1. INTRODUCTION
Approximately 3–5% of preschool-aged children will begin stuttering. In some cases the onset of stuttering is dramatic: a previously fluent child will wake up one morning unable to utter a fluent sentence. An accompanying puzzle is the phenomenon of natural recovery: that same child may stutter severely for weeks or months and then gradually return, without intervention, to normally fluent speech.
Preschool-aged children who begin to stutter will recover naturally approximately three-quarters of the time (Yairi & Ambrose, 1999), but predictor variables and causal factors are poorly understood. This study was driven by the hypothesis that breastfeeding could confer a measure of dose-related protection against persistent stuttering. The following sections will describe the rationale for this hypothesis, summarize the existing literature on breastfeeding and speech-language development, and briefly review the etiology of stuttering.
1.1 Human Milk and Neurodevelopment
Fluent speech, with its 140,000 neuromuscular events per second (Darley, Aronson, & Brown, 1975), depends on normal neurodevelopment, and neurodevelopment can be influenced by diet in early infancy. It is widely recognized that the neurological system changes rapidly during the first two years of life; the question less often considered is what, precisely, is required to build a brain. A newborn's brain weighs, on average, 350 g; a year later it will weigh 1100 g (Lawrence & Lawrence, 2005). More than half the solid weight of that newly built tissue will be lipid, and dietary fat intake exerts a significant influence on its composition (Farquharson, Jamieson, Logan, Cockburn, & Ainslie Patrick, 1992). The rationale for this study is that the differing fatty acid profiles of human milk and infant formula have the potential to affect children predisposed to stuttering via two different mechanisms: first, by subtly altering the composition and thus the function of the brain itself, and second, by influencing gene expression (Oddy, 2006; Jump, 2004).
Human milk contains two fatty acids that have been identified as particularly important for early neurodevelopment: the omega-3 (subsequently abbreviated as n-3) fatty acid docosahexaenoic acid (DHA) and the omega-6 (n-6) fatty acid arachidonic acid (AA). DHA is the fatty acid most prevalent in the mammalian brain; DHA levels within the brain are determined by dietary levels (Innis, 2007). DHA and AA are present in both gray and white matter, including myelin (Nettleton, 1995). Across most of the lifespan the body can synthesize DHA and AA, as well as other long-chain fatty acids, from shorter-chain fatty acids. In early infancy, however, this synthesis process is not adequate for optimal brain development; research shows that the rate at which DHA is incorporated into brain tissue outstrips the rate at which it can be synthesized. In infants who lack a dietary source of DHA, other fatty acids are incorporated into the brain to compensate for the dearth of DHA (Farquharson, Jamieson, Logan, Cockburn, & Ainslie Patrick, 1992). Thus differences in early diet can have long-lasting effects. In her 2006 study of the long-term effects of breastfeeding among Australian children, Oddy (2006) states, “Dietary alterations of n-3 and n-6 [fatty acids] can trigger dramatic alterations in brain lipid composition associated with changes in physical properties of membranes, alterations in enzyme activities, receptors, carrier mediated transport and cellular interactions” (p. 181). Differences in the fatty acid composition of brain tissue could contribute to differences in cell-to-cell communication, alterations in the function of synaptic membranes, and subtle impairments in nerve conductance and neurotransmission (Oddy, 2006; Lauritzen, Hansen, Jørgensen, & Michaelsen, 2001).
In contrast to human milk, most varieties of infant formula do not contain DHA/AA. Supplementation of infant formula with DHA/AA began in 2001 in the US, but the increased cost of EFA-fortified formula means that many children continue to receive the unsupplemented version. Furthermore, the impact of formula supplementation on neurodevelopment is controversial. Studies of long-term neurodevelopmental effects have reported no clear benefit to supplemented formula, suggesting that modified fatty acid profiles are not the sole determinant of neurodevelopmental outcomes (de Jong, Kikkert, Fidler, & Hadders-Algra, 2010; Smithers, Collins, Simmonds, Gibson, McPhee, & Makrides, 2010; see also a 2003 meta-analysis by Koo). Indeed, it would be reductive to argue that fatty acids are exclusively responsible for the differences observed in populations of breastfed and formula-fed children. Readers are referred to Riordan (2005) and Lawrence (2005) for further information on the properties of human milk that may influence neurodevelopment, and are encouraged to keep in mind that these observed differences may well be a synergistic effect of multiple nutrients, or may be related to as-yetunidentified human milk constituents.
