Abstract
Cytochrome P450 (CYP) of chicken and other avian species have been studied primarily with microsomes or characterized by cloning and protein expression. However, the overall existing isoforms in avian CYP1-3 families or dominant isoforms in avian xenobiotic metabolism have not yet been elucidated. In this study, we aimed to clarify and classify all of the existing isoforms of CYP1-3 in avian species using available genome assemblies for chicken, zebra finch, and turkey. Furthermore, we performed qRT-PCR assay to identify dominant CYP genes in chicken liver. Our results suggested that avian xenobiotic-metabolizing CYP genes have undergone unique evolution such as CYP2C and CYP3A genes, which have undergone avian-specific gene duplications. qRT-PCR experiments showed that CYP2C45 was the most highly expressed isoform in chicken liver, while CYP2C23b was the most highly induced gene by phenobarbital. Considering together with the result of further enzymatic characterization, CYP2C45 may have a dominant role in chicken xenobiotic metabolism due to the constitutive high expression levels, while CYP2C23a and CYP2C23b can be greatly induced by chicken xenobiotic receptor (CXR) activators. These findings will provide not only novel insights into avian xenobiotic metabolism, but also a basis for the further characterization of each CYP gene.
Introduction and Aim
The cytochrome P450 (CYP) families 1–3 are the major xenobiotic-metabolizing enzymes that contribute to the bioactivation or inactivation of numerous xenobiotic compounds such as drugs and environmental chemicals [1]. Xenobiotic-metabolizing ability is an important factor in determining sensitivity to foreign chemical compounds because the metabolites are readily excreted into urine. Xenobiotic-metabolizing CYPs are known to be expressed primarily in liver. mRNA expression levels of CYPs are known to correlate with their protein levels and enzymatic activity, although the strength of the correlations depends on the CYP isoform [2], [3].
Approximately 10,000 avian species are currently known, including poultry species, such as chicken and quail, and wild bird species, such as water birds and raptors. Because these species have numerous opportunities for exposure to drugs, pesticides, and other environmental chemicals, their xenobiotic-metabolizing ability and its relationship with chemical sensitivity is an important field of research. We previously showed large differences in the CYP-mediated metabolic activity of warfarin in chicken, ostrich, mallard, and owl [4]. This study explained, in part, the inconsistency between high LD50 value in chicken and frequent reports of secondary poisoning incidents in wild bird species.
Studies of CYP-mediated xenobiotic-metabolizing abilities in avian species have primarily examined activities toward specific substrates using liver microsomes or CYP induction by specific agents [5], [6]. Recently, enzymatic characteristics and phylogenetic relationships within each family have been examined for the chicken CYP isoforms, i.e., CYP1, CYP2C, CYP2D49, and CYP3A37 [7], [8] [9], [10], [11]. However, the xenobiotic-metabolizing CYP genes in avian species have not been completely elucidated, and the importance of each isoform has not been evaluated in comparison with other isoforms. Knowledge of each isoform is needed to be integrated into the whole perspectives.
In this report, we aimed to elucidate the existing isoforms of the avian CYP1-3 families using the available genome databases for chicken (Gallus gallusdomesticus), zebra finch ( Taeniopygia guttata ), and turkey ( Meleagris gallopavo ). We also investigated the phylogenetic relationships in comparison with human CYPs. Avian species are evolutionarily divided into three groups [12]. The Palaeognathae (e.g., ostrich and emu) diverged first, followed by the Galloanserae (e.g., chicken, turkey, and most other poultry species) and the Neoaves, which includes 99.5% of all avian species. Thus, comparative analyses of chicken, zebra finch, and turkey can provide a comprehensive understanding of the common features and differences among most bird species, except for those of the Palaeognathae. We used quantitative real-time RT-PCR (qRT-PCR) methods to clarify basal CYP mRNA expression patterns and phenobarbital (PB) induction in chicken liver to identify the dominant isoforms. Furthermore, we compared the enzymatic activity of chicken CYP2C proteins, i.e. CYP2C23a, CYP2C23b and CYP2C45. These results on avian xenobiotic-metabolizing CYP isoforms will serve as the basis for future studies of avian xenobiotic metabolism and species differences in the resulting chemical sensitivities.
Materials and Methods
Animals
Eight male and eight female White Leghorn chickens were obtained from Hokkaido Central Chicken Farm (Hokkaido, Japan). They were housed in plastic cages and fed a standard diet (Nihon Nosan Kogyo Co., Yokohama, Japan) and water ad libitum. The animal room was maintained at 25 °C ± 2 °C under a 12-h light–dark cycle (starting at 07:00 h). They were divided into two groups, i.e. saline-treated and PB-treated groups with each four individuals. For saline-treated group and PB-treated group, saline or PB (80 mg/kg dissolved in saline) were intraperitoneally injected for three days, respectively. The chickens were sacrificed after 24 hours of the last dose, at the age of 8 weeks old, using carbon dioxide, and their livers were collected. The livers were immediately frozen in liquid nitrogen and stored at −80 °C until use. All experiments using animals were performed under the supervision and with the approval of the Institutional Animal Care and Use Committee of Hokkaido University (Permit number 10-0067).
Phylogeny and synteny analyses of CYP1-3 genes from three avian species
The CYP1-3 genes of chicken, zebra finch, and turkey were retrieved using an NCBI BLAST search. Because the first gene set contained CYP genes other than CYP1-3, we first analyzed the retrieved genes by neighbor-joining phylogeny to select the CYP1-3 genes for further analyses. We then separated the genes into CYP1-3 families and performed phylogenetic analysis. The deduced amino acid sequences were aligned using MUSCLE [13] and employed for model selection and construction of maximum likelihood trees (bootstrapping = 100) using MEGA5 [14]. Because preliminary nucleotide analysis provided very similar results for all CYP1-3 families, we focused our study on the amino acid sequence results. Gaps and missing data were excluded from the analyses by partial deletion with site coverage cutoff of 95%.
