Abstract
The immune response goes haywire during sepsis, a deadly condition triggered by infection. Richard S. Hotchkiss and his colleagues take the focus off of the prevailing view that the key aspect of this response is an exuberant inflammatory reaction. They assess recent human studies bolstering the notion that immunosuppression is also a major contributor to the disease. Many people with sepsis succumb to cardiac dysfunction, a process examined by Peter Ward. He showcases the factors that cause cardiomyocyte contractility to wane during the disease.
Sepsis, the systemic inflammatory response syndrome that occurs during severe infection, kills more than 210,000 people in the US annually1. Developing new therapies for sepsis has been particularly frustrating, and over 25 trials of new agents have failed1. This failure has been partly due to a lack of understanding of the pathogenic mechanisms driving sepsis.
Two recent studies in human subjects shed light on one of the most important and relatively underrecognized mechanisms of sepsis immunopathology. Limaye et al.2 and Luyt et al.3 provide evidence that the otherwise dormant viruses cytomegalovirus (CMV) and herpes simplex virus (HSV) are reactivated in critically ill individuals—adding strength to the concept that a key aspect of critical illness is immunosuppression2,3. Most experimental therapies for sepsis have focused on attenuating the initial inflammatory response, ignoring—and possibly exacerbating—the progressive development of immunosuppression4–7. Although these approaches have demonstrated modest benefits in select groups of patients, the majority of deaths occur in patients with sepsis who are immune suppressed5–7. These two new studies should direct researchers toward fresh diagnostic and therapeutic approaches to sepsis.
Limaye et al.2 examined the incidence of reactivation of CMV in 120 CMV-seropositive critically ill individuals, many of whom apparently had sepsis2. Before their illness, these people had normal immunity. CMV viremia, as assessed by real-time PCR, occurred in 40 subjects (33%), indicating that CMV reactivation occurs frequently in the critically ill. CMV reactivation was associated with prolonged stay and death. These findings dovetail with an earlier study by Luyt et al.3 who reported a 21% incidence of HSV bronchopneumonitis that was attributed to viral reactivation in critically ill, immunocompetent individuals requiring prolonged mechanical ventilation3.
It is likely that only a modest number of the subjects in these two studies had clinically important viral infections. Rather, these studies show that critically ill individuals who had normal immunity before hospitalization become profoundly immunocompromised during a protracted illness, thereby enabling reactivation of latent viruses that may become clinically relevant.
Although the investigators didn’t specifically state the incidence of sepsis in their study populations, many of the subjects in these two studies had bacterial or fungal sepsis during their intensive care unit (ICU) stay2,3. For example, a number of patients in the CMV trial were hospitalized in the burn ICU, in which a substantial fraction of individuals invariably develop sepsis because of loss of barrier function. The HSV study population included subjects requiring prolonged mechanical ventilation, and ventilator-associated pneumonia occurs in up to 85% of this population. Clearly, CMV or HSV reactivation occurred in the setting of sepsis in many of the subjects.
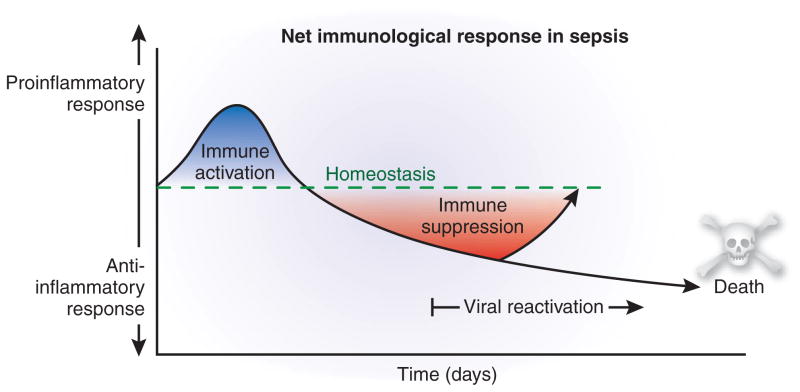
Sepsis initiates a complex immunologic response that varies over time (Fig. 1)4,5. Although recent studies show that both inflammatory and anti-inflammatory responses occur simultaneously in sepsis, the early net result is characterized by a hyperinflammatory response. The magnitude of this response varies depending on many factors, including the number and virulence of pathogens, and other disease conditions afflicting the patient4. With contemporary standard-of-care measures, the majority of patients survive the hyperinflammatory phase and enter a stage of protracted immunosuppression that has been termed ‘immunoparalysis’6,7. This immunosuppression in sepsis is manifested by loss of delayed type hypersensitivity response to positive control antigens, failure to clear the primary infection and development of new secondary infections6,7. Secondary, hospital-acquired infections include both virulent organisms such as Staphylococcus aureus and Clostridium difficile as well as organisms that are not particularly dangerous to nonimmunosuppressed individuals, such as Stenotrophomonas maltophila, Acinetobacter calcoaceticusbaumannii and Candida albicans. Infection with these latter organisms, as with CMV and HSV reactivation, highlights the marked immunosuppression in critically ill patients.
Figure 1.
