Abstract
As brain-resident immune cells, microglia (MG) survey the brain parenchyma to maintain homeostasis during development and following injury. Recent work in perinatal stroke, a leading cause of lifelong disability, has implicated MG as targets for therapeutic intervention during stroke progression. Although MG responses are complex, work in developing rodents suggests that MG limit brain damage and promote recovery after stroke. However, little is known about how energy-limiting conditions affect MG survival and mobility in developing brain tissues. Here, we used confocal time-lapse imaging to monitor MG viability and motility during hypoxia or oxygen-glucose deprivation (OGD) in neonatal hippocampal tissue slices derived from GFP-reporter mice (CX3CR1GFP/+). We found that MG in P5-P7 neonatal tissues remain viable for at least 6hr of hypoxia but begin to die after 2hr of OGD. Both hypoxia and OGD reduced MG motility. Unexpectedly, some MG retain or recover motility during OGD, and these active MG can contact and engulf dead cells. MG from younger neonates (P2-P3) are more resistant to OGD than those from older ones, indicating increasing vulnerability with developmental age. Finally, we show that transient (2hr) OGD reduces MG motility, migration, and viability. Although MG motility is rapidly restored after transient OGD, it remains below control levels for many hours. Together, these results show that MG in neonatal mouse brain tissues are vulnerable to both transient and sustained OGD, and many MG die within hours after onset of OGD. Preventing MG death may, therefore, provide a strategy for promoting tissue restoration after stroke.
Keywords: microglia, stroke, cell death, development
INTRODUCTION
Stroke is a leading cause of disability and death in the aged population but is also common in the very young. For example, cerebrovascular accidents occur in 20–30% of infants born at less than 35 weeks of gestation (Yager and Thornhill, 1997; Derugin et al., 2000). These early ischemic events often lead to long-term, devastating conditions including cerebral palsy, seizures, and cognitive disorders (Nelson, 2007). A better understanding of the cellular basis of brain responses to stroke could lead to more effective treatments in pre-term and term infants and children.
Classically, stroke research has focused on the acute effects of energy depletion on neurons. However, this neurocentric view is being complemented by an emerging glio-consciousness that recognizes roles for glial cells in the progression and potential recovery from stroke. As mediators of inflammation, brain-resident microglial cells (MG) have been thought to mostly exacerbate acute ischemic injury (see Lai and Todd, 2006; Yenari et al., 2010; Deng et al., 2011). However, several lines of evidence from experimental models of ischemia in rodent suggest that MG can improve outcome after stroke by supporting tissue homeostasis, rapidly clearing dead cells, and secreting trophic factors that promote cell survival and regeneration (for review, see Weinstein et al., 2010). For example, depleting endogenous MG in adult (Lalancette-Hebert et al., 2007) or neonatal (Faustino et al., 2011) brain in vivo, or in ex vivo slices (Montero et al., 2009), worsens stroke outcome. On the other hand, introduction of exogenous MG improves stroke outcome both in vivo (Kitamura et al., 2004; Kitamura et al., 2005; Hayashi et al., 2006; Imai et al. 2007) and in ex vivo preparations (Neumann et al., 2006). Moreover, MG can reduce neurotoxic effects in ex vivo slices by engulfing infiltrating neutrophils (Neumann et al., 2008). MG also release growth factors and cytokines, including TNF-α, which protect neurons (Lambertsen et al., 2009). Together, these studies indicate that activated MG may play important roles in tissue repair after stroke.
Although it is clear that ischemia induces rapid responses in MG in neonatal brain in vivo (Ivacko et al., 1996), there is little information on the consequent effects of ischemia on MG survival. Studies using cultured rodent MG report very different levels of cell death (15–88%) following simulated ischemia (Lyons and Kettenmann, 1998; Yenari and Giffard, 2001). However, such in vitro studies are potentially complicated by the possibility that cultured cells may develop resistance to hypoxia (Chock and Giffard, 2005). To date, MG viability and motility during ischemic conditions in developing brain tissue have remained largely uninvestigated.
To address these questions, we used oxygen-glucose deprivation as a model of stroke in neonatal mouse brain slices. Confocal time-lapse imaging in slices from GFP-reporter mice enabled us to directly monitor MG motility and survival during and following energy-limiting conditions. The results indicate that MG in neonatal brain tissues respond to stroke-like conditions in diverse ways: some MG are resistant and seem little affected, others show a transient or sustained reduction in cell motility, and many MG die.
MATERIALS AND METHODS
Animals and Preparation of Tissue Slices
Reporter mice expressing GFP under the control of the fractalkine receptor (CX3CR1) promoter were used for all experiments. Although only CX3CR1+/GFP mice were used in these experiments, it has been reported that in response to ischemia, wild type and CX3CR1+/− heterozygous mice show similar infarct volumes, though CX3CR1−/− null mice had less injury (Denes et al., 2008). This suggests that mice lacking one copy of CX3CR1 are not significantly different from wildtype mice in an ischemic context. In these mice, microglia (MG), as well as perivascular cells and meningeal cells that are easily distinguishable from parenchymal MG, are labeled green in the brain (Jung et al., 2000). Acutely isolated hippocampal slices were prepared from neonatal (P5-P7 unless otherwise stated) mice as detailed previously (Kurpius et al., 2007). Briefly, mice were swiftly decapitated, and brains were removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) with the following composition (in mM): NaCl 124; KCl 3; NaH2PO4 1.3; MgCl2 3; HEPES 10; CaCl2 3; glucose 10. Excised hippocampi were cut transversely (400µm thick) using a manual tissue chopper (Stoelting). Slices were maintained in HEPES-buffered aCSF. Animals were used in accordance with institutional guidelines, as approved by the animal care and use committee.
Hypoxia and Oxygen-Glucose Deprivation
Oxygen deprivation (hypoxia) was achieved by bubbling N2 into aCSF media for 15 minutes. Combined oxygen and glucose deprivation (OGD) was achieved by replacing 10mM glucose with 10mM sucrose in the aCSF medium followed by bubbling N2 into aCSF media for 15 minutes. In some cases, Sytox Orange (Molecular Probes, #S11368) or Topro-3 (Molecular Probes, #T3605) were added at 1:10,000 to aCSF (DMSO concentration at 0.0001%) before N2 bubbling. Only cells whose membranes are compromised (leaky) are labeled with Sytox or Topro-3. The volume of media used for each experiment ranged from 4–10 mL. The media was warmed briefly in a 37°C water bath and immediately applied to previously mounted slices, and the chamber was sealed and prepared for imaging. In some transient OGD experiments, the sealed chamber was maintained for two hours in a warm box (37°C) prior to subsequent mounting and imaging.
Time-Lapse Confocal Imaging
Four to twelve acutely excised tissue slices were mounted in a custom-built closed chamber containing ~3 mL HEPES-buffered aCSF. The chamber was then placed on the microscope stage and warmed to about 35°C by continuous, gentle warm air (Kurpius et al., 2007). Fluorescence images were captured using a Leica SP5 MP confocal/multiphoton imaging system with a xyz motorized stage on an inverted platform. For confocal microscopy, the following probes were imaged with the indicated laser lines: GFP (Argon 488 nm), Sytox Orange (HeNe 543 nm), Topro-3 (HeNe 633 nm). The confocal pinhole typically was opened to two Airy disc units to improve light collection and signal-to-noise. The chamber media was not changed during the course of imaging, except when performing transient OGD experiments. Pilot experiments showed that under control conditions with 12 slices in a chamber, neonatal MG remain actively motile for at least 8 hours of imaging without any media change. To capture a large (775 µm × 775 µm) field of view containing 40–60 MG, images were collected using a 20X/0.7 Plan Apo objective lens at a resolution of 1.4 pixels/µm. A typical time-lapse imaging experiment captured 15 confocal optical planes at 3µm z-step intervals spanning 45–60 µm in the axial (z) dimension from the slice surface. Stacks of confocal images were usually captured at 3–10 minute intervals. For all experiments, multisite imaging of several slices was employed. In some cases, this allowed us to image tissue slices from separate littermate animals simultaneously under identical conditions. Imaging sessions typically commenced about 30 min after tissue slicing and lasted several hours. Some transient OGD experiments were performed after several hours of incubation in the appropriate (control or OGD) media in a 37°C warm box.
