Abstract
Objectives
We compared the long-term outcomes of drug-eluting stents (DES) versus bare-metal stents (BMS) for treatment of bare-metal in-stent restenosis (ISR).
Background
There are no randomized trials or observational studies directly comparing the safety and efficacy of DES versus BMS for treatment of bare-metal ISR.
Methods
We examined data on all patients who underwent percutaneous coronary intervention (PCI) for ISR at Cleveland Clinic between 05/1999 and 06/2007. We compared the efficacy and safety of DES to BMS for treating bare-metal ISR. The primary end point was a composite of death, myocardial infarction (MI), or target lesion revascularization (TLR). The secondary endpoints were individual components of the primary endpoint.
Results
Of the 931 patients identified over 8 years, 706 had bare-metal ISR and met our study criteria. Of the 706 patients with bare-metal ISR, 362 were treated with DES and 344 with BMS. There were 230 cumulative events for a median follow-up of 3.2 years. After adjusting for 27 variables, DES were associated with lower primary endpoint compared to BMS for treatment of bare-metal ISR (21% versus 45%, adjusted hazard ratio [HR] 0.63; 95% confidence interval [CI], 0.42-0.95; p = 0.03). The individual secondary endpoint of death (8% versus 24%, p = 0.005) favored DES, but MI (3% versus 8%, p = 0.31), and TLR (13% versus 20%, p = 0.23) failed to reach statistical significance.
Conclusions
In our multivariate analysis of patients with bare-metal ISR, DES use was associated with significantly lower death, MI, or TLR when compared to BMS.
Keywords: in-stent restenosis, drug-eluting stents, bare-metal stents, vascular brachytherapy, revascularization
Introduction
In-stent restenosis (ISR) continues to be one of the most common adverse events after stenting, affecting 15-35% of lesions treated with bare-metal stents (BMS) (1-5). Bare-metal ISR is not a benign entity and has been associated with both poor survival and acute coronary syndromes (6-8). Currently, local vascular brachytherapy in conjunction with balloon angioplasty is the only U.S. Food and Drug Administration approved strategy to treat ISR (1,9-11). However, its use has been limited due to logistical and financial challenges, concerns of radiation exposure, evidence of edge restenosis, late “catch-up” restenosis phenomenon, and its association with late thrombosis (1,12,13). Other modalities such as atherectomy, cutting balloon angioplasty, and laser have not shown incremental advantage over balloon angioplasty for ISR (14-16). Treatment of bare-metal ISR with BMS improved both short- and long-term restenosis rates in vessels ≥3 mm when compared to balloon angioplasty alone (17,18). However, this strategy remains associated with a significant restenosis rate of 20% at 1 year and 25% at 4 years (17,18).
Drug eluting stents (DES) reduce the rate of restenosis by over 70% compared to BMS in native coronary lesions (19,20). Therefore, currently DES placement is believed to be the preferred percutaneous strategy for treating bare-metal ISR (1,21-24). However, to date no randomized controlled trials (RCT) have compared DES versus BMS for treating bare-metal ISR. Additionally, there are no observational studies that directly compare DES to BMS for treating bare-metal ISR.
Methods
Study population
We conducted a retrospective analysis on prospectively collected data from the percutaneous coronary intervention (PCI) registry at Cleveland Clinic, in patients who underwent PCI for ISR between 05/1999 through 06/2007. Baseline characteristics, angiographic data, and medications are collected at the time of PCI by trained research coordinators as part of this ongoing registry. The institutional review board waived requirements for informed consent for this institutional PCI registry.
Angiographic characteristics
We defined in-stent restenosis as any within stent or stent edge restenosis as previously established by Mehran and colleagues (25). Procedural and pharamacotherapy characteristics are captured prospectively. Similarly, information regarding balloon pre-dilation, stent size, stent length, maximum balloon dilatation for stent deployment, number of stents per case, residual stenosis, and other important angiographic features were also captured prospectively. Once DES were commercially available in 2003, the choice of stent type (DES versus BMS) was at the discretion of the operator performing the procedure.
