 | ||
|
| |||||||||||
| Glucose test for type 2 diabetes | Established benefit | 35.7 | 17.4 | 1.01 (0.99–1.02) | 0.18 | 1.14 (0.94–1.39) | 0.19 | 1.03 (1.02–1.04) | <0.001 | 1.88 (1.47–2.42) | <0.001 |
|
| |||||||||||
| Electrocardiogram | 13.3 | 9.1 | |||||||||
|
| |||||||||||
| Atrial fibrillation | No benefit | 0.99 (0.96–1.01) | 0.24 | 0.82 (0.61–1.09) | 0.17 | 1.03 (1.01–1.05) | 0.009 | 1.50 (1.10–2.06) | 0.01 | ||
|
| |||||||||||
| Heart attack | No benefit | 0.99 (0.97–1.01) | 0.36 | 0.80 (0.56–1.14) | 0.22 | 1.01 (0.98–1.04) | 0.42 | 1.52 (0.99–2.35) | 0.06 | ||
|
| |||||||||||
| Colonoscopy | 8.5 | 15.4 | |||||||||
|
| |||||||||||
| Colon cancer | Established benefit | 1.08 (0.98–1.19) | 0.14 | 1.12 (0.79–1.59) | 0.54 | 1.21 (1.13–1.30) | <0.001 | 2.17 (1.68–2.79) | <0.001 | ||
|
| |||||||||||
| Crohn's disease | No benefit | 0.98 (0.78–1.22) | 0.84 | 1.13 (0.76–1.68) | 0.54 | 1.17 (1.01–1.36) | 0.04 | 1.19 (0.88–1.60) | 0.26 | ||
|
| |||||||||||
| Cholesterol test | 50.9 | 26.3 | |||||||||
|
| |||||||||||
| Heart attack | Established benefit | 0.99 (0.98–1.02) | 0.92 | 0.80 (0.62–1.02) | 0.07 | 1.02 (1.00–1.04) | 0.04 | 1.36 (1.03–1.79) | 0.03 | ||
|
| |||||||||||
| Type 2 diabetes | No benefit | 1.01 (0.99–1.02) | 0.13 | 1.08 (0.89–1.30) | 0.44 | 1.00 (0.99–1.01) | 0.68 | 0.98 (0.80–1.21) | 0.88 | ||
|
| |||||||||||
| Chest radiography for lung cancer | No benefit | 8.0 | 7.3 | 1.01 (0.88–1.16) | 0.87 | 1.21 (0.70–2.08) | 0.49 | 1.02 (0.89–1.16) | 0.82 | 1.26 (0.72–2.19) | 0.42 |
|
| |||||||||||
| Cardiac stress test | 5.7 | 10.6 | |||||||||
|
| |||||||||||
| Atrial fibrillation | No benefit | 0.99 (0.96–1.02) | 0.44 | 0.81 (0.53–1.23) | 0.31 | 1.02 (1.00–1.05) | 0.02 | 1.36 (1.01–1.83) | 0.045 | ||
|
| |||||||||||
| Heart attack | Possible benefit | 0.99 (0.96–1.02) | 0.41 | 0.89 (0.48–1.64) | 0.71 | 1.03 (1.01–1.06) | 0.007 | 1.48 (0.97–2.26) | 0.07 | ||
|
| |||||||||||
| Blood test | 51.4 | 23.4 | |||||||||
|
| |||||||||||
| Celiac disease | No benefit | 0.96 (0.64–1.44) | 0.84 | 1.04 (0.83–1.30) | 0.76 | 0.99 (0.62–1.60) | 0.99 | 1.13 (0.88–1.47) | 0.34 | ||
|
| |||||||||||
| Colon cancer | No benefit | 0.99 (0.94–1.06) | 0.97 | 1.01 (0.83–1.24) | 0.92 | 1.07 (1.00–1.14) | 0.04 | 1.18 (0.94–1.49) | 0.14 | ||
|
| |||||||||||
| Crohn's disease | No benefit | 0.89 (0.77–1.02) | 0.08 | 0.91 (0.73–1.14) | 0.42 | 1.03 (0.89–1.20) | 0.67 | 0.98 (0.75–1.28) | 0.87 | ||
|
| |||||||||||
| Lupus | No benefit | 1.13 (0.83–1.52) | 0.44 | 0.99 (0.80–1.24) | 0.98 | 1.19 (0.89–1.60) | 0.24 | 1.10 (0.86–1.42) | 0.45 | ||
|
| |||||||||||
| Rheumatoid arthritis | No benefit | 1.06 (0.99–1.13) | 0.09 | 1.23 (0.98–1.54) | 0.07 | 1.04 (0.97–1.12) | 0.30 | 1.16 (0.90–1.50) | 0.24 | ||
|
| |||||||||||
| Breast self-examination for breast cancer | Possible benefit | 68.9 | 24.5 | 1.02 (0.98–1.06) | 0.40 | 1.13 (0.78–1.64) | 0.51 | 1.05 (1.01–1.09) | 0.02 | 1.90 (1.32–2.74) | 0.001 |
|
| |||||||||||
| Mammography for breast cancer | Established benefit | 39.0 | 13.8 | 0.99 (0.96–1.03) | 0.73 | 0.81 (0.55–1.18) | 0.27 | 1.06 (1.01–1.11) | 0.02 | 2.42 (1.60–3.67) | <0.001 |
|
| |||||||||||