NCBI’s MapViewer (http://www.ncbi.nlm.nih.gov/projects/mapview/) was used to visualize chromosomal synteny maps for each species. The latest genome assemblies were used; chicken Build 3.1, zebra finch Build 1.1, turkey Build 1.1, and human Build 37.3. UCSC BLAT (http://genome.ucsc.edu/index.html) and the Ensembl database (http://asia.ensembl.org/index.html) were used for additional confirmation of missing genes. Orthologous relationships were confirmed by synteny analysis. The gene sequences were submitted to the Cytochrome P450 Nomenclature Committee for nomenclature [15].
Quantitative real-time RT-PCR
Total RNA was extracted from the chicken livers using RNeasy Mini Kits (Qiagen, Valencia, CA). The purity and quantity of RNA were determined spectrophotometrically using NanoDrop ND-1000 (Thermo Scientific, DE, USA). A260/280 and A260/230 were generally ≥2. Total RNA (2 µg) was reverse transcribed using ReverTra Ace (Toyobo, Tokyo, Japan) in a final volume of 40 µl, according to manufacturer’s instructions. Gene-specific qRT-PCR primers (Table S1) were synthesized by Sigma-Aldrich (Tokyo, Japan). qRT-PCR was performed using the StepOnePlus Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The 10-µl PCR reaction mixture consisted of Fast SYBR Green Master Mix (Applied Biosystems), forward and reverse primers (200 nM each), and cDNA derived from 10 ng of total RNA. Plasmids containing each amplicon were used for the calibration curves. The plasmids were constructed with the PCR products and pCR2.1-TOPO vector using a TOPO TA Cloning Kit (Invitrogen, CA, USA). All samples, including cDNA derived from the chicken livers and the plasmid standards, were analyzed in duplicate using the following protocol: 95 °C for 20 s followed by 40 cycles of 95 °C for 3 s and 60 °C for 30 s. At the end of each PCR run, melt curve analysis was performed in the range of 60–95 °C. PCR products were confirmed to be single fragments by electrophoresis and direct sequencing methods. The glyceraldehyde-3-phosphate dehydrongenase (GAPDH) gene was used as an internal control. cDNAs obtained without reverse transcription were examined to confirm that the Ct value was >35.
Enzymatic characterization of chicken CYP2C isoforms using P450-Glo assay
Yeast microsomes expressing chicken CYP2C23a, CYP2C23b, CYP2C45 or rat CYP2C11 were prepared as described previously with several modifications [16]. In brief, the full-length insert encoding chicken CYP2C23a, CYP2C23b, CYP2C45 or rat CYP2C11 was amplified by PCR. The resulting fragments were subcloned into a pGYR1 expression vector containing yeast P450 reductase using In-Fusion HD Cloning Kit (Takara Bio, Japan). The AH22 strain of Saccharomyces cerevisiae was transformed with the expression plasmids by the lithium acetate method. The transformants with pGYR/CYP were selected by the complementation of lue2 auxotrophy. The selected transformants were cultivated in synthetic minimal medium (2% (w/v) D-glucose and 0.67% (w/v) yeast nitrogen base without amino acids) supplemented with 20 mg/l histidine. Microsomal fractions of yeast were prepared by the method of Oeda et al. [17]. The P450 content of yeast microsomes was estimated using the method of Omura and Sato [18]. The protein concentration of the microsomes was determined with the bicinchoninic acid (BCA) protein assay reagent (Nacalai Chemical Co., Japan) using bovine serum albumin (BSA) as a standard.
The activity of the cDNA-expressed proteins of chicken CYP2C23a, CYP2C23b, CYP2C45 and rat CYP2C11 were determined by P450-GloTM CYP2C8 assay and CYP2C9 assay (Promega Corporation, USA) utilizing a demethylation reaction with luciferin 6’ methyl ether (luciferin-ME; 100 µM) and a hydroxylation reaction with 6’ deoxyluciferin (luciferin-H; 100 µM) as the substrates. The assays were performed according to the manufacturer’s instructions with slight modifications. In brief, the test sample contained 1 pmol microsomal CYP, 50 mM phosphate buffer, and 100 µM of either luciferin-ME or luciferin-H. The samples were plated into 96-well white opaque polystyrene plate, pre-incubated at 37 °C for 10min, to which a NADPH regenerating system was added, then incubated for another 30min. After the reaction, 50 µl of reconstituted luciferin reagent was added and incubated at room temperature for further 30 min, and then the relative luminescence intensity was measured. Blank samples were incubated with the addition of phosphate buffer instead of NADPH regenerating system. The experiments were carried out in triplicate.
Calculation and statistics
Gene copy numbers were calculated for each cDNA sample using standard curves. The copy numbers were normalized with GAPDH copy numbers, and the normalized gene expression levels were compared as shown in the figures. The statistical analysis was performed using JMP 9 (SAS Institute, USA). Significant differences were evaluated by Student t test for mRNA induction, and tukey’s HSD test for CYP2C enzymatic activities.
Results and Discussion
Avian CYP1-3: annotation, nomenclature, and overview of the genes
In this study, we focused principally on the putatively functional genes with full-length and/or intact sequences. Based on phylogenetic and synteny analyses, the avian CYP1-3 genes were classified as shown in Tables S2–S4. The human CYP genes used in the analyses are shown in Table S5. The numbers of genes in each CYP1-3 family are shown in Table 1. The number of genes in each family did not differ much among the species, while CYP2 family appeared to include many more genes than the CYP1 and CYP3 families. Thomas [19] demonstrated that xenobiotic-metabolizing CYP genes have generally gone through rapid birth-death evolution (frequent gene duplications and losses). Our results showed that the avian CYP2 family genes, same as the human and mouse genes, have undergone frequent duplication events compared with the avian CYP1 and CYP3 family genes. However, the complement of CYP genes shown here may be incomplete and some genes could not be localized in synteny analysis. Due to the incompleteness of current assembly, annotation and locus information, there’s a possibility that our analysis could not fully cover the CYP complements. The information may be updated in future.
