Abstract
Background
Individual-based modeling is a growing technique in the HIV transmission and prevention literature, but insufficient attention has been paid to formally evaluate the quality of reporting in this field. We present reporting recommendations for individual-based models for HIV treatment and prevention, assess the quality of reporting in the existing literature, and comment on the contribution of this model type to HIV policy and prediction.
Methods
We developed reporting recommendations for individual-based HIV transmission mathematical models, and through a systematic search, used them to evaluate the reporting in the existing literature. We identified papers that employed individual-based simulation models and were published in English prior to December 31, 2012. Articles were included if the models they employed simulated and tracked individuals, simulated HIV transmission between individuals in a particular population, and considered a particular treatment or prevention intervention. The papers were assessed with the reporting recommendations.
Findings
Of 214 full text articles examined, 32 were included in the evaluation, representing 20 independent individual-based HIV treatment and prevention mathematical models. Manuscripts universally reported the objectives, context, and modeling conclusions in the context of the modeling assumptions and the model’s predictive capabilities, but the reporting of individual-based modeling methods, parameterization and calibration was variable. Six papers discussed the time step used and one discussed efforts to maintain internal validity in coding.
Conclusion
Individual-based models represent detailed HIV transmission processes with the potential to contribute to inference and policy making for many different regions and populations. The rigor in reporting of assumptions, methods, and calibration of individual-based models focused on HIV transmission and prevention varies greatly. Higher standards for reporting of statistically rigorous calibration and model assumption testing need to be implemented to increase confidence in existing and future modeling results.
Introduction
HIV transmission is influenced by numerous interactions between the biology of the virus and the behavior of individuals. The dynamics of transmission, treatment, and prevention are increasingly being represented by infectious disease mathematical models, which are accepted in the HIV literature as powerful predictive tools that motivate policy and inform clinical trial design [1]. Given the complexities of HIV transmission, models that represent individual-level behavior and partnering can be especially valuable. Individual-based simulations increase flexibility by allowing for heterogeneous individuals, interactions between individuals, correspondence to real life data, and a representation of the environment with which individuals interact [2,3]. This model type also allows individuals to have rationality in their actions (as opposed to completely random behavior) and simulates learning at individual and population levels [2]. However, individual-based models are difficult to parametrize, analyze, and generalize due to their complexity, leaving researchers to balance the advantages and disadvantages of this model type in the context of the problem of interest. As researchers begin large scale community randomized trials to assess the costs and benefits of HIV treatment and prevention interventions, there is a need for sophisticated and validated individual-level models to inform these studies’ designs.
The need for more consistent evaluation and comparison of mathematical models in the literature is a common theme in current modeling reviews and papers [4-6]. Individual-based model users have noted that there has been a decline in the reporting standards in the literature, and this may be contributing to its lack of use in many fields [4,7]. Previous reviews have aimed to provide the proper guidelines and documentation for mathematical models and simulations in the literature [5,6,8-11], but none have focused on the specific issues related to reporting of individual-based models in the context of HIV transmission and prevention.
In this paper, we describe the scope and quality of reporting of individual-based models in the HIV transmission and prevention literature. We first provide recommended reporting guidelines for individual-based mathematical models by tailoring previous mathematical modeling reporting guidelines to the individual-based model approach. We then apply these guidelines to existing HIV transmission and prevention individual-based models (found through a systematic search strategy) to assess the quality of reporting for this model type. We hope these guidelines will be a starting point for discussion with modelers to form standardized reporting guidelines for the united goal of improving the quality of the individual-based HIV modeling literature, and increasing their use among policy making consumers.
Methods
Reporting Recommendations
The following recommended reporting guidelines were constructed by expanding upon those presented in previous modeling reviews and from the experience of the authors (Table 1). The guidelines highlight the necessary components of general mathematical model reporting and the specific issues related to individual-based model reporting. We present each recommended guideline and provide the rationale for including the item with reference to the literature. Our guidelines do not rely on a specific reporting structure or article layout, as individual-based models are published in a wide variety of journal types. Instead our recommendations are organized into six sections according to different aspects of model development and presentation: 1) rationale, scope, and objective; 2) structure and features; 3) parameters; 4) assessment and validation; 5) presentation of results and conclusions; 6) authorship and funding (see Table 1).
Table 1. Recommended reporting guidelines for individual-based models of HIV transmission and prevention * .
| Topic | # |
Item
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| RATIONALE, SCOPE, AND OBJECTIVES | |||||||||
| Title and Abstract | 1 | Identify in the title or abstract that the analysis depends on an individual-based mathematical model. | |||||||
| Objective | 2 | State the objectives of the analysis with specific reference to the population(s), intervention(s), and time period(s) of interest. | |||||||
| Context | 3 | Justify the exploration of the policy question in the context of previous trials, cohorts, and modeling analyses for the time period(s) of interest. | |||||||
| Model Justification | 4 | Explain the need for an individual-based model in the context of the objectives by referencing necessary model features. | |||||||
| STRUCTURE AND FEATURES | |||||||||
| Structure | 5 | Describe the model’s structure in both words and figures and describe how it affords the ability to explore the question(s) of interest. | |||||||
| Assumptions | 6 | State the assumptions implicit in the model structure and justify with knowledge and data from the population of interest. | |||||||
| Validity of Sexual Behavior | 7 | Justify the validity of the necessary behavior accounted for in the model. | |||||||
| Validity of Biology | 8 | Justify the validity of the necessary biology accounted for in the model. | |||||||
| PARAMETERS | |||||||||
| Parameters | 9 | List fixed parameters and calibrated parameters with ranges justified by the literature. | |||||||
| Time step | 10 | State and justify the length of the time step used to advance model dynamics, if applicable. | |||||||
| Heterogeneity | 11 | Discuss how biological and behavioral heterogeneity is implemented in the model structure and whether this implementation allows for flexibility and specificity. | |||||||
| Interaction | 12 | Describe the parameters used to implement individual interaction in the model and justify the data used to parameterize these parameters. | |||||||
| ASSESSMENT AND VALIDATION | |||||||||
| Calibration | 13 | If the objective of the analysis is to describe or predict dynamics in a particular population of interest, describe the process used to calibrate the model dynamics to existing data including the statistical procedure, the types of outcome measures used, and the quality of the data used. | |||||||
| Sensitivity Analyses | 14 | Summarize the results of sensitivity analyses on the main model parameters, discuss whether the results support the robustness of findings, and describe future work needed. | |||||||
| Assumption Sensitivity | 15 | Discuss how the behavior and inference of the model changes when particular assumptions (e.g. alternative mixing patterns, different levels of heterogeneity for behavior and biology) are altered or deleted. | |||||||
| Stochastic Sensitivity | 16 | Summarize the impact of stochasticity on the model runs and justify through random seed variation and sample size variation. | |||||||
| Internal Validity | 17 | Describe the validity of the model programming by discussing how model bugs and program issues were checked and if modifications to model implementation were explored. | |||||||
| PRESENTATION OF RESULTS AND DISCUSSION | |||||||||
| Data Quality | 18 | Describe the quantity and quality of the data used to inform parameters for the population(s) of interest. | |||||||
| Data Conversion | 19 | Discuss issues related to the conversion of data to fit the time step used. | |||||||
| Results | 20 | Present key modeling results with uncertainty estimates and indicate how many parameter sets were run for each analysis. | |||||||
| Limitations and Strengths | 21 | Provide the key limitations and strengths of the modeling study. | |||||||
| Reproducibility | 22 | Discuss whether the model is able to reproduce the behavior of other populations or interventions of interest. | |||||||
| Discussion | 23 | Interpret the modeling analysis within realistic bounds, with reference to previous modeling studies, a discussion about the generalizability of the modeling results, and implications for future studies or models. | |||||||
| AUTHORSHIP AND FUNDING | |||||||||
| Authorship and Funding | 24 | List sources of funding and describe each author’s contribution to the modeling framework and conceptualization. | |||||||
Bolded guidelines are specific to individual-based models of HIV transmission and prevention. Non-bolded guidelines are adaptable across different types of models.
