Abstract
Non-viral lipid/polymeric vectors have widely been used as nanocarriers (NCs) for gene delivery. They possess large surface area to volume ratio and are able to interact with biomolecules through functional moieties, resulting in inadvertent biological impacts, in particular at genomic level. Thus, their genomic bio-signature needs to be investigated prior to use in vivo. Using high-throughput microarray and qPCR gene expression profiling techniques, we have reported the genomic impacts of lipid/polymeric NCs. Given the fact that the ultimate objectives of gene therapy may inevitably be impaired by nonspecific intrinsic genomic impacts of these NCs, here, we highlight their nonspecific genomic bio-signature. We envision that better understanding on the genotoxicity of gene delivery NCs, as guiding premise, will help us to develop much safer NCs and also to accelerate their translation into clinical use and to provide pivotal information on safety liabilities early in discovery and developments process prior to its inevitable consequences in vivo.
Keywords: Gene Delivery, Gene Therapy, Genotoxicity, Microarray, Nanotoxicology, Toxicogenomics
The principle of gene therapy possesses undeniable therapeutic advantages over the conventional therapeutic modalities that are basically dependent upon exploitation of small molecules or biological pharmaceuticals. These advantages are: a) specific or selective treatment of diseased cells/tissue, b) minimal adverse consequences, c) correction of the genetic cause of a disease, and d) long-term treatment after single application.1,2 To silence/suppress a target gene or to correct a genetic defect, the gene-based therapeutics such as antisense oligonucleotides (ASODNs), plasmid DNA, ribozymes, DNAzymes and siRNAs need to be shuttled to the target site.3-6 However, despite implementation of various strategies for delivery of gene medicines (e.g., viral and non-viral vectors as well as physical methods such as microinjection, electroporation, gene gun, ultrasound and hydrodynamic delivery),7-11 safe gene transfer into various target cells still faces major obstacles including: poor delivery efficiency, cellular toxicity, immunogenicity, oncogenicity, short-term transgenic expression and poor expression levels.1,12 For systemic gene delivery, viral and non-viral vectors have been widely used. Ideally, in either ex vivo or in vivo approach for gene therapy, only the therapy-intended gene expression changes should occur, nevertheless this is not always the case. Of the most commonly used gene delivery systems, viral vectors (e.g., retroviruses, lentiviruses, adenoviruses, and adeno-associated viruses) are known to be efficient delivery systems for nucleic acids while they can induce immunogenic responses,13-15 with serious consequences as seen in the disastrous death of Jesse Gelsinger, who suffered from ornithine transcarbamylase deficiency and went under gene therapy using adenoviral vector carrying the corrected gene. These problems decelerated human gene therapy modalities and highlighted use of safer non-viral vectors. It should be also noted that in vitro gene therapy of cells with ASODNs for a short period of time (3-6 h) were shown to induce desired effects, while in vivo gene therapy demands repeated administration through multiple injections for prolonged exposure of target cells to ASODNs.3 All these issues highlight the necessary use of safe biocompatible lipid/polymeric carries for gene delivery even though the transfection efficiency of viral vectors is greater than that of the non-viral vectors. Hence, several non-viral gene delivery nanosystems such as cationic polymer- or lipid-based formulations have been developed for nucleic acid delivery.16-18 These cationic nanostructures can readily condense DNA into complexes and form polyplexes/lipoplexes to be used for ex vivo and in vivo gene therapy.17,19 So far, many advanced nanomaterials (NMs) have been used as non-viral gene delivery system. While in vitro and in vivo preclinical applications of advanced NMs and NCs as molecular therapy continue to increase, their molecular signature (the so-called nanotoxicology) should effusively be investigated prior to their translation into human subjects. To disclose the nanotoxicological impacts of NMs and NCs, implementation of high throughput molecular profiling technologies appears to be inescapable. In fact, molecular impacts of these NMs may delineate the molecular toxicity mechanisms and pathways involved in repair, survival and/or cell death.20 To understand such molecular mechanism(s) of NMs/NCs in target cells, we need to look at the molecular pattern, in particular gene expression changes pattern using high throughput gene expression profiling techniques. Various techniques have been capitalized for high throughput gene expression profiling including: DNA microarray, serial analysis of gene expression (SAGE), single promoter-reporter biosensors sensitive to DNA damage in mammalian cells21 and next generation RNA sequencing.22 Of these approaches, DNA microarray technology, which has been established almost two decades ago,23 appears to be an effective method for large-scale global gene expression analysis,24 leading to emergence of genomic toxicity assessments, so-called as “Toxicogenomics”.25-29 We have utilized non-viral vectors for gene delivery,30-35 and widely used DNA microarray technology to determine intrinsic gene expression alteration potential of NMs/NCs used as gene delivery system.26-29, 33-38 Fig. 1 represents schematic illustration of DNA microarray steps.