Clinical End-points
The primary end point was a composite of all-cause mortality, myocardial infarction (MI), and target lesion revascularization (TLR). The secondary endpoints were individual components of the primary endpoint. Myocardial infarction was defined as occurrence of troponin elevation with electrocardiographic changes or angina. Peri-procedural MI was defined as peri-procedural rise in creatine kinase-MB ≥ 3 times the upper limit of normal (8.8 ng/ml) or MI requiring hospitalization. Patients were prospectively followed through review of hospital records and the Social Security Death Index. In general, data regarding revascularization, MI, and death are obtained prospectively. However, for the purposes of this analysis retrospective chart review was also performed in order to confirm all endpoints and to determine whether revascularization was target vessel or target lesion.
Statistical analysis
Baseline and angiographic characteristics of patients were compared using the Wilcoxon Rank sum test for continuous variables and the chi-square test for categorical variables. Unadjusted differences in outcome were tested using Kaplan-Meier curves. Subsequently, multivariable adjusted Cox proportional hazards analyses that accounted for baseline demographic features, angiographic variables, treatment assignment and other confounders (Table 1) were performed. In order to account for advances in PCI over time all multivariable models were adjusted for the procedural date. In total we adjusted for over 23 variables. A p-value of 0.05 was used as a cut-off for statistical significance. Analyses were performed with SAS, version 9.1 (SAS Institute, Cary, North Carolina).
Table 1.
Baseline and procedural characteristics based on stent used for treating bare-metal in-stent restenosis.
| Characteristics | Drug-eluting Stent (n = 362) | Bare-metal Stent (n = 344) | p-value |
|---|---|---|---|
| Age, years | 64±11 | 63±12 | 0.21 |
| Male, % | 271 (75) | 229 (67) | 0.02 |
| Body mass index | 30±5 | 30±6 | 0.35 |
| Heart rate | 70±14 | 71±13 | 0.25 |
| Left ventricular ejection fraction | 51±10 | 51±11 | 0.67 |
| Risk factors, % | |||
| Family history of premature coronary disease | 110 (30) | 138 (40) | 0.007 |
| Cigarette smoking | 38 (11) | 42 (12) | 0.47 |
| Diabetes mellitus | 144 (40) | 160 (47) | 0.07 |
| Insulin dependent diabetes mellitus | 47 (13) | 67 (20) | 0.02 |
| Non-insulin dependent diabetes mellitus | 95 (26) | 88 (26) | 0.84 |
| Medical history, % | |||
| Prior myocardial infarction | 197 (54) | 191 (56) | 0.77 |
| Peripheral arterial disease | 53 (15) | 43 (13) | 0.41 |
| Prior coronary artery bypass surgery | 159 (44) | 127 (37) | 0.06 |
| Stroke or transient ischemic attack | 44 (12) | 38 (11) | 0.65 |
| Chronic obstructive pulmonary disease | 45 (12) | 35 (10) | 0.34 |
| Clinical presentation, % | |||
| Acute myocardial infarction | 8 (2) | 11 (3) | 0.42 |
| Unstable angina | 170 (47) | 210 (61) | 0.0002 |
| New York Heart Association class, % | |||
| 3 | 46 (13) | 11 (3) | <0.0001 |
| 4 | 31 (9) | 5 (1) | <0.0001 |
| Medications, % | |||
| Aspirin | 362 (100) | 344 (100) | 1.00 |
| Clopidogrel | 362 (100) | 344 (100) | 1.00 |
| Heparin | 111 (31) | 192 (56) | <0.0001 |
| Glycoprotein IIb/IIIa inhibitors | 107 (30) | 258 (75) | <0.0001 |
| Beta-blockers | 129 (36) | 114 (33) | 0.49 |
| Angiotensin converting enzyme inhibitors | 182 (50) | 134 (39) | 0.003 |
| Statins | 298 (82) | 183 (53) | <0.0001 |
| Location of culprit lesion, % | |||
| Proximal left anterior descending artery | 51 (14) | 67 (19) | 0.06 |
| Mid or distal left anterior descending artery | 160 (44) | 167 (49) | 0.25 |
| Left circumflex artery | 158 (44) | 141 (41) | 0.48 |
| Right coronary artery | 153 (42) | 177 (51) | 0.01 |
| Angiographic characteristics | |||
| Reference vessel diameter, mm | 3.0±0.4 | 2.9±0.5 | <0.0001 |
| Stent length, mm | 37.7±21.8 | 20.2±15.8 | <0.0001 |
| Chronic total occlusion, % | 30 (8) | 12 (3) | 0.007 |
| Saphenous vein graft, % | 59 (16) | 48 (14) | 0.39 |
| Multivessel intervention | 104 (29) | 135 (39) | 0.003 |
| Number of diseased vessels, % | |||
| 1 | 225 (62) | 147 (43) | <0.0001 |
| 2 | 96 (27) | 122 (35) | 0.01 |
| 3 | 41 (11) | 75 (22) | 0.0002 |
| American College of Cardiology lesion score, % | |||
| A | 13 (4) | 24 (7) | 0.04 |
| B1 | 55 (15) | 83 (24) | 0.003 |
| B2 | 115 (32) | 112 (33) | 0.82 |
| C | 179 (49) | 125 (36) | 0.0004 |
| Procedural success, % | 356 (98) | 334 (97) | 0.27 |
Selected sub-groups that have been previously reported to gain the most benefit from DES, including diabetes, vessel size, and lesion length, were chosen for additional analysis (26,27).
