Abstract
Two haemoglobin-binding proteins, HmbR and HpuAB, contribute to iron acquisition by Neisseria meningitidis. These receptors are subject to high frequency, reversible switches in gene expression - phase variation (PV) - due to mutations in homopolymeric (poly-G) repeats present in the open reading frame. The distribution and PV state of these receptors was assessed for a representative collection of isolates from invasive meningococcal disease patients of England, Wales and Northern Ireland. Most of the major clonal complexes had only the HmbR receptor whilst the recently expanding ST-275-centred cluster of the ST-269 clonal complex had both receptors. At least one of the receptors was in an ‘ON’ configuration in 76.3% of the isolates, a finding that was largely consistent with phenotypic analyses. As PV status may change during isolation and culture of meningococci, a PCR-based protocol was utilised to confirm the expression status of the receptors within contemporaneously acquired clinical specimens (blood/cerebrospinal fluid) from the respective patients. The expression state was confirmed for all isolate/specimen pairs with <15 tract repeats indicating that the PV status of these receptors is stable during isolation. This study therefore establishes a protocol for determining in vivo PV status to aid in determining the contributions of phase variable genes to invasive meningococcal disease. Furthermore, the results of the study support a putative but non-essential role of the meningococcal haemoglobin receptors as virulence factors whilst further highlighting their vaccine candidacy.
Introduction
Neisseria meningitidis (the meningococcus) is a commensal of the human nasopharynx with an overall prevalence of approximately 10% [1]. It is a leading cause of meningitis and septicaemia globally and is associated with significant morbidity and mortality [2]. There are five main capsular serogroups associated with invasive meningococcal disease (IMD; serogroups A, B, C, W and Y) of which serogroups A, C, W and Y are preventable by means of efficacious glycoconjugate vaccines [3]. The polysaccharide of serogroup B meningococci (MenB), a leading cause of IMD in industrialized nations, is poorly immunogenic in humans [4]. MenB vaccine development has largely, therefore, focused upon sub-capsular antigens [2], [5]–[9]. Efforts to broaden coverage are, however, ongoing.
Within the human host, the essential nutrient iron is mainly sequestered within cells. Extracellular iron tends to be complexed with various proteins including transferrin, lactoferrin, haemoglobin (Hb) and Hb-haptoglobin, the respective availabilities of which differ according to anatomical site. For example, transferrin is mostly located in the serum, whilst lactoferrin is found in secretions [10]. Meningococci are able to acquire iron from these complexes via a variety of surface receptors [10]. As addition of free iron or transferrin is required to establish IMD in mouse models of infection [11], these receptors constitute potential virulence factors. The surface expression of the receptors also confers vaccine candidacy.
The TonB dependent HmbR and HpuAB systems are each able to bind Hb, whilst the latter also binds the Hb-haptoglobin complex [12], [13]. HmbR is an 89 kDa transmembrane protein, whilst HpuAB comprises a 37 kDa lipoprotein (HpuA) and an 85 kDa transmembrane protein (HpuB). Both systems undergo phase variation (PV) owing to homopolymeric (poly-G) tracts in the open reading frames of hmbR and hpuA [14]. These receptors are variably distributed among meningococci, with an overrepresentation of hmbR, and underrepresentation of the hpuAB-only genotype among IMD isolates [15], [16]. Notably, it has been demonstrated that the majority of isolates belonging to clonal complexes (CCs) with relatively high disease to carriage ratios possessed both receptor systems [16].
Analysis of PV status in two collections of isolates indicated that 91% of IMD isolates possessed one or both receptors in the PV ON configuration compared with only 71% among carriage isolates [16]. Both HmbR and HpuA (the surface exposed component of HpuAB) exhibit significant levels of amino acid sequence variation [16], [17]. Furthermore, the putative expression and immunogenicity of hmbR during IMD has been demonstrated by the presence of anti-HmbR antibodies in convalescent sera [10]. Collectively these findings suggest that the expression of at least one Hb receptor is important for IMD and that both receptors are subject to immune selective pressures, providing support for their potential role as both virulence factors and vaccine candidates.
In addition to hmbR and hpuA, meningococcal genomes contain multiple other phase variable genes, with homopolymeric tracts being a major mechanism for switching gene expression ON and OFF [18]. There have been few attempts to determine the PV status of these genes in IMD isolates even though several of them encode factors likely to affect survival of meningococci in systemic sites [18]. The study of the Hb receptors was one of the first to link a particular expression state with the disease state [16]. This and previous studies were, however, performed on isolates that had typically undergone several passages on blood-containing media following initial isolation, thus raising the possibility of in vitro selection of a PV state unrepresentative of that which prevails during IMD.
In this report we describe a methodology for direct analysis of the PV status of meningococcal genes in clinical specimens. This approach removes the potential bias associated with culture of meningococcal strains and is applicable to all genes whose PV is mediated by simple sequence repeat tracts. In addition, the study assessed the distribution and PV status of hmbR and hpuA among recent representative IMD cases from England, Wales and Northern Ireland in order to further explore their potential role as virulence factors and vaccine candidates.
Methods
Ethical approval
Favorable ethical approval for the study was provided by the National Research Ethics Service (research ethics committee reference number 11/NE/0235). The requirement for consent was waivered by the ethical review board as the study used anonymised samples that had already been collected for clinical need.
Isolates and clinical specimens
The study samples comprised 80 English, Welsh and Northern Irish MenB IMD isolates (received by the Health Protection Agency Meningococcal Reference Unit (MRU) between December 2008 and April 2011) and their corresponding clinical specimens (cerebrospinal fluid (CSF; n = 11) or blood or derivatives thereof (n = 69)). The isolates were selected to represent (in terms of CC) the diversity and distribution of recent English, Welsh and Northern Irish MenB IMD isolates as determined for the epidemiological year 2007/8 [19]. The CC distribution of the selected isolates is presented in table 1. Isolate/specimen pairs with non-culture real-time PCR ctrA cycle thresholds >35 [20] were excluded from the study in order to maximise the ability to detect the Hb receptor genes.
Table 1. Comparison of the clonal complex distribution of the study isolates and all English, Welsh and Northern Irish serogroup B invasive meningococcal disease isolates for the epidemiological year 2007/8.
| Clonal complex | Proportion of isolates (number) | |
| Study (80) | 2007/8 (539) | |
| 11 | 2.5% (2) | 1.1% (6) |
| 18 | 1.3% (1) | 1.7% (9) |
| 32 | 7.5% (6) | 5.9% (32) |
| 35 | 1.3% (1) | 1.5% (8) |
| 60 | 3.8% (3) | 2.0% (11) |
| 103 | 1.3% (1) | 0.2% (1) |
| 162 | 5.0% (4) | 1.9% (10) |
| 213 | 6.3% (5) | 9.5% (51) |
| 269 | 27.5% (22) | 33.0% (178) |
| 461 | 2.5% (2) | 2.2% (12) |
| 1157 | 3.8% (3) | 0.4% (2) |
| 41/44 | 26.3% (21) | 31.5% (170) |
| UA | 11.3% (9) | 7.2% (39) |
| other | n/a | 1.9% (10) |
Isolates used in the study were selected to represent the diversity and distribution (in terms of clonal complex) of all English, Welsh and Northern Irish invasive MenB isolates from 2007/8. Note that a proportion of the predominant CCs (cc269 and cc41/44) were forfeited to include lesser CCs. Other variations were due to the limited availability of multilocus sequence typed isolates with accessible clinical specimens. UA = unassigned STs. n/a = not applicable.
DNA extraction
DNA was extracted using the Qiagen DNeasy blood and tissue kit (Qiagen, Crawley, United Kingdom). Isolates were cultured overnight on Columbia agar plus 5% horse blood prior to DNA extraction as previously described [21]. DNA was extracted from a broad sweep of multiple colonies to avoid selection of unrepresentative phase variants. Extractions from clinical specimens were performed in accordance with the manufacturer's DNeasy Blood & Tissue Handbook (Qiagen, 2006 edition).
