Abstract
Introduction
Patients with type 2 diabetes mellitus (T2DM) often require intensification of basal insulin therapy. This retrospective, observational study compared real-world outcomes in US patients with T2DM treated with insulin glargine who added a rapid-acting insulin (RAI) (basal–bolus approach) with those who switched to premixed insulin (PMX).
Methods
The national US IMPACT® database was used to identify data from adult patients (≥18 years of age) with T2DM who added bolus RAI to insulin glargine (GLA + RAI) or who switched from GLA to PMX between 2001 and 2009. A stringent 1:1 propensity score-matching method was used to address the selection bias by matching GLA + RAI patients and PMX patients. Clinical and economic outcomes were determined for 1 year after the initial pharmacy claim for RAI or for PMX. Outcomes included treatment persistence and adherence, average insulin doses, glycated hemoglobin (A1C) levels, the prevalence and incidence of hypoglycemia, and health care costs/utilization. Analysis was carried out using an intent-to-treat approach.
Results
The study included data from 746 propensity-matched patients (n = 373 in each cohort). Treatment persistence and adherence were higher in the GLA + RAI cohort. There was no significant difference in A1C reduction from baseline and the number of patients achieving target A1C levels of <7% in each cohort. The incidence of hypoglycemic events was also similar in both groups. However, during follow-up, many patients (48.8%) who initially switched from insulin glargine to PMX crossed back over to use GLA and/or RAI as part of their regimen. Health care costs and utilization levels were not significantly different.
Conclusion
Clinical and economic outcomes were similar in T2DM patients who added RAI to GLA and in those who switched to PMX, but a basal–bolus strategy appears to be associated with better treatment persistence and adherence.
Keywords: type 2 diabetes mellitus, insulin glargine, rapid-acting insulin, premixed insulin, clinical outcomes, treatment persistence
Introduction
Diabetes mellitus is a progressive disease, and the majority of patients with type 2 diabetes mellitus (T2DM) eventually require insulin therapy, either alone or combined with oral antidiabetic drugs (OADs), in order to maintain glycemic control.1,2 Patients usually start insulin therapy with a single daily injection of an insulin analog, such as the intermediate-acting neutral protamine Hagedorn insulin, or the basal (long-acting) insulin analog insulin glargine (GLA) or insulin detemir.3 When these insulin analogs are no longer sufficient to maintain target glycated hemoglobin (A1C) levels and significant postprandial glucose excursions occur, guidelines recommend that mealtime injections of a rapid-acting insulin (RAI), such as insulin glulisine or insulin aspart, are added to the basal insulin regimen (“basal–bolus” strategy).3–5 Initiation with – or a switch to – two or three daily injections with premixed insulin (PMX) analogs (ie, fixed combinations of intermediate-acting insulin with regular insulin or an RAI) is an alternative approach, and can be considered based on A1C levels and patient attitude toward injection frequency.3–7
Due to the chronic nature of T2DM, an individualized, patient-centered approach should be the guiding principle of management.3 In this context, dose flexibility has been shown to be an important attribute of injectable treatment regimens for T2DM,8 and can contribute to increased patient satisfaction with therapy.9 Compared with PMX injections, basal–bolus therapy is a more complicated regimen for patients to adhere to; however, it allows for greater flexibility, especially with irregular mealtimes, and may improve patient satisfaction and treatment adherence.
Few clinical studies have been published that compare the possibilities of adding either a bolus insulin or switching to PMX when basal insulin alone no longer suffices. Intensification with basal–bolus therapy, compared with twice-daily PMX, improves glycemic control without an increase in the number of hypoglycemic events, but at the expense of more weight gain among basal–bolus users.10 Compared with basal insulin alone, PMX regimens have been associated with a greater reduction in A1C levels, but place significant restrictions on the timing of meals, and tend to cause more hypoglycemia and weight gain.11,12
When patients with T2DM require intensification of insulin therapy, choosing the most appropriate treatment strategy can be challenging. There are a lack of clinical data directly comparing outcomes in patients with T2DM managed using a basal–bolus strategy with outcomes in patients previously treated with basal insulin who switched to treatment with PMX insulin injections. Several clinical studies have reported that switching between different types of insulin therapy can improve glycemic control and does not have a detrimental effect on patient clinical or safety outcomes.13,14 However, investigators have acknowledged several limitations associated with trial results, including a short study duration, a small number of patients included, lack of control for trail participation, and lack of a control group.15
Randomized controlled trials (RCTs) remain the gold standard for research, as they permit the greatest internal validity. However, generalizability of RCT data and applicability to the broader population of patients in the “real world” (and to the individual patient) is nearly impossible, owing to the highly selective populations and tightly controlled conditions in such RCTs.16 While RCTs can inform on clinical and safety outcomes, they do not always provide the necessary insights into economic and/or humanistic outcomes. Well-designed observational studies are needed to provide insights into disease management in real-world practice settings.16,17 However, to date, very few studies have investigated the impact of insulin switching in real-world settings.18–20
The aim of this observational database study was to compare real-world clinical and economic outcomes in T2DM patients who were being treated with the basal insulin analog GLA and who intensified their treatment regimen by either adding mealtime injections of an RAI or switching to PMX injections.
Patients and methods
This was a retrospective cohort study of data from the Integrated Health Care Information Services (now OptumInsight) IMPACT® database, which contains data from approximately 50 US national managed-care plans. IMPACT® contains medical and pharmacy claims, eligibility data, and laboratory results for 107 million patients, 73% of whom had pharmacy benefits. Of those patients with pharmacy benefits, laboratory results were available for 18%.21 The database was used from July 1, 2000 to December 31, 2010. The subject-identification period was from January 1, 2001 to December 31, 2009.