Item 1: Title and Abstract – Identify in the title or abstract that the analysis depends on an individual-based mathematical model
Whether the structure of a mathematical model can be identified depends on how it is indexed in the literature, which relies on an informative title [12] and abstract. By indicating in the title or abstract that the analysis involves an individual-based mathematical model, authors alert readers to be aware of particular assumptions, structure, and details in the body of the paper.
Item 2: Objective – State the objectives of the analysis with specific reference to the population(s), intervention(s), and time period(s) of interest
Objectives should address the questions that the mathematical modeling exercise aims to answer, and reflect the efficacy, feasibility, and/or affordability of a particular HIV treatment or prevention intervention. A specific and focused purpose helps frame the paper’s methods and clarify the paper’s goals. The specificity of the objectives, especially with respect to the population, intervention, and time period of interest, justifies the model structure and indicates the data needs of the analysis. The information on setting and population will be essential for readers to assess the applicability and generalizability of the mathematical modeling results [12].
Item 3: Context – Justify the exploration of the policy question in the context of previous trials, cohorts, and modeling analyses for the time period(s) of interest
Authors should explain how their analysis adds to the existing literature by noting previous trials, studies, and modeling exercises that address similar questions. The political and social context of the tested intervention should also be discussed, to inform the readers of the potential consequences of this exploration [5]. The need for a new model should be justified if there is expansion on an existing model or model structure.
Item 4: Model Justification – Explain the need for an individual-based mathematical model in the context of the objectives by referencing necessary model features (e.g. a need for heterogeneity of individual behavior and/or biology, and/or the explicit modeling of the interaction between individuals)
The reasoning for the use of an individual-based model should be clarified early in the paper structure. Because our reporting guidelines are specific to mathematical analyses with a specific intervention, the need to justify a model’s design becomes necessary. Authors should emphasize the need for an individual-based model design by discussing the necessity of heterogeneity as well as explicit interaction of individuals in the context of the objectives, or other model necessary model features they have incorporated that are essential to their analysis [8,10,13]. .
Item 5: Structure – Describe the model’s structure in both words and figures and describe how it affords the ability to explore the question(s) of interest
By indicating the model structure in both words and figures, the authors are able to communicate effectively with the readers about the capability of the model structure and the validity of the model’s assumptions [8]. Figures are important for those unfamiliar with mathematical modeling, as they give a visual representation of what is happening inside of the model structure. Avoiding the “black-box” phenomenon will allow readers to better judge the quality of mathematical models in the literature and the subsequent results that arise from them [14]. Without a clear understanding of the structure of a given mathematical model, readers will have a difficult time piecing together which analyses and explorations are feasible.
Item 6: Assumptions – State the assumptions implicit in the model structure and justify with knowledge and data from the population of interest
The generalizability of the conclusions drawn from the model analysis are dictated by the model’s assumptions [13]. Data used to justify assumptions should be included, so readers are aware of all limitations of the modeling approach. Simplifying assumptions related to the interaction of individuals in the population and the progression of HIV should be highlighted, as these have a direct impact on the authors’ ability to make accurate inferences.
Item 7: Validity of Sexual Behavior – Justify the validity of the necessary behavior accounted for in the model
Many aspects of sexual behavior are important to the transmission and prevention of HIV in a population, and the level of detail inherent in individual-based models with respect to sexual behavior is much higher than any other type of modeling [15]. Authors should discuss all relevant behavioral processes including, but not limited to: relationship types, relationship durations, directionality in men who have sex with men (MSM) partnerships, number of sex acts per time step, presence or absence of risk groups, mixing pattern, mechanism of acquisition of partnerships, age of sexual debut, change in sexual behavior with aging or time, presence or absence of migration, and the presence or absence of testing and the treatment cascade. The elements of sexual behavior included in the model will depend on the analysis and population of interest and should be justified using data and information from the population of interest [8]. Authors should acknowledge poorly understood behaviors and limitations in data used to inform parameters.
Item 8: Validity of Biology - Justify the validity of the necessary biology accounted for in the model
The level of biological detail that an individual-based model should represent is dependent upon the aims of the analysis of interest. Many elements of individual- and community-level biology are important to the transmission and prevention of HIV, for example: the inclusion or exclusion of sexually transmitted infection, the tracking of virological markers (CD4/HVL), multiple HIV disease stages with differing transmission probabilities, impact of circumcision status on HIV transmission, presence or absence of opportunistic infections, the impact of treatment on health and future transmission events, and the presence or absence of resistance mutations. By describing the elements incorporated in the model, the readers are able to determine which aspects of HIV transmission and prevention can be assessed.
Item 9: Parameters - List fixed parameters and calibrated parameters with ranges justified by the literature
When individual-based models begin to represent sexual behavior and biology, the number of parameters needed to populate them grows dramatically [10]. By listing the main parameters examined in the analyses of interest, the readers are able to understand the necessary sources of the data. Further, including uncertainty ranges establishes the need for attaining parameter values through a fitting or calibration procedure (see Item 13) when data is not available from the literature. Where parameter values cannot be based on the literature or are not calibrated, data from similar populations or assumptions made about the population of interest should be provided [7,8].