Molecular analyses for detection of hmbR and hpuA and characterization of the homopolymeric tracts and closely flanking regions
Primers used in the study, and PCR/sequence analysis conditions can be viewed in table 2. A schematic diagram of the workflow for molecular analyses is provided in figure S1. Sequence and fragment analyses were performed on a 3130xL Genetic Analyzer installed with a 50 cm capillary array (Life Technologies Ltd, Paisley, United Kingdom).
Table 2. Primers used for PCR and sequence and fragment analyses for hmbR and hpuA.
| Target | PCR/Seq/fraga | Primer ID (direction) | Primer sequence (5′ to 3′) | Reference | Approx size (bases) |
| hmbR | PCR/Seq/fragb | hmbR-RF3 (fwd) | TGCCAACCTCTTTTACGAATGG | [25] | 400 |
| hmbR-RF4 (rev) | GCTACTGAACACGTCGTTCC | [35] | |||
| PCRc | hmbRzF (fwd) | CCACA(A/G)CTT(C/T)TTGGGTAAGATTGC | This study | 1000 | |
| hmbRzR (rev) | GACGCTACTTTGTCCACATTCAGACG | This study | |||
| PCRd | hmbReF (fwd) | AAAT(C/T)AACGA(C/T)AACCACCGCATCG | This study | 600 | |
| hmbRyR (rev) | GGCATTCAATTCCTGAGGCGTCA | This study | |||
| PCRe | exl3-seqF (fwd) | GGCGGAGTGCAAAATGATGC | [15] | 600 | |
| exl3-seqR (rev) | GCCATCTTTTAATTTAGCCGC | [15] | |||
| hpuA | PCR/Seq/fragb | hpuAC (fwd) | ATGCGATGAAATACAAAGCCC | [25] | 350 |
| hpuA350Rev (rev) | GGATGAAAGGGCGTATTGCGC | [25] | |||
| PCRf | hpuAFnest2 (fwd) | CAAATCCGCCAACGAAGCGAT(C/T)AA | This study | 2000 | |
| P26.85 (rev) | GGGAAACGCTTGGGCGATGG | [36] | |||
| PCRg | Hpu-pmt (fwd) | CCGATTTTTGCACCGACCCAC | This study | 600 | |
| hpuR-Seq3 (rev) | GAGGTCGATTTCGCCGTTGG | This study | |||
| PCRh | hpuA-for1 (fwd) | GCAACAATGCCTTGTCATCC | [16] | 1000–3000 | |
| hpuA-rev13 (rev) | TGATCGAAATGGGCGTACTC | [16] |
Purpose; conditions
Characterisation of homopolymeric tract and closely flanking regions; PCR - [MgCl2] = 2.5 mM, 25 (up to 45 for direct non-culture fragment analysis) cycles of (95°C – 30 seconds, 53°C – 30 seconds, 72°C – 60 seconds). Sequence analysis annealing temperature = 53°C. For fragment analysis, primers hmbR-RF3 and hpuA350Rev were FAM-labeled.
‘Round 1’ nested PCR for non-culture characterization of homopolymeric tract and closely flanking regions; [MgCl2] = 3 mM, 45 cycles of (95°C – 30 seconds, 56°C – 30 seconds, 72°C – 60 seconds).
d‘Round 2’ nested PCR for non-culture characterization of homopolymeric tract and closely flanking regions; [MgCl2] = 3 mM, 25 cycles of (95°C – 30 seconds, 56°C – 30 seconds, 72°C – 60 seconds).
eConfirmation of presence of exl3 (that replaces hmbR); [MgCl2] = 3 mM, 35 cycles of (95°C for 30 seconds, 56°C for 30 seconds, 72°C for 60 seconds).
f’Round 1’ nested PCR for non-culture characterization of homopolymeric tract and closely flanking regions; [MgCl2] = 3 mM, 45 cycles of (95°C – 30 seconds, 57°C – 30 seconds, 72°C – 150 seconds).
g‘Round 2’ nested PCR for non-culture characterization of homopolymeric tract and closely flanking regions; [MgCl2] = 3 mM, 25 cycles of (95°C – 30 seconds, 59°C – 30 seconds, 72°C – 60 seconds).
Confirmation of absence of hpuA; [MgCl2] = 3 mM, 35 cycles (95°C – 30 seconds, 53°C – 30 seconds, 72°C – 180 seconds).
Seq = sequence analysis
Frag = fragment analysis
Detection of hmbR and hpuA genes
The presence/absence of hmbR and hpuA among cultures was determined in accordance with Tauseef et al. [16]. Briefly, PCR and sequence analyses were performed using proximal, intragenically targeted primers (hmbR-RF3 and hmbr-RF4 (hmbR) and hpuAC and hpuA350Rev (hpuA)), closely flanking the respective homopolymeric tracts. For non-culture clinical specimens, sequence analysis (as above) was preceded by a nested PCR in which the initial PCR (‘round 1’, using extracted DNA as template) used remote intragenically targeted primers (hmbRzF and hmbRzR (hmbR), or hpuAFnest2 and P26.85 (hpuA)), and the second PCR (‘round 2’, using round 1 PCR product as template) used intermediate primers (hmbReF and hmbRyr (hmbR) or Hpu-pmt and hpuR-Seq3 (hpuA)).
The absence of hmbR was confirmed by PCR in which intragenically targeted primers (exl3-seqF and exl3-seqR) were used to detect the exl3 gene that replaces hmbR in the respective isolates, in accordance with Harrison et al. [15]. The absence of hpuA was confirmed by PCR amplification of the corresponding locus using extragenically targeted primers (hpuA-for1 and hpuA-rev13), in accordance with Tauseef et al. [16].
Characterization of homopolymeric tracts and flanking regions
Homopolymeric tracts and flanking sequences were characterised by a combination of sequence analysis (above) and fragment analysis of corresponding FAM labeled PCR products (amplified using primers hmbR-RF3-FAM and hmbr-RF4 (hmbR), and hpuAC and hpuA350Rev-FAM (hpuA)) against a GeneScan 500 LIZ size standard (Life Technologies Ltd) [16]. Non-culture fragment analysis of clinical specimens initially made use of a nested approach incorporating the respective round 1 PCRs (above). In order to reduce putative strand slippage artefacts generated for longer homopolymeric tracts, the total number of PCR cycles (45 plus 25 cycles for the nested protocol) was reduced by using a direct, non-nested protocol in conjunction with 25 to 45 cycle repeats (adjusted to maintain sensitivity while minimizing the number of cycles). The sensitivity of the non-nested, non-culture fragment analysis was further enhanced, where necessary, by increasing the pre-electrophoresis sample injection time on the sequence analyser (from a standard time of 6 seconds to between 18 and 50 seconds) and/or ethanol/sodium acetate precipitation of FAM-labeled PCR products that were then dissolved in formamide prior to electrophoresis.
Fragment sizes were determined using Peak Scanner software (v1.0, Life Technologies Ltd). These were compared with the corresponding sequence data in order to associate corresponding homopolymeric tract lengths (figure S2 (a) and (b)). Where multiple fragment peaks (differing in the number of homopolymeric tract repeats) were obtained, the relative amounts of the respective primary (1°) and secondary (2°) products were estimated by calculating the ratio of the corresponding peak areas (obtained using the Peak Scanner software; figure S2 (c)).
In order to exclude the potential effect of non-PV mutations on expression status, full length allele sequences were obtained from whole genome sequence data obtained using Illumina sequence technology (Illumina, California, USA).