Inclusion and exclusion criteria
Data from adult patients ≥18 years of age with a confirmed diagnosis of T2DM were identified for inclusion in the analysis. A T2DM diagnosis was defined as one or more inpatient visits, or two or more physician visits, at least 30 days apart with a primary or secondary diagnosis of T2DM (International Classification of Diseases, ninth revision, clinical modification [ICD-9-CM] codes: 250.x0 or 250.x2). This is different from the Healthcare Effectiveness Data and Information Set definition of diabetes,22 but has been validated to identify T2DM using administrative database analysis.23 The first pharmacy claim for an RAI or PMX analog was classed as the index date. Eligible patients were required to have continuous health-plan coverage for both medical and pharmacy benefits for at least 6 months prior to the index date (baseline period) and for at least 1 year post-index date (follow-up period). For inclusion, patients were also required to have used at least one prescription of GLA in each quarter during the 6-month baseline period as well as A1C test values available at baseline. Patients were eligible for inclusion whether or not they had been treated with OADs or glucagon-like peptide 1 during the baseline period. Patients who had used insulin of any type other than GLA in the baseline period were excluded.
Patients were assigned to one of two cohorts according to study drug use: patients who added an RAI to the GLA + RAI group, and patients who switched from basal GLA to PMX analog (either insulin aspart or insulin lispro) to the PMX group.
Outcomes
Clinical and economic outcomes were measured during a 1-year follow-up period and included measures of treatment persistence and adherence, daily insulin usage, glycemic control (assessed using A1C levels), hypoglycemia, health care utilization, and health care cost.
Treatment persistence was defined as the number of days for which a patient continuously remained on the study drug during the follow-up period without discontinuation or switching after study drug initiation. However, in the GLA + RAI group, only persistence to GLA was determined because patients were not likely to be on RAI continually. The standard measure of treatment persistence is based on days’ supply (as noted on pharmacy claims) and is challenging for use with insulin therapies, as insulin use is subject to dose titration and self-monitoring. Days of supply are often noted on pharmacy claims as 30 days, and do not take into account any factors that may affect usage, such as dose titration. Additionally, the mode of insulin administration may play a role as well, eg, package sizes of pen devices for insulin administration and vials (for insulin administration using a vial and syringe). For example, insulin pen fills often started with a volume of 15 mL (five pens, 3 mL each), while insulin vial fills often started with a volume of 10 mL. To take all this into account, an empirical method was used based on previous published literature.24–28 We defined the expected medication-coverage days for a specific volume of insulin dispensed (eg, 10 mL) as the 90th percentile of the time between the initial fill with this specific volume until the next fill among all patients who had at least one refill during the follow-up period. These volume-specific expected medication-coverage days were then applied back to all the insulin fills during the follow-up period with the same volume to estimate how long the patient would be covered by that fill. Patients who did not have continuous medication coverage during the follow-up period were considered nonpersistent. Sensitivity analyses were also conducted using the 75th and 95th percentiles of the time of medication coverage.
The standard measure of treatment adherence is the medication possession ratio (MPR), which can be calculated as:
However, the traditional MPR is challenging for use with insulin therapies, because it is based on days’ supply (as noted on pharmacy claims) and because insulin is subject to dose titration and self-monitoring. Additionally, the traditional MPR does not account for differences in the package sizes of insulin medications. Treatment adherence in this study was therefore measured using both the traditional MPR and the adjusted MPR, which uses the total number of days of drug supply during the follow-up period divided by the total number of days in the follow-up period and multiplied by the average days between prescription refills divided by average days of drug supply for patients:
The daily average consumption (DACON) of insulin was calculated as the total number of insulin units dispensed prior to the last refill of the study drug, divided by the total number of days between initiation and last refill in the entire follow-up period.21
Glycemic control was assessed from A1C levels at the end of the 1-year follow-up period, change in A1C from baseline, and the percentage of patients who reached A1C levels of <7%. The incidence of hypoglycemia was determined during the 1-year follow-up period. Hypoglycemia was defined as patients having a health care encounter (outpatient, inpatient, or emergency department [ED] visit) with a primary or secondary ICD-9-CM diagnosis code for hypoglycemia (250.8, diabetes with other specified manifestations; 251.0, hypoglycemic coma; 251.1, other specified hypoglycemia; or 251.2, hypoglycemia, unspecified).29 The setting of the hypoglycemic event (outpatient, ED, or hospital) was considered to be an indicator of the severity of the event.
Economic outcomes in the two treatment cohorts were compared using measures of health care resource utilization as well as health care costs. General health care resource utilization included outpatient visits, outpatient pharmacy costs, ED visits, inpatient admissions, length of hospital stay (days), and endocrinologist visits. Diabetes-related health care resource utilization included all claims associated with a code for a primary or secondary diagnosis of diabetes (ICD-9-CM 250.xx). Total all-cause health care costs were calculated from the amounts of adjudicated claims paid by the health care plan to cover outpatient costs, ED costs, inpatient costs, total outpatient pharmacy costs, and total costs for all non-pharmacy-related health care costs. Diabetes-related health care costs were calculated using the costs from medical claims with a primary or secondary diagnosis of diabetes (ICD-9-CM 250.xx), antidiabetic medications, blood glucose meters, lancets, and test strips.
