Abstract
The prevalence of Toxoplasma gondii (TOXO) infection in schizophrenia (SCZ) is elevated compared to controls (odds ratio=2.73). TOXO infection is associated with psychomotor slowing in rodents and non-psychiatric humans. Latency of the acoustic startle response, an index of neural processing speed, is the time it takes for a startling stimulus to elicit the reflexive response through a three-synapse subcortical circuit. We report a significant slowing of latency in TOXO seropositive SCZ vs. seronegative SCZ, and in TOXO seropositive controls vs. seronegative controls. Latency was likewise slower in SCZ subjects than in controls. These findings indicate a slowing of neural processing speed with chronic TOXO infection; the slowest startle latency was seen in the TOXO seropositive SCZ group.
Keywords: schizophrenia, Toxoplasma gondii, acoustic startle, latency
1. Introduction
Infectious agents figure prominently among environmental factors linked with schizophrenia (SCZ; Yolken and Torrey, 2008). Toxoplasma gondii (TOXO), an obligate intracellular parasite, has arguably the most compelling link (Yolken and Torrey, 2008).
Although infection is typically asymptomatic in immune competent individuals, a chronic infection commonly develops wherein TOXO cysts form in muscle and widely distributed areas of brain (Gonzalez et al., 2007; Vyas et al., 2007). TOXO infected rodents show impaired psychomotor performance (Hutchison et al., 1980; Witting, 1979) and learning (Piekarski, 1981; Witting, 1979). Psychomotor slowing (Havlicek et al., 2001) and impaired learning (Yolken et al., 2009) are likewise reported in TOXO seropositive humans without psychiatric illness. Asymptomatic people with chronic TOXO infection have increased rates of motor vehicle accidents (Flegr et al., 2002; Flegr et al., 2009; Kocazeybek et al., 2009; Yereli et al., 2006).
Patients with SCZ show elevated prevalence of TOXO infections. A recent meta-analysis of 23 methodologically sound studies reported a risk for TOXO seropositivity 2.7 times higher in SCZ patients than the general population (Torrey et al., 2007).
The acoustic startle reflex has been extensively studied in SCZ. It is a simple reflex elicited by a sudden intense stimulus. Latency of the startle response is the time required for a startling stimulus to elicit the reflexive response. It is preattentive and mediated by a simple three-synapse subcortical circuit (Koch, 1999), hence providing an index of neural processing speed. Several studies indicate that latency is prolonged in SCZ (Braff et al., 1978; Braff et al., 1999; Geyer and Braff, 1982; Braff et al., 1999; Hasenkamp et al., 2010; Swerdlow et al., 2006), although there are also negative findings reported (Braff et al. 1992; Parwani et al., 2000). Latency is highly heritable in SCZ and healthy control families (Hasenkamp et al., 2010).
In light of the psychomotor slowing seen with TOXO infection, we hypothesized that neural processing speed as indexed by acoustic startle latency might be slowed in SCZ subjects who are chronically infected with TOXO. Hence we conducted a study of startle latency and TOXO seropositivity in SCZ subjects and healthy controls.
2. Materials and Methods
2.1. Subjects
The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). 183 adult SCZ patients and 137 healthy controls (CON) met study inclusion criteria and signed a consent form approved by the Institutional Review Board at Emory University and the Atlanta VA Research and Development Committee. Some subjects were also included in an overlapping study (Hasenkamp et al., 2010); hence inclusion and exclusion criteria were similar. Diagnosis was confirmed by the Structured Clinical Interview for DSM-IV, Axis-I (SCID-I; First, 2001). Exclusion criteria for all subjects were current substance dependence, positive urine toxicology, history of sustained loss of consciousness, and major neurological or medical illness. CON subjects were excluded for any Axis I disorder and psychotic disorder in first-degree relatives. Subjects were screened to ensure normal hearing acuity using a Grason-Stadler audiometer (Model GS1710).