Item 10: Time Step - State and justify the length of the time step used to advance model dynamics, if applicable
The time step in an individual-based model is determined by the level of detail desired and computational limitations [13]. A short time step will provide greater detail on sexual behavior and biology, but will cause longer run times. Explicitly stating the time step, where appropriate, will make clear which processes the model describes well and makes the necessary data conversions for parameters transparent (see Item 19).
Item 11: Heterogeneity - Discuss how biological and behavioral heterogeneity is implemented in the model structure and whether this implementation allows for flexibility and specificity
One of the main strengths of the individual-based model structure is the ability to represent heterogeneity in behavior and biology [3] and, as such, methods used to implement this heterogeneity need to be detailed. Specific details on the discrete categories or continuous distributions used should be reported in the manuscript or supplementary material. Emphasizing the particular elements of behavior and biology that vary across individuals allows readers to understand how accurately these processes reflect reality and highlights strengths of the modeling exercise.
Item 12: Interaction - Describe the parameters used to implement individual interaction in the model and justify the data used to parameterize these parameters
HIV transmission that occurs within a particular partnership needs to be modeled accurately. Partnership formation and dissolution, the number of sexual acts in each partnership, the directionality of MSM partnerships, and the types of partnerships should be described in detail. By putting emphasis on the interaction between individuals in an individual-based model, authors will clearly describe the level of interaction between agents and consequently justify the need for this interaction.
Item 13: Calibration - If the objective of the analysis is to describe or predict dynamics in a particular population of interest, describe the process used to calibrate the model dynamics to existing data including the statistical procedure, the types of outcome measures used, and the quality of the data used
The process through which the model’s predictions, with regard to particular outcomes, are matched to data in the population of interest is called calibration [9]. There are many methods through which calibration can be statistically rigorous [5,9] and other methods through which it can be performed less rigorously. Calibration may be the most important piece of reported information for inferring a model’s ability to make accurate inferences about a particular population; therefore, details on the algorithms used and the outcomes calibrated should be provided. Although a model only needs to be calibrated once to reflect dynamics in a particular population, the calibration process should be repeated if the population, time period, or outcome of interest change. Models that have previously been calibrated should cite relevant previous manuscripts, and briefly describe the process. Authors should also make note of any effort to avoid over-fitting the model to data [16].
Item 14: Sensitivity Analyses - Summarize the results of sensitivity analyses on the main model parameters, discuss whether the results support the robustness of findings, and describe future work needed
As with any statistical analysis, it is essential to understand the sensitivity of the results to perturbations in parameters that are directly related to the intervention of interest [7]. For example, a model investigating how vaccine coverage impacts HIV incidence should vary vaccine coverage and efficacy to understand how model structure and parameterization impact the results. Sensitivity analyses may identify potential areas of model improvement and these discoveries should be noted. Less important results can be reported in supplemental material or an appendix.
Item 15: Assumption Sensitivity - Discuss how the behavior and inference of the model changes when particular assumptions (e.g. alternative mixing patterns, different levels of heterogeneity for behavior and biology) are altered or deleted
After describing the assumptions inherent in the model structure (see Item 6), the authors should discuss how the model’s behavior was dependent on these assumptions [8]. Assumption sensitivity analyses will reinforce the necessity of individual-based model structure and highlight which aspects of the intervention other model types would not capture. This type of sensitivity analysis will allow the authors to explore the generalizability of the model results to situations where the assumptions are violated [7,9]. Modelers will usually alter the assumptions most relevant for the analysis being performed, but altering other assumptions will improve the plausibility of the model results. Assumption sensitivity analyses can be performed in many different ways, one of which compares the results from the individual-based model to a deterministic model with simplified dynamics.
Item 16: Stochastic Sensitivity - Summarize the impact of stochasticity on the model runs and justify through random seed variation and sample size variation
Microsimulation models are often stochastic, meaning there is a certain level of randomness inherent to each model run [9,13]. Stochasticity can affect large simulation models in different ways, depending on which processes rely on random number generation. Authors should describe how stochasticity affects model results and what the authors have done to understand these effects, including increasing sample size or seeding the population differently [7].
Item 17: Internal Validity - Describe the validity of the model programming by discussing how model bugs and program issues were checked and if modifications to model implementation were explored
The internal validity of the model should be discussed, highlighting the steps taken to debug the model program and check the validity of the model structure [7,10]. This information should be placed in an appendix or supplemental material. Publications on previously published models should refer back to the methods of the original manuscript, but should not necessarily repeat the internal validity checks, unless the program code was changed. It is good practice to have two individuals program the model independently and compare the implementation to avoid bugs [7], though we acknowledge limited personnel or resources may make this infeasible. Model flowcharts, debugging runs, and other measures can also prevent bugs in the implementation of the code.
Item 18: Data Quality - Describe the quantity and quality of the data used to inform parameters for the population(s) of interest
As mentioned in Item 9, the number of parameters needed to populate an individual-based model is large, and it may be difficult to find data for the parameters needed. A discussion of the data quality for the population and region of interest will allow readers to understand the limitations to modeling in this population [10] and may encourage public health researchers and social scientists to collect additional relevant data.
Item 19: Data Conversion - Discuss issues related to the conversion of data to fit the time step used
The time step used is not often motivated by the data available, but rather by the goal of realism set by the authors. Data often has to be converted to the proper time step (e.g. number of sexual acts per partnership per time step). Any additional assumptions required for conversion should be discussed.
Item 20: Results - Present key modeling results with uncertainty estimates and indicate how many parameter sets were run for each analysis
Authors should report their results with uncertainty estimates. Particularly for individual-based models, authors should disclose the number of parameter sets used or runs averaged to get the results. Discussing uncertainty in results will help the authors anchor their conclusions (see Item 23) and give the readers a better understanding of the capabilities of the model.
Item 21: Limitations and Strengths - Provide the key limitations and strengths of the modeling study
The strengths and limitations of the methods used should be highlighted [12]. The limitations of an individual-based model are often dependent on the assumptions made, the computational power available, and the data used to inform the parameters. Detailed models of sexual behavior and transmission should emphasize the strengths of these details, while recognizing the potential weaknesses in data used to inform these processes.
Item 22: Reproducibility - Discuss whether the model is able to reproduce the behavior of other populations or interventions of interest
The generalizability of results (discussed in Item 23) should be emphasized along with the generalizability of the model structure. Some models are flexible enough to describe the behavior of many populations, while others are best suited to the dynamics in a single population. Describing whether the software and programming needed for implementing the model structure can be used to answer other questions of interest should be noted. A description of the generalizability of the model may encourage collaboration with other modeling groups or authors who have interest in using the model structure to answer alternative questions.