Image Processing
Images were collected and collated using Leica LAS AF software. Image stacks were assembled using Leica LAS AF software or ImageJ (Wayne Rasband, NIH). All images were processed using the “Smooth” filter in ImageJ to reduce noise. In all cases, comparisons were made on images processed identically. All movies generated represent the same xyz tissue volume, although they may differ in lengths of time.
Analysis of Microglial Cell Death
MG cell death events were identified by concomitant loss of GFP and gain of Sytox. Counts were made manually for the duration of a time-lapse sequence for each field of view analyzed. GFP-expressing parenchymal MG, but not GFP-expressing perivascular or meningeal cells, were counted. Because a few cells moved in or out of focus, the total number of MG cells was calculated as the sum of the number of GFP-positive MG cells at the end of the experiment (which represents the number of living MG cells) plus the number of dying MG cells counted during the sequence. Percent cell death was calculated as follows: (# of dead MG cells / total # of MG cells) × 100.
Analysis of Microglial Motility
We developed a simple measure of MG cell motility using ImageJ. First, 3D image stacks were combined to make 2D projection images for each time-point. Next, to account for any x-y tissue drift during the imaging session, 2D projection images were registered using the StackReg plugin (Thevenaz et al., 1998) running in ImageJ. Registered images were then smoothened to reduce background noise. To define the cell boundary, an arbitrary threshold was applied uniformly to all images in a given time sequence. To generate difference images, the absolute difference between two sequential thresholded images in a time series was calculated using the ‘Difference’ tool of the ‘Image Calculator’ feature of ImageJ. This is summarized in Supplemental Figure 1. Sequential difference images in a time sequence (i.e., image 1 and image 2) were used to generate a motility index (MI), which is a percent change in area calculated as follows:
MI = (Area of difference between image 1 and image 2/Total suprathreshold area of image 1) × 100.
MI was used for both single cell and multiple cell analysis.
Statistical Analysis
Data from several slices and different animals were pooled and analyzed. Each animal represents a separate experiment. All results are reported as mean ± standard deviation of the population. Statistical significance was assessed using Student's t test at the significance level P < 0.05.
RESULTS
Prolonged OGD but not hypoxia induces MG cell death in developing brain tissue slices
We showed previously that microglia (MG) are viable and sustain high levels of motility and migration for many hours in acutely isolated neonatal hippocampal slices (Stence et al., 2001; Kurpius et al., 2006; Kurpius et al., 2007). Therefore, we used this preparation to study effects of stroke-like conditions on MG motility and viability in live neonatal brain tissues. To directly assess the vulnerability of MG to hypoxic-ischemic injury, we used two-channel time-lapse confocal imaging of acutely excised hippocampal slices from GFP-reporter mice bathed in Sytox-containing artificial cerebrospinal fluid (aCSF). Sytox is a membrane impermeable DNA-binding dye that labels only dead and dying cells with compromised membranes. Because MG in these mice constitutively express GFP (Jung et al., 2000), these cells can be easily identified and a progressive loss of GFP can be used to assess MG cell death as it occurs in real time. We focused our analyses in hippocampal area CA1 because this area is especially vulnerable during ischemia (Lehotsky et al., 2009).
Under control imaging conditions, many non-MG cells (lacking GFP expression) in acutely isolated neonatal (P5-P7) hippocampal slices take up Sytox during the first several hours of imaging (Dailey and Waite, 1999), presumably as a result of traumatic injury induced by tissue slicing (Fig. 1A). Already by 2 hr, many cells lacking GFP were labeled by Sytox and others became labeled over the subsequent 4 hr (Fig. 1A arrows and arrowhead). However, under these control conditions we never observed death of a MG cell as determined by loss of GFP and subsequent uptake of Sytox (n = 740 MG cells, 14 slices, 3 animals).
Figure 1. OGD induces microglial cell death in acutely isolated neonatal hippocampal slices.
A, Time-lapse imaging shows that many non-microglial cells die under control conditions, as shown by Sytox labeling of nuclei of non-GFP expressing cells. Several dying cells are evident within the first two hours after tissue excision. More non-microglial cells die during the next several hours (arrows and arrowhead), but no GFP-expressing microglia die (assessed by loss of GFP and gain of Sytox) during this period of time. B, Images from a time-lapse movie of a microglial cell that dies during OGD. The microglial cell loses GFP and within minutes becomes labeled with Sytox. Note that the cell loses GFP before showing obvious signs of blebbing. See Supplemental Movie 1. C, Quantification shows that, for the dying microglia in (B), the GFP signal rapidly decreases in the soma as Sytox signal subsequently increases in the nucleus. D-D’, Some microglia become unhealthy looking after prolonged OGD (D’), showing rounded soma (arrow) and beaded branches (arrowheads), but retain GFP and fail to take up Sytox (red).
When hippocampal slices were imaged continuously for 6 hr under conditions of hypoxia or oxygen-glucose deprivation (OGD), we found that many MG cells rapidly lost GFP signal (within 5–10 min) and subsequently gained Sytox (Fig. 1B, C and Supplemental Movie 1). However, some unhealthy looking MG with beaded branches and/or rounded soma maintained their GFP fluorescence and failed to take up Sytox throughout the 6 hr of OGD (Fig. 1D, D’). Thus, we construed loss of GFP signal as an indicator of loss of membrane integrity coinciding with MG cell death.
Although MG death events were not observed during control conditions, they were frequently observed during severe energy depleting conditions (Fig. 2A). Under hypoxia alone, MG cell death was infrequent and not significantly higher than control conditions. We observed only 1.1 ± 1.8 MG cell death events per field of view (range: 0–7) during 6 hr of hypoxia, amounting to 1.9% of MG (n = 24 slices from 3 animals, Fig. 2A, B). A little over half (14 of 24) of the hypoxic slices had no MG cell death events. In contrast, during 6 hr of continuous OGD, the number of dying MG cells increased dramatically to 22.1 ± 3.8 per field of view (range: 14 to 27), corresponding to 44.0% of MG (n = 16 slices from 3 animals; Fig. 2A, B). This increase in MG cell death was highly significant when compared to both control and hypoxic conditions. MG death rarely occurred before 2 hr of continuous OGD and increased over the next several hours (Fig. 2C), peaking at about 5 hr (Fig. 2D). When slices were returned to normal oxygen-glucose conditions after 6 hr of OGD, unhealthy-looking MG (as in Fig. 1D’) did not regain normal morphology or motility (data not shown). Taken together, our results show that neonatal MG in brain tissues survive 6 hr of hypoxia but are vulnerable to 6 hr of OGD.
Figure 2. OGD, but not hypoxia, induces microglial cell death in acutely isolated neonatal hippocampal slices.
