Abstract
Background
Data on the cost-effectiveness of the behavioral treatment of obesity are not conclusive. The cost-effectiveness of treatment in primary care settings is particularly relevant.
Methods
We conducted a within-trial cost-effectiveness analysis of a primary care-based obesity intervention. Study participants were randomized to: Usual Care (quarterly visits with their primary care provider); Brief Lifestyle Counseling (Brief LC; quarterly provider visits plus monthly weight loss counseling visits; or Enhanced Brief Lifestyle Counseling (Enhanced Brief LC; all above interventions, plus choice of meal replacements or weight loss medication). A health care payer perspective was used. Intervention costs were estimated from tracking data obtained prospectively. Quality adjusted life years (QALYs) were estimated with the EuroQol-5D. We estimated cost per kilogram-year of weight loss and cost per QALY.
Results
Weight losses after 2 years were 1.7, 2.9, and 4.6 kg for Usual Care, Brief LC, and Enhanced Brief LC, respectively (p = 0.003 for comparison of Enhanced Brief LC vs. Usual Care). The incremental cost per kilogram-year lost was $292 for Enhanced Brief LC compared to Usual Care (95% CI $38 to $394). The incremental cost per QALY was $115,397, but the 95% CI were undefined. Comparison of short term cost per kg with published estimates of longer term cost per QALYs suggested that the intervention could be cost-effective over the long term (≥ 10 years).
Conclusions
A primary care intervention that included monthly counseling visits and a choice of meal replacements or weight loss medication could be a cost-effective treatment for obesity over the long term. However, additional studies are needed on the cost-effectiveness of behavioral treatment of obesity.
Keywords: Obesity, Reducing Diet, Primary Care, Costs, Cost-Effectiveness
Introduction
Obesity accounts for over nine percent of health care expenditures in the United States1 and is one of the leading causes of disability.2 There is an increased focus on treating obesity in primary care settings,3–5 which is highlighted by the recent decision from the Centers for Medicare and Medicaid Services to reimburse providers for intensive behavioral weight loss counseling conducted in primary care settings.6
A sizeable body of evidence supports the clinical efficacy of obesity treatment,7–9 but the cost-effectiveness of non-surgical treatment remains a partially open question. For example, two groups of investigators conducted economic analyses of the Diabetes Prevention Program and arrived at different conclusions about the cost-effectiveness of the intervention.10–12 Questions regarding the cost-effectiveness of treatment are particularly relevant to primary care settings, given the potentially higher costs of conducting treatment in health care environments, in contrast to programs who use lay personnel to deliver weight loss interventions.
We conducted an economic analysis of a clinical trial of obesity treatment that was implemented in six primary care practices. The study showed that quarterly primary care provider (PCP) visits in conjunction with brief monthly counseling visits provided by a medical assistant, combined with either meal replacements or weight loss medication, produced greater weight loss than did quarterly PCP visits alone.13
Methods
Participants
The clinical trial Practice-based Opportunities for Weight Reduction (POWER) at the University of Pennsylvania (POWER-UP) was a randomized comparison of three interventions, conducted in six primary care practices owned by the University of Pennsylvania Health System.13 Study participants (n = 390) had a body mass index of 30–50 kg/m2, weight of ≤ 400 pounds, plus abdominal obesity (elevated waist circumference), and at least one of the four other criteria for metabolic syndrome – either impaired fasting glucose/diabetes, elevated blood pressure/hypertension, low HDL cholesterol, or elevated triglycerides.14 Participants also had no serious or life threatening medical conditions. The methods for POWER-UP13 and two related trials15,16 have been described in detail previously.17
In POWER-UP, participants assigned to Usual Care (n = 130) received weight loss advice during quarterly visits with their primary care provider (PCP). Participants assigned to Brief Lifestyle Counseling (Brief LC; n = 131) received quarterly visits with their PCP, as well as brief monthly counseling visits with a weight loss coach, who was typically a medical assistant from the practice. Participants assigned to Enhanced Brief Lifestyle Counseling (Enhanced Brief LC; n = 129) received quarterly PCP visits, monthly coaching visits, as well as a choice of “enhanced” therapy – either meal replacements (Slim-Fast) or weight loss medication (orlistat or sibutramine). (Sibutramine was removed from the U.S. market in October 2010, towards the end of POWER-UP.)
Design of Cost-Effectiveness Analysis
We conducted an economic analysis to assess the cost-effectiveness of all three interventions (Usual Care, Brief LC, and Enhanced Brief LC). All resource use and other outcome data (e.g., weight data and quality-adjusted life years [QALYs]) for the economic analysis were collected prospectively. All 390 individuals from the clinical trial contributed data to the cost-effectiveness analysis.
Time Horizon
The time horizon for the study was the 2 years that each participant was enrolled in the trial (i.e., within-trial cost-effectiveness). All costs, kilogram-years, and QALYs during the second year were discounted at 3 percent.
Perspective
We took both a narrow health care payer perspective, which included all costs attributable to the intervention, as well a broader health care payer perspective that added the costs of concomitant medications and estimates of participants’ other health care use. The broader payer perspective was included to assess whether costs incurred for the intervention would be offset by reductions in other health care utilization.
Outcome Measures and Data Sources
Intervention Costs
We estimated the costs of the various components of the intervention including weight loss visit costs (primary care physicians plus coaches), weight loss medications, weight loss meal replacements, primary care physician training and supervision, coach training and supervision, and other intervention costs (e.g., pedometers, scales).