Phenotypic analyses
The ability to utilise Hb (and therefore the existence of at least one haemoglobin receptor in the ON state) was investigated among the isolates in accordance with Tauseef et al. [16]. Meningococcal suspensions (10–15 µL at optical density (650 nm) = 0.2) were spread-plated onto Mueller Hinton (MH) agar containing 40 µM desferal and incubated overnight (37°C with 5% CO2) in the presence of Hb impregnated discs (10 µL at 10 mg mL-1). Sterile water and transferrin (10 µL at 50 mg mL-1) and/or ferric nitrate (10 µL at 16.2 mg mL-1) impregnated discs and parallel cultures on plain Mueller Hinton agar (no desferal or nutritional supplements) served as controls. Nutritional supplements were obtained from Sigma-Aldrich (Dorset, UK).
Results
Distribution of hmbR and hpuA
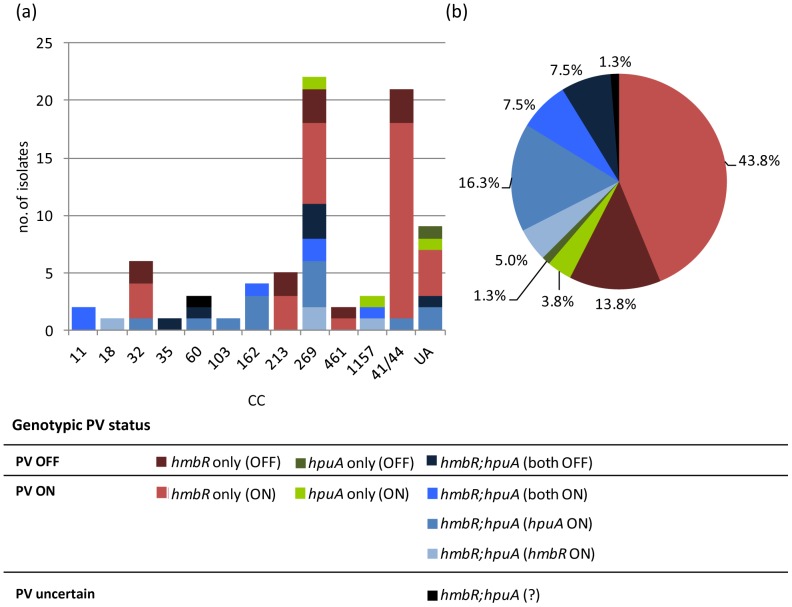
All of the 80 isolates possessed genes for at least one of the Hb receptors (figure 1). Among these, 57.5% possessed hmbR only, 5.0% possessed hpuA only, and 37.5% possessed both genes (designated hmbR:hpuA). The majority of isolates belonging to less prevalent CCs (<5 isolates) possessed both genes. Three of the most prevalent CCs, cc41/44, cc213 and cc32, comprised predominantly ‘hmbR-only’ isolates (95.2%, 83% and 100%, respectively). Contrastingly, the ST-269 clonal complex (cc269) was split between isolates comprising an hmbR-only (45.5%; all centered around sequence type (ST)-269 by eBURST analysis [19], table S1) or an hmbR:hpuA genotype (50%; of which 90.9% were centered around ST-275 [19]).
Figure 1. Clonal complex distribution of the combinations of hmbR and hpuA genes among a representative panel of English, Welsh and Northern Irish invasive serogroup B meningococcal isolates collected from 2008–2011.
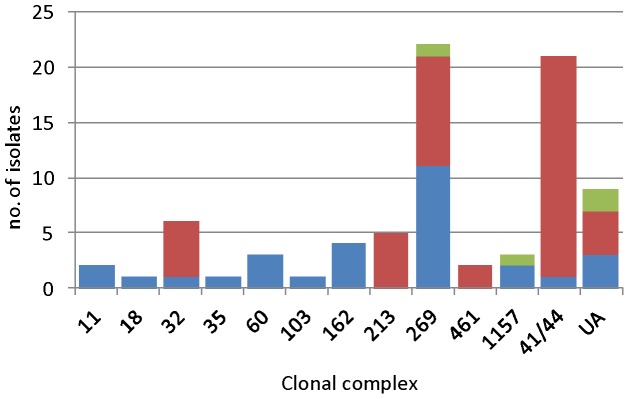
Blue bars indicate hmbR:hpuA, red bars indicate hmbR-only and green bars indicate hpuA-only. UA = unassigned STs.
Sequence analysis of homopolymeric tract regions of hmbR and hpuA among IMD isolates
Homopolymeric tracts are susceptible to strand slippage during PCR amplification and this susceptibility increases with tract length and the number of PCR cycles [22]. Affected PCR products comprise a heterogeneous population of amplicons with tract lengths varying around that of the template DNA. Upon sequence analysis this manifests itself as a series of superimposed, laterally shifted and progressively weakening chromatogram traces that occur immediately after the homopolymeric tract (in the direction of the respective chromatogram).
In the present study, dideoxy sequencing was performed on PCR products spanning the homopolymeric tracts and flanking regions. Among the isolates, alleles with >10 G repeats tended to yield the aforementioned heterogeneous products indicative of strand slippage during PCR and/or heterogeneous template DNA (and therefore a heterogeneous plate population). For tracts exceeding approximately 15 G repeats, the downstream sequence (with respect to the direction of the respective chromatogram) tended to be unreadable and could only be discerned from the antiparallel chromatogram.
Some inter-isolate sequence variation occurred within or adjacent to the repeat tracts. For example, one of the 76 hmbR sequences (obtained from isolate i4), contained a substitution within the homopolymeric tract in which the second to last G repeat was substituted for an A (figure 2a). Thus, whilst the absolute number of consecutive G repeats (‘absolute tract length’) was 9, the overall length of the corresponding region (‘effective tract length’; described by defined flanking regions on a pairwise nucleotide alignment; figure 2) was 11 bases. Similarly, nine of the 34 hpuA gene sequences had alterations in the sequence at the 5′ end of the repeat tract (examples of which are provided in figure 2b). One isolate (isolate i11) had a ‘G to T’ substitution thereby reducing the absolute tract length, whilst eight others (isolates i15, i17, i19, i59, i60, i61, i90 and i91) had a ‘C to G’ substitution thereby increasing the absolute tract length. In each case the effective tract length remained unaffected by the respective mutation. Since phase variation status is dependent on the overall length of the prototypical tract region, the effective tract length was utilised for predicting expression state in the subsequent analyses. Effective/absolute tract lengths can be viewed in table S1.
Figure 2. Sequence diversity in the homopolymeric tract regions of hmbR and hpuA.
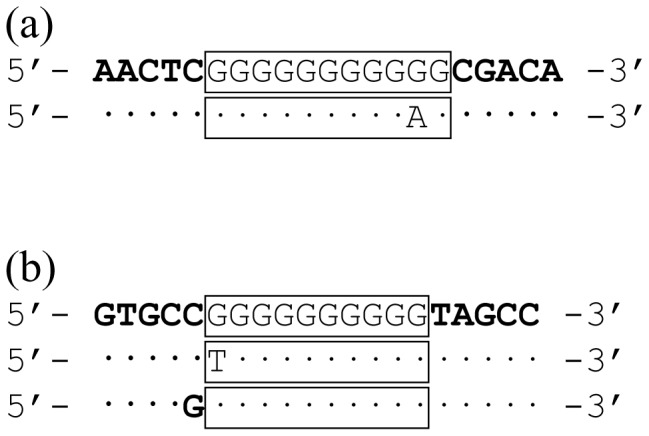
Due to polymorphisms in the homopolymeric tract and flanking regions, the phase variation expression status could not be reliably correlated with the absolute number of consecutive G repeats (absolute tract length), rather it corresponded with an ‘effective tract length’ between defined flanking regions. In the examples provided, tracts are illustrated as pairwise nucleotide alignments in which the defined flanking regions are highlighted using bold type whilst the effective tract length is denoted by a box. Pairwise nucleotide identities are denoted by dots. For hmbR (a) the defined flanking regions were AACTC and CGACA, respectively. In the upper example the effective and absolute tract lengths are the same (11 Gs vs 11 bases). The lower tract has a G to A substitution at the second to last G giving an absolute tract length of 9 Gs whilst the effective tract length remains 11 bases. For hpuA (b) the defined flanking regions were GTGC(C/G) and TAGCC, respectively. In the upper example the effective and absolute tract length are the same (10 Gs vs 10 bases). The middle example has a G to T substitution at the first G giving an absolute tract length of 9 Gs whilst the effective tract length remains 10 bases. The lower example has a C to G substitution immediately before the prototypical tract region giving an absolute tract length of 11 Gs whilst the effective tract length remains 10 bases.