| Prostate-specific antigen test for prostate cancer | Possible benefit | 22.6 | 18.6 | 1.02 (0.99–1.04) | 0.15 | 1.51 (1.05–2.17) | 0.03 | 1.06 (1.04–1.09) | <0.001 | 2.43 (1.71–3.45) | <0.001 |
Percentages for breast self-examination and mammography include only women, and those for prostate-specific antigen testing include only men. Additional information regarding the rationale behind the assessment of clinical benefit, current guidelines for screening tests, and relevant references are provided in Table 1 in the Supplementary Appendix.
The values in this category are the percentages of subjects who reported actually completing the listed screening test since receiving their genomic risk results.
The values in this category are the percentages of subjects who reported having an intention to increase the frequency of the listed screening test since receiving their genomic risk results. However, the percentages do not reflect the number of subjects who said they intended to complete a screening test after receiving their genomic results. Thus, the proportions of subjects in this category are generally lower than the proportions of subjects who reported actually completing the screening test.
Orange color coding represents a condition for which the subject's estimated lifetime risk was more than 20% above average or for which the overall lifetime risk was more than 25%.
Effect of testing and Genomic Risk
There were no significant associations between composite measures of risk and follow-up scores on anxiety level, dietary fat intake, and exercise behavior (Tables 6 and 7 in the Supplementary Appendix). There also was no significant association between composite measures of risk and the total number of screening tests actually completed after genetic testing. However, we did observe significant associations between composite measures of risk and the total number of screening tests that subjects intended to complete with greater frequency after genetic testing (correlation with mean estimated lifetime risk, rs = 0.046; P = 0.04; correlation with proportion of orange-coded conditions, rs = 0.028; P = 0.21), as well as test-related distress (correlation with average estimated lifetime risk, β = 0.117; P<0.001; correlation with proportion of orange-coded conditions, β = 0.050; P = 0.02) (Table 6 in the Supplementary Appendix). Associations between follow-up scores on behavioral measures and condition-specific risk estimates are shown in Tables 6, 7, and 8 and Figure 3 in the Supplementary Appendix.
Sharing Results
A total of 10.4% of subjects reported discussing their results with a Navigenics board-certified genetic counselor, and 26.5% reported sharing their results with their physician. Speaking with a genetic counselor was not associated with test-related distress or changes in anxiety level, dietary fat intake, or exercise behavior at follow-up. In addition, the sharing of results with a physician was not associated with test-related distress or a change in anxiety level but was associated with lower fat intake (β = −0.040, P = 0.009) and increased exercise activity (β = 0.049, P = 0.003) (Fig. 4 in the Supplementary Appendix).