Concomitant Medication and Other Health Care Costs
Concomitant medication use was assessed by reviewing medication lists with patients. Concomitant medication use also was confirmed through review of medical records. Other health care use was assessed with a questionnaire that asked about visits to physicians, visits to other health care providers, emergency room visits and hospitalizations. We assigned costs to medications by use of the “Big 4” Federal Supply Schedule ─ the pharmaceutical price list used by the Coast Guard, the Department of Defense, the Public Health Service, and the Veterans Administration.18 Costs of other health care use were assigned using cost schedules from publicly available sources, following the methods used in the economic evaluation of the Diabetes Prevention Program.19 All costs were updated to 2011, the year in which the trial concluded.20
Weight Loss
Certified staff members used a digital scale [Tanita BWB-800] to measure weight at baseline and at 6, 12, 18, and 24 months.13
Quality Adjusted Life Years (QALYs)
We used the EuroQoL (EQ5D) 3-level health state classification system and scoring rule to assess quality-adjusted life years (QALYs).21 One QALY represents one year of life lived in perfect health, while zero represents death.
Cost Per Kilogram-Year and Cost Per QALY
We estimated three ratios comparing the incremental cost of the weight loss intervention per incremental kilogram-year of weight loss during the two years of the trial, one ratio each for the three pairwise comparisons (Brief LC versus Usual Care, Enhanced Brief LC versus Usual Care, and Enhanced Brief LC versus. Brief LC). The cost per kilogram-year is defined the cost of losing 1 kg of weight for one year. Incremental kilogram-years were calculated as the difference in the area under the kilogram loss curves. One reason our primary ratios report on changes in kilograms instead of changes in QALYs is that the primary benefits of weight loss are related to avoided long-term disability and death and are unlikely to be observable in a 2-year study. The primary reason for using kilogram-years of weight loss (similar to pack years in smoking) rather than kilograms or pounds lost as the measure of change in weight is to standardize the results across studies with different lengths of follow-up.22,23 Had we not standardized, our ratio would reflect 2 years of costs divided by the mean weight reduction during the 2 years. The resulting ratio would not be comparable with ratios from single year studies that reflect 1 year of costs and possibly the same number of kilograms lost in year 1. In addition, we calculated a second set of ratios that expanded the numerator to include concomitant medication and other health care costs. We also calculated 2 sets of ratios with these same numerators but with incremental QALYs as the denominator.
Analysis
Cost Data
We separately performed multivariable analysis on five different types of costs: weight loss visits; weight loss medications; meal replacements; concomitant medications; and other healthcare costs. (We allocated mean primary care provider training costs, lifestyle coach training costs, and other intervention costs without use of multivariable analysis.) With the exception of weight loss visit costs (for which everyone had a non-zero cost), we estimated costs by use of two-part multivariable models (i.e., first part logit models to estimate the probability that the participant had any costs; second part generalized linear models (GLM) to estimate non-zero costs). Selection of link functions and families for the GLM models was guided by the fit of the data.24
Weight Loss
We followed the analysis of weight loss performed for the clinical trial and used a linear mixed effects model.
QALY Scores
Between 10% and 40% of QALY scores were missing at the different follow-up time points. We addressed missing data by use of inverse probability weighting in the logistic regression first part models and the GLM second part models that we developed to predict these scores.25,26 Weights were defined as the reciprocal of the estimated probability of being observed (i.e., generated from logistic regression with missingness status as the dependent variable). QALYs were calculated as the area under the discounted QALY score curve.
Sampling Uncertainty
Standard errors were estimated by use of a non-parametric bootstrap within the multivariable framework. We combined data on point estimates and standard errors to calculate p-values for the point estimates, 95% confidence intervals for the cost-effectiveness ratios, and the fraction of the distribution of the cost-effectiveness ratios that was acceptable at thresholds of $100 and $200 per kilogram-year and at thresholds of $50,000 and $100,000 per QALY.24 We also plotted the distribution of the cost-effectiveness ratio on the cost-effectiveness plane and illustrated the point estimate and 95% confidence intervals for the comparisons of Brief LC vs. Usual Care and Enhanced Brief LC vs. Usual Care.
Long-term Cost per QALY or per Life Year Gained
We did not directly project costs, weight losses, or QALYs beyond the 2 years of follow up. However, we attempted to put our within trial cost-effectiveness ratios into perspective by comparing our weight loss intervention cost per kilogram-year of weight loss ratios with those of published studies that reported both a within-trial weight loss intervention cost per kilogram (or kilogram-year) lost ratio (or the data from which such a ratio can be calculated) and a long-term cost per year of life saved or per QALY ratio.
Results
Characteristics of Study Participants
Baseline characteristics of study participants and weight losses are shown in Table 1. Participants were mostly female, and the majority was non-Hispanic white. They took an average of 3.3 medications and had an average of 2.4 self-reported medical conditions. At 1 year, participants assigned to Enhanced Brief LC lost significantly more weight than those in either of the other two groups. After two years, those in Enhanced Brief LC lost significantly more weight than those assigned to Usual Care, but not significantly more than those assigned to Brief LC.
Table 1.
Baseline demographic and clinical characteristics of study participants.*
| Usual Care (n = 130) |
Brief Lifestyle Counseling (n = 131) |
Enhanced Brief Lifestyle Counseling (n = 129) |
|
|---|---|---|---|
| Age | 51.7 ± 12.1 | 52.0 ± 12.2 | 51.0 ± 10.1 |
| Gender – number (%) | |||
| Female | 98 (75.4) | 110 (84.0) | 103 (79.8) |
| Male | 32 (24.6) | 21 (16.0) | 26 (20.2) |
| Race – number (%) | |||
| White | 81 (62.3) | 75 (57.3) | 74 (57.4) |
| Black | 46 (35.4) | 52 (39.7) | 52 (40.3) |
| Other | 3 (2.3) | 4 (3.1) | 3 (3.4) |
| Hispanic ethnicity – number (%) | |||
| Yes | 6 (4.6) | 6 (4.6) | 6 (4.7) |
| No | 124 (95.4) | 125 (95.4) | 123 (95.3) |
| Weight (kg) | 111.2 ± 20.0 | 106.3 ± 17.3 | 105.4 ± 17.2 |
| BMI | 39.0 ± 4.8 | 38.5 ± 4.6 | 37.8 ± 4.7 |
| Number of medications | 3.5 ± 2.7 | 3.3 ± 2.8 | 3.3 ± 2.7 |
| Number of co-morbid medical conditions | 2.4 ± 1.0 | 2.5 ± 1.2 | 2.2 ± 1.1 |
| Baseline EQ-5D* | 0.72 ± 0.30 | 0.78 ± 0.22 | 0.77 ± 0.26 |
Adapted from Wadden et al13 Values are mean ± SD, except for weight losses, which are mean ± SE. There were no significant differences for any baseline characteristics. The EQ-5D is a measure of quality-adjusted life years (QALYs). For weight loss, values with different superscripts are significantly different from each other.