Characterisation of homopolymeric tract lengths and PV status of hmbR and hpuA among IMD isolates
Owing to the heterogeneity of sequences containing relatively long homopolymeric tracts, the homopolymeric tract lengths of all 80 isolates were determined from a combination of sequence and fragment analyses.
Fragment analysis separates heterogeneous amplicon species according to length. The area below the corresponding fragment peaks provides an indication of the relative proportions of the respective amplicon species. In the present study the two peaks with the largest and second largest area were designated 1° and 2°, respectively (figure S2 (c)).The heterogeneity of the PCR products and the proportion of putatively artifactual fragments (as indicated by the ratio of the areas for the 1° and 2° peaks) increased as a function of tract length. For the default PCRs (25 cycle repeats), the mean 1° to 2° peak-area ratio for hmbR ranged from 30.4 for G8 tracts to 1.14 for G15 tracts (figure 3a). For hpuA the mean 1° to 2° peak area ratios ranged from 12.6 for G4 tracts to 1.10 for G14 tracts (figure 3b). A single isolate (isolate i15) possessed hpuA with a tract of approximately 19 G repeats by sequence analysis. On fragment analysis, this yielded >7 fragment peaks. The four largest peaks had area ratios (adjacent peaks) of 1.56, 1.07 and 2.02, respectively. The largest peak corresponded to a tract length of 16 G repeats which appeared incorrect when viewing the sequence analysis. To reduce the effect of strand slippage during PCR, hpuA fragment analysis was repeated for all isolates with ≥13 tract G repeats using a reduced number of PCR repeat cycles (from 25 to 20 cycles). The 1° peak was maintained for all isolates except isolate i15. In addition, the respective 1° to 2° peak-area ratios were seen to increase (figure 3c), thus supporting the initial results and predicted fragment sizes. The 1° peak of isolate i15 shifted to the right, however, suggesting that the original 1° peak was artifactual and that the isolate had an effective tract length of 18+ repeats which was more consistent with the chromatograms. The 1° to 2° peak area ratio for this isolate remained low (1.05), however, and so the exact number of repeats remained uncertain.
Figure 3. Influence of homopolymeric tract length on the heterogeneity of PCR products.
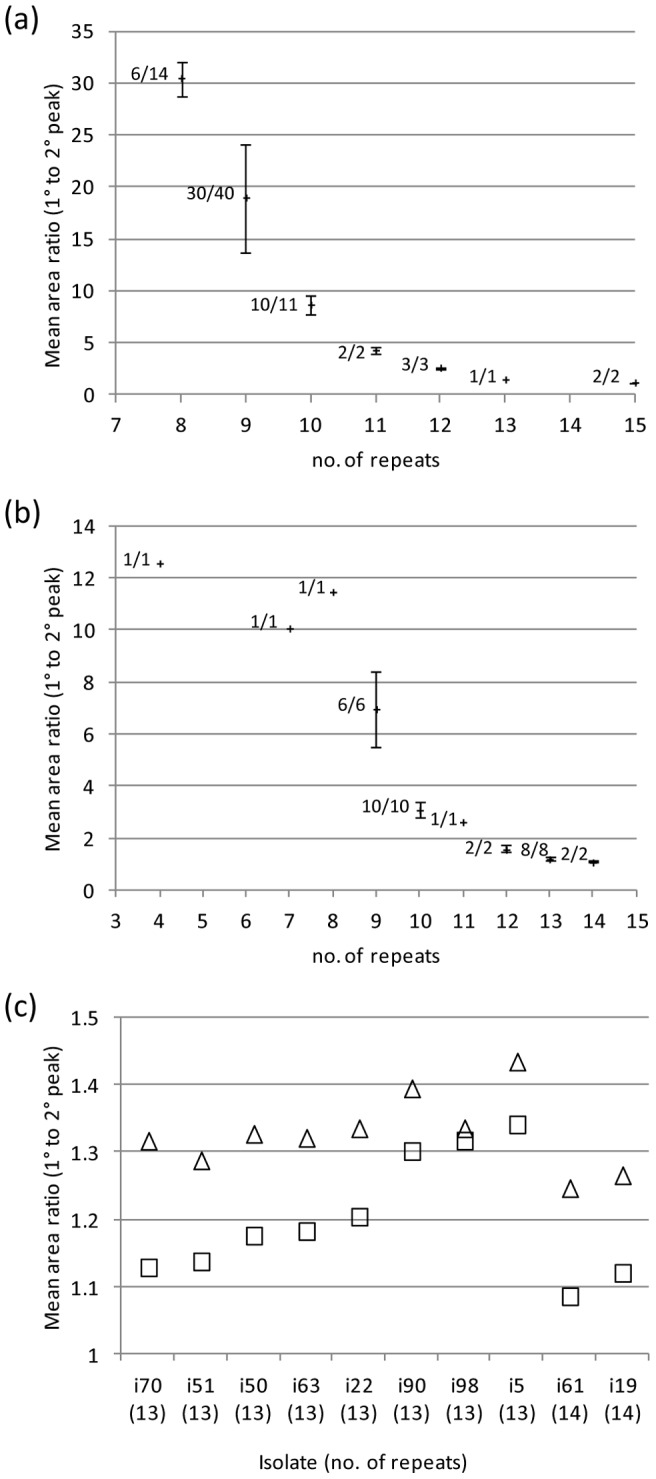
For a proportion of the isolates, amplification across the homopolymeric repeat tracts of hmbR and hpuA generated multiple peaks on fragment analysis. The relative amounts of the respective primary (1°) and secondary (2°) products were estimated by calculating the ratio of the corresponding peak areas using Peak Scanner software (Life Technologies Ltd). Charts (a) and (b) illustrate a decrease in mean peak area ratio with increasing absolute homopolymeric tract length (corresponding to the 1° peak) for hmbR and hpuA, respectively. The proportions of isolates exhibiting heterogeneous fragments are indicated beside markers. Hairs indicate standard deviations. All PCRs utilised 25 cycle repeats. Chart (c) compares the mean peak area ratios obtained using 25 (squares) or 20 (triangles) PCR cycle repeats for hpuA+ isolates possessing 13 to 14 G repeats. In each case the mean peak area ratio increased when using fewer PCR cycle repeats.
Distribution of predicted effective tract lengths and genotypic PV status among isolates
The hmbR gene was considered ON for effective tract lengths divisible by 3, whilst hpuA was considered ON for effective tract lengths of 4, 7, 10 and so on (figure 4). Among the hmbR+ isolates, 59.2% of the tracts were in the ON configuration. The predominant effective tract length for this gene was 9 (51.3% overall) which accounted for 86.7% of effective hmbR tracts in the ON configuration. Among the hpuA+ isolates 64.7% of the effective tracts were in the ON configuration. The predominant effective tract lengths among these were 10 (11/34, 32.4% overall) and 13 (9/34, 26.5% overall), collectively accounting for 90.9% of effective hpuA tracts in the ON configuration.
Figure 4. Distribution of effective homopolymeric tract lengths for hmbR and hpuA among a representative panel of English, Welsh and Northern Irish invasive serogroup B meningococcal isolates collected from 2008–2011.