Fig. 1.
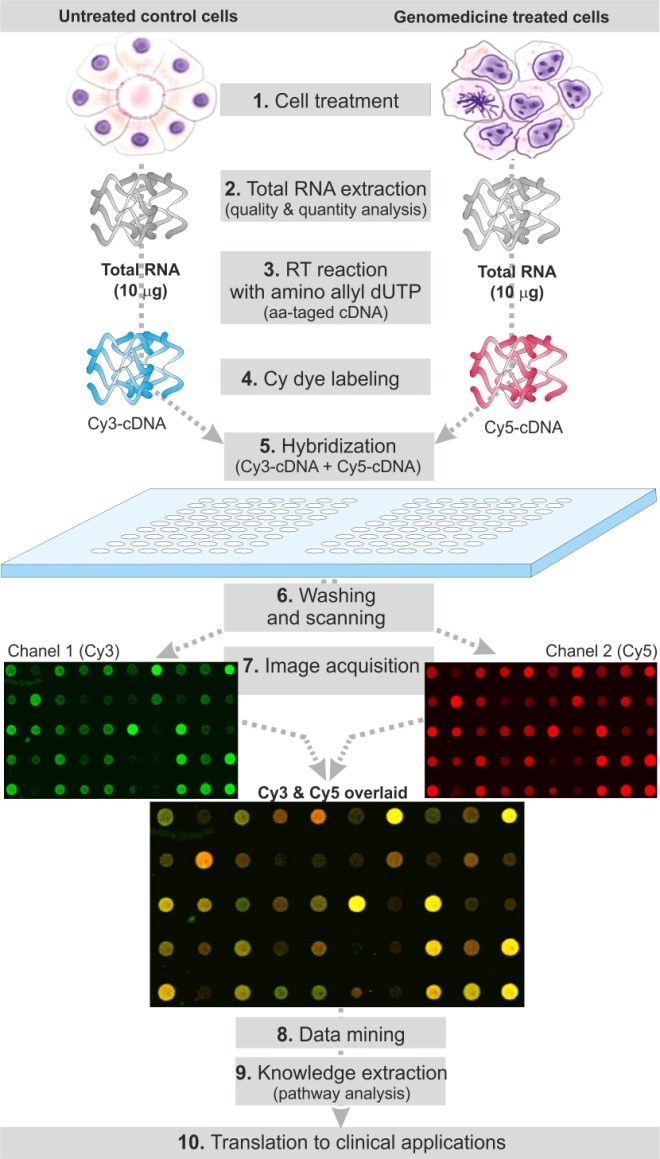
Schematic representation of DNA microarray procedure. The cultivated cells, at 40-50% confluency, are treated with a designated non-viral vector/genomedicine (1). The total RNA is extracted from the treated (T) and untreated (UT) cells and qualitatively and quantitatively analyzed (2). Reverse transcription (RT) is performed using total RNA (10 μg) and designated ratio of aminoallyl-dUTP:dTTP and RT products (cDNA) are purified (3). The T and UT cDNAs are labeled with cyanine dyes, i.e. Cy3 and Cy5, and the Cy3- and Cy4-lalbeled cDNAs are purified and quantitatively analyzed (4). The labeled cDNAs are hybridized on a glass slide array overnight (5). The slide arrays are washed and scanned for both Cy3 and Cy5 channels (6). Image acquisition is performed– Cy3 (green), Cy5 (red) and superimposed images are shown as examples (7). Data mining (8) and knowledge extraction (e.g., gene ontology, pathway analysis) are accomplished using designated software (9). The resultant data are translated into clinical applications (10).