Discussion
In a selected group of subjects who underwent direct-to-consumer genomewide risk profiling with a commercially available test, prospective short-term assessment of those who completed follow-up did not show measurable changes in anxiety level, dietary fat intake, or exercise behavior after genetic testing. We observed no indication of test-related distress in 90.3% of the subjects and no evidence of increased use of screening tests. Generally speaking, our findings support the null hypothesis (that provision of the results of a direct-to-consumer genomic risk test does not affect health-related behavior), but the potential effects on the population at large are still unknown.
Direct-to-consumer genomic risk testing recently attracted national attention when one company that offers such testing, Pathway Genomics,3 announced it would sell its DNA saliva kits at Walgreen stores nationwide.22 This announcement led the Food and Drug Administration to consider whether the federal regulatory approval of consumer genomic tests is warranted.23 The controversy has been complicated in part by a lack of prospective data regarding the effect of testing on consumers and on the clinical validity and utility of the tests. Our study was not designed to test the clinical validity and utility of the Navigenics Health Compass, nor does it shed any light on these critically important characteristics. Our focus was on evaluating the effect of testing in a selected group of consumers. We found no evidence that learning the results of genomic risk testing had any short-term psychological, behavioral, or clinical effects on the study subjects. The subjects in our study are probably representative of the current population of persons who purchase these tests, although they probably are not representative of the population at large. Subjects who might have been harmed psychologically by testing may have declined to participate or may have dropped out of the study. The failure of a large percentage of subjects (44%) to complete the study is notable.
Half the study subjects reported that they intended to undergo one or more screening tests more frequently in the future, but this finding is in part driven by a small number of subjects who reported their intention to undergo a large number of screening or medical tests more frequently (Fig. 2 in the Supplementary Appendix). Moreover, in general, the likelihood that reported intentions will translate into actual increases in use is extremely low. This may be a good thing, given that the majority of the screening tests we assessed are considered inappropriate for asymptomatic persons (Table 1 in the Supplementary Appendix). In most instances, the use of such tests would probably result in a waste of health care resources.20
The uncertain clinical validity and utility of genomewide testing also suggest that screening decisions that are based on the results of such tests may be ill considered.20,24 The data from these tests provide highly limited information, since they are derived from genomewide association studies that have yielded variants accounting in most cases for less than 10% of the heritability of the diseases studied. Thus, such studies are underpowered to provide meaningful estimates of genetic risk. Regardless of the accuracy of the risk estimates, however, the question we were attempting to address is the extent to which the reported risk had an effect on behavior. There is also evidence that different genomewide testing companies and laboratories produce discrepant risk estimates, with some indicating increased risk and others indicating decreased risk (as compared with average risk) for the same condition in the same person.24 We did not determine whether some of the subjects underwent testing with other companies or laboratories; if they did, they may have received discordant results, which could have resulted in confusion and adverse psychological effects. Furthermore, subjects may have been aware of the lack of established clinical validity and utility of direct-to-consumer genotyping platforms; such knowledge may have influenced the results of this study.
Only 10% of the subjects who completed follow-up reported speaking with a genetic counselor about their results, despite the fact that counseling services were provided free of charge. Perhaps this finding has to do with the high educational level and possibly greater-than-average scientific acumen of the subjects in our study. On the other hand, 26% of the subjects reported sharing their results with their physician. A recent survey showed that only 10% of physicians thought they had the necessary training and knowledge in genomics25 to use genetic testing in treating patients. Together, these findings point to a void with respect to physician knowledge of and education in genomics. We also observed that speaking with a genetic counselor or sharing results with a physician was not associated with changes in anxiety levels after genetic testing.
There are several limitations of our study. First, we studied a sample of convenience that consisted of subjects who elected to undergo testing, and our longitudinal cohort design did not include a control group. Second, the subjects in our study are clearly not representative of the broader U.S. population, and we therefore cannot draw conclusions about the effect of genomewide testing on the population at large. Third, 44% of subjects who elected to undergo testing did not complete follow-up. Fourth, our recruitment strategy allowed enrollment of spouses and family members; thus, some observations are probably not independent. Fifth, our study relied on brief, Web-based, self-reported assessment of health behavior, which can be less reliable than in-person assessment and less sensitive than other measures. Sixth, our findings are based on a single, short-term follow-up assessment and do not speak to the long-term effects of testing. Seventh, since we did not obtain data on screening behavior before genetic testing, a possible explanation for the negative results in terms of clinical effect is that subjects were already engaging in risk-reduction behavior before the start of the study.