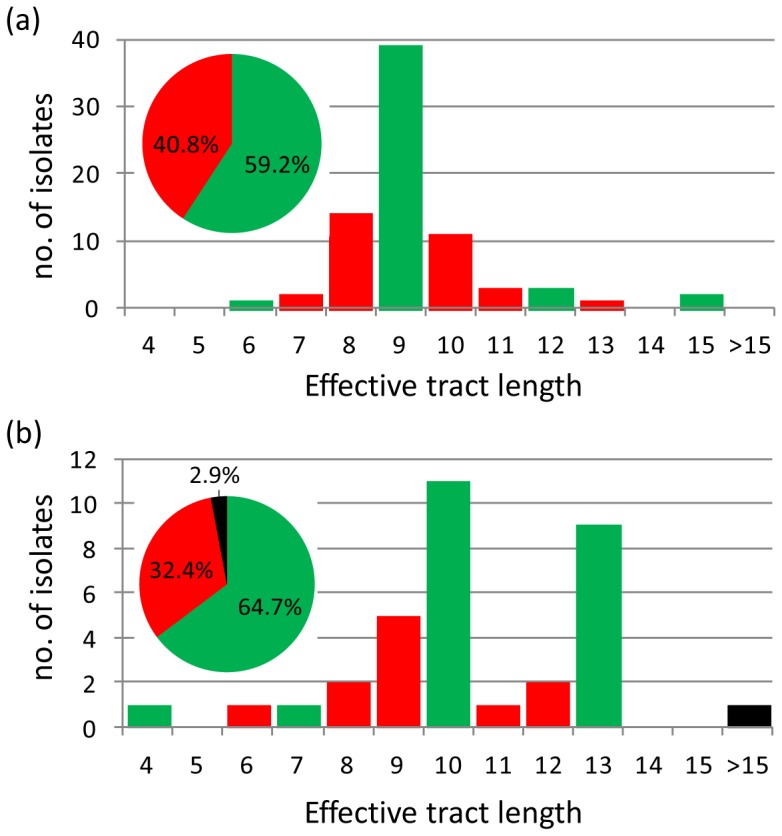
Bar charts show the number of isolates with a particular ‘effective homopolymeric tract length’ for hmbR (a) and hpuA (b). Green bars indicate a predicted ON expression state, red bars indicate a predicted OFF expression state, and black bars indicate an indeterminate expression state for a single isolate with a homopolymeric tract length of >15 G repeats. Inset are pie charts depicting the proportion of isolates representing each expression state for the respective genes.
The overall distribution of the combined hmbR:hpuA PV statuses and the distribution of hmbR:hpuA PV statuses with respect to CC were examined (Figure 5). Excluding isolate i15, 61/79 (77.2%) isolates were genotypically predicted to have at least one system in the ON state. These included approximately 76.9% of isolates among the minor CCs and unassigned STs, and approximately 75.9% of isolates among the major CCs (≥5 isolates) including 85.7% (18/21) of cc41/44, 66.7% (4/6) of cc32, 60% (3/5) of cc213 and 72% (16/22) of cc269 isolates (of which seven were centered on ST-269 and nine were centered on ST-275).
Figure 5. Distribution of genotypic phase variable expression status of hmbR:hpuA genotypes among a representative panel of English, Welsh and Northern Irish invasive serogroup B meningococcal isolates collected from 2008–2011.
This figure shows the number (a) or percentage (b) of isolates with particular combinations of PV expression states. In graph (a), there is further separation based on clonal complex. UA = unassigned STs. hmbR only = isolates that possess hmbR but not hpuA. hpuA only = isolates that possess hpuA but not hmbR. hmbR:hpuA = isolates that possess both hmbR and hpuA.
Phenotypic analyses
In order to correlate the genotypic and phenotypic expression states, the ability to utilise Hb as the sole iron source (indicating the presence of at least one system in the ON configuration) was assessed by growing isolates on iron-restricted media supplemented with different iron sources (figure S3). Excluding isolate i15, phenotyping was indeterminate for 8/79 fully genotyped isolates due to poor growth on MH agar or uncertainty regarding the status of the growth observed (data not shown). Among the remaining 71 isolates, 70 exhibited consistent genotypic/phenotypic PV statuses regarding the expression of at least one Hb receptor. The remaining isolate (hmbR-only) was genotypically ON (effective tract length = 9) but phenotypically OFF. Full length sequence analysis of the respective gene (obtained using Illumina sequence technology [Illumina, California, USA]) revealed the presence of a nonsense mutation downstream of the homopolymeric tract, which was consistent with the phenotypic OFF status (data not shown). Full length sequence analysis of hmbR and hpuA among the remaining isolates was consistent with their respective PV statuses. Genomic sequence data are available on the Pubmlst.org isolate database (http://pubmlst.org/neisseria), corresponding isolate/genome ids are listed in table S1.
The overall distribution, and the distribution with respect to CC, of isolates with or without at least one genotypically ON system (with phenotypic support where available) is provided in figure 6. Among all of the isolates, 66.3% (53/80) were confirmed to have at least one receptor in the ON state. A further 8.8% (7/80) were genotypically predicted to be ON but were phenotypically indeterminate. The absence of either receptor in the ON state was confirmed for 22.5% (18/80) of the isolates whilst a further 1.3% (1/80) were genotypically OFF but phenotypically indeterminate. Excluding the indeterminate isolates, these results indicated that 66.7% (12/18) of cc269 isolates, 83.3% (15/18) of cc41/44, 66.7% (4/6) of cc32 isolates and 60% (3/5) of cc213 isolates expressed at least one receptor.
Figure 6. Distribution of the genotypic (phase variable status on/off) and phenotypic expression of at least one of the haemoglobin receptors, HmbR and HpuA, among a representative panel of English, Welsh, and Northern Irish invasive serogroup B meningococcal isolates collected from 2008–2011.
geno ON = at least one receptor gene is in an ON phase variable state. geno OFF = both receptor genes are in an OFF phase variable state. pheno ON/OFF = able/unable to grow using haemoglobin as the sole iron source and therefore phenotypically expressing at least one/no haemoglobin receptor/s. geno indeterminate = genotypic phase variable state could not be determined owing to ambiguous sequence/fragment analysis. pheno indeterminate = phenotypic state could not determined owing to poor growth on MH agar or uncertainty regarding the status of the growth observed (isolate in ON configuration vs relatively large number of ON phase variants against an OFF background). The single isolate with a geno ON pheno OFF status possessed a nonsense mutation downstream of the homopolymeric tract. UA = unassigned STs.
Comparison of PV status between isolates and corresponding clinical specimens
Clinical specimens with real-time PCR ctrA cycle threshold values ≤35 were selected for the study in order to maximise the ability to detect the Hb receptor genes. A nested PCR protocol, consisting of one round of 45 cycles and a second round of 25 cycles, was initially performed using primers spanning the repeat tracts and giving final products of approximately 400–450 (hmbR) and 300–350 (hpuA) nucleotides. Only 2/76 and 1/34 of the clinical specimens failed to yield hmbR and hpuA products, respectively (corresponding real-time PCR ctrA cycle threshold values ranged from 31.13 to 34.53). A further 69/74 and 19/33 clinical specimens, respectively, yielded 1° fragment peaks corresponding to fragments within 0.41 (mean = 0.12) and 0.42 (mean = 0.29) bases of their respective isolates i.e. corresponding to the same amplicon length. The remaining clinical specimens (for which the corresponding isolates each possessed ≥10 tract repeats) yielded 1° fragment peaks up to 2.25 (mean 1.57) and 1.94 (mean 1.23) bases shorter than those of their respective isolates. All but two of these were brought within range (up to 0.39 bases, mean 0.07) of their respective isolates by eliminating the first round PCR and reducing the total number of cycle repeats to between 25 and 45 cycles. The remaining two specimens did not yield detectable products for the reduced number of PCR cycles. Thus, the PV status was matched for all isolate/specimen pairs for which non-culture PCR products could be obtained using adequately low numbers of PCR cycles.
The hmbR and hpuA PV status for individual isolates and their corresponding specimens can be viewed alongside phenotypic outcomes in table S1.