Table 1. CYP1-3 gene numbers in each species.
| Family | Chicken | Finch | Turkey | Human |
|---|---|---|---|---|
| 1 | 4 | 3 | 3 | 3 |
| 2 | 21 | 16 | 18 | 16 |
| 3 | 2 | 2 | 2 | 4 |
| Total | 27 | 21 | 23 | 23 |
CYP1
The CYP1 phylogeny was constructed with 243 amino acid residues using the JTT matrix-based model (Figure S1). Human CYP2A13 was used as an outgroup. CYP1A4/5 and CYP1B1 genes were confirmed to be completely conserved among the three avian species and human by phylogeny and synteny analyses (Figure S1). CYP1C1 was found to be a pseudogene with stop codons in zebra finch, turkey, and human CDSs. Only chicken CYP1C1 was a full-length sequence without stop codons. Although the avian CYP1A has been named CYP1A4/5, it is orthologous to human CYP1A1/2 [20].
CYP2
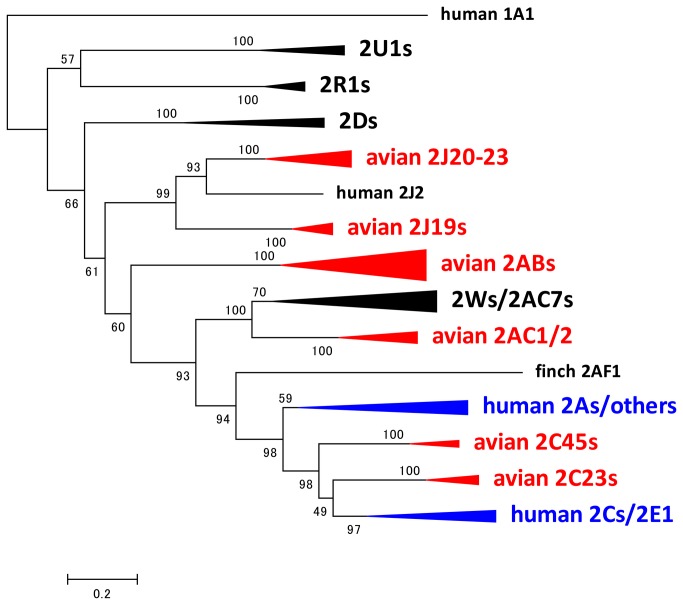
The CYP2 phylogeny was constructed with 441 amino acid residues using the JTT matrix-based model (Figure 1 for a compressed phylogeny, and Figure S2 for a full phylogeny). Human CYP1A1 was used as an outgroup. The phylogeny was generally consistent with the previously reported CYP2 phylogeny [9]. Some avian characteristics were found in the CYP2 family, i.e., multiple genes in the avian CYP2J, CYP2AB, CYP2AC, and CYP2W subfamilies, whereas humans have only a single gene, a single pseudogene in these subfamilies, absence of CYP2B and CYP2E; and a unique duplication pattern of CYP2C genes as described below.
Figure 1. Compressed phylogeny of CYP2 genes.
Phylogeny of CYP2 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 20 substitutions per 100 residues. The triangles indicate genes in the same subfamily. The size of the triangles indicates the number of genes included in the branch. Blue triangle and red triangle indicate avian specific branch and human specific branch, respectively. The detailed phylogeny is shown in Figure S2.
CYP2D, CYP2R, and CYP2U
There were single genes in the CYP2D, CYP2R, and CYP2U subfamilies in all species. Synteny was fully shared for CYP2R1 and CYP2U1 among birds and humans (Figure 2). The CYP2D cluster was also conserved among the species, except that the human CYP2D cluster contains CYP2D6 and two other CYP2D pseudogenes between TCF20 and NDUFA6. Because CYP2R and CYP2U have essential functions in the metabolism of the endogenous substrates vitamin D and arachidonic acid, these genes may be strongly conserved [21], [22](Chuang, 2004 #66).
Figure 2. Typical fully shared synteny of CYP2D, 2R and CYP2U.
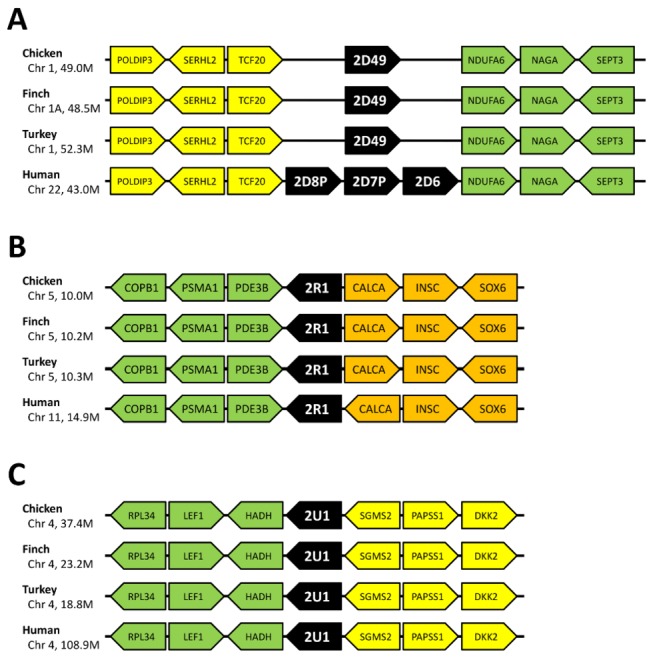
Synteny was well conserved for the following genes (A) CYP2D genes. (B) CYP2R1, (C) CYP2U1. CYP2R1 and CYP2U1 are known to metabolize endogenous compounds in mammal, while chicken CYP2D49 has been reported to metabolize bufuralol, a beta blocker drug.
CYP2ABFGST cluster
In human and mouse, the CYP2ABFGST clusters are known to be located on chromosomes 19 and 7A3, respectively [15]. Kirischian et al. [23] previously suggested that CYP2A, CYP2B, CYP2F, CYP2G, and CYP2S were mammalian-specific. In our investigation, neither the CYP2ABFGST cluster nor any of the genes included in the cluster, such as CYP2A and CYP2B, were found with the search tools in any of the avian species (data not shown). The anole lizard is known to possess the CYP2G gene. Thus, the CYP2ABFGST cluster may have been lost only in the avian lineage.