Item 23: Discussion – Interpret the modeling analysis within realistic bounds, with reference to previous modeling studies, a discussion about the generalizability of the modeling results, and implications for future studies or models
The discussion section of a modeling paper should emphasize the capability of the model to represent real world dynamics, while keeping the conclusions grounded upon the model assumptions. The generalizability of the results should be discussed, with a particular focus on the assumptions that allow for generalizability of the findings [12]. Future modeling and non-modeling studies should be proposed with insight as to how this body of work would contribute to the HIV literature as a whole.
Item 24: Authorship and Funding - List sources of funding and describe each author’s contribution to the modeling framework and conceptualization
Listing of funding sources allows other modelers to better understand what types of funding sources are applicable to modeling projects and grants. Additionally, by listing all the authors’ contributions, additional modeling teams or task forces can be composed based on capabilities of authors on previous projects and analyses.
Search
Following the development of these reporting guidelines, we systematically reviewed the current individual-based HIV transmission and prevention literature to better understand the quality of the reporting in this field and ways in which it can be improved.
We searched PubMed, EMBASE, BIOSYS, and Web of Science for modeling papers published in English prior to December 31, 2012. The search used the following terms modified to the particular search language of each of the databases: an HIV infection term to capture papers related to HIV, transmission and prevention terms to capture papers examining these particular interventions, and simulation terms to capture models. To avoid confusion over vocabulary or classification of the modeling papers, a broad number of search terms were used to capture individual-based simulation models. Details on search terms can be found in Text S1.
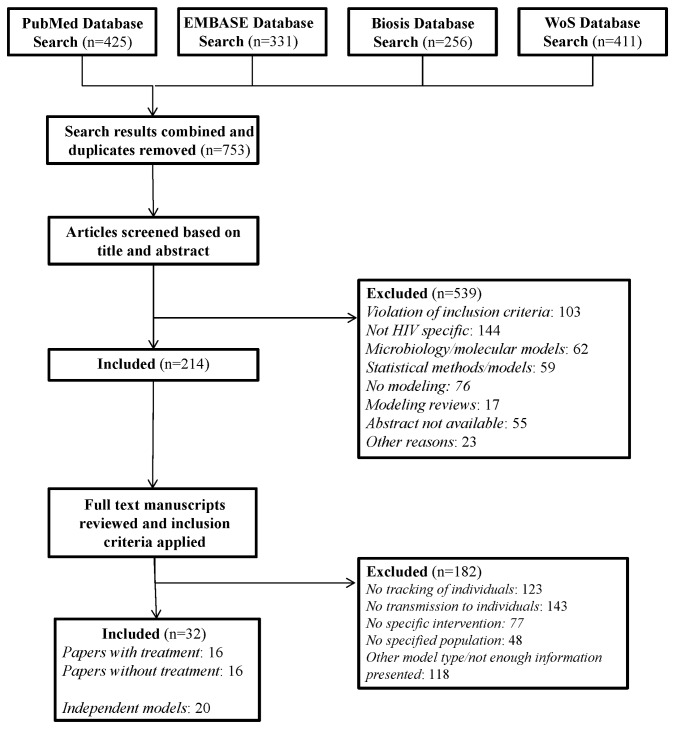
Following the removal of duplicates across databases, all titles and abstracts were screened for exclusion. If the title and abstract did not provide enough information to evaluate the inclusion criteria or the information provided suggested the model was relevant, the full text was examined and evaluated based on the inclusion criteria. Two authors (NA and KR) conducted the search (Figure 1).
Figure 1. Cascade of papers excluded and included in the systematic review of individual-based HIV transmission, treatment, and prevention models in the literature.
Inclusion and Exclusion Criteria
Papers were included in the evaluation if they described a model that tracked individual characteristics and histories and simulated HIV transmission between specific individuals. They had to simulate a population denoted either geographically and/or behaviorally (e.g. men who have sex with men in the Southern United States) and examine the effects of a particular intervention (e.g. circumcision rollout). These inclusion criteria allowed us to capture models that simulated HIV transmission and prevention realistically, while eliminating models that were exploratory or theoretical in nature.
We excluded mathematical models that did not account for the interaction between individuals, as we believe that behavior is an essential element of individual-based models aiming to replicate HIV transmission. Papers that represented the probability of acquiring HIV using mass action equations or the proportion of individuals infected were not considered to model direct interaction. We also excluded modeling reviews, conference abstracts, and unpublished studies, as they did not provide enough information on model structure to warrant evaluation.
Multiple papers utilizing the same model to answer different questions of interest were included and evaluated, acknowledging that details of model structure could be included in previously published papers. For each paper identified in the systematic review, the quality of reporting was evaluated based on the described recommendations. Assessment of the included models was independently undertaken by two authors (NA and KR).
Results
Characteristics of Studies
The search criteria identified 1,423 citations, of which 753 were unique records (Figure 1). After initial screening of abstracts and titles, 214 citations were reviewed in full text, and 32 citations were included in the systematic review and evaluation. The characteristics of the included studies are summarized in Table 2 .
Table 2. Information on the 32 papers describing individual-based HIV treatment models that passed the inclusion criteria.