Costs, Kilogram-Years, and QALYs
Costs, kilogram-years of weight loss, and QALYs are shown in Table 2. Intervention costs were highest for Enhanced Brief LC ($3092), followed by Brief LC ($1323), and by Usual Care ($837) (with all p values < 0.05). Usual Care had significantly higher concomitant medication costs than did Enhanced Brief LC, with Brief LC not significantly different from the other two groups. There were no significant differences between the three groups’ other health care costs. Mirroring the patterns for intervention cost, kilogram-years of weight loss were highest for Enhanced Brief LC followed by Brief LC and then by Usual Care (all differences statistically significant). There were no significant differences between the 3 groups’ QALYs.
Table 2.
Mean costs and quality-adjusted life years by intervention group.*
| Usual Care | Brief Lifestyle Counseling |
Enhanced Brief Lifestyle Counseling |
|
|---|---|---|---|
| Category | Mean (SE) | Mean (SE) | Mean (SE) |
| Weight losses (kg) | |||
| Year 1 | 2.3 ± 0.6a | 3.4 ± 0.6a | 7.1 ± 0.6b |
| Year 2 | 1.7 ± 0.7a | 2.9 ± 0.7a,b | 4.6 ± 0.7b,c |
| Weight loss intervention costs ($) | |||
| Weight loss visits | 274 (9)a | 581 (23)b | 656 (22)c |
| Weight loss medications | 0 (0)a | 0 (0)a | 708 (79)b |
| Meal replacements | 0 (0)a | 0 (0)a | 958 (91)b |
| Training/supervision: PCPs** | 443 -- | 443 -- | 443 -- |
| Training/supervision: Coaches** | 83 -- | 124 -- | 124 -- |
| Other intervention costs† | 37 -- | 175 -- | 203 -- |
| Intervention subtotal | 837 (9)a | 1323 (23)b | 3092 (85)c |
| Other health care costs ($) | |||
| Health care providers | 3472 (558) | 3116 (476) | 3061 (484) |
| Concomitant medications | 4831 (225)a,b | 4781 (239)b | 4541 (229)b,c |
| Intervention and other health care costs subtotal ($) | 9139 (622)a | 9219 (532)a | 10694 (576)b |
| Other outcomes | |||
| Kilogram-years lost‡ | 3.14 (0.74)a | 5.55 (0.96)b | 10.87 (1.13)c |
| Quality-adjusted life years§ | 1.629 (0.017) | 1.603 (0.035) | 1.642 (0.023) |
Values with different superscripts are significantly different from each other at p<0.05, while values sharing a superscript are not significantly different from each other. For example, in the row labeled “concomitant medications,” the values for Usual Care and Enhanced Brief LC are significantly different from each other, but the value for Brief LC is not significantly different from the other two values.
There are no estimate of variance for training and supervision, as we allotted these costs equally among participants in each intervention arm.
Exercise DVDs, thera-bands, scales, pedometers, Calorie King books
Cost of losing one kg for one year
This estimate is for the total number of quality-adjusted life years (QALYs) over the two years of the trial; the range of possibly QALYs is 0 (worst health) to 2 (perfect health).
Incremental Costs and Outcomes, Cost-Effectiveness Ratios and Acceptability Cut-Offs
Table 3 shows the incremental costs, kilogram-years of weight loss, and QALYs, as well as incremental cost-effectiveness ratios and their 95% CI, and the percentage of the distribution of the incremental cost-effectiveness ratio that was acceptable (i.e., below a cutoff that should be acceptable to health care payers). Ratios of incremental intervention costs to incremental kilogram-years of weight loss ranged from $201 (Brief LC vs. Usual Care) to $333 (Enhanced Brief LC vs. Brief LC). Thus, if health care payers were willing to pay $100 per kilogram-year, there was little chance that either Enhanced Brief LC or Brief LC was cost-effective, relative to Usual Care. If payers were willing to pay $200 per kilogram-year, Brief LC and Usual Care were a toss-up, but there was little chance that Enhanced Brief LC was cost-effective, relative to Brief LC or to Usual Care.
Table 3.
Incremental costs, kilogram years of weight loss, quality-adjusted life years (QALYs), cost-effectiveness ratios, and acceptability cutoffs.
| Comparison | Incremental cost | Incremental kg-years | Incremental cost-effectiveness ratio (ICER) | 95% CI for ICER | % of ICERs acceptable at $100/kg-year |
% of ICERs acceptable at $200/kg-year |
|---|---|---|---|---|---|---|
| Weight loss intervention costs: cost per kilogram-year | ||||||
| BLC vs. UC | 486 | 2.42 | 201 | 101 to 6876 | 0.022 | 0.496 |
| EBLC vs. UC | 2255 | 7.73 | 292 | 219 to 437 | 0 | 0.004 |
| EBLC vs. BLC | 1769 | 5.31 | 333 | 217 to 720 | 0 | 0.008 |
| Weight loss intervention and other health care costs: cost per kilogram-year | ||||||
| BLC vs. UC | 80 | 2.42 | 33 | −1974 to 2373 | 0.598 | 0.724 |
| EBLC vs. UC | 1554 | 7.73 | 201 | 38 to 394 | 0.111 | 0.495 |
| EBLC vs. BLC | 1474 | 5.31 | 277 | 72 to 657 | 0.046 | 0.242 |
| Weight loss intervention: cost per QALY | ||||||
| BLC vs. UC | 486 | −0.026 | −18,962 | −∞ to −4,906 and 10,206 to ∞* | 0.171 | 0.206 |
| EBLC vs. UC | 2255 | 0.013 | 167,401 | −∞ to −55,374 and 33,395 to ∞* | 0.125 | 0.371 |
| EBLC vs. BLC | 1769 | 0.039 | 45,262 | −∞ to −47,170 and 15,256 to ∞* | 0.538 | 0.709 |
| Weight loss intervention and other health care costs: cost per QALY | ||||||
| BLC vs. UC | 80 | −0.026 | −3134 | Undefined † | 0.247 | 0.243 |
| EBLC vs. UC | 1554 | 0.013 | 115,397 | −∞ to −25,084 and 11,568 to ∞* | 0.278 | 0.471 |
| EBLC vs. BLC | 1474 | 0.039 | 37,714 | −∞ to −32,244 and 6736 to ∞* | 0.595 | 0.733 |
Randomization assignments are Usual Care (UC), Brief Lifestyle Counseling (BLC), and Enhanced Brief Lifestyle Counseling (EBLC).