CYP2C/2E
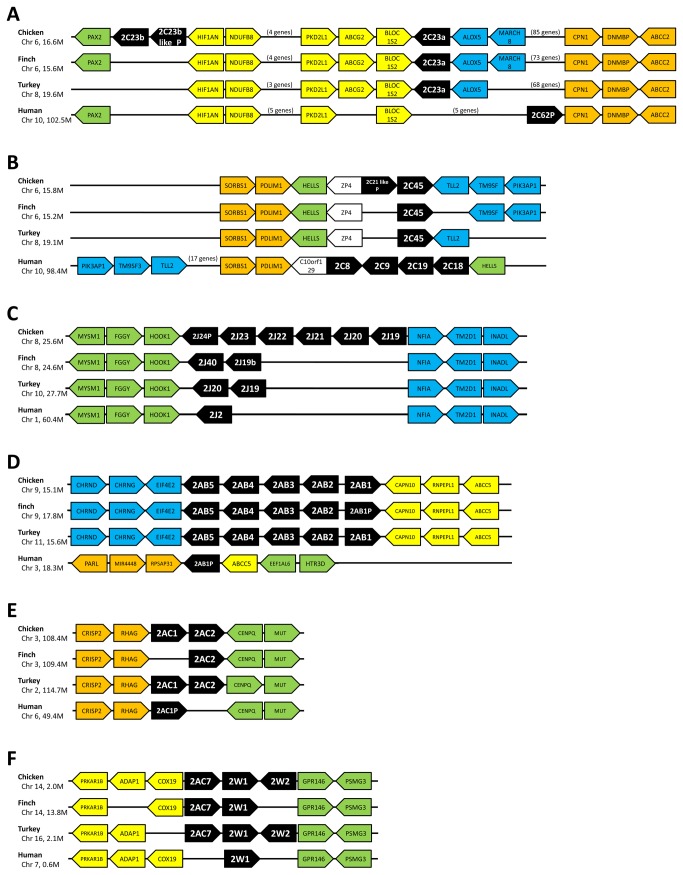
Avian CYP2C genes were formerly called CYP2H genes because they were assumed to be avian-specific. However, Kubota et al. [9] reported that the genes corresponded to mammalian CYP2C genes by phylogeny and synteny analyses. In our phylogeny, the avian CYP2C subfamily also comprised a clade with the human CYP2C and CYP2E clade. Human CYP2Cs and CYP2E1 together formed a single clade, which is clearly distinct from the avian CYP2C23 and CYP2C45 clade. The synteny of CYP2C23 and CYP2C45 are shown separately in Figure 3, although their positions are <1 Mb apart. In the CYP2C23 locus, CYP2C23a was located between BLOC1S2 and ALOX5 in the three bird species, whereas only chicken possessed another gene, CYP2C23b and CYP2C23b-like pseudogene between PAX2 and HIF1AN. The positional relations of PAX2, HIF1AN, BLOC1S1, and CPN1 were also conserved in human, but the CYP2C62 pseudogene was located next to CPN1. In turkey, a CYP2C23-like partial sequence existed between ALOX5 and CPN1. This sequence is assumed to have stop codons in CDS. In birds, >70 genes are present between BLOC1S2 and CPN1 genes; however, in human, only six genes including CYP2C62p are present between the BLOC1S2 and CPN1 genes.
Figure 3. Synteny of CYP2 family genes.
(A) Synteny of CYP2C23 genes. Only chicken had two CYP2C23 genes, whereas the other two birds possessed only one CYP2C23a gene. Avian CYP2C23 genes have been reported to be orthologous to human CYP2C62P, rat CYP2C23 and mouse CYP2C44. (B) Synteny of CYP2C45 genes. CYP2C45 genes were generally conserved among bird species. Although both avian CYP2C45 genes and human CYP2C genes located nearby SORBS1-PDLIM1 cluster, phylogeny did not suggest that avian CYP2C45 genes are related to human CYP2C genes. These results implied that the avian and human CYP2C genes having gone through independent duplication events in each lineage. (C) Synteny of CYP2J genes. Two of the zebra finch CYP2J genes were not localized to the chromosome and are not shown in this figure. (D) Synteny of CYP2AB genes. Five avian CYP2AB genes were fully conserved among bird species, while human have only one pseudogene. (E) Synteny of CYP2AC1 and CYP2AC2 genes. While avian species possessed one or two intact CYP2AC genes, human possessed only one pseudogene. The synteny was generally shared among bird species and human. (F) Synteny of CYP2AC7 and CYP2W genes. The gene orders around CYP2AC7 and CYP2W genes were generally conserved among species, although bird species possessed another CYP2W gene and CYP2AC7 gene in addition to CYP2W1. CYP2AC7 genes were thought to have diverged from CYP2W genes, and became a parent gene for CYP2AC1 and CYP2AC2.
The synteny of CYP2C45 was the most disrupted among the genes examined in this study. In chicken, CYP2C45 with a CYP2C21-like pseudogene was located 0.8 Mb upstream of the CYP2C23 locus. The avian CYP2C45 genes were located between SORBS1-PDLIM-HELLS-ZP4 and TLL2-TM9SF3. In contrast, CYP2Cs, i.e., CYP2C8, CYP2C9, CYP2C18, and CYP2C19, were located between SORBS1-C10orf129 and HELLS in human, and the TM9SF3-TLL2 cluster was located on the opposite side compared with the avian species. The human CYP2E1 gene was located between OR7M1P and SYCE1 at 135.3 Mb on chromosome 10, whereas CYP2C genes were located at 96.4–101.9 Mb on the same chromosome. The corresponding region and clusters to human CYP2E1 locus were not found in avian species.
From the above analyses, CYP2C genes and the cluster did not share clear synteny between avian species and human, whereas the synteny was shared among the avian species. Thus, clear orthologous relationships are not suggested between the avian and human CYP2C genes. This was also supported by the phylogeny. Rather, it is suggested that a single CYP2C ancestor gene duplicated independently in the avian and human lineages to form the current CYP2C gene complements. The three CYP2C23 genes, including one pseudogene only in chicken, suggest that duplication of the CYP2C23 gene may have occurred once or twice in early chicken history. This implies that the avian CYP2C genes loci have undergone frequent alteration in the genome structure.