Immunologic response in sepsis over time. Although both pro- and anti-inflammatory responses are activated early in sepsis, the proinflammatory response predominates. As sepsis progresses, the anti-inflammatory response becomes predominant, and it is during this later phase that secondary infections and viral reactivation occur. Early deaths during the early proinflammatory response phase are due to cytokine storm–mediated events, whereas later deaths during the anti-inflammatory phase are due to failure to control pathogens.
Multiple cellular mechanisms underlie immunosuppression in patients with sepsis, including activation of T regulatory cells and myeloid-derived suppressor cells8,9. Additionally, an early and ongoing immunopathology in sepsis is the apoptotic depletion of cells of both the innate and adaptive immune system10. Uptake of apoptotic cells further impairs host immunity by inducing an anti-inflammatory phenotype in phagocytic cells that consume the cellular corpses11. Prevention of this sepsis-induced apoptosis apparently attenuates the immunosuppressive cascade and leads to sustained immunity10. From this perspective, sepsis may be simply a race to the death between the host immune system and the pathogens, and pathogens gain an early advantage by inducing the death of billions of immune effector cells.
Additional mechanistic insights from these two studies can be obtained by comparison to studies of individuals with congenital immune deficiencies that confer susceptibility to particular classes of pathogens. HSV and CMV reactivation in critically ill patients suggests a T cell defect, and studies in ICU patients, especially those with sepsis, report extensive loss of CD4+ and CD8+ T cells. Natural killer cell counts are almost certainly reduced in sepsis, as well, and may also have a role in viral reactivation. Individuals with sepsis probably have multiple, interrelated immunologic defects owing to the extensive cross-talk between the specialized cells of the immune system that coordinate function to eradicate specific pathogens. For example, T cells make interferon-γ, a powerful macrophage activator, and, therefore, T cell dysfunction may result in macrophage defects.
Perhaps the most important implication of these two studies is that newer antibiotics alone are unlikely to substantially affect sepsis mortality. The fundamental problem in critically ill individuals is loss of immune competence; eradicating a particular class of pathogens will probably result in superinfection with other microorganisms. In addition to developing clinical practices to avoid infections, attention should be directed toward methods to enhance or restore immune function in critically ill individuals. Although there is risk of exacerbating the early hyperinflammatory phase of sepsis, methods to quantify the immune competence of each patient and appropriately time immunotherapy should minimize this danger.
Immunotherapy is being aggressively pursued in cancer research12. Similar to patients with sepsis, many people with cancer are immunosuppressed as a result of their underlying disease, and similar mechanisms, such as activation of myeloid-derived suppressor cells and T regulatory cells, may be involved in both disorders13. In this regard, a number of the immune-enhancing agents being investigated in cancer, such as antibody to programmed death-1 (ref. 14), agonistic antibody to CD40 (ref. 15) and interleukin-7 (R.S.H., J. Unsinger, D. Hildeman and C. Caldwell, unpublished data) are reported to improve survival in animal models of sepsis.
Research over the last few years has provided insight into the mediators of immune suppression during sepsis; the findings of Limaye et al.2 and Luyt et al.3 highlight the profound impact of prolonged critical illness on immune function and should stimulate basic research and development of methods to enhance immunity. In our opinion, reengaging or preserving host immune function will be the next major advance in the management of patients with sepsis.
Contributor Information
Richard S. Hotchkiss, Email: hotch@wustl.edu, Departments of Anesthesiology, Medicine and Surgery, Washington University School of Medicine, St. Louis, Missouri 63110, USA
Craig M. Coopersmith, Departments of Anesthesiology and Surgery, Washington University School of Medicine, St. Louis, Missouri 63110, USA
Jonathan E. McDunn, Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri 63110, USA
Thomas A. Ferguson, Department of Ophthalmology, Washington University School of Medicine, St. Louis, Missouri 63110, USA
References
- 1.Deans KJ, Haley M, Natanson C, Eichacker PQ, Minneci PC. J Trauma. 2005;58:867–874. doi: 10.1097/01.ta.0000158244.69179.94. [DOI] [PubMed] [Google Scholar]
- 2.Limaye AP, et al. J Am Med Assoc. 2008;300:413–422. [Google Scholar]
- 3.Luyt CE, et al. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Karl IE. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Osuchowski MF, Welch K, Siddiqui J, Remick DG. J Immunol. 2006;177:1967–1974. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 6.Adib-Conquy M, Cavaillon JM. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 7.Monneret G. Adv Sepsis. 2005;4:42–49. [Google Scholar]
- 8.Ni Choileain N, et al. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 9.Delano MJ, et al. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Nicholson DW. Nat Rev Immunol. 2006;6:813–822. doi: 10.1038/nri1943. [DOI] [PubMed] [Google Scholar]
- 11.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 12.Cheever MA. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 13.Stewart TJ, Abrams SI. Oncogene. 2008;27:5894–5903. doi: 10.1038/onc.2008.268. [DOI] [PubMed] [Google Scholar]
- 14.Huang X, et al. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwulst SJ, et al. J Immunol. 2006;177:557–565. doi: 10.4049/jimmunol.177.1.557. [DOI] [PubMed] [Google Scholar]