A, Representative images of slices with GFP-expressing microglia at the beginning and end of six hour long imaging experiments under control (left), hypoxia (middle), and OGD (right) conditions. All microglia under control conditions remain viable during this time (assessed by retention of GFP). Under hypoxia, only one microglia cell in the field of view (arrow) dies, as assessed by loss of GFP (arrowhead). In contrast, several microglia die during 6hr of sustained OGD (arrows and arrowheads), and some of the surviving microglia have rounded somata (dashed circles). B, The percent of dying microglia was determined by the method in (A) for control, hypoxia, and OGD conditions. No microglial cell death was observed in control slices. Microglial cell death increased significantly during OGD but not hypoxia. C, Graph shows a cumulative increase in microglial cell death during 6 hours of sustained OGD. D, Graph shows average number of microglia that die in each field of view per hour of OGD. Significance in C-D is shown relative to the first hour. *P ≤ 0.05; ***P ≤ 0.000005
Both hypoxia and OGD inhibit MG motility
MG process motility is important for MG functions including regulation of synaptic remodeling, migration to sites of injury, and phagocytosis of dead cell debris [for review, see Parkhurst and Gan (2010)]. These MG functions are critical for tissue recovery after stroke. To investigate the effect of energy-limiting conditions on MG motility in neonatal tissues, motility was quantified by calculating changes in cell morphology in sequential images of time-lapse movies to obtain a motility index (MI) (See Methods and Supplemental Fig. 1). Under control conditions, many MG were continually motile, repeatedly extending and retracting processes for the duration of the imaging period, as evident in difference images (Fig. 3A, top row). In contrast, both hypoxia and OGD caused a persistent reduction in MG process dynamism, especially in the stratum radiatum (SR) region of the hippocampus (Fig. 3A, middle and bottom rows, respectively). Quantitative analysis of MG motility over large fields of view containing tens of MG cells in the SR of area CA1 confirmed these qualitative impressions (Fig. 3B). Under control conditions, MG process motility generally increased over the first couple of hours as MG responded to neighboring cells damaged during tissue excision. Thereafter, MG process motility was maintained at a high level for up to 6 hr (n = 6 slices from 3 animals). Conversely, hypoxia (n = 6 slices from 3 animals) and OGD (n = 5 slices from 2 animals) caused a significant reduction in MG process motility over the first two hours of imaging, and this low level was maintained for the rest of the imaging period (Fig. 3B). When averaged over the duration of the 6 hr imaging period, the mean MG motility index was significantly lower in hypoxia- and OGD-treated slices relative to control (Fig. 3C). There was no significant difference between hypoxia- and OGD-treated slices (Fig. 3C and Supplemental Movie 2), indicating that MG viability and motility are differentially affected by hypoxia and OGD.
Figure 3. Both hypoxia and OGD reduce overall microglial process motility in stratum radiatum of hippocampal area CA1.
A, Representative difference images showing microglial motility in the CA1 stratum radiatum during control (top), hypoxia (middle), or OGD (bottom). Microglia maintain their process motility under control conditions, but loss of signal in these difference images shows that motility is substantially reduced under both hypoxic and OGD conditions. See Supplementary Movie 2. B, Quantitative analysis of motility (motility index) of microglia in several hippocampal slices per condition shows an increasing and sustained high level of motility in control condition but rapid and persistent depression of microglia motility in hypoxia- and OGD-treated slices. C, The average motility index over the 6 hr of treatment was significantly reduced in both the hypoxic and OGD groups relative to control. ***P ≤ 0.000005
Some MG are resistant to OGD
Although the motility of most MG in the SR layer of hippocampal area CA1 was inhibited by OGD, we noticed that some MG in the SR and in other layers—stratum pyramidale and stratum oriens (SP and SO)—were resistant to OGD. Individual MG cells fell into one of three general categories of motility behavior, which we termed Type 1, 2, and 3 (Fig. 4A). Type 1 MG cells were persistently motile throughout the imaging period. These cells showed a high level of process dynamism that was evident in difference images as large white patches around the cell margin (Fig. 4A, top row). Type 2 MG cells were initially quiescent but became motile later in the imaging period, either after initial branch retraction (e.g., during control conditions) or after a transient stall in process motility (e.g., during the early stages of hypoxic or OGD conditions) (Fig. 4A, middle row). Finally, Type 3 cells showed little process dynamism throughout the imaging period (Fig. 4A, bottom row).
Figure 4. Microglial motility can persist or spontaneously recover under hypoxia or OGD.
A, Difference images showing individual microglial cells that were persistently motile (Type 1 cells), transiently immotile (Type 2 cells), or persistently immotile (Type 3 cells). Type 1 and Type 2 microglial cells showed high levels of motility around the edges of cell body and processes (red arrows). Conversely, Type 3 cells retained their branch processes (numbered in the raw image), and the position and shape of the soma was essentially unchanged (dashed red lines). B, Graph showing the distribution of Type 1, 2, and 3 microglial cells after 6 hr in control, hypoxia, or OGD conditions. The percent of Type 1 (persistently motile) cells was significantly reduced during hypoxia and OGD. Type 3 (immotile) cells were never observed during control conditions. The total number of cells analyzed per condition is shown above each bar. C, Image of a slice during hypoxia with microglia (green) and Sytox-labeled dead cells (red) showing representative distribution of Type 1 (white arrows), Type 2 (white arrowheads), and Type 3 (yellow arrowheads) microglial cells. Note that many of the active microglial cells (Types 1 and 2) are found in or near the neuron-populated SP. SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
Under control conditions, all MG cells were active, with 91.1% of MG falling into the Type 1 (persistently motile) category. The percent of Type 1 MG in the CA1 region of hippocampus dropped significantly to 37.9 % during hypoxia and to 30.3 % during OGD (Fig. 4B). Whereas the percent of Type 2 (transiently immotile) cells remained largely unchanged during hypoxia relative to control (9.9 % and 8.9 % under control and hypoxic conditions, respectively), it was halved during OGD conditions (4.7 %). Conversely, the percent of Type 3 (immotile) MG increased from 0 % under control conditions to 52.2 % during hypoxia and 65.0 % during OGD. Although the majority of MG were immotile during hypoxia and OGD, to our surprise, some MG remained active under these conditions, showing little or no inhibition of motility during OGD (47.8 % during hypoxia and 35.0 % during OGD). These OGD-resistant MG were usually located in the stratum oriens (SO) or stratum pyramidale (SP) (Fig. 4C).
To determine whether the residual motility of MG during OGD can support important MG functions such as phagocytosis, we assessed MG engagement with neighboring Sytox-labeled dead and dying cells in tissue slices. Time-lapse sequences showed that some MG that recovered motility or remained active during OGD could engulf nearby dead cells (Fig. 5 and Supplemental Movie 3). Thus, while energy-depleting conditions largely inhibit MG motility, a level of motility is retained or restored in some tissue MG that enable them to engage and clear dead and dying cells.
Figure 5. OGD-resistant microglia can contact and phagocytize dead cells during OGD.
A, Hippocampal slice during OGD showing MG (green) amongst Sytox-labeled dead cells (red) in the stratum radiatum (SR) and stratum pyramidale (SP). B, Time-lapse sequence of boxed region in (A). A Sytox-labeled dead cell (arrow) is contacted and eventually engulfed by a nearby type 2 MG cell during continuous OGD. Images in A and B are projection images of 6 image planes (18 µm deep). Time is shown in hr:min. See Supplemental Movie 3.
MG viability and migration during OGD differ developmentally
Although earlier studies suggested that the immature brain is more resistant to ischemia than the mature brain, more recent studies have challenged this concept (Schaller, 2007). To determine whether MG viability and motility are developmentally sensitive, we compared MG in tissue slices from younger (P2-P3) and older (P6-P7) neonates. We found that MG in younger neonates are in general more resistant to sustained OGD, based on several parameters. First, the number of dying MG per field of view was significantly lower in tissues from younger neonates (2.2 ± 1.5 MG cell death events in 20 slices from 3 animals) than in tissues from older neonates (20.9 ± 4.1 MG cell death events in 11 slices from 2 animals). Because tissue slices from younger mice had fewer total MG, we determined the percent MG cell death in the hippocampal MG population and confirmed that this was also significantly lower in tissues from younger neonates (6.1 ± 4.2 %) than older ones (42.7 ± 10.9 %) (Fig. 6A; P < 0.0005). Furthermore, the timing of earliest MG cell death was significantly later in tissues from younger (199 ± 26 minutes per slice) versus older (123 ± 40 minutes per slice) neonates (Fig. 6B; P < 0.0005).