Statistical analyses
The analysis was conducted using an intent-to-treat approach, and all patients were observed for 12 months after the index date. To minimize the potential for selection bias in this retrospective observational study, stringent propensity score matching (PSM)30 using a 1:1 ratio was used to match patients in the GLA + RAI group with those in the PMX group to remove observed differences in baseline demographic and clinical characteristics, including age, baseline A1C, copayments, initial year, region, health plan, diabetes education, baseline comorbidities (ie, hypertension, hyperlipidemia, congestive heart failure, peripheral vascular disease, renal disease, retinopathy, neuropathy, nephropathy, chronic pulmonary disease, cancer, mental illness), baseline diabetes medications (eg, metformin, sulfonylureas, thiazolidinediones, OADs), concomitant nondiabetes medications (eg, statins, angiotensin-receptor blockers), health care utilizations, and health care costs.
Baseline characteristics, clinical outcomes, and economic parameters were summarized and compared among matched patients. P-values were determined using either Student’s t-test or χ2 test as appropriate. Kaplan–Meier curves were used to examine time to treatment discontinuation in both the GLA + RAI and PMX cohorts.
Results
Patient baseline characteristics
Data from a total of 2,012 patients were included in the study (1,637 in the GLA + RAI cohort and 375 in the PMX cohort). After PSM, data from a total of 746 matched patients were included in the analyses (n = 373 in each cohort). Overall, 42.2% of patients were women, the mean patient age was 56.4 years, mean baseline A1C was 9.5%, mean number of OADs at baseline was 1.7, and the mean baseline daily dose of GLA was 40.7 U/day (40.1 in the GLA + RAI cohort, 41.3 in the PMX cohort). Baseline demographic and clinical characteristics, health care costs, and health care utilizations were balanced after PSM (Table 1).
Table 1.
Clinical and economic characteristics at baseline
| Characteristics | GLA + RAI (n = 373) |
PMX (n = 373) |
P-value |
|---|---|---|---|
| Age, years, mean (SD) | 56.68 (10.11) | 56.07 (10.04) | 0.4035 |
| Male, n (%) | 212 (56.84) | 219 (58.71) | 0.6038 |
| A1C, %, mean (SD) | 9.54 (1.84) | 9.45 (1.84) | 0.5235 |
| Charlson Comorbidity Index, mean (SD) | 0.77 (1.31) | 0.69 (1.33) | |
| Comorbidity, n (%) | |||
| Hypertension | 277 (74.26) | 277 (74.26) | 0.4378 |
| Hyperlipidemia | 267 (71.58) | 271 (72.65) | 1.0000 |
| Myocardial infarction | 13 (3.49) | 6 (1.61) | 0.7440 |
| Congestive heart failure | 40 (10.72) | 34 (9.12) | 0.1038 |
| Peripheral vascular disease | 23 (6.17) | 31 (8.31) | 0.4624 |
| Renal disease | 31 (8.31) | 26 (6.97) | 0.2583 |
| Retinopathy | 69 (18.50) | 62 (16.62) | 0.4908 |
| Neuropathy | 66 (17.69) | 61 (16.35) | 0.5006 |
| Nephropathy | 33 (8.85) | 37 (9.92) | 0.6262 |
| Chronic pulmonary disease | 43 (11.53) | 33 (8.85) | 0.6155 |
| Cancer | 16 (4.29) | 19 (5.09) | 0.2261 |
| Oral antidiabetic drugs, n (SD) | 1.79 (1.06) | 1.71 (0.97) | 0.3144 |
| Medication, n (%) | |||
| Metformin | 239 (64.08) | 227 (60.86) | 0.3642 |
| SUs | 220 (58.98) | 218 (58.45) | 0.8818 |
| DPP-4 | 30 (8.04) | 29 (7.77) | 0.8921 |
| GLP-1 | 36 (9.65) | 37 (9.92) | 0.9019 |
| TZDs | 142 (38.07) | 141 (37.80) | 0.9399 |
| Meglitinides | 30 (8.04) | 17 (4.56) | 0.0501 |
| Alpha-glucosidase | 5 (1.34) | 6 (1.61) | 0.7613 |
| Baseline hypoglycemia, n (%) | |||
| Any hypoglycemia | 23 (6.17) | 22 (5.90) | 0.8778 |
| Any inpatient/ED-related hypoglycemia | 10 (2.68) | 7 (1.88) | 0.4617 |
| Health care utilization, n (%) | |||
| Any hospitalization | 51 (13.67) | 48 (12.87) | 0.7461 |
| Any diabetes-related hospitalization | 42 (11.26) | 40 (10.72) | 0.8149 |
| Health care cost, $ | |||
| Total cost, mean (SD) | 9,232 (15,922) | 9,072 (14,921) | 0.8871 |
| Total diabetes-related cost, mean (SD) | 3,602 (6,872) | 3,595 (5,950) | 0.9873 |
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin; SD, standard deviation; A1C, glycated hemoglobin; DPP, dipeptidyl peptidase; GLP, glucagon-like peptide; SUs, sulfonylureas; TZDs, thiazolidinediones; ED, emergency department.