| Authors | Year | Journal | Model Name | Population of Interest | Treatment Modeled? | Intervention |
|---|---|---|---|---|---|---|
| Adams et al. [17] | 1998 | Simulation | HIVSIM | Men who have sex with men (MSM) or heterosexual adults | No | Vaccine |
| Amirfar et al. [18] | 2006 | JAIDS | 15-25 year old South African adolescent and adult females and their infants | Yes | Vaccine/PMTCT | |
| Atkinson, J [48]. | 1996 | Comput Biomed Res | Injection drug users (IDU) | No | Behavioral | |
| Bendavid et al. [37] | 2010 | Arch Intern Med | Heterosexual adult South Africans | Yes | Testing/Treatment | |
| Bernstein et al. [25] | 1998 | Interfaces | SimulAIDS | Heterosexual adults in a generic east African city | No | Behavioral/STD testing |
| Beyrer et al. [26] | 2012 | Lancet | MSM residing in high (urban USA), middle (urban Peru), and low income countries | Yes | Behavioral | |
| Bracher et al. [27] | 2004 | Stud Fam Plann | Heterosexual adults in rural southern Malawi | No | Behavioral | |
| Enns and Brandeau [33] | 2011 | Health Care Manag Sci | Heterosexual Tanzanian adults | Yes | Behavioral | |
| Freeman et al. [40] | 2009 | Vaccine | STDSIM | Heterosexual adults in Sub-Saharan Africa | No | STD vaccine |
| Gray et al. [19] | 2003 | AIDS | Gray | Heterosexual adult discordant Ugandan couples | Yes | Treatment/Vaccine |
| Gray et al. [23] | 2007 | AIDS | Gray | Heterosexual adult discordant Ugandan couples | No | Male circumcision |
| Gray et al. [20] | 2011 | Vaccine | MSM in NSW, Australia | Yes | Vaccine | |
| Hallett et al. [39] | 2011 | PLoS Med | Stable HIV-1 serodiscordant heterosexual couples in South Africa | Yes | PrEP | |
| Hallett et al. [28] | 2011 | Sex Transm Infect | MSM in the Netherlands | Yes | Behavioral/Testing | |
| Hoare et al. [36] | 2012 | Sex Health | MSM in Victoria, Australia | Yes | Testing | |
| Hontelez et al. [21] | 2011 | Vaccine | STDSIM | Heterosexual adults in a rural South African setting | Yes | Vaccine |
| Hontelez et al. [38] | 2012 | AIDS | STDSIM | Heterosexual adults in Sub-Saharan Africa | Yes | Treatment |
| Korenromp et al. [41] | 2000 | AIDS | STDSIM | Heterosexual adults in Mwanza, Tanzania | No | STD treatment |
| Korenromp et al. [45] | 2002 | AIDS | STDSIM | Heterosexual adults in Rakai, Uganda | No | STD treatment |
| Korenromp et al. [43] | 2005 | J Infect Dis | STDSIM | Heterosexual adults in Rakai and Masaka, Uganda and Mwanza, Tanzania | No | STD treatment |
| Marshall et al. [15] | 2012 | PLoS One | IDU/NIDU/MSM in the New York metropolitical statistical area | Yes | Behavioral/Testing | |
| McCabe et al. [46] | 2010 | PLoS One | Pregnant women and infants in the United States | Yes | PMTCT | |
| McCreesh et al. [34] | 2011 | Sex Transm Infect | Heterosexual adults in rural South-West Uganda | Yes | Behavioral | |
| Rauner et al. [47] | 2005 | J Oper Res Soc | Pregnant women and infants in Tanzania | Yes | PMTCT | |
| Robinson et al. [29] | 1995 | AIDS | SimulAIDS | Heterosexual adults in rural south-west Uganda | No | Behavioral/STD treatment |
| Van der Ploeg et al. [30] | 1998 | Interfaces | STDSIM | Heterosexual adults in Nairobi, Kenya | No | Behavioral/STD treatment |
| van Vliet et al. [31] | 2001 | Bull World Health Organ | STDSIM | Heterosexual adults | No | Behavioral |
| Vieira et al. [22] | 2010 | Ann. Oper Res | Urban heterosexual, MSM, and bisexual adult Brazilians | Yes | Treatment/Vaccine/Behavioral | |
| Vissers et al. [32] | 2011 | Epidemiol Infect | STDSIM | Heterosexual adults in Tanzania | No | Behavioral |
| White et al. [44] | 2004 | JAIDS | STDSIM | Heterosexual adults in Rakai and Masaka, Uganda and Mwanza, Tanzania | No | STD treatment |
| White et al. [24] | 2008 | AIDS | STDSIM | Heterosexual adults in Sub-Saharan Africa | No | Male circumcision |
| Wilson et al. [35] | 2011 | Sex Transm Infect | MSM in Melbourne, Australia | No | Testing |
Individual-based microsimulation models were first published in the 1980’s, though the oldest model to fit our inclusion criteria was published in 1995. The included analyses were published in a number of different journals (Table 2), ranging from computationally focused to medical journals. About half of them (n=15) were published in journals that had high eigenfactor and article influence (>90th percentile respectively). More than half of the studies (n=22) explored HIV transmission in heterosexual populations, eight studies analyzed HIV epidemics among MSM, and two studies focused on injection drug users (IDUs). Most studies (n=21) were populated with data from African countries. The interventions of interest in these models varied, with many examining the presence of a vaccine (n=6), behavioral interventions (n=13), and HIV testing and/or antiretroviral treatment (n=8) with several analyses featuring multiple interventions (n=9).
Evaluation
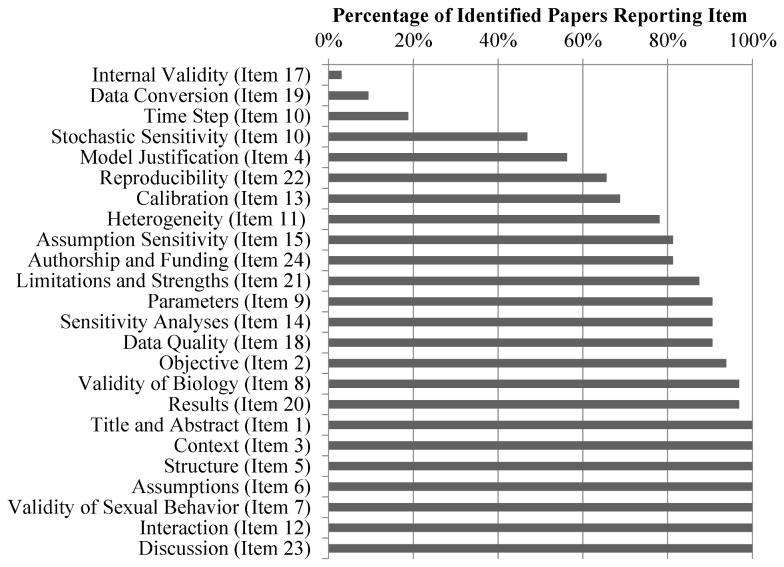
The number and percentage of papers that complied with the recommended reporting guidelines are reported in Table 3 and Figure 2. Table S1 provides the details of this evaluation for each paper.
Table 3. Evaluation of reporting quality in individual-based models in the HIV transmission and prevention literature (N=32).
| Item | Total Number of Papers |
|---|---|
| n (%) | |
| 1. Title and Abstract | 32 (100.0) |
| 2. Objective | 30 (93.8) |
| 3. Context | 32 (100.0) |
| 4. Model Justification | 18 (56.3) |
| 5. Structure | 32 (100.0) |
| 6. Assumptions | 32 (100.0) |
| 7. Validity of Sexual Behavior | 32 (100.0) |
| 8. Validity of Biology | 31 (96.9) |
| 9. Parameters | 29 (90.6) |
| 10. Time step | 6 (18.8) |
| 11. Heterogeneity | 25 (78.1) |
| 12. Interaction | 32 (100.0) |
| 13. Calibration | 22 (68.8) |
| 14. Sensitivity Analyses | 29 (90.6) |
| 15. Assumption Sensitivity | 26 (81.3) |
| 16. Stochastic Sensitivity | 15 (46.9) |
| 17. Internal Validity | 1 (3.1) |
| 18. Data Quality | 29 (90.6) |
| 19. Data Conversion | 3 (9.4) |
| 20. Results | 31 (96.9) |
| 21. Limitations and Strengths | 28 (87.5) |
| 22. Reproducibility | 21 (65.6) |
| 23. Discussion | 32 (100.0) |
| 24. Authorship and Funding | 26 (81.3) |
Figure 2. Bar chart of the percentage of identified papers that complied with each reporting guideline item.