Results
Baseline characteristics
A total of 706 patients with bare-metal ISR met our study criteria during the 8 year study period. Of these 362 were treated with DES and 344 were treated with BMS. Baseline and target lesion characteristics, according to stent type, are presented in Table 1. In general, patient and procedural characteristics were similar between both groups. However, patients who received DES were more likely to be male, have diabetes, have a worse New York Heart Association class, have greater use of angiotensin-converting enzyme inhibitors or statin therapy, have more complex lesions or chronic total occlusions, and have greater total stent length (Table 1). Patients who were treated with BMS were more likely to present with unstable angina or multi-vessel disease and have greater use of heparin and glycoprotein IIb/IIIa inhibitors (Table 1). For the 2 groups (DES or BMS) the mean balloon pressure was 14mm Hg. Additionally, the rate of post dilatation using a non-compliant balloon in the DES group was 58% and in the BMS group was 55% with a p-value of 0.34.
Clinical outcomes
DES versus BMS for treating bare-metal ISR
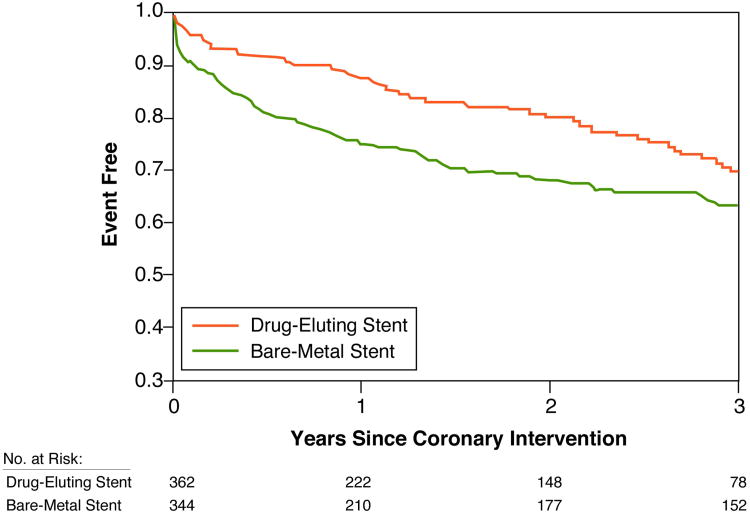
Among 706 patients treated for bare-metal ISR, 230 cumulative events (death, MI or TLR) occurred during a median follow-up of 3.2 years (IQR: 1.6-4.9 years) (Figure 1). Treatment of bare-metal ISR with DES was associated with a lower composite endpoint compared with those who were treated with BMS (adjusted HR, 0.63; 95% CI, 0.42-0.95; p = 0.03) (Table 2). Analysis of secondary endpoints revealed that all-cause mortality was lower with DES than BMS (8% versus 24%, adjusted HR, 0.37; 95 percent CI, 0.18-0.74; p = 0.005) (Table 2). Similarly the rates of MI (3% versus 8%, adjusted HR 0.54; 95% CI, 0.16-1.78; p = 0.31) and TLR (13% versus 20%, adjusted HR 0.67; 95% CI, 0.35-1.29; p = 0.23) trended towards favoring DES, but failed to reach statistical significance (Table 2).
Figure.
Kaplan Meier curves of bare-metal ISR treated with DES versus BMS (n = 706). The composite endpoint of death, myocardial infarction, or target lesion revascularization is represented as a function of time and favors DES. ISR = in-stent restenosis; DES = drug-eluting stents; BMS = bare-metal stents.
Table 2.