Treatment persistence and adherence
Treatment persistence and adherence outcomes are correlated and reflect two aspects of patients’ medication-taking behavior: persistence refers to the patient continuing the treatment for the prescribed duration, while adherence (compliance) describes how likely patients are to take their medication as prescribed.31 A higher proportion of patients in the GLA + RAI cohort were treatment-persistent compared with the PMX cohort (52.01% versus 43.16%, respectively; P = 0.0156) (Figure 1A). Sensitivity analysis using the 75th and 95th percentiles yielded similar results (Figure 1A). Treatment adherence (adjusted MPR) was higher among patients in the GLA + RAI cohort compared with patients in the PMX cohort (0.77 versus 0.66, P < 0.0001) (Figure 1B).
Figure 1.
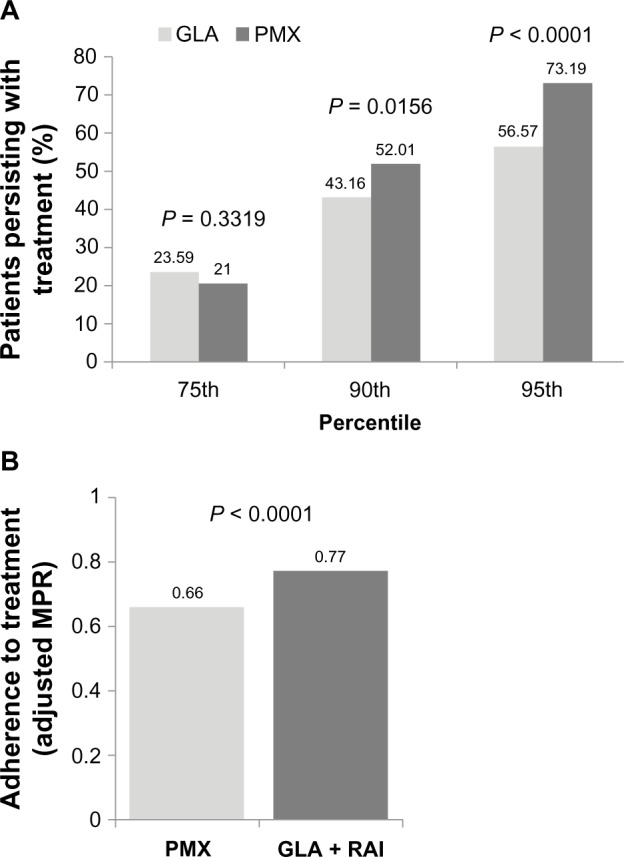
Treatment persistence (A) and adherence (B) in the basal–bolus (GLA + RAI) and premix insulin (PMX) cohorts over 1-year of follow-up. In addition to the 90th percentile data, sensitivity analyses involving the 75th and 95th percentile are shown for persistence data.
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin; MPR, medication possession ratio.
As treatment persistence is defined as the number of days patients remained on the index drug without discontinuation or switching, patients switching from one type of PMX to another would be considered nonpersistent with their index drug. To account for the fact that patients switching between PMX insulin could be considered persistent with their index drug class, the persistence analysis was repeated to include patients who switched their type of PMX during follow-up (8.8% of the PMX cohort). When these PMX-to-PMX patients were included as persistent users, the persistence rate in the PMX group increased from 43.16% to 45.31%, but remained lower than persistence in the GLA + RAI cohort (P = 0.0671).
The number of days that patients persisted with their treatment was significantly higher in the GLA + RAI cohort than in the PMX cohort (274 days versus 249 days, P = 0.002). Kaplan–Meier analysis of survival curves showed that patients in the GLA + RAI cohort were less likely to discontinue treatment early compared with those in the PMX cohort (P = 0.0055) (Figure 2).
Figure 2.
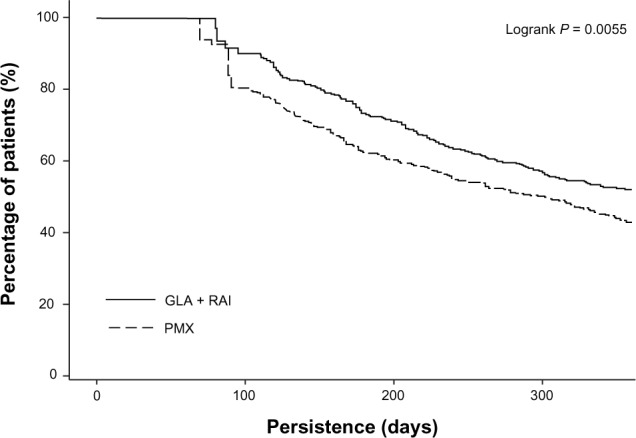
Kaplan–Meier curve for the time to treatment discontinuation.
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin.
The insulin-usage pattern was also determined for both groups over the 1-year follow-up period. In the PMX cohort, almost half of the patients (48.8%, n = 182) also used GLA or an RAI during the follow-up period. Of these, 40.1% (n = 73) switched back to GLA, 44.5% (n = 81) used GLA and a PMX alternately or concomitantly, 9.9% (n = 18) used an RAI and a PMX concomitantly, and 5.5% (n = 10) used an RAI only. In the GLA + RAI cohort, only 5% of patients (n = 19) used a PMX at any time during the follow-up period.
Clinical outcomes
Among patients with A1C data available at the end of the 1-year follow-up (207 patients in the GLA + RAI cohort and 234 patients in the PMX cohort), the reduction from baseline A1C was similar (GLA + RAI −0.79% PMX −0.71%; PI= 0.6455) (Figure 3). The proportion of patients reaching A1C levels <7% was 15.5% in the GLA + RAI group and 13.7% in the PMX group (P = 0.5956).
Figure 3.