CYP2J
Humans have only one CYP2J gene, whereas avian species have multiple CYP2J genes. We searched for the CYP2J genes not only in GenBank but also in Ensembl and UCSC BLAT. We found that chicken has six genes including one partial sequence. Zebra finch has four genes, although two of them are not localized on the chromosome. Turkey has two genes and one partial gene. Synteny analysis showed that CYP2J genes were conserved on the chromosome, i.e., between MYSM1-FGGY-HOOK1 and NFIA-TM2D1-INADL (Figure 3). The phylogeny suggested independent duplication events in the early chicken and zebra finch lineages.
CYP2AB
The CYP2AB clade was constructed with only bird genes because humans have only a CYP2AB1 pseudogene, and it was excluded from phylogenetic analysis. The CYP2AB genes were divided into two clades, the CYP2AB1, CYP2AB4, and CYP2AB5 clade and the CYP2AB2 and CYP2AB3 clade. CYP2AB1 through CYP2AB5 genes were found in all avian species, although zebra finch CYP2AB1 was found to be a pseudogene with a stop codon in CDS. Turkey CYP2AB3 was not found in GenBank but was found in Ensembl.
In synteny analysis, avian CYP2AB genes were found to be fully conserved between CHRND-CHRNG-EIF4E2 and CAPN10-RNPEPL1-ABCC5, although the human CYP2AB1 pseudogene was found between PARL-MIR4448-RPSAP31 and ABCC5-EEF1AL6-HTR3D on chromosome 3 (Figure 3). In human, the CHRND-CHRNG-EIF4E2 cluster and CAPN10-RNPEPL1 cluster were found at 233.4 Mb and 241.5 Mb, respectively, on chromosome 2.
CYP2AC1/2
CYP2AC1 and CYP2AC2 formed a clade with a sister clade of CYP2W and CYP2AC7 in the phylogeny. CYP2AC1 and 2AC2 genes were found between RHAG and CENPQ in each species, and the synteny was clearly conserved including in human (Figure 3). In human, only a single CYP2AC1p was found in the corresponding locus. Zebra finch possessed only one CYP2AC2 gene, and chicken and turkey possessed two CYP2AC genes, CYP2AC1 and CYP2AC2. This situation with fully conserved synteny and few duplication events is similar to that of CYP2D, CYP2R, and CYP2U, although functions or physiological roles have not yet been reported for CYP2AC1 and CYP2AC2.
CYP2AC7/2W1
CYP2AC7 and CYP2W genes formed one clade in the phylogeny with a sister clade of CYP2AC1 and CYP2AC2 genes.
In synteny analysis, the CYP2AC7 and CYP2W genes were located between PRKAR1B-ADAP1-COX19 and GPR146-PSMG3 in birds and humans (Figure 3). In chicken and turkey, there were two CYP2W genes (CYP2W1 and CYP2W2) and one CYP2AC7 gene, whereas in zebra finch, there was only one CYP2W gene. Only one CYP2W1 gene was present in human. Duplication events only in the Galloanserae lineage are suggested for the CYP2W1 and CYP2W2 genes. CYP2AC7 may be a parent gene for CYP2AC1/2 genes.
CYP3
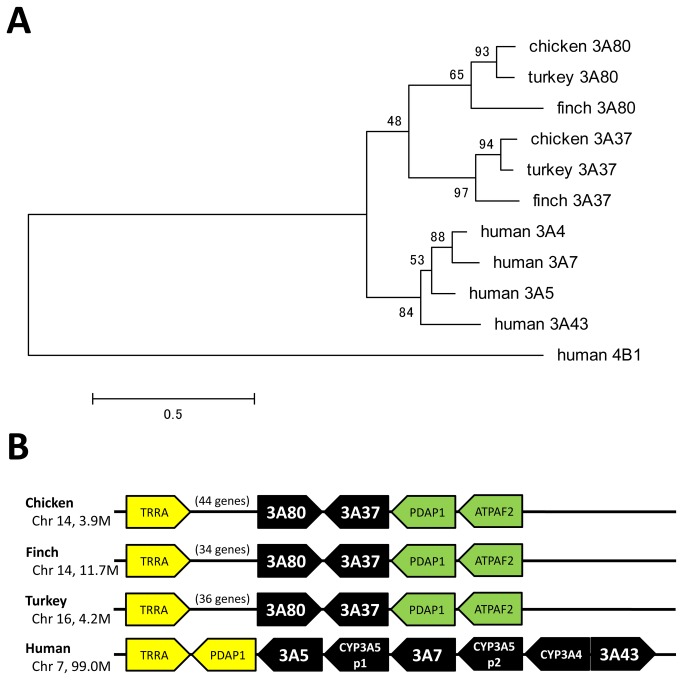
The phylogeny was constructed with 315 amino acid residues using the JTT matrix-based model (Figure 4). Human CYP4B1 was used as an outgroup. The CYP3 family was clearly divided into two clades, the bird CYP3A clade and human CYP3A clade, as shown in the phylogeny. Two CYP3A genes, CYP3A37 and CYP3A80, are present in birds, whereas four CYP3A genes are present in humans.
Figure 4. Phylogenetic tree and synteny of CYP3A genes.
(A) Phylogeny of CYP3 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 50 substitutions per 100 residues. (B) Avian species possessed two CYP3A genes, whereas humans possessed six genes including two pseudogenes. Although the loci of CYP3A genes were almost conserved between avian species and human, none of the avian CYP3A genes showed clear correspondence to any of the human CYP3A genes.