Depending on NCs surface charge and functional moieties, they can intrinsically induce profound biological effects in target cells. It is not clearly understood how the internalized nanoparticles (NPs) can elicit inadvertent cellular impacts such as mutagenicity, inflammatory response and DNA damage.39 Hence, it is important to shed some lights on the genocompatibility and toxicogenomics of NCs. Surprisingly, little is known upon intrinsic bio-signature of gene delivery NCs. Based on our microarray screening findings, most of non-viral gene delivery NCs appear to interfere with the main objectives of such therapy by masking/stimulating nonspecific genes due to their own genomic impacts.26-28,37,38,40,41 Non-viral polycations are principally able to condense, enhance the delivery and improve the biological end-point of nucleic acids; however, they often exert cytotoxicity which is dependent on delivery system/target cells.42 Thus, both transfection efficiency evaluation and safety assessment are essential for gene transfer with these gene therapy vectors.43-45 Fig. 2 represents the scatter plots of gene expression changes induced by cationic polymer (PolyfectTM) alone or as polyplex in A431 cells. As shown in Fig. 2, a large number of various genes can be upregulated by cationic NCs, while complexed with DNA as polyplex the alteration in gene expressions was reduced perhaps because of reduction in surface charge of polymeric carrier. A number of factors may affect the efficacy and safety of non-viral vector-mediated gene transfer, in particular their structural properties and type of target cells and tissue.46-48 We have tested various polymeric and lipidic NCs and found that the gene expression changes correlate with surface charge and molecular structure and degree of biodegradation of NCs. For example poly (lactic-co-glycolic acid) (PLGA), as a biodegradable polymer, was found to be safer than cationic non-biodegradable polymers.49 Further, the branched molecular structures appear to induce greater genomic impacts than the linear ones as previously reported for the cytotoxic impacts of branched and linear polyethylenimine nanostructures in A431 cells.40 Surprisingly, despite these facts, little attention has been paid for validation of gene therapy strategies through proof-of-concept studies solely for the influence of carriers of genomedicines. Given the fact that various target cells may display different responses, the transfection efficiency and safety of vectors should also be optimized carefully based on types of target cells and target organs.50 Once target cells were transfected, specific attention should be given to the genotoxicity potentials of gene-based medicines. In fact, the definitions and purposes of gene therapy have been sharply focused, but the actual implementation of the task has not kept pace due to many unanticipated roadblocks.51
Fig. 2.
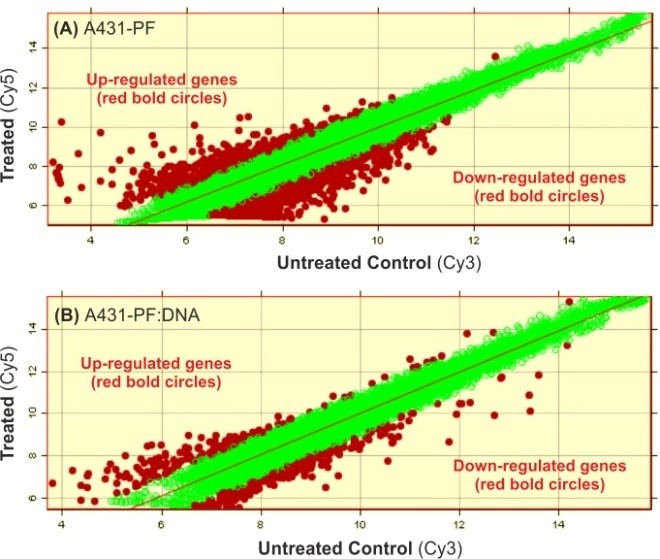
Scatter plots of gene expression changes induced by cationic polymer alone or as polyplex in A431 cells. Panels A and B respectively represent the genomic impact of Polyfect™ (PF) and its complex with scrambled DNA. PF:DNA Polyplex induced significantly lower gene expression. Each circle conveys a designated gene. Red circles represent up-regulated or down-regulated genes, and green circles show unchanged genes.