Figure 6. Developmental sensitivity of microglial viability and motility in neonatal hippocampal slices.
Hippocampal slices from younger (P2/P3) or older (P6/P7) neonates were imaged for 6 hours during OGD. A–B, Quantitative analysis showed that microglia in the younger tissues were much less likely to die (A) and survived longer (B) under OGD. C, The fraction of microglia that were motile during OGD was much higher in the younger tissues. D-E, The most active microglia during OGD had a higher peak velocity (D) and travelled farther (E) in the younger tissues. F-G, Two representative examples of the most active microglial cells showing migration tracks (colored lines in 6 hr images) during sustained OGD in a P2 (F) and P6 (G) tissue slice. Arrowheads in G indicate four microglial cells that died during the imaging session. None of the microglia in this field of view died in the P2 slice. Time is shown as hr:min. See Supplemental Movie 4. SO: stratum oriens; SP: stratum pyramidale; SR: stratum radiatum.
The effects of OGD on MG motility and migration also differed with developmental age. The fraction of persistently motile (Type 1) MG decreased significantly from 82.2 ± 12.8 % in P2-P3 slices to 19.4 ± 13.9 % in P6-P7 slices (Fig. 6C; P < 0.0005). Also, we tracked the migration of surviving MG during OGD and found that the fastest MG in P2-P3 neonatal slices migrated at a significantly higher peak velocity (1.8 ± 0.4 µm/min per slice; n = 15 cells from 5 slices) than surviving MG in P6-P7 slices (0.34 ± 0.1 µm/min; n = 15 cells from 5 slices, Fig. 6D; P < 0.0005). Consequently, the total distance travelled by MG during 6 hr of OGD dropped significantly from 857 ± 239 µm to 298 ± 74 µm as development progressed (Fig. 6E; P < 0.0005). The migration tracks of two representative cells each in younger (P2) and older (P6) tissue slices, respectively, are shown (Fig. 6F,G and Supplemental Movie 4). These results indicate that MG are functionally more resilient to sustained OGD in younger neonatal tissues than older ones.
Transient (2hr) OGD has lasting effects on MG motility, migration, and survival
Thus far, our energy-limiting conditions were applied continuously for several hours. However, hypoxic-ischemic episodes in the brain can result from transient blood vessel occlusion followed by reperfusion. Therefore, we investigated the effect of transient OGD (tOGD) on MG process motility and survival. We used a 2 hr transient OGD period because we rarely observed MG cell death in neonatal (P6-P7) slices during this time period (Fig. 2C,D). The transient OGD imaging protocol consisted of baseline recordings for one hour, followed by recordings during OGD for two hours, and concluding with an hour of imaging during washout to mimic reperfusion.
As observed during sustained (6 hr) OGD, we found that most MG became immotile during transient (2 hr) OGD, but some maintained their motility throughout OGD (Fig. 7). As noted above, cells in the SP usually remain motile during OGD (Fig. 7B, top row) while those in the SR become immotile (Fig. 7B, bottom row). For those MG that were inhibited by tOGD, motility was reduced within 15 min of OGD exposure and was subsequently maintained at low levels throughout the 2 hr tOGD period. However, MG rapidly recovered upon washout. As early as 3 min into the washout period, the motility of extant processes was restored, and within 15 min of OGD washout, new MG processes were generated (Fig. 7B; see also Supplemental Movie 5). Quantitative analyses showed that the motility of persistently motile Type 1 MG (n = 12 cells from 3 slices) continued largely unchanged during the 4 hr imaging period (Fig. 7C,D). However, MG cells that become immotile during transient OGD had a lower MI to start with but recovered even beyond baseline levels after recovery from OGD (n= 12 cells from 4 slices) (Fig. 7C,D). These observations indicate that MG in developing brain tissues can resist or rapidly recover from short term (≤2hr) energy-limiting conditions.
Figure 7. Motile and immotile microglia during transient (2hr) OGD.
Microglial motility was monitored for 1 hr before, 2 hr during, and 1 hr after transient OGD (tOGD). A, Representative image of area CA1 in a P6 hippocampal slice subjected to tOGD. B, Top Row: Difference images of a Type 1 microglial cell in the SP (top box in Panel A) showing that motility is maintained during OGD. Bottom Row: Difference images of a representative Type 3 cell in the SR showing inhibition of motility during tOGD but rapid recovery of motility following washout. C, The motility indices of several cells that displayed motile (n=12) or immotile (n=12) phenotypes were averaged. Note that immotile cells started with a lower baseline motility level, were rapidly inhibited by OGD, but recovered motility immediately after OGD washout. D, The average motility was calculated for the baseline, OGD, and washout periods. Motility for ‘immotile cells’ was significantly depressed during OGD and enhanced immediately after OGD. Cell motility for persistently ‘motile cells’ did not change significantly throughout the observation period. See Supplemental Movie 5. ***P ≤ 0.000005. SP: stratum pyramidale; SR: stratum radiatum.
Thus far, our transient OGD studies focused solely on the motile responses of MG during and immediately following the insult. We extended these studies to investigate possible longer-term effects of transient OGD on MG motility, migration, and viability. For these studies, hippocampal slices were isolated from neonates, and half were carried under normal oxygen and glucose levels (control) while the other half were subjected to 2 hr OGD. After treatment, both control and tOGD slices were placed together in imaging chambers containing normal aCSF, and then imaged simultaneously by multi-site time-lapse imaging for several hours starting at 4 hr after the treatment period (Fig. 8A). Over this 6 hr post-treatment period, motility indices for OGD-treated slices (7 slices from 2 animals) were persistently lower than those for their control counterparts (11 slices from 2 animals) (Fig. 8B). Transient OGD reduced average motility levels to about two-thirds of normal for at least 6 hr (Fig. 8B,C). Given the lower MG motility levels in transient OGD-treated slices relative to control slices, we predicted that these MG would also migrate poorly. Indeed, analysis of MG migration showed that the most motile MG cells traveled much shorter distances in slices previously subjected to transient OGD (246 ± 56µm) relative to MG in control slices (490 ± 234µm; Fig. 8D).
Figure 8. Transient (2hr) OGD caused a prolonged alteration of microglia behavior.
Multiple slices from the same animals were exposed to either control conditions or transient OGD for 2 hr, returned to normoxic/normoglycemic conditions for 4 hr, then mounted and imaged simultaneously at 3 min intervals for several hours. This serves as an extended ex vivo “reperfusion” model. A, Representative time-lapse sequences (difference images) during the simulated reperfusion period from two daughter slices previously subjected to control conditions (top row) or tOGD (bottom row). Time is shown as hours after end of 2hr of OGD. B–C, Quantification of microglia motility shows a persistent and statistically significant reduction in microglial motility during the washout period following OGD. D, Microglial migration distance was significantly reduced during the 6 hr period following 2 hr tOGD. F, There was a significant increase in the number of MG that died in the 6 hr period following 2 hr tOGD. ***P ≤ 0.0005
We next assessed MG viability and found a significant increase in the number of MG cell death events following transient OGD (mean = 3.2 ± 1.9; 35 total events; range: 0 to 6 events per field of view in 11 slices from 2 animals) compared to control (mean = 0.17 ± 0.37; 2 total events in 12 slices from 2 animals) when assessed at 6 hr after the end of transient OGD (Fig. 8E; P < 0.0005). Taken together, these results suggest that even transient (2hr) OGD has persisting effects on the motility, migration, and viability of MG in neonatal brain tissues.