2.2. Acoustic Startle Measurement
Methodology for measuring the acoustic startle reflex was similar to that of Braff and colleagues (Braff et al., 1992), and to that used previously in our laboratory (Hasenkamp et al, 2008; Hasenkamp et al., 2010). Further description of methodology can be found in Supplementary Methods. Briefly, subjects were seated in an audiology booth and asked to look straight ahead and keep their eyes open during the test session. Acoustic stimuli were delivered binaurally through headphones (Maico, TDH-39-P). The startle session began with a 60 second (s) acclimation period consisting of 70 dB white noise that continued as background throughout the session. The pulse-alone stimuli were116 dB, 40-ms white noise bursts; the prepulse stimuli were 85 dB, 20-millisecond (ms) white noise bursts presented 30, 60, and 120 ms prior to the startle stimulus. The session began with a habituation block of six pulse alone stimuli (HAB1). The main part of the session consisted of three blocks each containing three pulse-alone trials and three prepulse+pulse trials at each of the three designated prepulse intervals (30-, 60- and 120-ms), for a total of 36 startle stimuli presented in a pseudorandom order. Finally, a second habituation block of six pulse alone stimuli was presented at the end (HAB2) of the session. Inter-trial intervals were 10–25 s (average 15 s).
2.3. TOXO assays
Specimens were tested for TOXO specific IgG using the Toxoplasma IgG EIA (Bio-Rad, Redmond, WA) according to manufacturer’s instructions. This EIA demonstrated 100% agreement with the established CDC serum panel tested before the study. See Supplement for further methods.
2.4. Statistical methods
Initial startle reactivity, habituation, and pulse-alone startle magnitude were analyzed with univariate ANCOVAs with between-subjects factors of TOXO status (seropositive vs. seronegative) and Diagnosis (SCZ vs. CON). Onset latency and prepulse inhibition of startle were analyzed using mixed model ANCOVAs with the same between-subjects factors and a within-subjects factor of Trial Type. Age was used as a covariate in both sets of ANCOVAs.
3. Results
3.1. Demographics
The mean age of the subjects was 44.9±10.3 years for the SCZ group and 37.7±14.2 years for the CON group (Independent samples t-test, t(318)=−7.27, p<0.001). Since increased age can increase risk for TOXO infection, age was used as a covariate in analyses. The SCZ group was 65% male; the CON group was 31% male (Fisher’s Exact Test, p < 0.001). 71.6% of the SCZ subjects were treated with second-generation antipsychotics, 10.9% were on a second-generation plus a first-generation agent, 9.8% were on a first-generation agent only, and 7.1% were on no antipsychotic medication.
3.2. Group Differences in TOXO serointensity
A two-part Poisson regression model for continuous data (Afifi et al., 2007) was fit to TOXO serointensity and indicated that SCZ subjects had more TOXO specific IgG than CON subjects (Wald Chi-Square on psychiatric Diagnosis, controlling for age=285.68, p<0.001), replicating prior work (Torrey et al., 2007). 11.5% of the SCZ group was TOXO positive; 9.5% of the CON group was seropositive. The groups did not differ by Fisher’s Exact Test (p = 0.71) on rates of TOXO seropositivity.