In short, NCs used for delivery of nucleic acids are able to inherently induce inadvertent alterations in gene expression. Therefore, since we literally look at the impact of active agents but not the vehicles, we need to be cautious upon inevitable bio-signature (molecular impacts) of NCs that may interfere with the main goal of gene therapy modality.
Acknowledgements
The authors express their sincere gratitude to Prof. Saghir Akhtar, Kuwait University.
Ethical issues
The authors declare no ethical issues.
Competing interests
The authors declare no conflict of interests.
References
- Rubanyi GM. The future of human gene therapy. Mol Aspects Med . 2001;22:113–42. doi: 10.1016/s0098-2997(01)00004-8. [DOI] [PubMed] [Google Scholar]
- Barar J, Omidi Y. Translational Approaches toward Cancer Gene Therapy: Hurdles and Hopes. BioImpacts . 2012;2:127–43. doi: 10.5681/bi.2012.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MD, Hussain M, Nawaz Q, Sayyed P, Akhtar S. The cellular delivery of antisense oligonucleotides and ribozymes. Drug Discov Today . 2001;6:303–15. doi: 10.1016/s1359-6446(00)00326-3. [DOI] [PubMed] [Google Scholar]
- Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet . 2000;1:91–9. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- Kerr D. Clinical development of gene therapy for colorectal cancer. Nat Rev Cancer . 2003;3:615–22. doi: 10.1038/nrc1147. [DOI] [PubMed] [Google Scholar]
- McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet . 2002;3:737–47. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- Cristiano RJ. Viral and non-viral vectors for cancer gene therapy. Anticancer Res . 1998;18:3241–5. [PubMed] [Google Scholar]
- Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol . 2001;38:177–92. doi: 10.1016/s1040-8428(01)00103-2. [DOI] [PubMed] [Google Scholar]
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand . 2003;177:437–47. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther . 2002;9:1647–52. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- Tamura T, Sakata T. Application of in vivo electroporation to cancer gene therapy. Curr Gene Ther . 2003;3:59–64. doi: 10.2174/1566523033347462. [DOI] [PubMed] [Google Scholar]
- Dass CR. Cytotoxicity issues pertinent to lipoplex-mediated gene therapy in-vivo. J Pharm Pharmacol . 2002;54:593–601. doi: 10.1211/0022357021778817. [DOI] [PubMed] [Google Scholar]
- Audouy SA, de Leij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res . 2002;19:1599–605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- Ferber D. Gene therapy. Safer and virus-free? Science . 2001;294:1638–42. doi: 10.1126/science.294.5547.1638. [DOI] [PubMed] [Google Scholar]
- Ferber D. Ferber DGene therapyRepair kits for faulty genes. Science . 2001;294:1639. doi: 10.1126/science.294.5547.1639. [DOI] [PubMed] [Google Scholar]
- Clark PR, Hersh EM. Cationic lipid-mediated gene transfer: current concepts. Curr Opin Mol Ther . 1999;1:158–76. [PubMed] [Google Scholar]
- Hirko A, Tang F, Hughes JA. Cationic lipid vectors for plasmid DNA delivery. Curr Med Chem . 2003;10:1185–93. doi: 10.2174/0929867033457412. [DOI] [PubMed] [Google Scholar]
- Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev . 2002;54:715–58. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- Aissaoui A, Oudrhiri N, Petit L, Hauchecorne M, Kan E, Sainlos M. et al. Progress in gene delivery by cationic lipids: guanidinium-cholesterol-based systems as an example. Curr Drug Targets . 2002;3:1–16. doi: 10.2174/1389450023348082. [DOI] [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H, Aubrecht J, Kleinjans JC, Ahr HJ. Application of toxicogenomics to study mechanisms of genotoxicity and carcinogenicity. Toxicol Lett . 2009;186:36–44. doi: 10.1016/j.toxlet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Aubrecht J, Caba E. Gene expression profile analysis: an emerging approach to investigate mechanisms of genotoxicity. Pharmacogenomics . 2005;6:419–28. doi: 10.1517/14622416.6.4.419. [DOI] [PubMed] [Google Scholar]