The modeling papers almost universally described the context in which the analyses were performed and the objectives of the simulation studies. More than half of the papers (n=17) were not distinguished in the title as a mathematical model or a simulation analysis, but all papers did note the use of a model in the abstract. Fifty six percent of the papers (n=18) justified the use of an individual-based model as the necessary method to answer the question of interest. Nine of the models included a graphical representation of the model structure, while the rest described the structure in plain language. Some of the papers (n=9) referred to an appendix or a previous paper for more detail on the model structure. Nineteen percent (n=6) state and justify the length of the time step used and 78% of the papers (n=25) describe how heterogeneity is implemented in the model structure or recognize that heterogeneity was used in the individual-based modeling framework. Sixty nine percent (n=22) describe the process through which the model was calibrated to data, although six of the reviewed papers did not aim to accurately represent the dynamics in a population, but rather understand general trends, and so would not be expected to perform a calibration procedure. Nearly half (n=15) of the papers summarize the impact of stochasticity on the model results and discuss the magnitude of stochasticity in the model behavior. Sensitivity analyses describing how the model behaved when assumptions were altered or deleted were performed for 81% of the papers (n=26). One paper described the validity of the model programming, debugging procedure, and other details related to the implementation and validation of the programming. Three papers discussed the issue of converting data to fit the time step restrictions in the model structure. Nearly all papers (n=31) presented the modeling results clearly; eleven of these papers presented uncertainty estimates around their effect estimates and predicted values. All of the papers provided interpretations of the modeling results within realistic bounds without overinflating the usefulness of the results.
Discussion
We found that individual-based models in the HIV transmission and prevention literature are able to answer a wide range of questions related to specific populations and interventions (Table 2). The models examined how a variety of HIV interventions such as vaccination [17-22], circumcision [23,24], condom usage [25-32], reduction in concurrency [25,26,29,32-34], HIV testing [15,28,35-37], anti-retroviral treatment [19,22,37-39], STD control [25,29,30,40-45], and prevention of mother to child transmission [18,46,47] can affect HIV incidence and prevalence in a wide variety of settings including North America [15,26,46], Australia [20,35,36] and sub-Saharan Africa [18,19,21,23-25,27,29,30,32-34,37-45,47]. These analyses were able to discuss the effects of interventions in less researched and accessible populations like MSM [15,17,20,22,26,28,35,36] and IDU [15,48].
We found that the reporting of results from individual-based model analyses was very strong with respect to the basics of public health research and other model analyses (e.g. stating the objective, giving context from the literature, and providing grounded conclusions) but lacking in the description of methods particular to individual-based models. Authors may feel uncomfortable giving detailed descriptions of the methods in a paper aimed at a general public health audience because the technical details may make the paper harder to read or understand. However, detailed reporting is essential to ensure that the quality of the literature remains high and the results are reproducible. To this extent, emphasis on reporting items related to structure (Item 5), assumptions (Item 6), calibration (Item 13) and strengths and limitations (Item 21) will be most important for individuals trying to understand published individual-based models. The more frequently modeling methods are included in public health analyses, the more widely the methods will be accepted and valued. Additionally, providing detail and transparency in methods will encourage collaboration among mathematical modelers, while making individual-based modeling more accessible to those unfamiliar with the process.
As evidenced by the diversity of journals the models were published in (Table 2), individual-based models are valued for their ability to represent existing epidemics for a wide variety of populations and regions. Just over half of the articles included in this review noted that the analysis relied on mathematical modeling in the title, which suggests that authors might feel that denoting a study as a modeling study could deter readers. However, as this practice becomes more common, modeling papers will be read with the same clarity and readiness as other types of analyses. As more journals recognize the utility of modeling, distinguishing model analyses from classic public health analyses becomes more important.
Individual-based models are used widely in other fields, including ecology [49], meteorology [50], and traffic monitoring [51], and it is encouraging to see their influence growing in the HIV literature. Individual-based modeling is user-friendly and highly visual, allowing for collaboration and understanding across multidisciplinary teams [52]. Strengths of individual-based models include their ability to model the interaction between individuals with great detail and to reflect heterogeneity in behavior and biology. Many authors noted the inability to completely parametrize their models from the literature, tractably analyze models without uncertainty, and the need for sophisticated methods of calibration to help increase their confidence in their findings. Deterrents to individual-based modeling in the HIV transmission literature include the lack of biological and behavioral data in many populations, which prevents parameterization of this complex model type, as noted in some reviewed papers [26,39].
The creation of individual-based models is a complex and arduous process, yet standards for reporting them are relatively non-existent. We aimed to provide guidelines to strengthen the reporting of results in this field and an overview of individual-based models examining HIV-related interventions. The existing literature is broad and thorough; however, more information is needed on the rigor of calibration and the rationale for the use of individual-based modeling. Future work in this field should aim to make the literature accessible to a general audience by using clear language that non-modelers and non-mathematicians can understand. The clearer the presentation, the more widely modeling literature will be read and applied in the future. By collaborating with other interested parties or modeling groups, we hope to develop a consensus statement on the reporting of individual-based models in the HIV treatment and prevention literature.
Supporting Information
Search terms for systematic review.
(DOCX)
Tabled evaluation of all eligible systematically identified individual-based HIV transmission models based on the reporting recommendations.
(DOCX)
Acknowledgments
The authors would like to thank Carol Ann Mita for her assistance in the systematic search. The authors would also like to thank Marc Lipsitch, Rochelle Walensky, and the reviewers for their helpful comments on the draft.