3.3. Startle latency
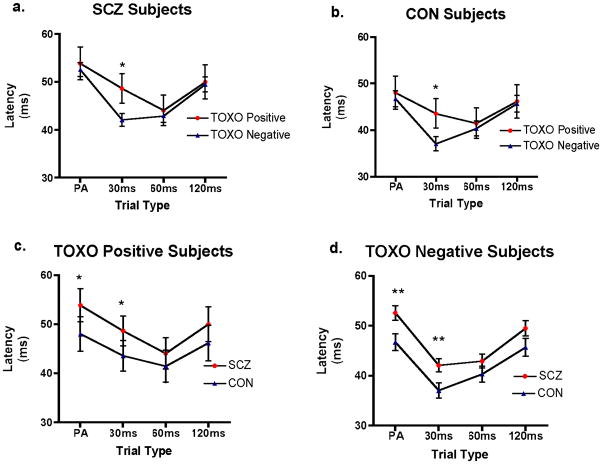
In planned comparisons within each trial type, TOXO positive subjects had longer latency in the 30ms prepulse+pulse trials than TOXO negative subjects (for both SCZ and CON subjects analyzed separately, p=0.04). SCZ subjects had longer latency than CON in the pulse-alone and the 30ms prepulse+pulse trials (for both TOXO positive and TOXO negative groups analyzed separately, p=0.01). In order to further investigate main effects and interactions, a mixed model ANCOVA on onset latency with between-group factors of TOXO status (seropositive vs. seronegative) and Diagnosis (SCZ vs. CON) and within subjects factor of Trial Type (pulse-alone trials, prepulse+pulse trials with interstimulus intervals of 30, 60, and 120 ms) and age as a covariate was performed. The model was significant for Trial Type (F(3,486)=3.21, p=0.02) and Diagnosis (F(1,162)=6.24, p=0.01). Other factors and interactions for the overall model were not significant, although age reached trend-level significance (F(1,162)=3.45, p=0.07). We estimated separate a similarly structured exploratory ANCOVA model with the added covariate of sex. In this model sex was not significant, nor were there significant interactions with this covariate.
3.4. Analysis of other startle variables
In a univariate model, initial startle reactivity (startle magnitude in the first six pulse-alone trials) did not differ between TOXO positive and TOXO negative subjects, between SCZ and CON subjects, nor was the interaction of TOXO status and Diagnosis significant. Similarly, habituation (percent decline in startle magnitude between HAB1 and HAB2) and startle magnitude to pulse-alone trials in BLOCKS 1–3 did not differ between TOXO positive and TOXO negative subjects, between SCZ and CON subjects, nor were interactions of TOXO status and Diagnosis significant. Finally, in a mixed model ANCOVA, prepulse inhibition differed significantly between trial types as expected (F(2,474)=8.05, p<0.001) but the model was not significant for the between-group factors of TOXO status, Diagnosis, nor for the interaction of these two factors with Trial Type.
4. Discussion
Prolonged acoustic startle latency is associated with TOXO seropositivity in SCZ and CON subjects. Prolonged latency in SCZ reported previously (Braff et al., 1978; Braff et al.,1999; Geyer and Braff, 1982; Hasenkamp et al., 2010; Swerdlow et al., 2006) was also confirmed in this dataset. The association of TOXO seropositivity and startle latency, a measure of neural processing speed, is conceptually consistent with findings of psychomotor slowing in rodents and nonpsychiatric TOXO positive humans. TOXO seropositivity was associated with prolonged latency in both SCZ and CON subjects but the interaction of TOXO status and diagnostic group (SCZ vs. CON) was not significant in our mixed model ANCOVA. However, the group with the slowest latency were those SCZ subjects who were TOXO seropositive.
Regarding mechanism, TOXO cysts are widely distributed in brains of infected animal hosts (Gonzalez et al., 2007; Vyas et al., 2007). Although the persistence of TOXO cysts in the brain after initial infection in humans with and without SCZ must remain speculative, evidence indicates that mice and rats remain chronically infected with TOXO (Dubey, 1997) and cysts were seen in infected guinea pigs for up to five years, the longest period observed (Lainson, 1959). Regarding possible mechanisms whereby TOXO cysts could affect startle latency, the TOXO genome contains two genes that code for the enzyme tyrosine hydroxylase, the rate limiting enzyme in dopamine synthesis (Gaskell et al., 2009). TOXO derived tyrosine hydroxylase is active in the host (Yolken et al., 2009), leading to increased dopamine synthesis (Webster and McConkey, 2010). A common dopamine D3 receptor polymorphism determining D3 receptor gain of function is associated with latency differences in human subjects (Roussos et al., 2008). Earlier work demonstrated that D1 or D2 agonists prolong latency (Naudin et al., 1990; Svensson, 1990). Hence slowed latency is linked to the pharmacology of dopamine. Thus the TOXO organism could lead to a slowing of startle latency by inducing a state of hyperdopaminergia.