Unadjusted and multivariable adjusted hazard ratios for the primary and secondary endpoints by treatment strategy for bare-metal in-stent restenosis (drug-eluting stents versus bare-metal stents).
| Drug-eluting stent Events (%) | Bare-metal stent Events (%) | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Total Population (n=706) | n=362 | n=344 | ||
|
| ||||
| Composite of death, MI, or TLR (n=230) | 76 (21) | 154 (45) | - | - |
| Unadjusted | - | - | 0.69 (0.52-0.92) | 0.01 |
| Adjusted | - | - | 0.63 (0.42-0.95) | 0.03 |
|
| ||||
| All cause mortality (n=112) | 29 (8) | 83 (24) | - | - |
| Unadjusted | - | - | 0.48 (0.31-0.75) | 0.001 |
| Adjusted | - | - | 0.37 (0.18-0.74) | 0.005 |
|
| ||||
| Myocardial infarction (n=36) | 10 (3) | 26 (8) | - | - |
| Unadjusted | - | - | 0.49 (0.23-1.03) | 0.06 |
| Adjusted | - | - | 0.54 (0.16-1.78) | 0.31 |
|
| ||||
| Target lesion revascularization (n=116) | 47 (13) | 69 (20) | - | - |
| Unadjusted | - | - | 0.72 (0.49-1.05) | 0.09 |
| Adjusted | - | - | 0.67 (0.35-1.29) | 0.23 |
CI = Confidence interval; MI = Myocardial infarction; TLR = Target lesion revascularization.
Sub-group analysis
When we limited our analysis to the post-2003 era (after DES became commercially available), DES treatment still remained associated with a lower primary endpoint compared to BMS for bare-metal ISR (Table 3). Patients without diabetes and those with vessel size less than 3.5 mm had better outcomes with DES compared to BMS (Table 3). However, DES use did not demonstrate benefit in patients with diabetes or vessels greater than 3.5 mm (Table 3). Lesion length also did not influence outcomes based on type of stent used (Table 3).
Table 3.
Multivariable adjusted hazard ratios for composite of death, myocardial infarction, or target lesion revascularization in selected sub-groups.
| Drug-eluting stent Events (%) | Bare-metal stent Events (%) | Hazard Ratio (95% CI) | p-value | |
|---|---|---|---|---|
| PCI after 2003 (n=421) | 76 (21) | 29 (49) | 0.59 (0.35-1.00) | 0.05 |
|
| ||||
| Diabetes Mellitus | - | - | - | - |
| Present (n = 304) | 35 (24) | 78 (49) | 0.93 (0.51-1.71) | 0.83 |
| Absent (n = 402) | 41 (19) | 76 (41) | 0.52 (0.29-0.93) | 0.03 |
|
| ||||
| Vessel diameter | - | - | - | - |
| ≥ 3.5 mm (n = 112) | 11 (17) | 23 (48) | 0.77 (0.17-3.54) | 0.73 |
| < 3.5 mm (n = 594) | 65 (22) | 131 (44) | 0.63 (0.40-0.97) | 0.04 |
|
| ||||
| Lesion length | - | - | - | - |
| ≥15 mm (n = 336) | 44 (23) | 68 (47) | 0.67 (0.35-1.28) | 0.22 |
| < 15 mm (n = 370) | 32 (19) | 86 (43) | 0.66 (0.37-1.19) | 0.17 |
CI = Confidence interval; PCI = Percutaneous coronary intervention.
Discussion
Numerous trials have evaluated the safety and efficacy of DES, however there are no published RCT or observational studies directly comparing DES to BMS for treating bare-metal ISR. In our single center cohort of 931 consecutive patients who presented with ISR, over a period of 8 years, we methodically addressed this issue by a conducting multivariable analysis.
We examined the long term outcomes of patients who were treated with DES or BMS for bare-metal ISR. Our analysis demonstrates that that for treatment of bare-metal ISR, DES use was associated with a lower composite endpoint of all-cause mortality, MI, or TLR, when compared to BMS at over 3 years of follow-up. To our knowledge, this is the first and largest study to directly compare the safety and efficacy of DES versus BMS in patients undergoing PCI for bare-metal ISR. Our results also indicated a decrease in mortality with DES. While we used multiple adjustments the possibility of selection bias and confounding cannot be excluded. Importantly, however, DES was not associated with increased MI rate and led to a lower incidence of revascularization.