The confidence intervals for the weight loss intervention cost per QALY confidence intervals and weight loss and other healthcare cost per QALY represent exclusion intervals. We can be 95% confident of value when our willingness to pay falls outside the confidence interval. For example, if willingness to pay is greater than -$55,374 and less than $33,395, we can be 95% confident that compared to usual care the intensive intervention yields QALYs at a weight loss intervention cost that exceeds our willingness to pay. Otherwise, we cannot be 95% confident that the value of the 2 therapies differs.
When the 95% confidence interval is undefined, there is no value of willingness to pay for which we can be 95% confident that the intervention provides units of outcome (e.g., QALYs) at a price that is below our willingness to pay. This occurs when cost-effectiveness ratios fall into all four quadrants of the cost-effectiveness plane.
When the analytic approach switched from kilogram-years of weight loss to within-trial QALYs, there was still limited evidence that either Brief LC or Enhanced Brief LC was cost-effective relative to Usual Care. The point estimate for QALYs for Brief LC was smaller than that for Usual Care (yielding a point estimate for the comparison of cost and effects that indicated that Brief LC was dominated – more costly and less effective – by Usual Care). Only 20% of the distribution of the resulting cost-effectiveness ratio indicated that Brief LC was acceptable. The ratios for Enhanced Brief LC versus Usual Care were both greater than $100,000 per QALY, with at most 47% of the distribution acceptable (Enhanced Brief LC versus Usual Care when we were willing to pay $100,000 per QALY).
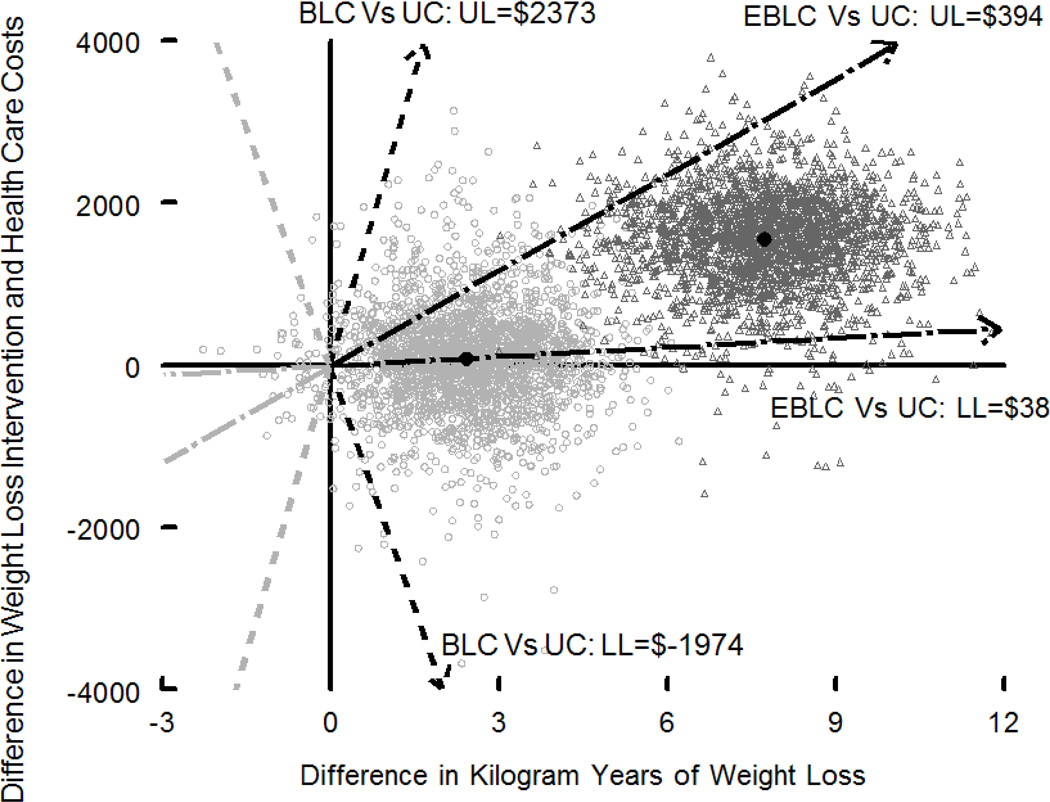
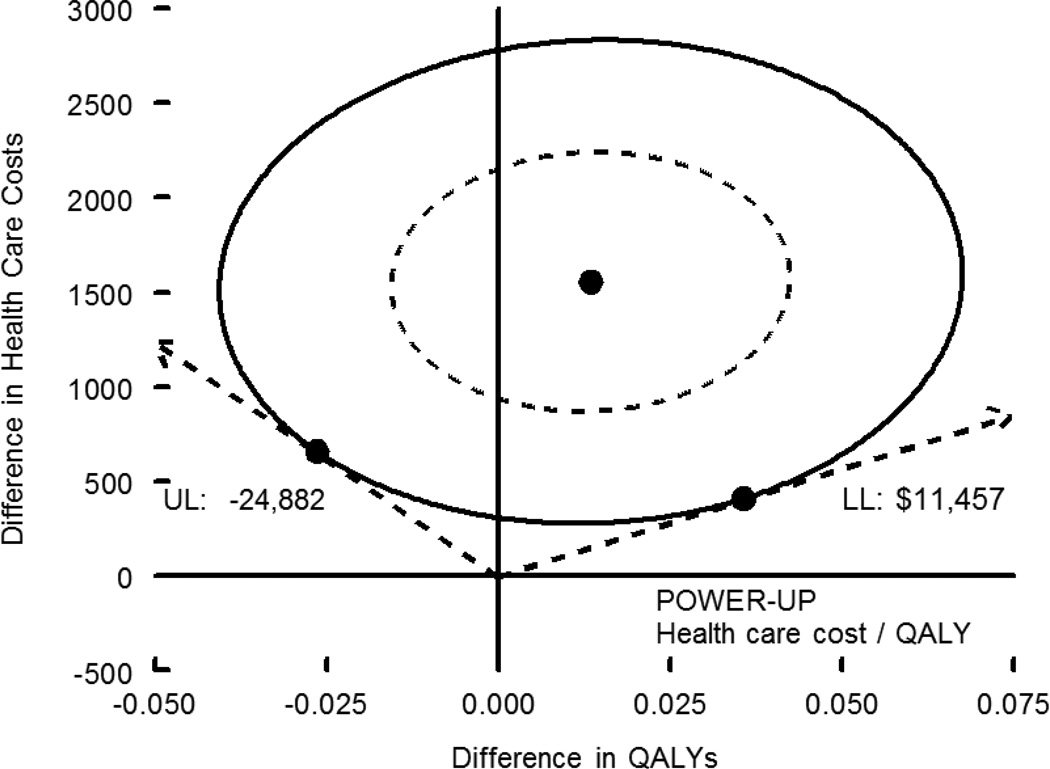
Figures 1 and 2 show the point estimate and 95% confidence interval of the cost-effectiveness ratio for kilogram-years (Figure 1) and for QALYs (Figure 2). Figure 1 shows Enhanced Brief LC and Brief LC, while Figure 2 shows only Enhanced Brief LC relative to Usual Care, due to space constraints of the “clouds” (i.e., the bootstrap replicates of the cost-effectiveness ratio, forming a “cloud”) for the 95% CI. To be comparable to other published estimates, Figure 1 includes only intervention costs, while Figure 2 includes both intervention plus other health care costs. The cost/kg-year for Enhanced Brief LC vs. Usual Care was $292 ($38, $394). The cost/QALY for Enhanced Brief LC vs. Usual Care was $115,397, and the 95% CI were undefined due to the broad spread of cost-effectiveness ratios in the bootstrap analysis.
Figure 1.