Another potential mechanism for prolonged latency stems from the host immune response to TOXO infection. TOXO replication requires host tryptophan; the host cytokine interferon gamma shunts tryptophan degradation along the kynurenine pathway into kynurenine and kynurenic acid (KYNA), thereby depleting tryptophan. KYNA is an inhibitor at the N-methyl-D-aspartate subtype of glutamate receptor and the alpha7 nicotinic acetylcholine receptor (Schwarcz and Hunter, 2007). It is possible that KYNA induced antagonism at either of these receptors results in slowing of neural processing by an as yet unknown mechanism.
A limitation of the study is that we did not assess personality differences or differences in socioeconomic status between subject groups and therefore cannot rule out these factors as potential confounds. We had speculated that TOXO seropositivity would differentially prolong latency in SCZ subjects specifically, but this was not supported by our data. However, given the known association of TOXO infection with elevated risk of SCZ, and since the slowest latency was seen in our TOXO seropositive SCZ group, it is possible that slowing of neural processing in the context of TOXO infection may be a marker of a pathophysiological process in SCZ. Further research is needed to understand the connections between SCZ risk, TOXO infection, and slowing of startle latency as an index of slowed neural processing.
Supplementary Material
Figure 1. Effects of TOXO status and diagnosis on startle latency.
Each panel shows startle latency in milliseconds (ms) on the Y-axis and Trial Type on the X-axis. Stars indicate p<0.05 (*) or p<0.01 (**) for planned comparisons between groups displayed as lines for the Trial Type designated. (a) TOXO positive vs. TOXO negative SCZ subjects; (b) TOXO positive vs. TOXO negative CON subjects; (c) TOXO positive SCZ vs. TOXO positive CON subjects; (d) TOXO negative SCZ vs. TOXO negative CON subjects.
Acknowledgments
Infrastructure support was provided by the Mental Health and R&D Services of the Atlanta Department of Veterans Affairs Medical Center, and the Centers for Disease Control and Prevention. At Emory University the following also provided infrastructure support: the Department of Psychiatry and Behavioral Sciences in the Emory University School of Medicine, the Department of Psychology, and Rollins School of Public Health.
Funding and Support
Funded by the Department of Veterans Affairs Merit Review Program (ED). Additional salary support was provided to ED from NIDA (R01DA018294-01A2) and BDP (NIMH (1R21MH083138-01A1).
Contributors
ED and BDP designed the study. Subjects and data were collected by ED, WH, RG. TOXO assays were conducted by BP, HNR, SH, PPW, MCF. Data analysis was conducted by NB, WH, MCF, SH, ED. JLJ and MHH consulted on study design, TOXO assays, and data analysis. The first draft was written by ED and BDP. All authors edited and approved the manuscript.
Conflict of Interest
Dr. Duncan has received grant support from Bristol-Myers Squibb Company, Ortho-McNeil Janssen Scientific Affairs, GlaxoSmithKline not related to the content of this study. No other authors have any disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bradley D. Pearce, Email: bpearce@emory.edu.
Sydney Hubbard, Email: sydney.hubbard@gmail.com.
Hilda N. Rivera, Email: igi2@cdc.gov.
Patricia P. Wilkins, Email: pma1@cdc.gov.
Marylynn C. Fisch, Email: MFisch@live.com.
Myfanwy H. Hopkins, Email: myfanwy.hopkins@emory.edu.
Wendy Hasenkamp, Email: whasenk@gmail.com.
Robin Gross, Email: regross@emory.edu.
Nancy Bliwise, Email: nbliwis@emory.edu.
Jeffrey L. Jones, Email: JLJ1@CDC.GOV.
Erica Duncan, Email: erica.duncan@va.gov.