Discussion
Studies have shown that the majority of IMD isolates possess genes for at least one Hb receptor and that the expression of at least one Hb receptor may be important for virulence [16]. This, in conjunction with their surface expression, makes them attractive vaccine candidates. To further evaluate these qualities, studies are required of recent isolates that are epidemiologically representative of specific countries or regions of interest. In addition, the PV status of isolates has to be compared to the prevailing in vivo state in order to account for any putative phase variable changes associated with in vitro selective pressures occurring during and after initial isolation. These twin objectives were achieved in this study by comparing the distribution and PV status of the Hb receptors in isolates, and their respective specimens, representative of recent IMD in England, Wales and Northern Ireland. Another major outcome of this study was the development of a general approach for studying the contributions of phase variable genes to meningococcal pathogenesis. This is an important contribution as this species contains multiple phase variable genes with key roles in host interactions and in disease progression [18].
Consistent with previous findings [16], [17], hmbR (either alone or in conjunction with hpuA) was highly represented among these IMD isolates (present in 95%) whilst the presence of hpuA as the sole receptor was rare (5%). Interestingly, with the exception of cc461, most of the relatively infrequent CCs (cc11, cc18, cc35, cc60, cc103, cc162 and cc1157) mainly comprised isolates possessing alleles for both receptors. This relatively small collection of isolates exhibited trends consistent with a previous report [16]. The majority of isolates belonging to more prevalent CCs (cc41/44, cc213, cc32 and the ST-269-cluster of cc269) possessed hmbR only. This finding highlights the importance of the HmbR protein as a putative virulence factor for the major disease-associated meningococcal strains currently circulating in the UK. A novel finding was that the recently expanding, and broadly antigenically divergent ST-275-cluster of cc269 [19] (constituting approximately half of cc269 isolates in the present study) mainly comprised isolates with both receptors. The increasing prevalence of these isolates could suggest that immunity against the HmbR protein in these isolates has engendered a selection bias for dual Hb receptor strains due to their ability to maintain iron acquisition from Hb while avoiding adaptive immune responses. Longitudinal studies of carriage isolates combined with serology to correlate the PV states of the two receptors with the onset of receptor-specific immunity may serve to shed light on this possibility.
Owing to polymorphisms in or around the homopolymeric repeat tracts, the assertion that tract lengths of 9, 12, 15 and 18 (hmbR) or 7, 10, 13, 16 and 19 (hpuA) G repeats correspond with the ON expression status [16] did not always hold true for the present isolate panel. Rather, the ‘effective tract length’ between defined up/downstream nucleotides was a more appropriate indicator. With the exception of a single hmbR-only isolate with a nonsense mutation downstream of the hmbR homopolymeric tract, the predicted PV statuses obtained in this way were supported by the phenotypic status of all isolates that were compatible with the growth media and exhibited non-ambiguous growth status. Western or dot blot analyses using suitable antisera may circumvent the limitations associated with the poor growth of some isolates by enabling direct detection of the respective antigens. These analyses may, however, be susceptible to the influence of low-level phase variants. Immunogold labeling could also be considered since this has the potential to discriminate and quantify such phase variants.
This study also highlighted the need to minimise the number of PCR cycles in order to avoid misleading results arising through strand slippage during amplification. The greater susceptibility to such errors observed among longer homopolymeric tracts and the apparent propensity for contraction of the homopolymeric tract have previously been documented for homopolymeric (poly-T) tracts [22]. As such, the exact tract length for a study isolate with 18+ tract repeats could not be confidently ascertained. Nonetheless, tracts of at least 15 bp in length were successfully quantified using 25 PCR repeat cycles. It should be noted that whilst heterogeneity may have constituted genuine background PV within the respective DNA templates for some isolates or clinical specimens, it is difficult to separate this cause from PCR slippage. Hence, in the present study only the predominant genotype/phenotype was ascertained and considered as representative of a particular strain or specimen.
Despite having undergone a small number of passages on blood containing media during and after initial isolation, the PV statuses of the isolates were highly consistent with those obtained for the corresponding specimens and hence were representative of the disease-associated meningococcal genotype and phenotype. This suggests that the findings of previous studies of these phase variable loci in disease-associated isolates are an accurate reflection of the in vivo state [16].
The proportion of isolates genotypically predicted to express at least one Hb receptor (76.3%) was lower than that previously described for IMD isolates (91%) [16]. This is likely to be due to the different distribution of lineages among the respective isolate panels. Tauseef et al. [16] utilised 90 IMD strains from the strain panel used to evaluate multilocus sequence typing (MLST) [23]. This includes, for example, three CCs primarily associated with serogroup A (cc1, cc4 and cc5, 29/90, 32.2% overall), none of which were included in the present panel. Similarly, cc269 and cc41/44 constituted 27.5% and 26.3% of the present panel, respectively, but only 1% and 12% of the ‘MLST strain panel’. It is interesting to note, however, that the PV OFF status was not associated with any single lineage, with CCs 32, 35, 60, 213, 461, 41/44 and both cc269 clusters all including ≥24% OFF isolates. Thus the results were not skewed by any single CC.
In light of the relative prevalence of hmbR and absence of the ‘hpuA-only’ genotype among IMD isolates, it is surprising to note that 19/29 (excluding isolate i15) hmbR:hpuA isolates possessed hpuA in an ON configuration versus only 10/29 for hmbR. As speculated for cc269, this may provide further indication of the development of immunity in the UK population against the corresponding HmbR proteins, resulting in a more frequent occurrence of these strains and the expression of HpuAB during IMD. Alternatively, there may be selection for HpuAB during systemic spread of meningococci due to a higher availability of Hb-haptoglobin complexes (the ligand for HpuAB) as opposed to free haemoglobin (the ligand for HmbR). Preferential selection of HpuAB over HmbR has previously been noted during a laboratory acquired meningococcal infection in which the pre-infection organism (hmbr:hpuA) was ON for hmbR and OFF for hpuA [24]. The isolate in question was a so-called mutator, however, the above factors may still have been responsible for the ultimate PV statuses.
Unfortunately, due to the small numbers of specimens, it was not possible to detect whether a particular phase variation state for IMD isolates is required in the CSF as opposed to the blood but this may be an area for more extensive examinations of the roles of these and other phase variable genes.
The current study indicates that ON phase variants occur at a similar frequency in IMD isolates (76%) as previously detected for carriage (71%) isolates [16]. The observed difference between IMD and carriage isolates was less pronounced than that previously detected [16]. Therefore the association of the expression of at least one Hb receptor with IMD may be less robust than initially speculated [16]. However, the carriage isolates previously described were obtained from a localised study of first year university students and included few MenB isolates and multiple isolates arising from clonal expansion [25]. It would be interesting, therefore, to compare the present isolate panel with a comparable, contemporaneous and geographically diverse panel of English, Welsh and Northern Irish MenB carriage isolates to see if the apparent invasive/carriage isolate mismatch still applies. Nonetheless, it seems that expression of at least one Hb receptor may facilitate, but is not essential for, IMD. This is suggestive of broader redundancy of iron uptake mechanisms during IMD, possibly due to the action of Tbp [10].