Cost-effectiveness (cost per kilogram-kg) of the Enhanced Brief LC (EBLC) and Brief LC (BLC) interventions, relative to Usual Care (UC). The figure shows the point estimate and 95% confidence intervals for the cost-effectiveness ratio (difference in costs [intervention costs plus health care costs] divided by difference in kilogram-years). The lighter open circles show bootstrap replicates of the incremental cost-effectiveness ratio comparing Brief LC and Usual Care. The darker open triangles show bootstrap replicates of Enhanced Brief LC and Usual Care. The point estimates for the ratio of weight loss intervention cost per kilogram years of weight loss were $201 for Brief LC vs. Usual Care and $292 for Enhanced Brief LC vs. Usual Care. The dashed arrows show the 95% CI of the cost-effectiveness ratio for Brief LC vs. Usual Care. The dashed and dotted arrows show the 95% CI of the cost-effectiveness ratio for Enhanced Brief LC vs. Usual Care. A total of 2500 bootstrap replicates were done for each comparison.
Figure 2.
Cost-effectiveness (cost per QALY) of the Enhanced Brief LC and Brief LC interventions, relative to Usual Care (UC). The figure shows the point estimate and 95% confidence intervals for the cost-effectiveness ratio (difference in costs [intervention costs plus health care costs] divided by difference in QALYs). The point estimate of cost per QALY was $115,397. The 95% confidence interval include all values of willingness to pay between the lower limit of $11,457 and the Y axis (positive infinity) on the right side of the graph, as well as all values between the upper limit of -$24,882 and the Y axis (negative infinity) on the left side of the graph.
Comparison of Cost per Kilogram-Year with Longer Term Cost per QALY/per Life Year
Table 4 reports the results of four studies which reported (or from which we were able to estimate) a cost per kilogram or per kilogram-year as well as a longer term cost per QALY or per life year (LY) gained.19,27–31 Our estimates of $201, $292, and $333 weight loss intervention cost per kilogram year ratios generally fall at the upper end of the cost per kilogram range for these studies. Assuming that the relationship between short term cost per kilogram and long-term cost per QALY observed in these studies is also applicable to our ratio, we would expect that our long term cost per QALY ratio for Brief LC would fall somewhere in the range between $7,000 and $12,600; for Enhanced Brief LC the cost/QALY might be as high as $22,000. Even if the relationship between our cost per QALY and cost per kilogram lost ratios was twice that in the other studies, our point estimate would be less than $26,000 (Brief LC) and $44,000 (Enhanced Brief LC) per QALY.
Table 4.