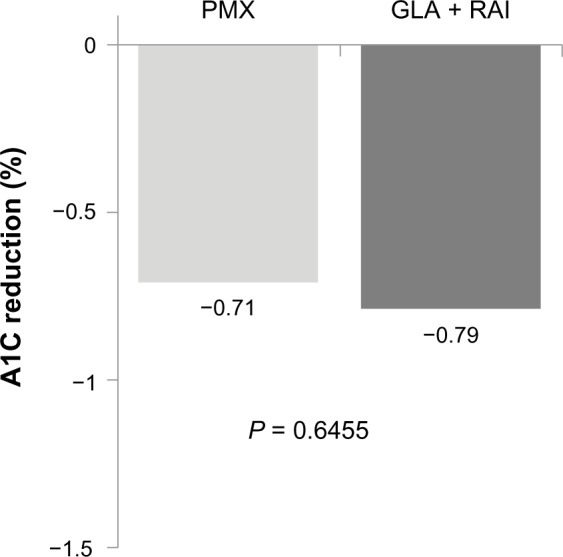
A1C reduction among patients in the basal–bolus (GLA + RAI, n = 207) and premixed insulin (PMX, n = 234) cohorts who had baseline and follow-up data available.
Abbreviations: A1C, glycated hemoglobin; GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin.
The DACON of insulin for patients in the GLA + RAI cohort was 40.3 U/day of GLA (median 35.6 U/day) and 33.8 U/day of RAI (median 25.8 U/day). In the PMX cohort, the average DACON was 55.6 U/day (median 49.4 U/day).
Data from patients’ medical claims showed that the incidence and prevalence of hypoglycemia-related events were similar across the GLA + RAI and PMX cohorts (Figure 4A and B). The prevalence of any hypoglycemic event (ie, in any clinical setting) in the GLA + RAI and PMX groups was 11.26% and 11.80%, respectively (P = 0.8186). The prevalence of inpatient/ED-related hypoglycemia (as a proxy for severe hypoglycemia) in the two groups was 5.1% versus 4.3%, respectively (P = 0.6035). The overall hypoglycemia event rate was 21 events/100 patient-years in the GLA + RAI group compared with 35 events per 100 patient-years in the PMX group (P = 0.2033), and the inpatient/ED-related hypoglycemia event rates in the two groups were five versus six events per 100 patient-years, respectively (P = 0.7889).
Figure 4.
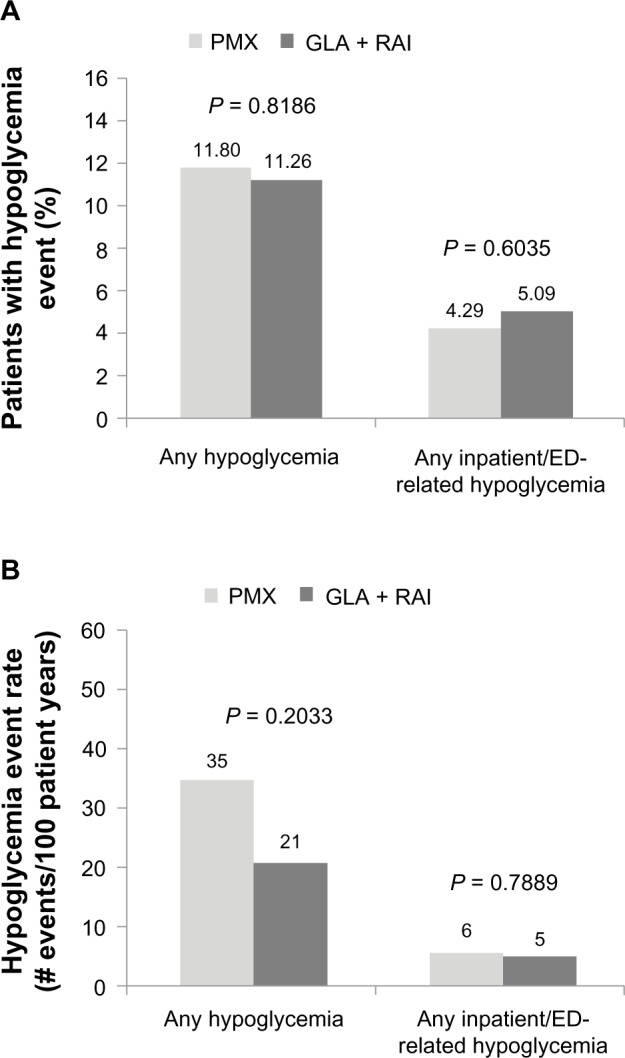
Hypoglycemia prevalence (A) and incidence (B) at the end of the 1-year follow-up.
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin; ED, emergency department.