DISCUSSION
We investigated in real time the viability and motility of microglia (MG) in developing mouse brain tissue slices during energy-limiting conditions. We show that virtually all MG survive for at least 6 hr during sustained hypoxia, whereas nearly half of the MG die within 6 hr of sustained oxygen and glucose deprivation (OGD). Additionally, both hypoxia and OGD reduced the number of motile MG as well as the level of motile activity. However, some OGD-resistant MG persist and can engage and phagocytize nearby dead cells. During sustained OGD, MG from early neonates survive better and are more motile than those from older neonates. Finally, we show that even transient (2 hr) OGD significantly reduces MG viability and has long-lasting effects on MG motility and migration.
Microglial Viability during OGD
MG cell death studies have been performed in cultured MG where varying degrees of susceptibility to 6 hr of OGD were reported (Lyons and Kettenmann, 1998; Yenari and Giffard, 2001). However, cell metabolism may change during long-term cell culture, and the extent to which these results reflect cell behaviors in living tissues and in vivo has been unclear. MG have been detected in the ischemic core 24 hours after the onset of permanent middle cerebral artery occlusion (MCAO) in both adult (Mabuchi et al., 2000) and neonatal (Wen et al., 2004) rat brain, suggesting that some MG may survive for some time under permanent ischemia. Recent data has suggested that after 24 hours of transient MCAO, the density of MG decreases by about 40% in the ischemic core relative to the penumbra (Faustino et al., 2011). However, clear indication of the degree of MG cell death in living brain tissues during ischemia is lacking. Indeed, it is difficult to estimate the fraction of surviving MG from such in vivo studies where individual MG are not followed due to possible proliferation of surviving MG, migration from surrounding tissues, or infiltration of hematogenous cells of monocyte lineage.
We used an ex vivo neonatal acute brain slice system because it retains the native tissue organization of neurons and glia and allows for greater accessibility for imaging. It also avoids potential problems associated with changes in cell metabolism in long-term cell and tissue culture preparations, or infiltration and redistribution of cells in vivo. Using two-channel time-lapse imaging, we monitored MG viability in developing brain tissues and, in line with previous in vitro cell culture studies (Lyons and Kettenmann, 1998; Yenari and Giffard, 2001), show that MG cell death is rapidly induced by OGD but not hypoxia. Additionally, we determined the timing of MG cell death and showed that neonatal MG begin to die after just two hours of OGD. MG in neonates were recently shown to help protect the developing brain during stroke (Faustino et al., 2011). In light of this, our observation of MG death both during sustained OGD and following transient OGD suggests that enhancing MG survival during ischemic injury could serve as a neuroprotective strategy for neonatal stroke.
To enhance MG survival, an understanding of the modes and mechanisms of MG cell death during OGD is critical. During ischemia, both necrotic and apoptotic modes of cell death have been reported (Mehta et al., 2007), and anti-apoptotic treatment is protective in both adults (Li et al., 2012; Lee et al., 2004; Fong et al., 2010) and neonates (Nijboer et al., 2011, Carlsson et al., 2011). Whether MG death during OGD is necrotic, apoptotic, or necroptotic (Vandenabeele et al., 2010) has not yet been determined. However, during in vivo neonatal ischemia, MG reportedly activate an apoptosis executioner caspase, caspase-3 (Manabat et al., 2003; Peng et al., 2010, Ness et al., 2008). It is presently unclear whether this caspase-3 activation indicates MG commitment to apoptotic cell death, or MG activation that does not lead to cell death (Burguillos et al., 2011).
With regard to mechanisms that lead to cell death during ischemia, other CNS cell types are vulnerable to ischemic insult via calcium dysregulation, resulting in calcium overload and excitotoxicity (Szydlowska and Tymianski, 2010). Indeed calcium overload has been shown to induce MG cell death (Nagano et al., 2006), so it is possible that this mechanism contributes to MG death during OGD. Both glutamatergic and purinergic signaling have been shown to mediate excitotoxic death in neurons, astrocytes, and oligodendrocytes (Matute, 2011). Although, to the best of our knowledge, glutamate receptors are not expressed in neonatal MG, it has been reported that MG in the adult hippocampus express glutamate receptors a few days after transient ischemia (Gottlieb and Matute, 1997). However, glutamate failed to elicit electrophysiological or morphological responses in hippocampal (Wu and Zhuo, 2008) and retinal (Fontainhas et al., 2011) MG, suggesting that in the resting/surveillant state MG lack functional glutamate receptors and transporters. Thus, during early stages of ischemia, MG may be less vulnerable to glutamate excitotoxicity because they may only express glutamate receptors after some delay following activation (Matute et al., 2006; Matute, 2011).
On the other hand, it is well established that MG express several P2X and P2Y type purinergic receptors in the resting state. Recently, both the P2X4 and P2X7 receptors have been implicated in ATP-induced cell death in macrophages (Hanley et al., 2012; Kawano et al., 2012). Both of these receptors are detected in MG as early as E16 (Xiang and Burnstock, 2005), and they are upregulated in hippocampal MG in response to ischemia (Cavaliere et al., 2003; Cavaliere et al., 2005). It is tempting to speculate that, with high levels of extracellular ATP during OGD (Dale and Frenguelli, 2009), purinergic receptor activation could mediate a type of excitotoxic MG cell death. Whether calcium influx induced by glutamate- or ATP-dependent signaling mediates OGD-induced MG death remains to be determined.
Microglial Motility during OGD
MG motility has been extensively studied during normal and pathological states (for reviews, see Parkhurst and Gan, 2010; Ohsawa and Kohsaka, 2011). Baseline MG motility may monitor and remove synapses with reduced synaptic activity after ischemia (Wake et al., 2009) and contribute to maintenance of the blood brain barrier (Parkhurst and Gan, 2010). However, while several studies of MG motility have been performed in adult brain, motility in neonatal brain in vivo remains little studied under normal or stroke-like conditions. The present work is the first to report on the effects of OGD on MG motility in neonatal brain tissues and shows that both hypoxia and OGD significantly inhibit motility of neonatal MG.
Studies in adult CNS have differed with respect to effects of blood flow loss on MG motility. In the post-mortem spinal cord of adult mice, MG motility is retained for a period of time independent of blood flow (Dibaj et al., 2010). However, recent two-photon in vivo imaging in the cortex of adult mice showed that disruption of blood flow by photothrombosis, transient artery occlusion, or global ischemia abolished MG motility (Masuda et al., 2011). This latter study reported that restoration of blood flow after a 30 minute occlusion restored MG motility. While our results are consistent with Masuda et al. (2011) in that they show a reversible loss of MG motility, we extended these findings by showing that neonatal MG can regain motility and migration during OGD, albeit at reduced levels.
Paradoxically, in P11-27 rat hippocampal slices there is an increase in levels of extracellular ATP during OGD and a burst of ATP release following OGD (Dale and Frenguelli, 2009). Since ATP acts as a MG chemotactic and chemokinetic signal (Honda et al., 2001, Davalos et al., 2005; Haynes et al., 2006), it is possible that this ATP burst promotes MG motility after OGD (see Fig. 7C). We showed that, despite an initial restoration of MG motility, it is significantly reduced several hours later. This persistent reduction of motility may result from low extracellular ATP levels after the ATP burst following transient OGD. Indeed, cultured astrocytes subjected to two hours of transient OGD show a dramatic increase in ATP release during the first hour of washout, but ATP release drops significantly below control levels 5 hours later (Iwabuchi and Kawahara, 2009). Similarly, there is evidence of “secondary energy failure” in the neonatal brain 4–6 hours into reperfusion after transient hypoxic-ischemic injury (Ten et al., 2010), which may also contribute to the reduced MG activity levels. Together, these studies suggest that changes in both extracellular and intracellular ATP levels following stroke could regulate MG motility.