In birds, the gene order around CYP3A37 and CYP3A80 was fully conserved among species because there were more than 34–44 genes between TRRA and CYP3A80-CYP3A37-PDAP1 (Figure 4). In contrast, in human, PDAP1 was located next to TRRA and the CYP3A genes were arranged next to them in tandem. This suggests that a chromosome inversion occurred around the locus of the CYP3A gene in either the avian or human lineage. The phylogenetic and synteny analyses suggested no clear orthology between any of the bird CYP3A genes and any of the human CYP3A genes. McArthur et al. [24] suggested independent CYP3A duplication events in early rodent history, primate history, and teleost history. Our results also suggested a single duplication event of the CYP3A gene in early bird history.
mRNA expression levels in chicken liver; basal expression and induction by PB
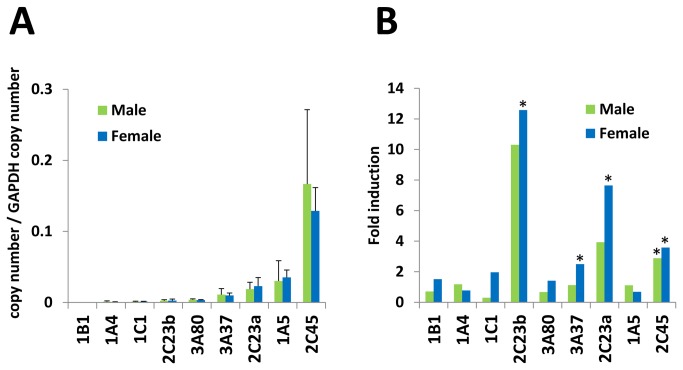
We focused on the isoforms that have been reported in the literature or are putatively predominant in liver. Ten isoforms, CYP1A4, CYP1A5, CYP1B1, CYP1C1, CYP2C23a, CYP2C23b, CYP2C45, CYP2D49, CYP3A37, and CYP3A80, were examined. We examined GAPDH and beta-actin for normalization and found that GAPDH was more stable, including in phenobarbital-induced chicken liver samples (data not shown). In the above gene set, CYP2C45 showed the highest expression level in both male and female chickens, followed by CYP1A5, CYP2C23a, and CYP2D49 (Figure 5). The CYP3A isoforms, i.e., CYP3A37 and CYP3A80, had the fifth and sixth highest expression levels in this gene set. In chicken, CYP2C45, which showed the highest mRNA expression, is suggested as a candidate for the most important isoform. In contrast, chicken CYP3A37 and CYP3A80 showed relatively low expression. Blevins et al. [25] have reported the age-related alteration in 7-benzyloxyquinoline-metabolizing activity, which is considered to be a function of CYP3A proteins. The enzymatic activity was highest in 8-week-old chickens, and at 20 weeks, the activity had declined to one-fifth of the activity at 8 weeks. Because our samples in this study were aged 8 weeks, older or adult chickens may have even lower mRNA expression levels of CYP3A genes. CYP3A37 genes have been cloned and characterized in chicken and turkey, while CYP3A80 have not been characterized in any literature, to our knowledge [11,26]. This is consistent with our result, which suggested CYP3A80 has far lower expression levels in chicken liver. Thus, CYP3A37 was suggested to be more important in avian xenobiotic metabolism than CYP3A80 because of the higher expression level. In our qRT-PCR, none of the genes examined showed sex differences in mRNA expression levels. However, age-related alterations may possibly affect mRNA expression patterns in chicken liver. This study is the first to report mRNA expression profiles of CYP1-3 genes in chicken liver. Further study is needed to elucidate the relationships among mRNA expression levels, protein expression levels, and enzymatic activity and their alterations by factors such as inducing agents, age and sex. We further investigated the mRNA induction by PB, a typical mammalian CAR activator. However, chicken do not possess CAR or PXR, but chicken xenobiotic receptor (CXR) as an orthologous receptor to them [27]. In chicken, PB is known to induce CYP2C genes and CYP3A genes [10,11,28,29]. Our result also showed significant induction of CYP2C23a, CYP2C23b, CYP2C45 and CYP3A37 in female chicken, although only CYP2C45 was significantly induced in male chicken. The rank order of the average induction levels were CYP2C23b > CYP2C23a > CYP2C45 > CYP3A37 in both male and female chicken.
Figure 5. Comparison of basal mRNA expression levels and induction by PB of CYP genes in chicken liver.
qRT-PCR was performed with cDNAs derived from chicken liver for following genes: CYP1A4, CYP1A5, CYP1B1, CYP1C1, CYP2C23a, CYP2C23b, CYP2C45, CYP2D49, CYP3A37, and CYP3A80. The transcripts of each gene were calculated using plasmid standards. The values represent the expression levels normalized with GAPDH gene transcripts. (A) basal expression levels in non-treated chicken liver. Data are expressed as an average ± SD (B) fold induction levels by PB are indicated by the ratio of the mRNA expression levels of PB-treated chicken liver and saline-treated chicken liver for each gene. The fold induction level is shown by an average, and the significant induction of genes in PB-treated chicken compared to saline-treated chicken is indicated by an asterisk. N=4 for each group.
Function and possible role of CYP2C isoforms in avian xenobiotic metabolism
As the results of mRNA expression levels suggested CYP2C genes may have important roles in chicken xenobiotic metabolism, we examined the function of the CYP2C isoforms using P450-Glo assay kit, with substrates of luciferin-H (CYP2C8 assay) and luciferin-ME (CYP2C9 assay). In comparison to rat CYP2C11 as a positive control, all the chicken CYP2C isoforms showed lower activity with luciferin-H, and higher activity in luciferin-ME (Table 2). Among the chicken CYP2C isoforms, the activity were significantly different to both substrates, suggesting the clear difference in substrate selectivity even between CYP2C23a and CYP2C23b which shared 93.4% of identity percentage of amino acid sequences. The rank order of the activity toward luciferin-H and luciferin-ME were both CYP2C23a > CYP2C23b > CYP2C45. Although the activities toward the P450-Glo substrates were lower than the other CYP2C isoforms, CYP2C45 may have larger contribution to xenobiotic metabolism in vivo because of the constitutive high expression of mRNA. On the other hand, CYP2C23a and CYP2C23b showed higher induction compared to CYP2C45 by phenobarbital and higher activity toward luciferin-H and luciferin-ME. They may be isoforms important under the exposure of xenobiotic chemicals.
Table 2. Activity of CYP2C proteins assayed with P450-Glo.
| Luciferin-H | Luciferin-ME | |||||||
|---|---|---|---|---|---|---|---|---|
| Chicken CYP2C23a | 196.3 | ± | 6.3 | 1 | 1869.0 | ± | 63.5 | a |
| Chicken CYP2C23b | 62.7 | ± | 3.8 | b | 236.3 | ± | 9.2 | b |
| Chicken CYP2C45 | 12.7 | ± | 3.8 | c | 142.7 | ± | 6.7 | b |
| Rat CYP2C11 | 262.7 | ± | 7.9 | d | 121.7 | ± | 6.8 | b |
Activity of CYP2C23a, CYP2C23b and CYP2C45 proteins were examined with P450-Glo assay. The assays were performed in triplicate. Data are expressed as an average ± SE, with a unit of relative luminescent unit. Different characters indicate significant differences.