Short-term estimate of cost per kilogram or cost per kilogram-year and longer-term estimate of cost per QALY or cost per life-year (LY) gained.
| Intervention | Estimate of short term cost per kilogram/kilogram year |
Longer term cost per QALY/LY |
Time frame for cost per QALY/per LY |
|
|---|---|---|---|---|
| Diabetes Prevention Program18,26,27* (2010 U.S. dollars) |
Intensive group behavioral lifestyle modification |
$159 / kg-year | $10,037 per QALY |
10 years |
| Roux29† (2001 U.S. dollars) |
Combined diet, exercise, and behavior modification |
$290 / kg | $12,600 per QALY |
Lifetime |
| Gustafson30┼ (2007 U.S. dollars) |
Intensive group behavioral lifestyle intervention |
$55 / kg $132 / kg-year |
$3,612 per LY |
Lifetime |
| Krukowski28≠ (2009 U.S. dollars) |
Internet intervention Internet plus in-person intervention |
$130 / kg $260 / kg year |
$7,177≠ per LY |
Lifetime |
Cost per kg calculated from weight losses at 1, 2, and 3 years in clinical trial (Knowler et al.) and from intervention costs reported in years 1–3 (Herman et al.)
Cost per kg calculated from reported direct medical program costs (Roux Table 1) and an estimated 8.6 kg of weight loss based on changes in BMI (Roux Table 1).
Discussion
In this study, the within-trial estimate of cost per kilogram was similar to other studies.19,27–34 The within trial estimates of cost per QALY, on the other hand, did not suggest that the Brief LC and Enhanced Brief LC interventions were cost-effective, relative to Usual Care. As the ultimate benefits of weight loss are the avoidance of long term disability and death, we did not expect the latter ratios to be acceptable. Our comparison of published cost per kilogram ratios with their projected long term cost per QALY ratios suggests that, had we used a decision model to project our results, they would have fallen in an acceptable range. Thus, if health care payers are willing to base health policy on the results of decision models, the POWER-UP results suggest that a primary care-based treatment model could be cost-effective over the long term.
Confidence intervals for the short-term cost per QALY ratios could not be estimated with precision. The Brief LC and Enhanced Brief LC interventions were more expensive than the Usual Care intervention, but these costs were somewhat offset by lower costs for concomitant medications, as shown in other trials.35–37 The most robust long-term estimate of cost per QALY comes from the Diabetes Prevention Program (DPP), and suggests that intensive behavioral intervention is cost-effective over the longer term (10 years to lifetime).27 Most other cost-effectiveness studies of obesity treatment also have reported cost-effectiveness ratios of less than $50,000 per QALY.29,30,38–43 The ongoing economic analysis of the Look AHEAD (Action for HEAlth in Diabetes) Trial will provide more data about the cost-effectiveness of intensive obesity treatment. A preliminary estimate of cost per kilogram-year, using cost data presented as an abstract44 and four year weight losses from Look AHEAD,45 suggest that the cost of that intervention may be higher (approximately $300 per kg-year) than in the DPP.
A major question underlying economic analyses of health care interventions is: will payers reimburse them? With regard to obesity, treatment has historically been inconsistently reimbursed or not reimbursed at all. The recent decision by Medicare to reimburse intensive treatment in primary care is welcome, although evaluation of effectiveness will certainly be needed. Reimbursement for obesity treatment in all forms has often been subjected to a “return on investment” (ROI) argument – that is, treatment must save the employer or health plan money in order to be reimbursed. Although some evidence suggests that employer-sponsored wellness programs can produce a ROI,46 only a minority of health care interventions are actually cost saving.47 With obesity treatment, the return on investment is likely to take longer to observe than most or payers are able or willing to wait.48 An alternative approach to obesity coverage decisions, which is used by the National Institute for Clinical Excellence (NICE) in the United Kingdom, is to subject all new treatments to analyses of clinical and cost-effectiveness. Orlistat, a pharmacologic treatment for obesity, underwent such an analysis, and despite the modest weight losses associated with the drug, was recommended by NICE to be used in clinical practice.49
A major goal of health services research in obesity is to help determine how obesity prevention and treatment can be delivered to the largest number of people at the lowest possible cost. The results of this analysis suggest that the interventions used in this clinical trial could be cost-effective over the long term. The primary care setting remains the place where patients with obesity and common weight related health-related conditions (e.g., diabetes, hypertension) are seen and treated the most often. Thus, PCPs have a unique opportunity to initiate obesity treatment and to refer for treatment. Whether on-site treatment, as reimbursed by Medicare, or offsite treatment, as with commercial weight loss programs50,51 or call centers,15 is the most cost-effective way to provide treatment, remains a question for future studies.
In conclusion, we found that a primary care-based intervention of monthly counseling, combined with either meal replacements or pharmacotherapy, was not clearly cost-effective using cost per QALY. However, the intervention appeared reasonably cost-effective in the short term using cost per kilogram and in comparison to published estimates.
Acknowledgements
This research was supported by grants from the National Heart, Lung, and Blood Institute (U01-HL087072) and the National Institute of Diabetes and Digestive and Kidney Diseases (K24-DK065018).
POWER-UP Research Group: Investigators and Research Coordinators
Academic investigators at the Perelman School of Medicine at the University of Pennsylvania were Thomas A. Wadden, Ph.D. (principal investigator), David B. Sarwer, Ph.D. (co-principal investigator), Robert I. Berkowitz, M.D., Jesse Chittams, M.S., Lisa Diewald, M.S., R.D., Shiriki Kumanyika, Ph.D., Renee Moore, Ph.D., Kathryn Schmitz, Ph.D., Adam G. Tsai, M.D., MSCE, Marion Vetter, M.D., and Sheri Volger, M.S., R.D.
Research coordinators at the University of Pennsylvania were Caroline H. Moran, B.A., Jeffrey Derbas, B.S., Megan Dougherty, B.S., Zahra Khan, B.A., Jeffrey Lavenberg, M.A., Eva Panigrahi, M.A., Joanna Evans, B.A., Ilana Schriftman, B.A, Dana Tioxon, Victoria Webb, B.A., and Catherine Williams-Smith, B.S.
POWER-UP Research Group: Participating Sites and Clinical Investigators
PennCare - Bala Cynwyd Medical Associates: Ronald Barg, M.D., Nelima Kute, M.D., David Lush, M.D., Celeste Mruk, M.D., Charles Orellana, M.D., and Gail Rudnitsky, M.D. (primary care providers); Angela Monroe (lifestyle coach); Lisa Anderson (practice administrator).