OGD-Resistant MG
Although most MG were inhibited by sustained 6 hr OGD, we found that about 30 % of MG in acute P5-P7 hippocampal slices resisted the motility inhibiting effects of OGD and either retained or regained motile activity during energy limiting conditions. The basis for this resistance is currently unclear, but “resistant” MG were frequently located near the pyramidal cell body layer (SP). It is possible that MG in proximity to the neuronal cell body layer experience higher levels of metabolites that confer resistance to OGD. Both neurons and astrocytes release ATP during OGD (Liu et al., 2008; Dale and Frenguelli, 2009; Iwabuchi and Kawahara, 2009), and both astrocytes and MG participate in regenerative ATP-induced ATP release (Davalos et al., 2005; Dou et al., 2012). It is, therefore, possible that even during OGD, the extracellular purine levels near the stratum pyramidale (SP) may facilitate resistance of MG in this region.
Given that these OGD-resistant MG are motile, we were curious as to their functional capacity to respond to injured cells. Time-lapse imaging showed that these MG can contact and engulf dead cells, thereby clearing the brain of potentially toxic cell debris, and indicating that the phagocytic signaling machinery is intact in MG and can be harnessed. Because even limited phagocytosis by MG may be important in protecting the neonatal brain during stroke (Faustino et al., 2011), enhancing MG resistance to ischemic injury may be beneficial in stroke therapy.
Developmental Sensitivity of MG to OGD during the Neonatal Period
Debate persists on the effects of age on ischemic susceptibility. There is conflicting data as to whether the developing brain is more susceptible to ischemic injury than the adult brain (Yager and Thornhill, 1997; Schaller, 2007). We attempted to inform this debate on a cellular level by investigating MG susceptibility during the early neonatal period. Using dissociated neonatal cultures, Chock and Giffard, 2005 showed that MG become less vulnerable to OGD with increasing time in culture. However, our results in acutely isolated slices indicate that MG viability and motility during OGD are better preserved in younger neonatal tissues than older ones. These differences may be accounted for by the differences in the preparations. Interestingly, previous work in rats of the same age reported similar resistance of neurons to hypoxiaischemia in younger than older neonates (Towfighi et al., 1997). Therefore, during the early postnatal period, MG, like neurons, become more vulnerable to ischemic injury as development progresses. Given the results of Chock and Giffard, 2005, it is possible that with age, MG by themselves are more vulnerable to ischemic insult. However, cell extrinsic factors released in tissues by other cells, including neurons, could promote MG resistance to OGD. It will be important to determine what feature(s)—intrinsic and/or extrinsic—of the early postnatal period promotes MG resistance to OGD and if such resistance could enhance neuronal survival.
This is the first report on MG viability and motility during stroke-like conditions in developing brain tissues. Our results indicate that both hypoxia and OGD inhibit MG motility, whereas OGD but not hypoxia induces MG cell death within the first few hours. The inhibitory effects of OGD on MG survival and motility increase with developmental age. Nevertheless, some surviving MG can retain or recover motility and phagocytize debris, thereby contributing to tissue restoration. It remains unclear what specific factors regulate MG cell death during OGD, and future studies will need to address these questions. Nevertheless, these results extend our understanding of MG survival and behavior in developing brain tissues, and suggest that survival of MG is an important consideration when developing therapeutic strategies for stroke in the perinatal brain.
Supplementary Material
Supplemental Figure 1: Method of generating ‘difference images’ for motility index analysis. First, confocal z-stacks for each time-point in a series were combined (maximum brightness) to obtain ‘projection images.’ The series of projection images was then ‘registered’ (using the StackReg plugin in ImageJ) to adjust for any lateral drift. For illustration purposes, images A and A’ represent two adjacent registered projection images in a time series taken at 3 min intervals (00:00 and 00:03, respectively). Projection images were then smoothened (average of 3×3 pixel neighborhood) to reduce background noise (B, B’). Then, an arbitrary threshold was applied to define the area of the cell (C, C’). The same threshold was applied to all images in a time series. D, A ‘difference image’ was generated from adjacent time-points (C and C’) using the Image Calculator ‘difference’ function in ImageJ, which calculates and displays the absolute value of differences between pixels in the two images. Thus, white areas indicate regions of change (either extension or retraction) in the cell. Note, for example, areas of change in processes (arrows). Dark regions indicate areas of little or no change in the cell, as for example in the cell soma (arrowhead). A video of this cell can be found in Supplemental Movie 5.
Supplemental Movie 1: Time-lapse movie of hippocampal slice during oxygen-glucose deprivation (OGD) with Sytox where GFP expressing microglia (MG) die as shown by loss of GFP followed by gain of Sytox. Length of movie: 45 minutes; interval between frames: 3 minutes.
Supplemental Movie 2: Time-lapse movie of difference images used to quantify the motility index of microglial cells during control (left), hypoxia (middle) and OGD (right) conditions. Notice that only during control conditions are there continuously obvious changes in microglial dynamics. Length of movie: 6 hours; interval between frames: 10 minutes.
Supplemental Movie 3: Time-lapse movie of a microglia (green) that is able to engulf a Sytox labeled dead cell (red) in the stratum radiatum of a hippocampus slice during OGD. Length of movie: 6 hours; interval between frames: 10 minutes.
Supplemental Movie 4: Time-lapse movie from a P2 slice (left) and a P6 slice (right) during OGD labeled with Topro-3 (yellow) that, like Sytox, labels the stratum pyramidale. In each movie, two representative GFP-expressing microglia (MG) are tracked (yellow and red lines through each movie) through time. Tracks of P2 MG are much more elaborate than those of P6 MG showing that OGD less effectively inhibits MG motility and migration in the P2 animal than the P6 one. Length of movie: 6 hours; interval between frames: 5 minutes.
Supplemental Movie 5: Time-lapse movie including sample microglial cells from the same slice in the stratum pyramidale (SP) [left] and stratum radiatum (SR) [right] monitored during 1 hour of baseline then 2 hours of OGD then an hour of washout. The SP’s cell motility remains largely unchanged before, during or after OGD. Although the cell in the SR is motile during baseline, its motility is severely limited by OGD and restored after OGD. Length of movie: 4 hours; interval between frames: 3 minutes.