Funding Statement
The authors acknowledge funding from the following sources: NIAID AI 007433 (http://www.niaid.nih.gov/researchfunding/pages/default.aspx), and RO1MH087328-03 (http://grants.nih.gov/grants/about_grants.htm). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stover J (2000) Influence of mathematical modeling of HIV and AIDS on policies and programs in the developing world. Sex Transm Dis 27: 572-578. doi:10.1097/00007435-200011000-00005. PubMed: 11099072. [DOI] [PubMed] [Google Scholar]
- 2. Gilbert N (2007) Agent-based models. Sage Publications, Incorporated. [Google Scholar]
- 3. Law AM, Kelton WD (1991) Simulation modeling and analysis. New York: McGraw-Hill. [Google Scholar]
- 4. Marshall B, Paczkowski M, Tempalski B, Pouget E, Friedman S et al. (2012) Combination interventions for the prevention of HIV among injection drug users: a complex systems dynamics model. J Int AIDS Soc 15: 108. [Google Scholar]
- 5. Delva W, Wilson DP, Abu-Raddad L, Gorgens M, Wilson D et al. (2012) HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation. PLOS Med 9: e1001239 PubMed: 22802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N (2011) Mathematical models in the evaluation of health programmes. Lancet 378: 515-525. doi:10.1016/S0140-6736(10)61505-X. PubMed: 21481448. [DOI] [PubMed] [Google Scholar]
- 7. Richiardi M, Leombruni R, Saam N, Sonnessa M (2006) A common protocol for agent-based social simulation. J Artif Soc Soc 9. [Google Scholar]
- 8. Fone D, Hollinghurst S, Temple M, Round A, Lester N et al. (2003) Systematic review of the use and value of computer simulation modelling in population health and health care delivery. J Public Health (Oxf) 25: 325-335. doi:10.1093/pubmed/fdg075. PubMed: 14747592. [DOI] [PubMed] [Google Scholar]
- 9. Rutter CM, Zaslavsky AM, Feuer EJ (2011) Dynamic Microsimulation Models for Health Outcomes A Review. Med Decis Mak 31: 10-18. doi:10.1177/0272989X10369005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Philips Z, Bojke L, Sculpher M, Claxton K, Golder S (2006) Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics 24: 355–371. doi:10.2165/00019053-200624040-00006. PubMed: 16605282. [DOI] [PubMed] [Google Scholar]
- 11. Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V et al. (2006) A standard protocol for describing individual-based and agent-based models. Ecol Modell 198: 115-126. doi:10.1016/j.ecolmodel.2006.04.023. [Google Scholar]
- 12. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC et al. (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340: c869. doi:10.1136/bmj.c869. PubMed: 20332511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Imhoff E, Post W (1998) Microsimulation methods for population projection. Population: An English Selection: 97-138. PubMed: 12157954. [PubMed] [Google Scholar]
- 14. Karplus WJ (1977) The spectrum of mathematical modeling and systems simulation. Math Comput Simulat 19: 3-10. doi:10.1016/0378-4754(77)90034-9. [Google Scholar]
- 15. Marshall BD, Paczkowski MM, Seemann L, Tempalski B, Pouget ER et al. (2012) A complex systems approach to evaluate HIV prevention in metropolitan areas: preliminary implications for combination intervention strategies. PLOS ONE 7: e44833. doi:10.1371/journal.pone.0044833. PubMed: 23028637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halpin B (1999) Simulation in sociology. Am Behav Sci 42: 1488-1508. doi:10.1177/0002764299042010003. [Google Scholar]
- 17. Adams AL, Barth-Jones DC, Chick SE, Koopman JS (1998) Simulations to evaluate HIV vaccine trial designs. Simul 71: 228-241. doi:10.1177/003754979807100403. [Google Scholar]
- 18. Amirfar S, Hollenberg JP, Abdool Karim SS (2006) Modeling the impact of a partially effective HIV vaccine on HIV infection and death among women and infants in South Africa. J Acquir Immune Defic Syndr 43: 219-225. doi:10.1097/01.qai.0000230526.79341.83. PubMed: 16951648. [DOI] [PubMed] [Google Scholar]
- 19. Gray RH, Li XB, Wawer MJ, Gange SJ, Serwadda D et al. (2003) Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS 17: 1941-1951. doi:10.1097/00002030-200309050-00013. PubMed: 12960827. [DOI] [PubMed] [Google Scholar]
- 20. Gray RT, Ghaus MH, Hoare A, Wilson DP (2011) Expected epidemiological impact of the introduction of a partially effective HIV vaccine among men who have sex with men in Australia. Vaccine 29: 6125-6129. doi:10.1016/j.vaccine.2011.06.061. PubMed: 21703320. [DOI] [PubMed] [Google Scholar]
- 21. Hontelez JA, Nagelkerke N, Bärnighausen T, Bakker R, Tanser F et al. (2011) The potential impact of RV144-like vaccines in rural South Africa: a study using the STDSIM microsimulation model. Vaccine 29: 6100-6106. doi:10.1016/j.vaccine.2011.06.059. PubMed: 21703321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vieira IT, Cheng RCH, Harper PR, de Senna V (2010) Small world network models of the dynamics of HIV infection. Ann Oper Res 178: 173-200. doi:10.1007/s10479-009-0571-y. [Google Scholar]
- 23. Gray RH, Li XB, Kigozi G, Serwadda D, Nalugoda F et al. (2007) The impact of male circumcision on HIV incidence and cost per infection prevented: a stochastic simulation model from Rakai, Uganda. AIDS 21: 845-850. doi:10.1097/QAD.0b013e3280187544. PubMed: 17415039. [DOI] [PubMed] [Google Scholar]
- 24. White RG, Glynn JR, Orroth KK, Freeman EE, Bakker R et al. (2008) Male circumcision for HIV prevention in sub-Saharan Africa: who, what and when? AIDS 22: 1841-1850. doi:10.1097/QAD.0b013e32830e0137. PubMed: 18753931. [DOI] [PubMed] [Google Scholar]
- 25. Bernstein RS, Sokal DC, Seitz ST, Auvert B, Stover J et al. (1998) Simulating the control of a heterosexual HIV epidemic in a severely affected east African city. Interfaces 28: 101-126. doi:10.1287/inte.28.3.101. [Google Scholar]
- 26. Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S et al. (2012) Global epidemiology of HIV infection in men who have sex with men. Lancet 380: 367-377. doi:10.1016/S0140-6736(12)60821-6. PubMed: 22819660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bracher M, Santow G, Watkins SC (2004) Assessing the potential of condom use to prevent the spread of HIV: A microsimulation study. Stud Fam Plann 35: 48-64. doi:10.1111/j.1728-4465.2004.00005.x. PubMed: 15067788. [DOI] [PubMed] [Google Scholar]