PennCare - Internal Medicine Associates of Delaware County: David E. Eberly, M.D., Albert H. Fink Jr., M.D., Kathleen Malone, C.R.N.P., Peter B. Nonack, M.D., Daniel Soffer, M.D., John N. Thurman, M.D., and Marc J. Wertheimer, M.D. (primary care providers); Barbara Jean Shovlin, Lanisha Johnson (lifestyle coaches); Jill Esrey (practice administrator).
PennCare - Internal Medicine Mayfair: Jeffrey Heit, M.D., Barbara C. Joebstl, M.D., and Oana Vlad, M.D. (primary care providers); Rose Schneider, Tammi Brandley (lifestyle coaches); Linda Jelinski (practice administrator).
Penn Presbyterian Medical Associates: Joel Griska, M.D., Karen J. Nichols, M.D., Edward G. Reis, M.D., James W. Shepard, M.D., and Doris Davis-Whitely, P.A. (primary care providers); Dana Tioxon (lifestyle coach); Charin Sturgis (practice administrator).
PennCare - University City Family Medicine: Katherine Fleming, C.R.N.P., Dana B. Greenblatt, M.D., Lisa Schaffer, D.O., Tamara Welch, M.D., and Melissa Rosato, M.D. (primary care providers); Eugonda Butts, Marta Ortiz, Marysa Nieves, and Alethea White (lifestyle coach); Cassandra Bullard (practice administrator).
PennCare - West Chester Family Practice: Jennifer DiMedio, C.R.N.P., Melanie Ice, D.O., Brandt Loev, D.O., John S. Potts, D.O., and Christine Tressel, D.O. (primary care providers); Iris Perez, Penny Rancy, and Dianne Rittenhouse (lifestyle coaches); Joanne Colligan (practice administrator).
Footnotes
Conflict of Interest
David Sarwer discloses that he has relationships with the following companies: Allergan, BaroNova, Enteromedics, Ethicon Endo-Surgery, and Galderma. The other authors declare no conflicts of interest. Thomas Wadden serves on the advisory boards of Novo Nordisk and Orexigen Therapeutics, which are developing weight loss medications, as well as of Alere and the Cardiometabolic Support Network, which provide behavioral weight loss programs.
References
- 1.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28:822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 2.Trogdon JG, Finkelstein EA, Hylands T, Dellea PS, Kamal-Bahl SJ. Indirect costs of obesity: a review of the current literature. Obes Rev. 2008;9:489–500. doi: 10.1111/j.1467-789X.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 3.Leblanc ES, O'Connor E, Whitlock EP, Patnode CD, Kapka T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S Preventive Services Task Force. Ann Intern Med. 2011;155:434–447. doi: 10.7326/0003-4819-155-7-201110040-00006. [DOI] [PubMed] [Google Scholar]
- 4.Rao G, Burke LE, Spring BJ, Ewing LJ, Turk M, Lichtenstein AH, et al. New and emerging weight management strategies for busy ambulatory settings: a scientific statement from the American Heart Association endorsed by the Society of Behavioral Medicine. Circulation. 2011;124:1182–1203. doi: 10.1161/CIR.0b013e31822b9543. [DOI] [PubMed] [Google Scholar]
- 5.Tsai AG, Wadden TA. Treatment of obesity in primary care practice in the United States: a systematic review. J Gen Intern Med. 2009;24:1073–1079. doi: 10.1007/s11606-009-1042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Medicaid and Medicare Services. [Accessed December 14, 2012];Decision memo for intensive behavioral therapy for obesity (CAG-00423N) Available at: http://www.cms.gov/medicare-coverage-database/details/nca-decisionmemo.aspx?&NcaName=Intensive%20Behavioral%20Therapy%20for%20Obesity&bc=ACAAAAAAIAAA&NCAId=253.
- 7.Buchwald H, Avidor Y, Braunwald E, Jenson MD, Poires W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 9.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;(12 Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26:2518–2523. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddy DM, Schlessinger L, Kahn R. Clinical outcomes and cost-effectiveness of strategies for managing people at high risk for diabetes. Ann Intern Med. 2005;143:251–264. doi: 10.7326/0003-4819-143-4-200508160-00006. [DOI] [PubMed] [Google Scholar]
- 12.Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med. 2005;142:323–332. doi: 10.7326/0003-4819-142-5-200503010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadden TA, Volger S, Sarwer DB, Vetter M, Tsai A, Berkwotiz R, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on the detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Appel LJ, Clark JM, Yeh HC, Wang NY, Coughlin JW, Daumit G, et al. Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 2011;365:1959–1968. doi: 10.1056/NEJMoa1108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett GG, Warner ET, Glasgow RE, Askew S, Goldman J, Ritzwoller D, et al. Obesity Treatment for Socioeconomically Disadvantaged Patients in Primary Care Practice. Arch Intern Med. 2012;172:565. doi: 10.1001/archinternmed.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeh HC, Clark JM, Emmons KE, Moore RH, Bennett GG, Warner ET, et al. Independent but coordinated trials: insights from the practice-based Opportunities for Weight Reduction Trials Collaborative Research Group. Clin Trials. 2010;7:322–332. doi: 10.1177/1740774510374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Department of Veterans Affairs. [Accessed November 11, 2012];Drug Pharmaceutial Prices: Federal Supply Schedule. http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx.