In the only RCT comparing BMS to balloon angioplasty for treatment of ISR, both the restenosis rate (27% vs. 49%, p = 0.007) and event-free survival (84% vs. 62%, p = 0.002) were better after BMS, in patients with vessels ≥3 mm (17). But this benefit was lost in the broader study population (17). Similarly, in an earlier observational study, Mehran and colleagues, showed that BMS did not reduce TLR or death at 1 year compared to balloon angioplasty even though the in-hospital death, Q-wave MI, or TLR were higher with balloon angioplasty than with BMS (5.6% versus 0.7%, p = 0.02) (28). More recently Alfonso and colleagues compared sirolimus-eluting stent (SES) to BMS in ISR by using the individual stent arms of 2 separate RCT (BMS versus balloon angioplasty and SES versus balloon angioplasty) (29). In this analysis, both angiographic late loss (0.13 vs. 1.04 mm, p < 0.001) and repeat revascularization at 1 year (10.5 vs. 19.6%, p < 0.05) favored SES over BMS (29). This advantage of SES over BMS was preserved even in large vessel ISR, an area where BMS has shown a signal for benefit compared to balloon angioplasty alone (17,29). This is in contrast to our sub-group analysis in which smaller vessels (<3.5 mm) benefit from DES but this advantage was lost in larger vessels (≥ 3.5 mm).
Mechanistic and intra-vascular ultrasound studies have shown that the poor performance of BMS treated bare-metal ISR lesions most likely stems from the enhanced stimulation of neointimal hyperplasia by BMS in an already restenotic lesion (25,27,30). The even more malignant neointimal proliferation seen in diabetes is the likely explanation why neither DES nor BMS benefited this population in our study.
Taken in totality, both the previous indirect analysis by Alfonso et al and our direct comparison with long term follow-up, support the superiority of DES over BMS in treating bare-metal ISR. However for patients needing urgent non-cardiac surgery or those who cannot tolerate prolonged dual antiplatelet therapy, BMS still may be a reasonable alternative to DES particularly in larger vessels.
Our study has several limitations. It is an observational study; therefore, unobserved biases may have played a role in our findings. Future RCT should better address such biases. Some differences were noted amongst the 2 groups and these may have played a role in the benefit seen with DES. However, multiple adjustments for over 23 variables were performed that should have accounted for these baseline differences. Our study population extends to a time when DES were not available, but subgroup analysis revealed that similar results were obtained regardless of the study period examined. One possible mechanism of benefit favoring DES treated patients is a prolonged course of dual antiplatelet therapy in this group. Although data regarding long-term dual antiplatelet therapy is not available, at our institution most operators prescribe 6 weeks of dual antiplatelet therapy for BMS and at least 2 years of dual antiplatelet therapy for DES.
Conclusion
Our study sheds new light on the outcomes of bare-metal ISR treatment with DES versus BMS. In this large cohort of patients with long term follow-up, we demonstrate that there is a considerable and durable advantage of DES over BMS in the treatment of bare-metal ISR. Given the cost, potential for stent thrombosis, and the need for dual antiplatelet therapy, RCT should directly compare DES versus BMS for treating bare-metal ISR.
Acknowledgments
Dr. Shishehbor is supported in part by the National Institutes of Health, National Institute of Child Health and Human Development, Multidisciplinary Clinical Research Career Development Programs Grant K12 HD049091 and the National Institutes of Health Loan Repayment Program.
Dr. Ellis discloses the following relationships: Research Grants - Boston Scientific; Consultant - Boston Scientific, Abbott Vascular, Cordis.
Footnotes
Disclosures: Drs. Singh, Filby, Gorodeski, Lincoff and Mr. El Sakr, have no conflicts of interest or disclosures to report.