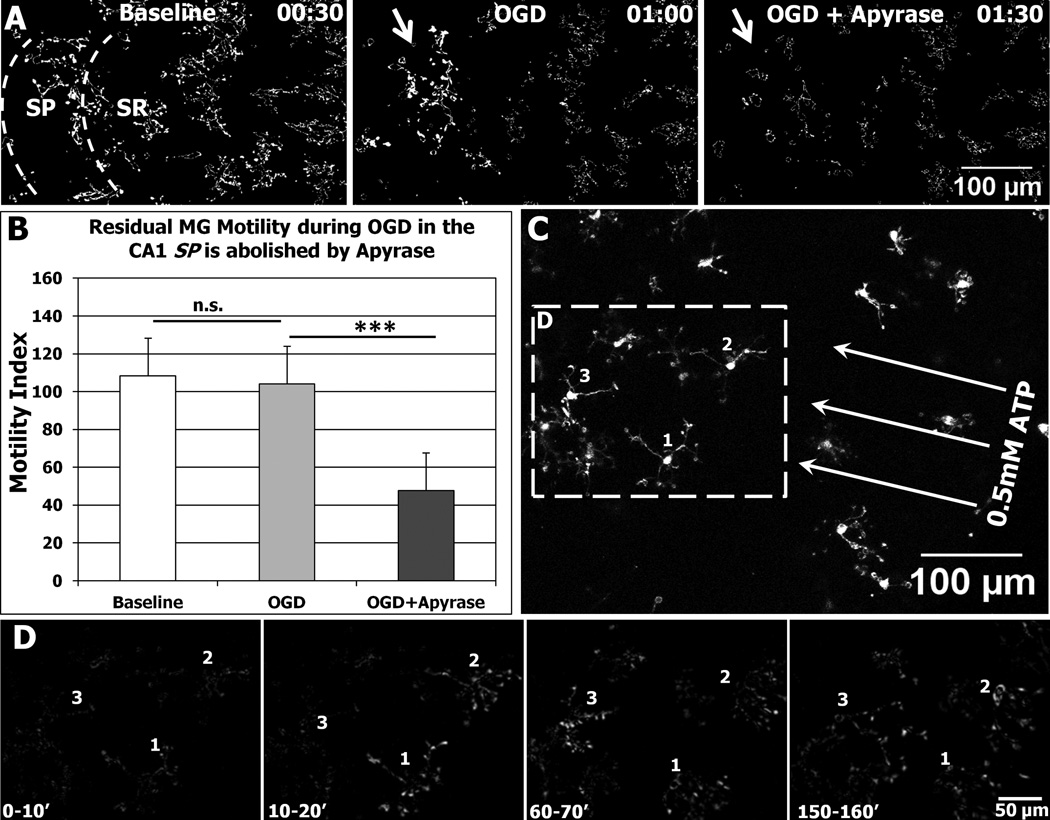
Figure 9.
Acknowledgements
Supported by grants to M.E.D. from the American Heart Association (0950160G) and National Institutes of Health (NS064006), and a P30 Center Grant from the NIH NIDCD (DC010362) to the University of Iowa. The authors thank Ms. Leah Fuller for assistance with mouse colony management and genotyping.
Footnotes
The authors declare that they have no conflict of interest.
REFERENCES
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- Carlsson Y, Schwendimann L, Vontell R, Rousset CI, Wang X, Lebon S, Charriaut-Marlangue C, Supramaniam V, Hagberg H, Gressens P, Jacotot E. Genetic inhibition of caspase-2 reduces hypoxic-ischemic and excitotoxic neonatal brain injury. Ann Neurol. 2011;70:781–789. doi: 10.1002/ana.22431. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Dinkel K, Reymann K. Microglia response and P2 receptor participation in oxygen/glucose deprivation-induced cortical damage. Neuroscience. 2005;136:615–623. doi: 10.1016/j.neuroscience.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Cavaliere F, Florenzano F, Amadio S, Fusco FR, Viscomi MT, D'Ambrosi N, Vacca F, Sancesario G, Bernardi G, Molinari M, Volonte C. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Chock VY, Giffard RG. Development of neonatal murine microglia in vitro: changes in response to lipopolysaccharide and ischemia-like injury. Pediatr Res. 2005;57:475–480. doi: 10.1203/01.PDR.0000155758.79523.44. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–230. doi: 10.1006/meth.1999.0775. 177. [DOI] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–179. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Deng YY, Lu J, Ling EA, Kaur C. Role of microglia in the process of inflammation in the hypoxic developing brain. Front Biosci (Schol Ed) 2011;3:884–900. doi: 10.2741/194. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, Vexler ZS. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Steffens H, Nadrigny F, Neusch C, Kirchhoff F, Schomburg ED. Long-lasting post-mortem activity of spinal microglia in situ in mice. J Neurosci Res. 2010;88:2431–2440. doi: 10.1002/jnr.22402. [DOI] [PubMed] [Google Scholar]
- Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J, Lou HF, Chen Z, Li XM, Luo QM, Duan S. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. Jan. 2012;10 doi: 10.1038/cr.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, Gertz K, Endres M, Bechmann I, Enjyoji K, Robson SC, Kettenmann H. The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia. 2008;56:331–341. doi: 10.1002/glia.20606. [DOI] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong WH, Tsai HD, Chen YC, Wu JS, Lin TN. Anti-apoptotic actions of PPAR-gamma against ischemic stroke. Mol Neurobiol. 2010;41:180–186. doi: 10.1007/s12035-010-8103-y. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Kronlage M, Kirschning C, Del Rey A, Di Virgilio Del Rey F, Leipziger J, Chessell IP, Sargin S, Filippov MA, Lindemann O, Mohr S, Konigs V, Schillers H, Bahler M, Schwab A. Transient P2X7 Receptor Activation Triggers Macrophage Death Independent of Toll-like Receptors 2 and 4, Caspase-1, and Pannexin-1 Proteins. J Biol Chem. 2012;287:10650–10663. doi: 10.1074/jbc.M111.332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Tomimatsu Y, Suzuki H, Yamada J, Wu Z, Yao H, Kagamiishi Y, Tateishi N, Sawada M, Nakanishi H. The intra-arterial injection of microglia protects hippocampal CA1 neurons against global ischemia-induced functional deficits in rats. Neuroscience. 2006;142:87–96. doi: 10.1016/j.neuroscience.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, Sano H, Sawada M. Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab. 2007;27:488–500. doi: 10.1038/sj.jcbfm.9600362. [DOI] [PubMed] [Google Scholar]
- Ivacko JA, Sun R, Silverstein FS. Hypoxic-ischemic brain injury induces an acute microglial reaction in perinatal rats. Pediatr Res. 1996;39:39–47. doi: 10.1203/00006450-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Iwabuchi S, Kawahara K. Possible involvement of extracellular ATP-P2Y purinoceptor signaling in ischemia-induced tolerance of astrocytes in culture. Neurochem Res. 2009;34:1542–1554. doi: 10.1007/s11064-009-9942-7. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, Takenouchi T, Kitani H, Kojima S. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun. 2012;419:374–380. doi: 10.1016/j.bbrc.2012.01.156. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Takata K, Inden M, Tsuchiya D, Yanagisawa D, Nakata J, Taniguchi T. Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci. 2004;94:203–206. doi: 10.1254/jphs.94.203. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Yanagisawa D, Inden M, Takata K, Tsuchiya D, Kawasaki T, Taniguchi T, Shimohama S. Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci. 2005;97:289–293. doi: 10.1254/jphs.sc0040129. [DOI] [PubMed] [Google Scholar]
- Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55:873–884. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- Kurpius D, Wilson N, Fuller L, Hoffman A, Dailey ME. Early activation, motility, and homing of neonatal microglia to injured neurons does not require protein synthesis. Glia. 2006;54:58–70. doi: 10.1002/glia.20355. [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG. Microglia in cerebral ischemia: molecular actions and interactions. Can J Physiol Pharmacol. 2006;84:49–59. doi: 10.1139/Y05-143. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, Nielsen HH, Haugaard LS, Wirenfeldt M, Nielsen M, Dagnaes-Hansen F, Bluethmann H, Faergeman NJ, Meldgaard M, Deierborg T, Finsen B. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwon HM, Kim YJ, Lee KM, Kim M, Yoon BW. Effects of hsp70.1 gene knockout on the mitochondrial apoptotic pathway after focal cerebral ischemia. Stroke. 2004;35:2195–2199. doi: 10.1161/01.STR.0000136150.73891.14. [DOI] [PubMed] [Google Scholar]
- Lehotsky J, Burda J, Danielisova V, Gottlieb M, Kaplan P, Saniova B. Ischemic tolerance: the mechanisms of neuroprotective strategy. Anat Rec (Hoboken) 2009;292:2002–2012. doi: 10.1002/ar.20970. [DOI] [PubMed] [Google Scholar]
- Li Z, Pang L, Fang F, Zhang G, Zhang J, Xie M, Wang L. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- Liu HT, Sabirov RZ, Okada Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008;4:147–154. doi: 10.1007/s11302-007-9077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons SA, Kettenmann H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J Cereb Blood Flow Metab. 1998;18:521–530. doi: 10.1097/00004647-199805000-00007. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, Hori M, Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 2003;34:207–213. doi: 10.1161/01.STR.0000047101.87575.3C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011;219:53–64. doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Montero M, Gonzalez B, Zimmer J. Immunotoxic depletion of microglia in mouse hippocampal slice cultures enhances ischemia-like neurodegeneration. Brain Res. 2009;1291:140–152. doi: 10.1016/j.brainres.2009.06.097. [DOI] [PubMed] [Google Scholar]