Health care utilization and cost outcomes
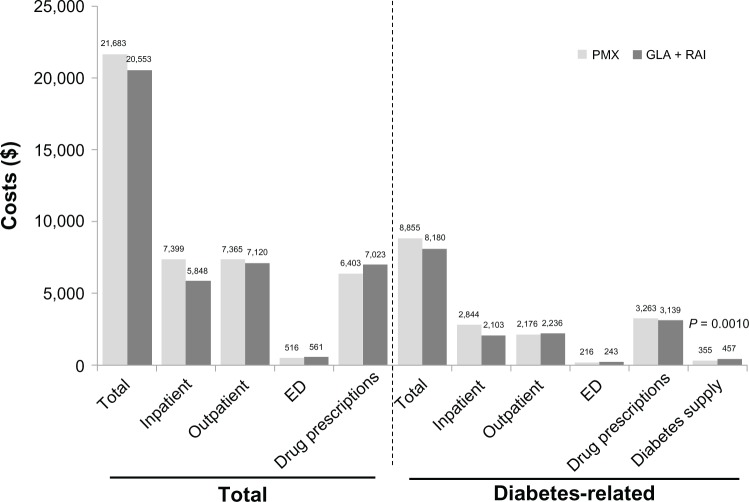
During 1 year of follow-up, all health care resource-utilization outcomes were similar across the GLA + RAI and PMX cohorts (Table 2). The mean total health care costs over 1 year were not significantly different between the GLA + RAI and PMX cohorts ($20,553 versus $21,683, respectively; P = 0.5964) (Figure 5). Mean 1-year total diabetes-related health care costs were also similar in both cohorts ($8,180 versus $8,855; P = 0.3603) (Figure 5).
Table 2.
Health care resource utilization over 1 year of follow-up
| Outcomes | GLA + RAI (n = 373) |
PMX (n = 373) |
P-value |
|---|---|---|---|
| Total health care utilization | |||
| Any hospitalization, n (%) | 90 (24.13) | 80 (21.45) | 0.3828 |
| Hospitalizations, n (SD) | 0.33 (0.74) | 0.32 (0.77) | 0.9228 |
| Hospitalization days, n (SD) | 1.91 (7.93) | 2.47 (7.79) | 0.3260 |
| Any ED visit, n (%) | 127 (34.05) | 122 (32.71) | 0.6979 |
| ED visits, n (SD) | 0.76 (1.87) | 0.79 (2.27) | 0.8189 |
| Any office visit, n (%) | 373 (100.00) | 372 (99.73) | 0.3170 |
| Office visits, n (SD) | 19.75 (14.25) | 20.32 (16.38) | 0.6133 |
| Any endocrinologist visit, n (%) | 171 (45.84) | 162 (43.43) | 0.5074 |
| Endocrinologist visits, n (SD) | 1.78 (2.49) | 1.70 (2.56) | 0.6740 |
| Diabetes-related health care utilization | |||
| Any hospitalization, n (%) | 84 (22.52) | 73 (19.57) | 0.3232 |
| Hospitalizations, n (SD) | 0.29 (0.68) | 0.28 (0.66) | 0.7845 |
| Hospitalization days, n (SD) | 1.60 (5.77) | 2.15 (7.05) | 0.2422 |
| Any ED visit, n (%) | 90 (24.13) | 89 (23.86) | 0.9317 |
| ED visits, n (SD) | 0.34 (0.77) | 0.39 (0.87) | 0.4480 |
| Any office visit, n (%) | 373 (100.00) | 370 (99.20) | 0.0826 |
| Office visits, n (SD) | 8.79 (5.36) | 8.94 (6.40) | 0.7192 |
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin; SD, standard deviation; ED, emergency department.
Figure 5.
Health care cost outcomes over 1 year of follow-up.
Abbreviations: GLA, insulin glargine; RAI, rapid-acting insulin; PMX, premixed insulin; ED, emergency department.
Discussion
In this real-world observational study of insulin treatment in patients with T2DM, an intent-to-treat analysis showed that both clinical and economic outcomes were similar for patients adding an RAI to GLA and for those switching from GLA to PMX. Both groups showed significant yet similar A1C reduction from baseline, but only 14%–15% reached A1C levels <7% at the end of the 1-year follow-up, mostly due to high A1C (9.5%) at baseline, patients’ persistence with their treatment, and less aggressive titration in the real-world setting compared to RCTs. However, in the current study, treatment persistence was better among patients adding an RAI to basal GLA than among patients switching to PMX. This could be attributed to the fact that almost half of the PMX cohort (182 patients) who switched to premixed analog insulin in the current study switched back to GLA or also used GLA and an RAI during the follow-up period. Previous studies showed significant glycemic improvement when patients switched from PMX to GLA-based insulin-treatment regimens.18,19
Importantly, these data provide information on insulin-switching patterns and the impact of insulin switching in real-life clinical practice from a large national US database. To our knowledge, this crossover in the usage of insulin treatments in everyday diabetes management has not been reported previously; further observational studies are required to better understand insulin-treatment patterns in patients with T2DM. In the clinical trial setting, the use of GLA plus an RAI has been reported to result in better diabetes-related quality of life with less fear of hypoglycemia compared with PMX in patients with T2DM requiring intensification of treatment.32 This might be explained by the increased flexibility of using basal–bolus treatment with GLA plus an RAI compared with fixed twice-daily injections of PMX, and may translate into the real-world setting, possibly explaining the high switching rate observed in the PMX cohort in our analysis.