- 28. Hallett TB, Smit C, Garnett GP, de Wolf F (2011) Estimating the risk of HIV transmission from homosexual men receiving treatment to their HIV-uninfected partners. Sex Transm Infect 87: 17-21. doi:10.1136/sextrans-2011-050102.58. PubMed: 20643658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson NJ, Mulder DW, Auvert B, Hayes RJ (1995) Modelling the impact of alternative HIV intervention strategies in rural Uganda. AIDS 9: 1263-1270. doi:10.1097/00002030-199511000-00008. PubMed: 8561980. [DOI] [PubMed] [Google Scholar]
- 30. Van der Ploeg CPB, Van Vliet C, De Vlas SJ, Ndinya-Achola JO, Fransen L et al. (1998) STDSIM: A microsimulation model for decision support in STD control. Interfaces 28: 84-100. doi:10.1287/inte.28.3.84. [Google Scholar]
- 31. van Vliet C, Meester EI, Korenromp EL, Singer B, Bakker R et al. (2001) Focusing strategies of condom use against HIV in different behavioural settings: an evaluation based on a simulation model. Bull World Health Organ 79: 442-454. PubMed: 11417040. [PMC free article] [PubMed] [Google Scholar]
- 32. Vissers DC, SJ DEV, Bakker R, Urassa M, Voeten HA, et al (2011) The impact of mobility on HIV control: a modelling study. Epidemiol Infect 139: 1845-1853. doi:10.1017/S0950268811000069. PubMed: 21299914. [DOI] [PubMed] [Google Scholar]
- 33. Enns EA, Brandeau ML (2011) Inferring model parameters in network-based disease simulation. Health Care Manag Sci 14: 174-188. doi:10.1007/s10729-011-9150-2. PubMed: 21373984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCreesh N, O’Brien K, Nsubuga R, Shafer A L, Bakker R, et al. (2011) Exploring the potential impact on hiv incidence of a reduction in concurrency in rural Uganda: A modelling study. Sex Transm Infect 87: A37. doi:10.1136/sextrans-2011-050109.36. [DOI] [PubMed] [Google Scholar]
- 35. Wilson DP, Fairley CK, Sankar D, Williams H, Keen P et al. (2011) Replacement of conventional HIV testing with rapid testing: mathematical modelling to predict the impact on further HIV transmission between men. Sex Transm Infect 87: 588-593. doi:10.1136/sextrans-2011-050002. PubMed: 21934115. [DOI] [PubMed] [Google Scholar]
- 36. Hoare A, Gray RT, Wilson DP (2012) Could implementation of Australia’s National Gay Men’s Syphilis Action Plan have an indirect effect on the HIV epidemic? Sex Health 9: 144-151. PubMed: 22498158. [DOI] [PubMed] [Google Scholar]
- 37. Bendavid E, Brandeau ML, Wood R, Owens DK (2010) Comparative Effectiveness of HIV Testing and Treatment in Highly Endemic Regions. Arch Intern Med 170: 1347-1354. doi:10.1001/archinternmed.2010.249. PubMed: 20696960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hontelez JAC, De Vlas SJ, Baltussen R, Newell ML, Bakker R et al. (2012) The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 26: S19-S30. doi:10.1097/QAD.0b013e3283558526. PubMed: 22781175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hallett TB, Baeten JM, Heffron R, Barnabas R, de Bruyn G et al. (2011) Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLOS Med 8: e1001123 PubMed: 22110407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freeman EE, White RG, Bakker R, Orroth KK, Weiss HA et al. (2009) Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine 27: 940-946. doi:10.1016/j.vaccine.2008.11.074. PubMed: 19071187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korenromp EL, Van Vliet C, Grosskurth H, Gavyole A, Van der Ploeg CP et al. (2000) Model-based evaluation of single-round mass treatment of sexually transmitted diseases for HIV control in a rural African population. AIDS 14: 573-593. doi:10.1097/00002030-200003310-00013. PubMed: 10780720. [DOI] [PubMed] [Google Scholar]
- 42. Korenromp EL, Bakker R, de Vlas SJ, Gray RH, Wawer MJ et al. (2002) HIV dynamics and behaviour change as determinants of the impact of sexually transmitted disease treatment on HIV transmission in the context of the Rakai trial. AIDS 16: 2209-2218. doi:10.1097/00002030-200211080-00014. PubMed: 12409743. [DOI] [PubMed] [Google Scholar]
- 43. Korenromp EL, White RG, Orroth KK, Bakker R, Kamali A et al. (2005) Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: A synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J Infect Dis 191: S168-S178. doi:10.1086/425274. PubMed: 15627227. [DOI] [PubMed] [Google Scholar]
- 44. White RG, Orroth KK, Korenromp EL, Bakker R, Wambura M et al. (2004) Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: A modeling study. J Acquir Immune Defic Syndr 37: 1500-1513. doi:10.1097/01.qai.0000127062.94627.31. PubMed: 15602129. [DOI] [PubMed] [Google Scholar]
- 45. Korenromp EL, Bakker R, Gray R, Wawer MJ, Serwadda D et al. (2002) The effect of HIV, behavioural change, and STD syndromic management on STD epidemiology in sub-Saharan Africa: Simulations of Uganda. Sex Transm Infect 78: i55-i63. doi:10.1136/sti.78.suppl_1.i55. PubMed: 12083448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCabe CJ, Goldie SJ, Fisman DN (2010) The Cost-Effectiveness of Directly Observed Highly-Active Antiretroviral Therapy in the Third Trimester in HIV-Infected Pregnant Women. PLOS ONE 5: e10154 PubMed: 20405011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rauner MS, Brailsford SC, Flessa S (2005) Use of discrete-event simulation to evaluate strategies for the prevention of mother-to-child transmission of HIV in developing countries. J Oper Res Soc 56: 222-233. doi:10.1057/palgrave.jors.2601884. [Google Scholar]
- 48. Atkinson J (1996) A simulation model of the dynamics of HIV transmission in intravenous drug users. Comput Biomed Res 29: 338-349. doi:10.1006/cbmr.1996.0025. PubMed: 8812079. [DOI] [PubMed] [Google Scholar]
- 49. Grimm V, Railsback SF (2005) Individual-based modeling and ecology. Princeton University Press. [Google Scholar]
- 50. Athanasiadis IN, Mitkas PA (2004) An agent-based intelligent environmental monitoring system. Manag Environ Qual 15: 238-249. doi:10.1108/14777830410531216. [Google Scholar]
- 51. Chu L, Liu HX, Oh J-S, Recker W (2003) A calibration procedure for microscopic traffic simulation. IEEE: 1574-1579. [Google Scholar]
- 52. Epstein JM (2009) Modelling to contain pandemics. Nature 460: 687-687. doi:10.1038/460687a. PubMed: 19661897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms for systematic review.
(DOCX)
Tabled evaluation of all eligible systematically identified individual-based HIV transmission models based on the reporting recommendations.
(DOCX)