In conclusion, in a selected group of subjects who chose to undergo direct-to-consumer genomewide testing, we found no short-term changes in psychological health, diet and exercise behavior, or use of screening tests. Potential effects of this type of genetic testing on the population at large are not known.
Supplementary Material
Acknowledgments
Supported in part by grants from the National Center for Research Resources of the National Institutes of Health (NIH) (1UL1RR025774-01, to Dr. Topol) and the NIH's National Human Genome Research Institute (1R21HG005747-01, to Dr. Bloss) and by the Scripps Genomic Medicine Division of Scripps Health.
We thank Laura Ornowski, M.S., of Scripps for her assistance in data collection; and Vance Vanier, M.D., Michele Cargill, Ph.D., and Elana Silver, M.S., of Navigenics, along with their genetic counselors and other staff, for their support of the project.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.deCODEme home page. http://www.decodeme.com.
- 2.Navigenics home page. http://www.navigenics.com.
- 3.Pathway Genomics home page. http://www.pathway.com.
- 4.23andMe home page. https://www.23andme.com.
- 5.Botkin JR, Smith KR, Croyle RT, et al. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A. 2003;118A:201–9. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- 6.Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10:19–32. doi: 10.1097/GIM.0b013e31815f524f. [DOI] [PubMed] [Google Scholar]
- 7.Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer's disease. N Engl J Med. 2009;361:245–54. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins VR, Meiser B, Ukoumunne OC, Gaff C, St John DJ, Halliday JL. The impact of predictive genetic testing for hereditary nonpolyposis colorectal cancer: three years after testing. Genet Med. 2007;9:290–7. doi: 10.1097/gim.0b013e31804b45db. [DOI] [PubMed] [Google Scholar]
- 9.Lerman C, Hughes C, Croyle RT, et al. Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Prev Med. 2000;31:75–80. doi: 10.1006/pmed.2000.0684. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Green RC. Genetic risk assessment for adult children of people with Alzheimer's disease: the Risk Evaluation and Education for Alzheimer's Disease (REVEAL) study. J Geriatr Psychiatry Neurol. 2005;18:250–5. doi: 10.1177/0891988705281883. [DOI] [PubMed] [Google Scholar]
- 11.Bloss CS, Ornowski L, Silver E, et al. Consumer perceptions of direct-to-consumer personalized genomic risk assessments. Genet Med. 2010;12:556–66. doi: 10.1097/GIM.0b013e3181eb51c6. [DOI] [PubMed] [Google Scholar]
- 12.SurveyMonkey home page. http://www.surveymonkey.com.
- 13.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory (form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 14.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–9. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 16.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 17.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Weiss DS, Marmar CR. The Impact of Event Scale–Revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD. New York: Guilford Press; 1997. pp. 399–411. [Google Scholar]
- 19.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale–Revised. Behav Res Ther. 2003;41:1489–96. doi: 10.1016/j.brat.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 20.McGuire AL, Burke W. An unwelcome side effect of direct-to-consumer personal genome testing: raiding the medical commons. JAMA. 2008;300:2669–71. doi: 10.1001/jama.2008.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimension Research home page. http://www.dimensionresearch.com/resources/resources_overview.html.
- 22.Pollack A. Start-up may sell genetic tests in stores. New York Times. 2010 May 11;:B2. [Google Scholar]
- 23.Idem. F.D.A. faults companies on unapproved genetic tests. New York Times. 2010 Jun 11;:B2. [Google Scholar]
- 24.Ng PC, Murray SS, Levy S, Venter JC. An agenda for personalized medicine. Nature. 2009;461:724–6. doi: 10.1038/461724a. [DOI] [PubMed] [Google Scholar]
- 25.Healy M. As genetic testing races ahead, doctors are left behind. Los Angeles Times. 2009 Oct 24;:4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.