Conclusions
In this study, we have established a comprehensive understanding of the existence and overall relationships of avian CYP1-3 genes in chicken, zebra finch, and turkey in comparison with human genes. We have revealed several remarkable characteristics of avian CYP1-3 genes, including the absence of clear orthology between avian and human CYP2C or CYP3A genes and the existence of CYP2J, CYP2AB, and CYP2AC duplication events in the early bird lineage. A common feature among birds and humans was also found, in that CYP2R1 and CYP2U1 were fully conserved despite independent avian and mammalian evolution, which is probably because of their essential functions in the metabolism of endogenous compounds. Because species differences in the avian and mammalian CYP1-3 genes may be important for their extrapolation to the fields of pharmacology and toxicology, detailed enzymatic and physiological characterization of the avian CYP1-3 genes is required.
In qRT-PCR assays, CYP2C45 showed the highest basal mRNA expression in chicken liver, and CYP2C23b gene was the most induced gene by PB followed by CYP2C23a and CYP2C45. In the enzymatic characterization of CYP2C isoforms utilizing P450-Glo substrates, the highest activity was observed in CYP2C23a and the lowest in CYP2C45. Because the contribution of each CYP isoform to the drug metabolism is greatly decided by its protein expression levels in liver, avian CYP2C45 may also be the dominant isoform in avian xenobiotic metabolism, and other CYP2C genes may have a role to respond to intake of xenobiotics. Based on these findings, further investigation is needed to characterize in detail the avian CYP isoforms, including their expression patterns in other organs, proteomic approaches to quantifying the proteins, functional analyses, and their relationships with chemical sensitivities.
Supporting Information
Phylogenetic tree and synteny of CYP1 family genes. (A) Phylogeny of CYP1 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 20 substitutions per 100 residues. (B) CYP1A4 and CYP1A5 genes (orthologues of human CYP1A1 and CYP1A2). (C) CYP1B1 genes.
(TIF)
Full phylogenetic tree of CYP2 family genes. Phylogeny of CYP2 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 50 substitutions per 100 residues. The compressed version of this phylogeny is shown in Figure 1.
(TIF)
Primers for qRT-PCR.
(XLS)
CYP 1-3 genes of chicken.
(XLSX)
CYP 1-3 genes of zebra finch.
(XLSX)
CYP 1-3 genes of turkey.
(XLSX)
CYP 1-3 genes of human.
(XLSX)
Acknowledgments
We thank D. Nelson of the Cytochrome P450 Nomenclature Committee for naming the new avian CYP genes. The authors would like to thank Enago (www.enago.jp) for the language review.
Funding Statement
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) awarded to Prof. M. Ishizuka (Nos. 24248056 and 24405004) and JSPS Fellowship for KPW (231864). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nebert DW, Russell DW (2002) Clinical importance of the cytochromes P450. Lancet 360: 1155-1162. doi:10.1016/S0140-6736(02)11203-7. PubMed: 12387968. [DOI] [PubMed] [Google Scholar]
- 2. Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S et al. (2011) Simultaneous Absolute Protein Quantification of Transporters, Cytochromes P450, and UDP-Glucuronosyltransferases as a Novel Approach for the Characterization of Individual Human Liver: Comparison with mRNA Levels and Activities. Drug Metab Dispos 40: 83-92. PubMed: 21994437. [DOI] [PubMed] [Google Scholar]
- 3. Temesvári M, Kóbori L, Paulik J, Sárváry E, Belic A et al. (2012) Estimation of Drug-Metabolizing Capacity by Cytochrome P450 Genotyping and Expression. J Pharmacol Exp Ther 341: 294-305. doi:10.1124/jpet.111.189597. PubMed: 22262920. [DOI] [PubMed] [Google Scholar]
- 4. Watanabe KP, Saengtienchai A, Tanaka KD, Ikenaka Y, Ishizuka M (2010) Comparison of warfarin sensitivity between rat and bird species. Comp Biochem Physiol C 152: 114-119. PubMed: 20346414. [DOI] [PubMed] [Google Scholar]
- 5. Gupta RP, Abou-Donia MB (1998) Cytochrome P450 enzymes in chickens: characteristics and induction by xenobiotics. Comp Biochem Physiol C 121: 73–83. PubMed: 9972452. [DOI] [PubMed] [Google Scholar]
- 6. Walker CH (1998) Avian forms of cytochrome P450. Comp Biochem Physiol C 121: 65-72. PubMed: 9972451. [DOI] [PubMed] [Google Scholar]
- 7. Cai H, Jiang J, Yang Q, Chen Q, Deng Y (2012) Functional Characterization of a First Avian Cytochrome P450 of the CYP2D Subfamily (CYP2D49). PLOS ONE 7: e38395. doi:10.1371/journal.pone.0038395. PubMed: 22675558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jönsson ME, Woodin BR, Stegeman JJ, Brunström B (2011) Cytochrome P450 1 genes in birds: evolutionary relationships and transcription profiles in chicken and Japanese quail embryos. PLOS ONE 6: e28257. doi:10.1371/journal.pone.0028257. PubMed: 22164255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kubota A, Stegeman JJ, Goldstone JV, Nelson DR, Kim EY et al. (2011) Cytochrome P450 CYP2 genes in the common cormorant: Evolutionary relationships with 130 diapsid CYP2 clan sequences and chemical effects on their expression. Comp Biochem Physiol C 153: 280-289. PubMed: 21130899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baader M, Gnerre C, Stegeman JJ, Meyer UA (2002) Transcriptional activation of cytochrome P450 CYP2C45 by drugs is mediated by the chicken xenobiotic receptor (CXR) interacting with a phenobarbital response enhancer unit. J Biol Chem 277: 15647-15653. doi:10.1074/jbc.M109882200. PubMed: 11867618. [DOI] [PubMed] [Google Scholar]