- 19.Herman WH, Brandle M, Zhang P, Williamson DF, Matulik MJ, Ratner RE, et al. Costs associated with the primary prevention of type 2 diabetes mellitus in the diabetes prevention program. Diabetes Care. 2003;26:36–47. doi: 10.2337/diacare.26.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Department of Labor Bureau of Labor Statistics. [Accessed November 1, 2012]; Consumer Price Index http://www.bls.gov/cpi/
- 21.Sullivan PW, Ghushchyan V. Preference-Based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 23.Spielman AB, Kanders B, Kienholz M, Blackburn GL. The cost of losing: an analysis of commercial weight-loss programs in a metropolitan area. J Am Coll Nutr. 1992;11:36–41. doi: 10.1080/07315724.1992.10718194. [DOI] [PubMed] [Google Scholar]
- 24.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. 1st edn. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 25.Lin DY. Linear regression analysis of censored medical costs. Biostatistics. 2000;1:35–47. doi: 10.1093/biostatistics/1.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Lin DY. Regression analysis of incomplete medical cost data. Statistics in Medicine. 2003;22:1181–1200. doi: 10.1002/sim.1377. [DOI] [PubMed] [Google Scholar]
- 27.Diabetes Prevention Program Research G. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krukowski RA, Tilford JM, Harvey-Berino J, West DS. Comparing behavioral weight loss modalities: incremental cost-effectiveness of an internet-based versus an in-person condition. Obesity. 2011;19:1629–1635. doi: 10.1038/oby.2010.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux L, Kuntz KM, Donaldson C, Goldie SJ. Economic evaluation of weight loss interventions in overweight and obese women. Obesity. 2006;14:1093–1106. doi: 10.1038/oby.2006.125. [DOI] [PubMed] [Google Scholar]
- 31.Gustafson A, Khavjou O, Stearns SC, Keyserling TC, Gizlice Z, Lindsley S, et al. Cost-effectiveness of a behavioral weight loss intervention for low-income women: the Weight-Wise Program. Prev Med. 2009;49:390–395. doi: 10.1016/j.ypmed.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AM, Conaway MR, Crowther JQ, Hazen KY, Nadler JL, Oneida B, et al. Translating lifestyle intervention to practice in obese patients with type 2 diabetes: Improving Control with Activity and Nutrition (ICAN) study. Diabetes Care. 2004;27:1570–1576. doi: 10.2337/diacare.27.7.1570. [DOI] [PubMed] [Google Scholar]
- 33.Wolf AM, Siadaty M, Yaeger B, Conaway MR, Crowther JQ, Nadler JL, et al. Effects of lifestyle intervention on health care costs: Improving Control with Activity and Nutrition (ICAN) J Am Diet Assoc. 2007;107:1365–1373. doi: 10.1016/j.jada.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 34.John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26:621–626. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson JW, Jhaveri MA. Reductions in medications with substantial weight loss with behavioral intervention. Curr Clin Pharmacol. 2010;5:232–238. doi: 10.2174/157488410793352030. [DOI] [PubMed] [Google Scholar]
- 36.Collins RW, Anderson JW. Medication cost savings associated with weight loss for obese non-insulin-dependent diabetic men and women. Prev Med. 1995;24:369–374. doi: 10.1006/pmed.1995.1060. [DOI] [PubMed] [Google Scholar]
- 37.Redmon JB, Bertoni AG, Connelly S, Feeney PA, Glasser SP, Glick H, et al. Effect of the look AHEAD study intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care. 2010;33:1153–1158. doi: 10.2337/dc09-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avenell A, Broom J, Brown TJ, Aucott L, Stearns SC, Smith WCS, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 39.Galani C, Schneider H, Rutten FF. Modelling the lifetime costs and health effects of lifestyle intervention in the prevention and treatment of obesity in Switzerland. Int J Public Health. 2007;52:372–382. doi: 10.1007/s00038-007-7014-9. [DOI] [PubMed] [Google Scholar]
- 40.McConnon A, Kirk SF, Cockroft JE, Harvey EL, Greenwood DC, Thomas JD, et al. The Internet for weight control in an obese sample: results of a randomised controlled trial. BMC Health Serv Res. 2007;7:206. doi: 10.1186/1472-6963-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai AG, Glick HA, Shera D, Stern L, Samaha FF. Cost-effectiveness of a low-carbohydrate diet and a standard diet in severe obesity. Obes Res. 2005;13:1834–1840. doi: 10.1038/oby.2005.223. [DOI] [PubMed] [Google Scholar]
- 42.Olsen J, Willaing I, Ladelund S, Jorgensen T, Gundgaard J, Sorensen J. Cost-effectiveness of nutritional counseling for obese patients and patients at risk of ischemic heart disease. Int J Technol Assess Health Care. 2005;21:194–202. [PubMed] [Google Scholar]
- 43.van Baal PH, van den Berg M, Hoogenveen RT, Vijgen SM, Engelfriet PM. Cost-effectiveness of a low-calorie diet and orlistat for obese persons: modeling long-term health gains through prevention of obesity-related chronic diseases. Value Health. 2008;11:1033–1040. doi: 10.1111/j.1524-4733.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang P, Lawlor MS, Davis C, et al. Costs associated with delivering a structured lifestyle intervention and diabetes education program in overweight and obese adults with type 2 diabetes: year 4 results from the Action for Health in Diabetes (Look AHEAD) Study. Diabetes Care. 2011;60:A68. [Google Scholar]
- 45.Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baicker K, Cutler D, Song Z. Workplace wellness programs can generate savings. Health Aff. 2010;29:304–311. doi: 10.1377/hlthaff.2009.0626. [DOI] [PubMed] [Google Scholar]
- 47.Russell LB. Preventing chronic disease: an important investment, but don't count on cost savings. Health Aff. 2009;28:42–45. doi: 10.1377/hlthaff.28.1.42. [DOI] [PubMed] [Google Scholar]
- 48.O'Grady MJ, Capretta JC. Assessing the economics of obesity and obesity interventions. Washington, D.C.: Campaign to End Obesity; Mar, 2012. [Google Scholar]
- 49.National Institute for Clinical Excellence. [Accessed April 20, 2012];NICE issues guidance on Orlistat for obesity. 2001 http://www.nice.org.uk/guidance/index.jsp?action=article&o=32161.
- 50.Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 51.Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet. 2011;378:1485–1492. doi: 10.1016/S0140-6736(11)61344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]