- Ramskold D, Kavak E, Sandberg R. How to analyze gene expression using RNA-sequencing data. Methods Mol Biol . 2012;802:259–74. doi: 10.1007/978-1-61779-400-1_17. [DOI] [PubMed] [Google Scholar]
- Saei AA, Omidi Y. A glance at DNA microarray technology and applications. BioImpacts . 2011;1:75–86. doi: 10.5681/bi.2011.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science . 1995;270:467–70. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Nuwaysir EF, Bittner M, Trent J, Barrett JC, Afshari CA. Microarrays and toxicology: the advent of toxicogenomics. Mol Carcinog . 1999;24:153–9. doi: 10.1002/(sici)1098-2744(199903)24:3<153::aid-mc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target . 2003;11:311–23. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Barar J, Akhtar S. Toxicogenomics of cationic lipid-based vectors for gene therapy: impact of microarray technology. Curr Drug Deliv . 2005;2:429–41. doi: 10.2174/156720105774370249. [DOI] [PubMed] [Google Scholar]
- Hollins AJ, Omidi Y, Benter IF, Akhtar S. Toxicogenomics of drug delivery systems: Exploiting delivery system-induced changes in target gene expression to enhance siRNA activity. JDrug Target . 2007;15:83–8. doi: 10.1080/10611860601151860. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Kafil V, Barar J. Toxicogenomics of nonviral cationic gene delivery nanosystems. In: Yuan X, editor. Non-viral gene therapy. Rijeka: InTech; 2011. p. 547-76.
- Najar AG, Pashaei-Asl R, Omidi Y, Farajnia S, Nourazarian AR. EGFR antisense oligonucleotides encapsulated with nanoparticles decrease EGFR, MAPK1 and STAT5 expression in a human colon cancer cell line. Asian Pac J Cancer Prev . 2013;14:495–8. doi: 10.7314/apjcp.2013.14.1.495. [DOI] [PubMed] [Google Scholar]
- Nourazarian AR, Najar AG, Farajnia S, Khosroushahi AY, Pashaei-Asl R, Omidi Y. Combined EGFR and c-Src antisense oligodeoxynucleotides encapsulated with PAMAM Denderimers inhibit HT-29 colon cancer cell proliferation. Asian Pac J Cancer Prev . 2012;13:4751–6. doi: 10.7314/apjcp.2012.13.9.4751. [DOI] [PubMed] [Google Scholar]
- Nourazarian AR, Pashaei-Asl R, Omidi Y, Najar AG. c-Src antisense complexed with PAMAM denderimes decreases of c-Src expression and EGFR-dependent downstream genes in the human HT-29 colon cancer cell line. Asian Pac J Cancer Prev . 2012;13:2235–40. doi: 10.7314/apjcp.2012.13.5.2235. [DOI] [PubMed] [Google Scholar]
- Fox S, Hollins A, Sohail M, Omidi Y, Southern E, Benboubetra M. et al. The design and activity of small interfering RNA (siRNA) as a potential therapeutic agent for the down-regulation of the epidermal growth factor receptor (EGFR) J Pharm Pharmacol . 2004;56:28. [Google Scholar]
- Hollins A, Omidi Y, Fox S, Griffiths S, Akhtar S. Polyethylenimine-mediated delivery of small interfering RNA targeting the epidermal growth factor receptor: a comparison of linear and branched polymer architecture. J Pharm Pharmacol . 2004; 56: 53. [Google Scholar]
- Hollins AJ, Benboubetra M, Omidi Y, Zinselmeyer BH, Schatzlein AG, Uchegbu IF. et al. Evaluation of generation 2 and 3 poly(propylenimine) dendrimers for the potential cellular delivery of antisense oligonucleotides targeting the epidermal growth factor receptor. Pharm Res . 2004;21:458–66. doi: 10.1023/B:PHAM.0000019300.04836.51. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Benboubetra M, Hollins A, Drayton R, Akhtar S. Dendrimeric delivery systems for siRNA and gene therapy intrinsically alter gene expression in human epithelial cells. J Pharm Pharmacol . 2004; 56: 52. [Google Scholar]