- 11. Ourlin JC, Baader M, Fraser D, Halpert JR, Meyer UA (2000) Cloning and Functional Expression of a First Inducible Avian Cytochrome P450 of the CYP3A Subfamily (CYP3A37). Arch Biochem Biophys 373: 375-384. doi:10.1006/abbi.1999.1566. PubMed: 10620362. [DOI] [PubMed] [Google Scholar]
- 12. Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL et al. (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320: 1763-1768. doi:10.1126/science.1157704. PubMed: 18583609. [DOI] [PubMed] [Google Scholar]
- 13. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792-1797. doi:10.1093/nar/gkh340. PubMed: 15034147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739. doi:10.1093/molbev/msr121. PubMed: 21546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson DR, Zeldin DC, Hoffman SMG, Maltais LJ, Wain HM et al. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenet Genomics 14: 1–18. PubMed: 15128046. [DOI] [PubMed] [Google Scholar]
- 16. Ikushiro S, Sahara M, Emi Y, Yabusaki Y, Iyanagi T (2004) Functional co-expression of xenobiotic metabolizing enzymes, rat cytochrome P450 1A1 and UDP-glucuronosyltransferase 1A6, in yeast microsomes. Biochim Biophys Acta 1672: 86-92. doi:10.1016/j.bbagen.2004.02.012. PubMed: 15110090. [DOI] [PubMed] [Google Scholar]
- 17. Oeda K, Sakaki T, Ohkawa H (1985) Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA 4: 203–210. doi:10.1089/dna.1985.4.203. PubMed: 3159557. [DOI] [PubMed] [Google Scholar]
- 18. Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. J Biol Chem 239: 2370–2378. PubMed: 14209971. [PubMed] [Google Scholar]
- 19. Thomas JH (2005) Rapid Birth-Death Evolution Specific to Xenobiotic Cytochrome P450 Genes in Vertebrates. PLOS Genet 3(5): e67 PubMed: 17500592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goldstone HMH, Stegeman JJ (2006) A Revised Evolutionary History of the CYP1A Subfamily: Gene Duplication, Gene Conversion, and Positive Selection. J Mol Evol 62: 708-717. doi:10.1007/s00239-005-0134-z. PubMed: 16752211. [DOI] [PubMed] [Google Scholar]
- 21. Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (2004) Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci USA 101: 7711-7715. doi:10.1073/pnas.0402490101. PubMed: 15128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chuang SS (2003) CYP2U1, a novel human thymus- and brain-specific cytochrome P450, catalyzes omega- and (omega-1)-hydroxylation of fatty acids. J Biol Chem 279: 6305-6314. doi:10.1074/jbc.M311830200. PubMed: 14660610. [DOI] [PubMed] [Google Scholar]
- 23. Kirischian N, McArthur AG, Jesuthasan C, Krattenmacher B, Wilson JY (2011) Phylogenetic and Functional Analysis of the Vertebrate Cytochrome P450 2 Family. J Mol Evol 72: 56-71. doi:10.1007/s00239-010-9402-7. PubMed: 21116621. [DOI] [PubMed] [Google Scholar]
- 24. McArthur AG, Hegelund T, Cox RL, Stegeman JJ, Liljenberg M et al. (2003) Phylogenetic Analysis of the Cytochrome P450 3 (CYP3) Gene Family. J Mol Evol 57: 200-211. doi:10.1007/s00239-003-2466-x. PubMed: 14562963. [DOI] [PubMed] [Google Scholar]
- 25. Blevins S, Siegel PB, Blodgett DJ, Ehrich M, Lewis RM (2012) Liver enzymes in White Leghorns selected for the sheep red blood cell immune response. Poult Sci 91: 322-326. doi:10.3382/ps.2011-01764. PubMed: 22252343. [DOI] [PubMed] [Google Scholar]
- 26. Rawal S, Mendoza KM, Reed KM, Coulombe RA Jr. (2009) Structure, Genetic Mapping, and Function of the Cytochrome P450 3A37 Gene in the Turkey (Meleagris gallopavo). Cytogenet Genome Res 125: 67-73. doi:10.1159/000218748. PubMed: 19617698. [DOI] [PubMed] [Google Scholar]
- 27. Handschin C, Podvinec M, Meyer UA (2000) CXR, a chicken xenobiotic-sensing orphan nuclear receptor, is related to both mammalian pregnane X receptor (PXR) and constitutive androstane receptor (CAR). Pro Natl Acad Sci 97:10769-10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goriya HV, Kalia A, Bhavsar SK, Joshi CG, Rank DN, Thaker AM (2005) Comparative evaluation of phenobarbital-induced CYP3A and CYP2H1 gene expression by quantitative RT-PCR in Bantam, Bantamized White Leghorn and White Leghorn chicks. J Vet Sci 6: 279–285. PubMed: 16293989. [PubMed] [Google Scholar]
- 29. Hansen AJ, May BK (1989) Sequence of a chicken phenobarbital-inducible cytochrome P450 cDNA: regulation of two P450 mRNAs transcribed from different genes. DNA 8: 179-191. doi:10.1089/dna.1.1989.8.179. PubMed: 2470563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree and synteny of CYP1 family genes. (A) Phylogeny of CYP1 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 20 substitutions per 100 residues. (B) CYP1A4 and CYP1A5 genes (orthologues of human CYP1A1 and CYP1A2). (C) CYP1B1 genes.
(TIF)
Full phylogenetic tree of CYP2 family genes. Phylogeny of CYP2 amino acid sequences from chicken, zebra finch, turkey, and human. The maximum likelihood tree was created using MEGA5 software. The numbers on the branches indicate the number of times per 100 bootstrap replicates that the branch appeared in the trees, estimated by a random resampling of the data. The scale bar represents 50 substitutions per 100 residues. The compressed version of this phylogeny is shown in Figure 1.
(TIF)
Primers for qRT-PCR.
(XLS)
CYP 1-3 genes of chicken.
(XLSX)
CYP 1-3 genes of zebra finch.
(XLSX)
CYP 1-3 genes of turkey.
(XLSX)
CYP 1-3 genes of human.
(XLSX)