To our knowledge this is the first real-world observational study comparing patients with T2DM on basal GLA treatment who added an RAI, with patients on basal GLA who switched to PMX when treatment intensification was required. A similar observational database study previously compared outcomes in patients initiating insulin therapy with basal insulin versus PMX treatment in patients with T2DM.33 In contrast to our findings, the earlier study reported that after 36 months, more patients remained on PMX treatment (83%) than on basal insulin therapy (67% GLA, 57% neutral protamine Hagedorn; P < 0.001).33 However, the study only examined treatment initiation and did not evaluate patients who were switching due to a need to intensify insulin treatments; the impact of adding an RAI was not analyzed. The improved treatment persistence with PMX was gained at the cost of a higher weight gain and insulin dosage, and was at odds with the greater glycemic control that was observed in patients using basal GLA compared to those on PMX.33
Several systematic reviews of published studies of insulin treatments for T2DM have been published.34,35 The Agency for Healthcare Research and Quality reported that whereas long-acting basal insulin analogs (administered alone) are more effective than PMX analogs in lowering fasting glucose, PMX is more effective than long-acting basal insulin in reducing postprandial glucose and A1C levels at the expense of greater weight gain and more hypoglycemia.34 However, there was insufficient clinical evidence to allow conclusions to be drawn regarding the effectiveness of PMX versus basal–bolus regimens in lowering either fasting or postprandial glucose.34 Our study, therefore, adds valuable clinical information to the outcomes associated with each of these types of insulin-intensification strategies, based on everyday clinical practice. Prospective controlled studies are also required to clarify the clinical benefits and weaknesses of basal–bolus versus PMX in patients uncontrolled on OADs or OADs plus basal insulin.
Our study has several potential limitations. This was a retrospective analysis of health care claims data, and as such cannot establish causality and may be subject to selection bias and other confounding factors. For example, the analysis assumed that a filled prescription equated to insulin use, but the existence of a claim for a filled prescription does not actually indicate whether or not the medication was consumed or whether it was taken as prescribed. Similarly, the presence of a diagnosis code on a medical claim form is not a 100% positive indicator of disease, as the diagnosis may be coded incorrectly or included as rule-out criteria rather than a confirmation of disease. Furthermore, blood glucose data are not included in the database, and identification of hypoglycemia events was based on ICD-9-CM codes in the claims data, and was therefore subject to coding error. The use of ICD-9-CM codes may over- or underestimate hypoglycemia. Differences in the characteristics of patients in each cohort could also potentially impact results. Although obesity was controlled for in this analysis, different weight distributions may have been present in each cohort, and hence may have affected the results. However, stringent PSM was used in the data analyses to attempt to minimize such effects. Additional limitations include the lack of information in the database on why patients switched their insulin treatment during the follow-up period, particularly in the PMX cohort. Dosing information was estimated based on filled pharmacy claims, which could introduce a bias to the results based on the frequency and the dose of treatment differences. Finally the number of daily RAI injections was not known in the real-world setting of this study, and may have affected treatment persistence.
It is also important to note that the analyses were based on observational health-claims data from a managed-care population, and may not be representative of other populations or applicable to all patients with T2DM. However, IMPACT® is a national US database comprising health-claims data from a large cohort of over 100 million US adults, and accordingly should represent a valid sample that is representative of clinical practice.
In summary, our retrospective analysis, using an intent-to-treat approach, showed that for US patients with T2DM, the clinical and economic outcomes associated with basal–bolus insulin treatment were similar to those in patients who switched to PMX. However, adding an RAI to GLA in a basal–bolus strategy was associated with increased treatment persistence compared with switching to PMX. Interestingly, we found that many patients who initially switched from GLA to PMX crossed back over to use GLA and/or an RAI as part of their regimen, possibly due to treatment effectiveness, quality of life, or treatment flexibility. Further studies are required to gain a better understanding of the impact of different treatment strategies on treatment persistence, long-term outcomes, and health care costs in order to help clinicians optimize outcomes for patients with T2DM.
Acknowledgments
This study was funded by Sanofi US, Inc. The authors received writing/editorial support in the preparation of this manuscript, provided by Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
Footnotes
Disclosure
Miao and Wei are employees of Sanofi US, Inc. Baser and Xie are employees at STATinMED, which received funding from Sanofi US, Inc to carry out this work. The authors report no other conflicts of interest in this work.
References
- 1.Shomali M. Add-on therapies to metformin for type 2 diabetes. Expert Opin Pharmacother. 2011;12(1):47–62. doi: 10.1517/14656566.2011.542150. [DOI] [PubMed] [Google Scholar]
- 2.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR. Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57) Diabetes Care. 2002;25(2):330–336. doi: 10.2337/diacare.25.2.330. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55(6):1577–1596. doi: 10.1007/s00125-012-2534-0. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association (ADA) Standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13(11):1020–1027. doi: 10.1111/j.1463-1326.2011.01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raskin P, Allen E, Hollander P, et al. Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 7.Raskin PR, Hollander PA, Lewin A, Gabbay RA, Bode B, Garber AJ. Basal insulin or premix analogue therapy in type 2 diabetes patients. Eur J Intern Med. 2007;18(1):56–62. doi: 10.1016/j.ejim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi: 10.1007/s10198-010-0224-8. [DOI] [PubMed] [Google Scholar]