- Omidi Y, Barar J, Heidari HR, Ahmadian S, Yazdi HA, Akhtar S. Microarray analysis of the toxicogenomics and the genotoxic potential of a cationic lipid-based gene delivery nanosystem in human alveolar epithelial a549 cells. Toxicol Mech Methods . 2008;18:369–78. doi: 10.1080/15376510801891286. [DOI] [PubMed] [Google Scholar]
- Barar J, Hamzeiy H, Mortazavi-Tabatabaei SA, Hashemi-Aghdam SE, Omidi Y. Genomic signature and toxicogenomics comparison of polycationic gene delivery nanosystems in human alveolar epithelial A549 cells. Daru . 2009;17:139–47. [Google Scholar]
- Barar J, Omidi Y. Cellular Trafficking and Subcellular Interactions of Cationic Gene Delivery Nanomaterials. J Pharm Nut Sci . 2011;1:68–81. [Google Scholar]
- Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BioImpacts . 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidi Y, Barar J. Induction of human alveolar epithelial cell growth factor receptors by dendrimeric nanostructures. Int J Toxicol . 2009;28:113–22. doi: 10.1177/1091581809335177. [DOI] [PubMed] [Google Scholar]
- Pedroso de Lima, Simoes S, Pires P, Faneca H, Duzgunes N. Cationic lipid-DNA complexes in gene delivery: from biophysics to biological applications. Adv Drug Deliv Rev . 2001;47:277–94. doi: 10.1016/s0169-409x(01)00110-7. [DOI] [PubMed] [Google Scholar]
- Bell H, Kimber WL, Li M, Whittle IR. Liposomal transfection efficiency and toxicity on glioma cell lines: in vitro and in vivo studies. Neuroreport . 1998;9:793–8. doi: 10.1097/00001756-199803300-00005. [DOI] [PubMed] [Google Scholar]
- Benns JM, Mahato RI, Kim SW. Optimization of factors influencing the transfection efficiency of folate-PEG-folate-graft-polyethylenimine. J Control Release . 2002;79:255–69. doi: 10.1016/s0168-3659(01)00513-2. [DOI] [PubMed] [Google Scholar]
- Romoren K, Thu BJ, Bols NC, Evensen O. Transfection efficiency and cytotoxicity of cationic liposomes in salmonid cell lines of hepatocyte and macrophage origin. Biochim Biophys Acta . 2004;1663:127–34. doi: 10.1016/j.bbamem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Bragonzi A, Conese M. Non-viral approach toward gene therapy of cystic fibrosis lung disease. Curr Gene Ther . 2002;2:295–305. doi: 10.2174/1566523023347832. [DOI] [PubMed] [Google Scholar]
- Schatzlein AG. Non-viral vectors in cancer gene therapy: principles and progress. Anti-Cancer Drugs . 2001;12:275–304. doi: 10.1097/00001813-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Tranchant I, Thompson B, Nicolazzi C, Mignet N, Scherman D. Physicochemical optimisation of plasmid delivery by cationic lipids. J Gene Med . 2004; 6 Suppl 1(1):S24–35. doi: 10.1002/jgm.509. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Davaran S. Impacts of biodegradable polymers: Towards biomedical applications. In: Sharma SK, Mudhoo A, editors. Handbook of applied biopolymer technology: Synthesis, degradation and applications. Cambridge: Royal Society of Chemistry; 2011. p. 388-418.
- Omidi Y, Hollins AJ, Drayton RM, Akhtar S. Polypropylenimine dendrimer-induced gene expression changes: the effect of complexation with DNA, dendrimer generation and cell type. J Drug Target . 2005;13:431–43. doi: 10.1080/10611860500418881. [DOI] [PubMed] [Google Scholar]
- Verma IM. Gene therapy: hopes, hypes, and hurdles. Mol Med . 1994;1:2–3. [PMC free article] [PubMed] [Google Scholar]