References
- 1.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O'Neill WW, Schaff HV, et al. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines. J Am Coll Cardiol. 2008;51(2):172–209. doi: 10.1016/j.jacc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331(8):496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 3.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331(8):489–95. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 4.Doyle B, Rihal CS, O'Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, Bresnahan J, Holmes DR., Jr Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007;116(21):2391–8. doi: 10.1161/CIRCULATIONAHA.107.707331. [DOI] [PubMed] [Google Scholar]
- 5.Singh IM, Filby SJ, Sakr FE, Gorodeski EZ, Lincoff AM, Ellis SG, Shishehbor MH. Clinical outcomes of drug-eluting versus bare-metal in-stent restenosis. Catheter Cardiovasc Interv. 2009 doi: 10.1002/ccd.22278. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006;151(6):1260–4. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Schuhlen H, Kastrati A, Mehilli J, Hausleiter J, Pache J, Dirschinger J, Schomig A. Restenosis detected by routine angiographic follow-up and late mortality after coronary stent placement. Am Heart J. 2004;147(2):317–22. doi: 10.1016/j.ahj.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lee MS, Pessegueiro A, Zimmer R, Jurewitz D, Tobis J. Clinical presentation of patients with in-stent restenosis in the drug-eluting stent era. J Invasive Cardiol. 2008;20(8):401–3. [PubMed] [Google Scholar]
- 9.Teirstein PS, Massullo V, Jani S, Popma JJ, Mintz GS, Russo RJ, Schatz RA, Guarneri EM, Steuterman S, Morris NB, et al. Catheter-based radiotherapy to inhibit restenosis after coronary stenting. N Engl J Med. 1997;336(24):1697–703. doi: 10.1056/NEJM199706123362402. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Teirstein PS, Moses JW, Tripuraneni P, Lansky AJ, Jani S, Wong SC, Fish D, Ellis S, Holmes DR, et al. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344(4):250–6. doi: 10.1056/NEJM200101253440402. [DOI] [PubMed] [Google Scholar]
- 11.Waksman R, Raizner AE, Yeung AC, Lansky AJ, Vandertie L. Use of localised intracoronary beta radiation in treatment of in-stent restenosis: the INHIBIT randomised controlled trial. Lancet. 2002;359(9306):551–7. doi: 10.1016/s0140-6736(02)07741-3. [DOI] [PubMed] [Google Scholar]
- 12.Baierl V, Baumgartner S, Pollinger B, Leibig M, Rieber J, Konig A, Krotz F, Sohn HY, Siebert U, Haimerl W, et al. Three-year clinical follow-up after strontium-90/yttrium-90 beta-irradiation for the treatment of in-stent coronary restenosis. Am J Cardiol. 2005;96(10):1399–403. doi: 10.1016/j.amjcard.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 13.Costa MA, Sabate M, van der Giessen WJ, Kay IP, Cervinka P, Ligthart JM, Serrano P, Coen VL, Levendag PC, Serruys PW. Late coronary occlusion after intracoronary brachytherapy. Circulation. 1999;100(8):789–92. doi: 10.1161/01.cir.100.8.789. [DOI] [PubMed] [Google Scholar]
- 14.vom Dahl J, Dietz U, Haager PK, Silber S, Niccoli L, Buettner HJ, Schiele F, Thomas M, Commeau P, Ramsdale DR, et al. Rotational atherectomy does not reduce recurrent in-stent restenosis: results of the angioplasty versus rotational atherectomy for treatment of diffuse in-stent restenosis trial (ARTIST) Circulation. 2002;105(5):583–8. doi: 10.1161/hc0502.103347. [DOI] [PubMed] [Google Scholar]
- 15.Albiero R, Silber S, Di Mario C, Cernigliaro C, Battaglia S, Reimers B, Frasheri A, Klauss V, Auge JM, Rubartelli P, et al. Cutting balloon versus conventional balloon angioplasty for the treatment of in-stent restenosis: results of the restenosis cutting balloon evaluation trial (RESCUT) J Am Coll Cardiol. 2004;43(6):943–9. doi: 10.1016/j.jacc.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 16.Giri S, Ito S, Lansky AJ, Mehran R, Margolis J, Gilmore P, Garratt KN, Cummins F, Moses J, Rentrop P, et al. Clinical and angiographic outcome in the laser angioplasty for restenotic stents (LARS) multicenter registry. Catheter Cardiovasc Interv. 2001;52(1):24–34. doi: 10.1002/1522-726x(200101)52:1<24::aid-ccd1007>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Alfonso F, Zueco J, Cequier A, Mantilla R, Bethencourt A, Lopez-Minguez JR, Angel J, Auge JM, Gomez-Recio M, Moris C, et al. A randomized comparison of repeat stenting with balloon angioplasty in patients with in-stent restenosis. J Am Coll Cardiol. 2003;42(5):796–805. doi: 10.1016/s0735-1097(03)00852-0. [DOI] [PubMed] [Google Scholar]