- 9.Peyrot M, Rubin RR, Polonsky WH, Best JH. Patient reported outcomes in adults with type 2 diabetes on basal insulin randomized to addition of mealtime pramlintide or rapid-acting insulin analogs. Curr Med Res Opin. 2010;26(5):1047–1054. doi: 10.1185/03007991003634759. [DOI] [PubMed] [Google Scholar]
- 10.Fritsche A, Larbig M, Owens D, Häring HU. Comparison between a basal-bolus and a premixed insulin regimen in individuals with type 2 diabetes-results of the GINGER study. Diabetes Obes Metab. 2010;12(2):115–123. doi: 10.1111/j.1463-1326.2009.01165.x. [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357(17):1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 12.Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 13.Schiel R, Müller UA. Efficacy and treatment satisfaction of once-daily insulin glargine plus one or two oral antidiabetic agents versus continuing premixed human insulin in patients with type 2 diabetes previously on long-term conventional insulin therapy: the SWITCH Pilot Study. Exp Clin Endocrinol Diabetes. 2008;116(1):58–64. doi: 10.1055/s-2007-985367. [DOI] [PubMed] [Google Scholar]
- 14.Reaney M, Cypryk K, Tentolouris N, et al. Resource utilisation and clinical data before and after switching between short-acting human insulin and rapid-acting insulin analogues in patients with type 2 diabetes: the SWING study. Diabetes Res Clin Pract. 2012;97(2):231–241. doi: 10.1016/j.diabres.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Davies M, Sinnassamy P, Storms F, Gomis R. Insulin glargine-based therapy improves glycemic control in patients with type 2 diabetes sub-optimally controlled on premixed insulin therapies. Diabetes Res Clin Pract. 2008;79(2):368–375. doi: 10.1016/j.diabres.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Lakey WC, Barnard K, Batch BC, Chiswell K, Tasneem A, Green JB. Are current clinical trials in diabetes addressing important issues in diabetes care? Diabetologia. 2013;56(6):1226–1235. doi: 10.1007/s00125-013-2890-4. [DOI] [PubMed] [Google Scholar]
- 17.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer H, Klinge A. Patients with type 2 diabetes inadequately controlled on premixed insulin: effect of initiating insulin glargine plus oral antidiabetic agents on glycaemic control in daily practice. Int J Clin Pract. 2007;61(12):2009–2018. doi: 10.1111/j.1742-1241.2007.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharplin P, Gordon J, Peters JR, Tetlow AP, Longman AJ, McEwan P. Switching from premixed insulin to glargine-based insulin regimen improves glycaemic control in patients with type 1 or type 2 diabetes: a retrospective primary-care-based analysis. Cardiovasc Diabetol. 2009;8:9. doi: 10.1186/1475-2840-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blak BT, Smith HT, Hards M, Curtis BH, Ivanyi T. Optimization of insulin therapy in patients with type 2 diabetes mellitus: beyond basal insulin. Diabet Med. 2012;29(7):e13–e20. doi: 10.1111/j.1464-5491.2012.03586.x. [DOI] [PubMed] [Google Scholar]
- 21.Baser O, Bouchard J, DeLuzio T, Henk H, Aagren M. Assessment of adherence and healthcare costs costs of insulin device (FlexPen®) versus conventional vial/syringe. Adv Ther. 2010;27(2):94–104. doi: 10.1007/s12325-010-0009-6. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Quality Assurance HEDIS 2013 Available from: http://www.ncqa.org/HEDISQualityMeasurement/HEDISMeasures/HEDIS2013.aspxAccessed July 19, 2013
- 23.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res Clin Pract. 2010;89(2):189–195. doi: 10.1016/j.diabres.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract. 2011;17(6):845–852. doi: 10.4158/EP10401.OR. [DOI] [PubMed] [Google Scholar]
- 25.Xie L, Zhou S, Wei W, Gill J, Pan C, Baser O. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2013;15(3):230–236. doi: 10.1089/dia.2012.0253. [DOI] [PubMed] [Google Scholar]
- 26.Xie L, Wei W, Pan C, Du J, Baser O. A real-world study of patients with type 2 diabetes initiating basal insulins via disposable pens. Adv Ther. 2011;28(11):1000–1011. doi: 10.1007/s12325-011-0074-5. [DOI] [PubMed] [Google Scholar]
- 27.Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14(6):673–680. doi: 10.3111/13696998.2011.605818. [DOI] [PubMed] [Google Scholar]
- 28.Levin P, Wei W, Wang L, Pan C, Douglas D, Baser O. Combination therapy with insulin glargine and exenatide: real-world outcomes in patients with type 2 diabetes. Curr Med Res Opin. 2012;28(3):439–446. doi: 10.1185/03007995.2012.654850. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Campbell CR, Fonseca V, Shi L. Impact of hypoglycemia associated with antihyperglycemic medications on vascular risks in veterans with type 2 diabetes. Diabetes Care. 2012;35(5):1126–1132. doi: 10.2337/dc11-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 31.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 32.Polonsky WH, Rosenstock J, Gilmore AS, Wei W, Chaudhs SL, Riddle MC. Patient reported outcomes using twice-daily insulin aspart premixed vs insulin glargine plus 1 prandial insulin glulisine or step-wise addition of glulisine to glargine in type 2 diabetes uncontrolled with oral agents [abstract] Diabetes. 2011;60(Suppl 1):A616. [Google Scholar]
- 33.Gordon J, Pockett RD, Tetlow AP, McEwan P, Home PD. A comparison of intermediate and long-acting insulins in people with type 2 diabetes starting insulin: an observational database study. Int J Clin Pract. 2010;64(12):1609–1618. doi: 10.1111/j.1742-1241.2010.02520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agency for Healthcare Research and Quality Comparative Effectiveness, Safety, and Indications of Insulin Analogues in Premixed Formulations for Adults with Type 2 Diabetes: Executive Summary Rockville (MD)AHRQ; 2008Available from: http://effectivehealthcare.ahrq.gov/ehc/products/18/108/2008_0915InsulinAnaloguesExecSum.pdfAccessed July 19, 2013 [PubMed] [Google Scholar]
- 35.Ilag LL, Kerr L, Malone JK, Tan MH. Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparison. Clin Ther. 2007;29(6 Pt 1):1254–1270. doi: 10.1016/j.clinthera.2007.07.003. [DOI] [PubMed] [Google Scholar]