The predominance of hmbR-only isolates among the major CCs and the lack of expression of either hmbR or hpuA among approximately one fifth of the isolates may reduce the potential coverage of an HmbR/HpuA-containing meningococcal vaccine for MenB in England, Wales and Northern Ireland. Given the necessity of iron for IMD [10], however, a vaccine containing HmbR, HpuA and TbpB as its primary components might circumvent any redundancy in iron uptake mechanisms within the bloodstream. A caveat to this is the prospect that mutants may escape by bypassing the need for the lipoprotein components of the respective systems (HpuA and TbpB) for sufficient iron acquisition [26]; the use of several antigenic components would, nonetheless, reduce the likelihood of vaccine escape. Other MenB vaccines have not focused on potential redundancy-mediated escape in this way [27], [28]. A further benefit to a vaccine incorporating HmbR, HpuA and TbpB is that non-bactericidal antibodies against these receptors may interfere with the process of iron uptake, thereby abrogating virulence [29]. Such effects are not accounted for when measuring serum bactericidal antibody activity which is the correlate of protection for MenB. The vaccine potential of Tbp (either the whole receptor, TbpBA, or the lipoprotein component, TbpB alone) is well documented though the immune responses elicited have been relatively poorly cross-protective, necessitating the use of >1 antigenic variant for broad protection [30]-[33]. Such a need for multiple antigens or antigenic variants has been demonstrated for other subcapsular antigens, including those of the recently licensed 4CMenB vaccine [34] or Pfizer's investigational bivalent recombinant fHbp vaccine [8]. Universal vaccine coverage of MenB is, however, yet to be achieved [9]. Improved coverage for such vaccines might be sought, for example, by incorporating additional or relatively cross-protective antigens/antigenic variants and/or improved adjuvants.
The demonstration of a strong correlation between the PV states of hmbR and hpuA for IMD isolates and corresponding clinical specimens provides two strategies for determining the in vivo states of these phase variable loci during IMD. For specimens with permissive amounts of meningococcal DNA, direct PCR of repeat tracts can be utilised to determine the in vivo PV state. For cases where the DNA content of specimens is low, analysis of PV state in a minimally-passaged meningococcal isolate will provide a strong indication of the in vivo state. This latter method facilitates studies of phase variable genes as it removes the requirement for ethical approval and because clinical specimens are finite and not always readily available. Both of these methods will indicate the predominant PV state present in the majority of IMD cases. Further studies may be required to determine if meningococcal pathogenesis is associated with a mix of PV states either within or between different anatomical sites. It should also be noted that, in the present study, isolates were cultured on horse blood-containing agar prior to DNA extraction. Alternative media and/or phase variable target genes may affect and/or be affected by relevant selective pressures during culturing that must initially be accounted for (by similar parallel non-culture analyses) on a case by case basis.
Phase variable genes are likely to make significant contributions to IMD and possibly to escape of vaccine-induced immune responses since these genes often encode vaccine antigens (e.g. PorA and NadA), complement resistance factors (e.g. LgtA – one of the enzymes contributing to LPS sialylation), adhesins and invasins (e.g. Opc, Opa and MspA), as well as the haemoglobin-binding proteins described herein. Thus, dissection of the contributions of phase variable genes and other hypermutable loci to IMD and other bacterial diseases is achievable using these methodologies and could provide novel insights into bacterial disease processes.
Supporting Information
Workflow of PCR, sequence analyses and fragment analyses performed in the study. The steps described applied to both hmbR and hpuA unless otherwise stated. aPrimers hmbR-RF3 and hmbR-RF4 (for hmbR) or hpuAC and hpuA350Rev (for hpuA). For fragment analyses primers hmbR-RF3 and hpuA350Rev were FAM-labelled. bPrimers hmbRzF and hmbRzR (for hmbR) or hpuAFnest2 and P26.85 (for hpuA). cPrimers hmbReF and hmbRyR (for hmbR) or Hpu-pmt and hpuR-Seq3 (hpuA). dPrimers exl3-seqF and exl3-seqR. ePrimers hpu-for1 and hpuArev13. fNegative reactions (-; failure to amplify products) indicated the absence of the respective gene and prompted confirmatory PCRs using genomic DNA template. gPositive reactions (+; amplification of products) indicated the presence of the gene and prompted sequence analysis of respective PCR product and fragment analysis using a genomic DNA template. M = size markers. pos = positive control. neg = negative control. Gels are depicted schematically.
(PDF)
Characterisation of homopolymeric tracts and flanking regions. (a) Chromatogram of the hmbR homopolymeric tract and closely flanking regions for isolate i21. The chromatogram indicates the presence of nine homopolymeric G repeats (black peaks). (b) Fragment analysis for the corresponding FAM-labelled PCR product for isolate i21. The fragment peak (blue) corresponds to a fragment size (S) of 430.22 bases as compared with the flanking GeneScan 500 LIZ size standard fragments (orange peaks; 400 and 450 bases, respectively). (c) Multiple fragments obtained for hmbR for isolate i2 (homopolymeric tract length = 12 G repeats). Neighbouring peaks represent amplicons differing by a single homopolymeric tract repeat. The primary (1°) peak (corresponding to a fragment length of 433.53 bases) has an area (A) of 1724.2, and the secondary (2°) peak (corresponding to a fragment length of 433.53 bases) has an area of 695.9. The peak area ratio for the 1° and 2° peaks is 2.48 (1724.2/695.9).
(PDF)
Example of positive and negative results in phenotypic analysis of ability to grow on Hb as the sole iron source. On Mueller Hinton (MH) agar + desferal (iron chelater), isolate i16 exhibited good growth on haemoglobin (Hb) indicating expression of at least one Hb receptor. Isolate i106 did not grow using Hb as the sole iron source indicating that no Hb receptor was expressed. Controls: both isolates grew on transferrin (Tf) as a sole iron source and on untreated MH agar. Neither isolate grew on H20 supplement on iron depleted MH agar.
(PDF)
Genotypic and phenotypic phase variation status of hmbR and hpuA among a representative panel of English, Welsh and Northern Irish invasive serogroup B meningococcal isolates and their corresponding clinical specimens. aPresence of at least one gene in the ON configuration (ON = at least one gene present in ON configuration; OFF = no genes in ON configuration). bAbility to utilise Hb as sole iron source. cIsolate contained >18 homopolymeric tract repeats. dPoor growth on Mueller Hinton agar or uncertainty regarding growth status. eSpecimen did not yield detectable PCR products at reduced number of PCR cycles. fIndicates whether ST is centred upon ST-269 (269) or ST-275 (275) by eBURST analysis. gisolate/genome id on the pubmlst database (http://pubmlst.org/perl/bigsdb/bigsdb.pl?db=pubmlst_neisseria_isolates). n/a = not applicable (hmbR-/hpuA-) same = same as that of corresponding isolate.
(PDF)
Acknowledgments
This publication made use of the Meningitis Research Foundation Meningococcus Genome Library (http://www.meningitis.org/research/genome) developed by Public Health England, the Wellcome Trust Sanger Institute and the University of Oxford as a collaboration.