References
- Afifi AA, Kotlerman JB, Ettner SL, Cowan M. Methods for improving regression analysis for skewed continuous or counted responses. Annual Rev Public Health. 2007;28:95–111. doi: 10.1146/annurev.publhealth.28.082206.094100. [DOI] [PubMed] [Google Scholar]
- Braff D, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiat. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;14:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff D, Swerdlow N, Geyer M. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiat. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Tissue cyst tropism in Toxoplasma gondii: a comparison of tissue cyst formation in organs of cats, and rodents fed oocysts. Parasitology. 1997;115 ( Pt 1):15–20. doi: 10.1017/s0031182097008949. [DOI] [PubMed] [Google Scholar]
- Flegr J, Havlicek J, Kodym P, Maly M, Smahel Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect Dis. 2002;2:11. doi: 10.1186/1471-2334-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J, Klose J, Novotna M, Berenreitterova M, Havlicek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4(3):e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19(1):1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, Colasante C, Pino S, Hernandez L. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats: A behavioral analysis. Behav Brain Res. 2007;177(1):70–79. doi: 10.1016/j.bbr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Epstein MP, Green A, Wilcox L, Boshoven W, Lewison B, Duncan E. Heritability of acoustic startle magnitude, prepulse inhibition, and startle latency in schizophrenia and control families. Psychiat Res. 2010;178(2):236–243. doi: 10.1016/j.psychres.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Norrholm SD, Green A, Lewison B, Boshoven W, Keyes M, Duncan E. Differences in startle reflex and prepulse inhibition in European-Americans and African-Americans. Psychophysiology. 2008;45(5):876–882. doi: 10.1111/j.1469-8986.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlicek J, Gasova ZG, Smith AP, Zvara K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001;122(Pt 5):515–520. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- Hutchison WM, Aitken PP, Wells BW. Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasit. 1980;74(5):507–510. doi: 10.1080/00034983.1980.11687376. [DOI] [PubMed] [Google Scholar]
- Kocazeybek B, Oner YA, Turksoy R, Babur C, Cakan H, Sahip N, Unal A, Ozaslan A, Kilic S, Saribas S, Aslan M, Taylan A, Koc S, Dirican A, Uner HB, Oz V, Ertekin C, Kucukbasmaci O, Torun MM. Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci Int. 2009;187(1–3):103–108. doi: 10.1016/j.forsciint.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiology. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Lainson R. A note on the duration of Toxoplasma infection in the guinea-pig. Ann Trop Med Parasitol. 1959;53:120–121. doi: 10.1080/00034983.1959.11685908. [DOI] [PubMed] [Google Scholar]
- Naudin B, Canu S, Costentin J. Effects of various direct or indirect dopamine agonists on the latency of the acoustic startle response in rats. J Neural Transm. 1990;82:43–53. doi: 10.1007/BF01244833. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan E, Bartlett E, Madonick S, Efferen T, Rajan R, Sanfilipo M, Chappell P, Chakravorty S, Gonzenbach S, Ko G, Rotrosen J. Impaired prepulse inhibition of acoustic startle in schizophrenics. Biol Psychiat. 2000;47(7):662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Piekarski G. Behavioral alterations caused by parasitic infection in case of latent toxoplasma infection. Zentralbl Bakteriol Mikrobiol Hyg A. 1981;250(3):403–406. [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Bitsios P. The dopamine D(3) receptor Ser9Gly polymorphism modulates prepulse inhibition of the acoustic startle reflex. Biol Psychiat. 2008;64(3):235–240. doi: 10.1016/j.biopsych.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophrenia Bull. 2007;33(3):652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson L. The role of the dopaminergic system in the modulation of the acoustic startle response in the rat. Eur J Pharmacol. 1990;175:107–111. doi: 10.1016/0014-2999(90)90160-8. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiat. 2006;63(12):1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophrenia Bull. 2007;33(3):729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. P Natl Acad Sci USA. 2007a;104(15):6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasit (Praha) 2010;57(2):95–104. doi: 10.14411/fp.2010.012. [DOI] [PubMed] [Google Scholar]
- Witting PA. Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z Parasitenkd. 1979;61(1):29–51. doi: 10.1007/BF00927085. [DOI] [PubMed] [Google Scholar]
- Yereli K, Balcioglu IC, Ozbilgin A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int. 2006;163(1–2):34–37. doi: 10.1016/j.forsciint.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatr. 2008;13(5):470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.