- Nagano T, Kimura SH, Takai E, Matsuda T, Takemura M. Lipopolysaccharide sensitizes microglia toward Ca(2+)-induced cell death: mode of cell death shifts from apoptosis to necrosis. Glia. 2006;53:67–73. doi: 10.1002/glia.20260. [DOI] [PubMed] [Google Scholar]
- Ness JM, Harvey CR, Washington JD, Roth KA, Carroll SL, Zhang J. Differential activation of c-fos and caspase-3 in hippocampal neuron subpopulations following neonatal hypoxia-ischemia. J Neurosci Res. 2008;86:1115–1124. doi: 10.1002/jnr.21573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KB. Perinatal ischemic stroke. Stroke. 2007;38:742–745. doi: 10.1161/01.STR.0000247921.97794.5e. [DOI] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J, Gunzer M, Gutzeit HO, Ullrich O, Reymann KG, Dinkel K. Microglia provide neuroprotection after ischemia. FASEB J. 2006;20:714–716. doi: 10.1096/fj.05-4882fje. [DOI] [PubMed] [Google Scholar]
- Neumann J, Sauerzweig S, Ronicke R, Gunzer F, Dinkel K, Ullrich O, Gunzer M, Reymann KG. Microglia cells protect neurons by direct engulfment of invading neutrophil granulocytes: a new mechanism of CNS immune privilege. J Neurosci. 2008;28:5965–5975. doi: 10.1523/JNEUROSCI.0060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CT, Culmsee C, van Bel F, Hagberg H, Kavelaars A. Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol. 2011;70:255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Kohsaka S. Dynamic motility of microglia: Purinergic modulation of microglial movement in the normal and pathological brain. Glia. 2011;59(12):1793–1799. doi: 10.1002/glia.21238. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Gan WB. Microglia dynamics and function in the CNS. Curr Opin Neurobiol. 2010;20:595–600. doi: 10.1016/j.conb.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sola A, Moore J, Wen T. Caspase inhibition by cardiotrophin-1 prevents neuronal death in vivo and in vitro. J Neurosci Res. 2010;88:1041–1051. doi: 10.1002/jnr.22269. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Bechade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. J Neurochem. 2011;119:901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- Schaller BJ. Influence of age on stroke and preconditioning-induced ischemic tolerance in the brain. Exp Neurol. 2007;205:9–19. doi: 10.1016/j.expneurol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Ten VS, Yao J, Ratner V, Sosunov S, Fraser DA, Botto M, Sivasankar B, Morgan BP, Silverstein S, Stark R, Polin R, Vannucci SJ, Pinsky D, Starkov AA. Complement component c1q mediates mitochondria-driven oxidative stress in neonatal hypoxic-ischemic brain injury. J Neurosci. 2010;30:2077–2087. doi: 10.1523/JNEUROSCI.5249-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Ruttimann UE, Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans Image Process. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Mauger D, Vannucci RC, Vannucci SJ. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Koerner IP, Moller T. Microglia in ischemic brain injury. Future Neurol. 2010;5:227–246. doi: 10.2217/fnl.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TC, Rogido M, Genetta T, Sola A. Permanent focal cerebral ischemia activates erythropoietin receptor in the neonatal rat brain. Neurosci Lett. 2004;355:165–168. doi: 10.1016/j.neulet.2003.10.078. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–2032. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. Expression of P2X receptors on rat microglial cells during early development. Glia. 2005;52:119–126. doi: 10.1002/glia.20227. [DOI] [PubMed] [Google Scholar]
- Yager JY, Thornhill JA. The effect of age on susceptibility to hypoxic-ischemic brain damage. Neurosci Biobehav Rev. 1997;21:167–174. doi: 10.1016/s0149-7634(96)00006-1. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Giffard RG. Ischemic vulnerability of primary murine microglial cultures. Neurosci Lett. 2001;298:5–8. doi: 10.1016/s0304-3940(00)01724-9. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Method of generating ‘difference images’ for motility index analysis. First, confocal z-stacks for each time-point in a series were combined (maximum brightness) to obtain ‘projection images.’ The series of projection images was then ‘registered’ (using the StackReg plugin in ImageJ) to adjust for any lateral drift. For illustration purposes, images A and A’ represent two adjacent registered projection images in a time series taken at 3 min intervals (00:00 and 00:03, respectively). Projection images were then smoothened (average of 3×3 pixel neighborhood) to reduce background noise (B, B’). Then, an arbitrary threshold was applied to define the area of the cell (C, C’). The same threshold was applied to all images in a time series. D, A ‘difference image’ was generated from adjacent time-points (C and C’) using the Image Calculator ‘difference’ function in ImageJ, which calculates and displays the absolute value of differences between pixels in the two images. Thus, white areas indicate regions of change (either extension or retraction) in the cell. Note, for example, areas of change in processes (arrows). Dark regions indicate areas of little or no change in the cell, as for example in the cell soma (arrowhead). A video of this cell can be found in Supplemental Movie 5.
Supplemental Movie 1: Time-lapse movie of hippocampal slice during oxygen-glucose deprivation (OGD) with Sytox where GFP expressing microglia (MG) die as shown by loss of GFP followed by gain of Sytox. Length of movie: 45 minutes; interval between frames: 3 minutes.
Supplemental Movie 2: Time-lapse movie of difference images used to quantify the motility index of microglial cells during control (left), hypoxia (middle) and OGD (right) conditions. Notice that only during control conditions are there continuously obvious changes in microglial dynamics. Length of movie: 6 hours; interval between frames: 10 minutes.
Supplemental Movie 3: Time-lapse movie of a microglia (green) that is able to engulf a Sytox labeled dead cell (red) in the stratum radiatum of a hippocampus slice during OGD. Length of movie: 6 hours; interval between frames: 10 minutes.
Supplemental Movie 4: Time-lapse movie from a P2 slice (left) and a P6 slice (right) during OGD labeled with Topro-3 (yellow) that, like Sytox, labels the stratum pyramidale. In each movie, two representative GFP-expressing microglia (MG) are tracked (yellow and red lines through each movie) through time. Tracks of P2 MG are much more elaborate than those of P6 MG showing that OGD less effectively inhibits MG motility and migration in the P2 animal than the P6 one. Length of movie: 6 hours; interval between frames: 5 minutes.
Supplemental Movie 5: Time-lapse movie including sample microglial cells from the same slice in the stratum pyramidale (SP) [left] and stratum radiatum (SR) [right] monitored during 1 hour of baseline then 2 hours of OGD then an hour of washout. The SP’s cell motility remains largely unchanged before, during or after OGD. Although the cell in the SR is motile during baseline, its motility is severely limited by OGD and restored after OGD. Length of movie: 4 hours; interval between frames: 3 minutes.