Funding Statement
This study was supported by the Meningitis Research Foundation, grant number 1002.0 entitled “Examination of meningococcal haemoglobin receptors as potential vaccine targets”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cartwright KA, Stuart JM, Jones DM, Noah ND (1987) The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica . Epidemiol Infect 99: 591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang Q, Tzeng YL, Stephens DS (2012) Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol 4: 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pace D, Pollard AJ, Messonier NE (2009) Quadrivalent meningococcal conjugate vaccines. Vaccine 27 Suppl 2B30–41. [DOI] [PubMed] [Google Scholar]
- 4. Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, et al. (1972) Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis 126: 514–521. [DOI] [PubMed] [Google Scholar]
- 5. Arnold R, Galloway Y, McNicholas A, O'Hallahan J (2011) Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine 29: 7100–7106. [DOI] [PubMed] [Google Scholar]
- 6.Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, et al. (1991) Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann 14: : 195–207; discussion 208–110. [PubMed] [Google Scholar]
- 7. Bai X, Findlow J, Borrow R (2011) Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert Opin Biol Ther 11: 969–985. [DOI] [PubMed] [Google Scholar]
- 8. Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, et al. (2010) Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 28: 6086–6093. [DOI] [PubMed] [Google Scholar]
- 9. Vogel U, Taha MK, Vazquez JA, Findlow J, Claus H, et al. (2013) Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 13: 416–425. [DOI] [PubMed] [Google Scholar]
- 10. Perkins-Balding D, Ratliff-Griffin M, Stojiljkovic I (2004) Iron transport systems in Neisseria meningitidis . Microbiol Mol Biol Rev 68: 154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holbein BE (1981) Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun 34: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohde KH, Dyer DW (2004) Analysis of haptoglobin and hemoglobin-haptoglobin interactions with the Neisseria meningitidis TonB-dependent receptor HpuAB by flow cytometry. Infect Immun 72: 2494–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stojiljkovic I, Hwa V, de Saint Martin L, O'Gaora P, Nassif X, et al. (1995) The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol 15: 531–541. [DOI] [PubMed] [Google Scholar]
- 14. Lewis LA, Gipson M, Hartman K, Ownbey T, Vaughn J, et al. (1999) Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol Microbiol 32: 977–989. [DOI] [PubMed] [Google Scholar]
- 15. Harrison OB, Evans NJ, Blair JM, Grimes HS, Tinsley CR, et al. (2009) Epidemiological evidence for the role of the hemoglobin receptor, hmbR, in meningococcal virulence. J Infect Dis 200: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tauseef I, Harrison OB, Wooldridge KG, Feavers IM, Neal KR, et al. (2011) Influence of the combination and phase variation status of the haemoglobin receptors HmbR and HpuAB on meningococcal virulence. Microbiology 157: 1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans NJ, Harrison OB, Clow K, Derrick JP, Feavers IM, et al. (2010) Variation and molecular evolution of HmbR, the Neisseria meningitidis haemoglobin receptor. Microbiology 156: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bayliss CD, Field D, Moxon ER (2001) The simple sequence contingency loci of Haemophilus influenzae and Neisseria meningitidis . J Clin Invest 107: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucidarme J (2012) Ph.D. thesis. Potential coverage of an investigational, multicomponent, meningococcal vaccine with a focus on the ST-269 clonal complex. University of Manchester, Manchester, United Kingdom.
- 20. Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, et al. (2006) Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol 55: 887–896. [DOI] [PubMed] [Google Scholar]
- 21. Lucidarme J, Comanducci M, Findlow J, Gray SJ, Kaczmarski EB, et al. (2009) Characterization of fHbp, nhba (gna2132), nadA, porA, sequence type (ST), and genomic presence of IS1301 in group B meningococcal ST269 clonal complex isolates from England and Wales. J Clin Microbiol 47: 3577–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riepsamen AH, Gibson T, Rowe J, Chitwood DJ, Subbotin SA, et al. (2011) Poly(T) variation in heteroderid nemotode mitochondrial genomes is predominantly an artefact of amplification. J Mol Evol 72: 182–192. [DOI] [PubMed] [Google Scholar]
- 23. Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, et al. (1998) Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A 95: 3140–3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omer H, Rose G, Jolley KA, Frapy E, Zahar JR, et al. (2011) Genotypic and phenotypic modifications of Neisseria meningitidis after an accidental human passage. PLoS One 6: e17145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala'Aldeen DA, et al. (2011) Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol 49: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen CJ, McLean D, Thomas CE, Anderson JE, Sparling PF (2002) Point mutations in HpuB enable gonococcal HpuA deletion mutants to grow on hemoglobin. J Bacteriol 184: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, et al. (2006) The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 177: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, et al. (2010) The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog 6: e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pintor M, Ferrón L, Gómez JA, Powell NB, Ala'Aldeen DA, et al. (1996) Blocking of iron uptake from transferrin by antibodies against the transferrin binding proteins in Neisseria meningitidis . Microb Pathog 20: 127–139. [DOI] [PubMed] [Google Scholar]
- 30. Ala'Aldeen DA, Stevenson P, Griffiths E, Gorringe AR, Irons LI, et al. (1994) Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun 62: 2984–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, et al. (1993) Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine 11: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 32. Rokbi B, Renauld-Mongenie G, Mignon M, Danve B, Poncet D, et al. (2000) Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect Immun 68: 4938–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weynants VE, Feron CM, Goraj KK, Bos MP, Denoel PA, et al. (2007) Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis . Infect Immun 75: 5434–5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Serruto D, Bottomley MJ, Ram S, Giuliani MM, Rappuoli R (2012) The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 30 Suppl 2B87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin P, van de Ven T, Mouchel N, Jeffries AC, Hood DW, et al. (2003) Experimentally revised repertoire of putative contingency loci in Neisseria meningitidis strain MC58: evidence for a novel mechanism of phase variation. Mol Microbiol 50: 245–257. [DOI] [PubMed] [Google Scholar]
- 36. Lewis LA, Gray E, Wang YP, Roe BA, Dyer DW (1997) Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis . Mol Microbiol 23: 737–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Workflow of PCR, sequence analyses and fragment analyses performed in the study. The steps described applied to both hmbR and hpuA unless otherwise stated. aPrimers hmbR-RF3 and hmbR-RF4 (for hmbR) or hpuAC and hpuA350Rev (for hpuA). For fragment analyses primers hmbR-RF3 and hpuA350Rev were FAM-labelled. bPrimers hmbRzF and hmbRzR (for hmbR) or hpuAFnest2 and P26.85 (for hpuA). cPrimers hmbReF and hmbRyR (for hmbR) or Hpu-pmt and hpuR-Seq3 (hpuA). dPrimers exl3-seqF and exl3-seqR. ePrimers hpu-for1 and hpuArev13. fNegative reactions (-; failure to amplify products) indicated the absence of the respective gene and prompted confirmatory PCRs using genomic DNA template. gPositive reactions (+; amplification of products) indicated the presence of the gene and prompted sequence analysis of respective PCR product and fragment analysis using a genomic DNA template. M = size markers. pos = positive control. neg = negative control. Gels are depicted schematically.
(PDF)
Characterisation of homopolymeric tracts and flanking regions. (a) Chromatogram of the hmbR homopolymeric tract and closely flanking regions for isolate i21. The chromatogram indicates the presence of nine homopolymeric G repeats (black peaks). (b) Fragment analysis for the corresponding FAM-labelled PCR product for isolate i21. The fragment peak (blue) corresponds to a fragment size (S) of 430.22 bases as compared with the flanking GeneScan 500 LIZ size standard fragments (orange peaks; 400 and 450 bases, respectively). (c) Multiple fragments obtained for hmbR for isolate i2 (homopolymeric tract length = 12 G repeats). Neighbouring peaks represent amplicons differing by a single homopolymeric tract repeat. The primary (1°) peak (corresponding to a fragment length of 433.53 bases) has an area (A) of 1724.2, and the secondary (2°) peak (corresponding to a fragment length of 433.53 bases) has an area of 695.9. The peak area ratio for the 1° and 2° peaks is 2.48 (1724.2/695.9).
(PDF)
Example of positive and negative results in phenotypic analysis of ability to grow on Hb as the sole iron source. On Mueller Hinton (MH) agar + desferal (iron chelater), isolate i16 exhibited good growth on haemoglobin (Hb) indicating expression of at least one Hb receptor. Isolate i106 did not grow using Hb as the sole iron source indicating that no Hb receptor was expressed. Controls: both isolates grew on transferrin (Tf) as a sole iron source and on untreated MH agar. Neither isolate grew on H20 supplement on iron depleted MH agar.
(PDF)
Genotypic and phenotypic phase variation status of hmbR and hpuA among a representative panel of English, Welsh and Northern Irish invasive serogroup B meningococcal isolates and their corresponding clinical specimens. aPresence of at least one gene in the ON configuration (ON = at least one gene present in ON configuration; OFF = no genes in ON configuration). bAbility to utilise Hb as sole iron source. cIsolate contained >18 homopolymeric tract repeats. dPoor growth on Mueller Hinton agar or uncertainty regarding growth status. eSpecimen did not yield detectable PCR products at reduced number of PCR cycles. fIndicates whether ST is centred upon ST-269 (269) or ST-275 (275) by eBURST analysis. gisolate/genome id on the pubmlst database (http://pubmlst.org/perl/bigsdb/bigsdb.pl?db=pubmlst_neisseria_isolates). n/a = not applicable (hmbR-/hpuA-) same = same as that of corresponding isolate.
(PDF)