- 18.Alfonso F, Auge JM, Zueco J, Bethencourt A, Lopez-Minguez JR, Hernandez JM, Bullones JA, Calvo I, Esplugas E, Perez-Vizcayno MJ, et al. Long-term results (three to five years) of the Restenosis Intrastent: Balloon angioplasty versus elective Stenting (RIBS) randomized study. J Am Coll Cardiol. 2005;46(5):756–60. doi: 10.1016/j.jacc.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 19.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 20.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 21.Holmes DR, Jr, Teirstein P, Satler L, Sketch M, O'Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E, et al. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295(11):1264–73. doi: 10.1001/jama.295.11.1264. [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Ellis SG, O'Shaughnessy CD, Martin SL, Satler L, McGarry T, Turco MA, Kereiakes DJ, Kelley L, Popma JJ, et al. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295(11):1253–63. doi: 10.1001/jama.295.11.1253. [DOI] [PubMed] [Google Scholar]
- 23.Ellis SG, O'Shaughnessy CD, Martin SL, Kent K, McGarry T, Turco MA, Kereiakes DJ, Popma JJ, Friedman M, Koglin J, et al. Two-year clinical outcomes after paclitaxel-eluting stent or brachytherapy treatment for bare metal stent restenosis: the TAXUS V ISR trial. Eur Heart J. 2008;29(13):1625–34. doi: 10.1093/eurheartj/ehn231. [DOI] [PubMed] [Google Scholar]
- 24.Dibra A, Kastrati A, Alfonso F, Seyfarth M, Perez-Vizcayno MJ, Mehilli J, Schomig A. Effectiveness of drug-eluting stents in patients with bare-metal in-stent restenosis: meta-analysis of randomized trials. J Am Coll Cardiol. 2007;49(5):616–23. doi: 10.1016/j.jacc.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Mehran R, Dangas G, Abizaid AS, Mintz GS, Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW, Leon MB. Angiographic patterns of in-stent restenosis: classification and implications for long-term outcome. Circulation. 1999;100(18):1872–8. doi: 10.1161/01.cir.100.18.1872. [DOI] [PubMed] [Google Scholar]
- 26.Alfonso F, Perez-Vizcayno MJ, Hernandez R, Bethencourt A, Marti V, Lopez-Minguez JR, Angel J, Iniguez A, Moris C, Cequier A, et al. Long-term clinical benefit of sirolimus-eluting stents in patients with in-stent restenosis results of the RIBS-II (Restenosis Intra-stent: Balloon angioplasty vs. elective sirolimus-eluting Stenting) study. J Am Coll Cardiol. 2008;52(20):1621–7. doi: 10.1016/j.jacc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Singh M, Gersh BJ, McClelland RL, Ho KK, Willerson JT, Penny WF, Holmes DR., Jr Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: insights from the Prevention of Restenosis With Tranilast and Its Outcomes (PRESTO) trial. Circulation. 2004;109(22):2727–31. doi: 10.1161/01.CIR.0000131898.18849.65. [DOI] [PubMed] [Google Scholar]
- 28.Mehran R, Dangas G, Abizaid A, Lansky AJ, Mintz GS, Pichard AD, Satler LF, Kent KM, Waksman R, Stone GW, et al. Treatment of focal in-stent restenosis with balloon angioplasty alone versus stenting: Short- and long-term results. Am Heart J. 2001;141(4):610–4. doi: 10.1067/mhj.2001.113998. [DOI] [PubMed] [Google Scholar]
- 29.Alfonso F, Perez-Vizcayno MJ, Hernandez R, Fernandez C, Escaned J, Banuelos C, Bethencourt A, Lopez-Minguez JR, Angel J, Cequier A, et al. Sirolimus-eluting stents versus bare-metal stents in patients with in-stent restenosis: results of a pooled analysis of two randomized studies. Catheter Cardiovasc Interv. 2008;72(4):459–67. doi: 10.1002/ccd.21694. [DOI] [PubMed] [Google Scholar]
- 30.Kim SW, Mintz GS, Escolar E, Ohlmann P, Pregowski J, Tyczynski P, Hassani SE, Pichard AD, Satler LF, Kent KM, et al. An intravascular ultrasound analysis of the mechanisms of restenosis comparing drug-eluting stents with brachytherapy. Am J Cardiol. 2006;97(9):1292–8. doi: 10.1016/j.amjcard.2005.11.055. [DOI] [PubMed] [Google Scholar]