Though the mechanism is incompletely understood, research does show that diet affects the composition of neural tissue. Farquharson, Jamieson, Logan, Cockburn, & Ainslie Patrick (1992) obtained necropsy gray matter samples from infants who died of SIDS, comparing exclusively breastfed babies with exclusively formula-fed babies. All of the formula-fed babies had significantly less DHA in their cerebrocortical tissue than their breastfed counterparts; those fed formula were observed to have a corresponding increase in n-6 series fatty acids.
Because of the ethical concerns raised by manipulation of the nutrient content of an infant’s diet, animal studies can provide some additional information on this topic. Rodent studies are particularly useful for this purpose because of similarities between rodent pups' prenatal/postnatal brain growth patterns and those of human infants. Lim, Hoshiba, and Salem (2005) compared adult rats given varying types of milk during infancy. In findings that paralleled those of Farquharson, Jamieson, Logan, Cockburn, & Ainslie Patrick in their study of human infants (1992), they reported that early diets high in n-6 fatty acids were clearly associated with brain tissue high in n-6 fatty acids. In the rat pups, this difference in neural tissue composition was associated with significant performance deficits, a finding that raises questions about the potential long-term ramifications of early diets high in n-6 fatty acids in human populations.
Neurotransmission can be affected by fatty acid profiles; in addition, the fatty acid composition of a cell membrane can have significant effects on the expression of genes within that cell. Oddy reports: “n-6 and n-3 fatty acids directly govern the transcription rate of specific genes.… This means that the n-6 and n-3 in our cell membranes exert a significant influence on the way a given genetic profile is expressed” (p. 180). For researchers studying genetically mediated phenomena, including certain forms of speech-language impairment, this is an observation of critical import.
Research into the impact of human milk on speech-language development has examined children from infancy onward. A 1999 study by Vestergaard et al. points to a connection between breastfeeding and early pre-speech development. In this prospective cohort study, babies who were exclusively breastfed for a longer period produced variegated babbling at earlier ages. The effect persisted after control for multiple potential confounding variables, including social class, maternal education, prenatal smoking, birthweight, gestational age, and number of prior illnesses.
Taylor and Wadsworth (1984) studied the long-term effects of breastfeeding on a variety of domains. One of the questions in their survey asked parents whether their 5-year-olds had a “stammer, stutter, or other [speech] problem.” Based on this single question, they found no correlation between infant feeding choices and stuttering.
Research into children’s language skills has shown a consistent association between breastfeeding and improved language development (Dee, Li, Lee, and Grummer-Strawn, 2007; Oddy, 2006; Gibson-Davis & Brooks-Gunn, 2006). Tomblin, Smith, and Zhang (1997) found that children breastfed for <9 months faced a significant increase in their risk of specific language impairment (SLI). All of these authors reported significant effects after control for potential confounding variables.
Covariate control is a persistent problem with breastfeeding research in cultures where middle-class educated women are most likely to breastfeed their children: it is difficult to determine whether outcomes of interest are related to breastfeeding, to maternal education, to socioeconomic advantage, or to synergistic effects of multiple factors. This difficulty was circumvented in a series of studies by Kramer and colleagues. They selected more than 17,000 pregnant women who planned to breastfeed after their children were born, and randomized them to two groups of healthcare providers (HCPs): one with extensive training in supporting the breastfeeding dyad, one with only the usual training. The children under the care of knowledgeable HCPs breastfed significantly longer than the control children, and the investigators have followed these children longitudinally to assess group differences across a variety of domains. In a 2008 study, they reported a statistically significant verbal IQ difference of 0.5 SD between the two groups of children at age 6.5.
Some researchers have also investigated a link between diet in early infancy and subsequent diagnosis with an autistic spectrum disorder. Tanoue and colleagues (1989) compared age at weaning for children with autism and typically developing controls, and concluded that breastfeeding duration was significantly longer in the control group. In 2006 Schultz and colleagues corroborated these findings, reporting an odds ratio of 4.41 for children without a dietary source of long-chain fatty acids.
Taken together, these studies indicate that it is reasonable to assess for an association between breastfeeding in infancy and later communication skills. Research into the long-term effects of breastfeeding in other domains lends further support to this claim, since a number of genetically linked conditions occur less frequently in breastfed individuals (Léon-Cava, Lutter, Ross, & Martin, 2002).
1.2 Stuttering Etiology
To elucidate further the rationale underlying our hypothesis, we offer a brief review of stuttering etiology. Stuttering is known to have multiple etiologic strands: a genetic component underlies subtle neurological differences in people who stutter. Observers have long remarked that stuttering appears to run in families, and researchers have begun to elucidate the nature of stuttering's genetic component.
In very early stuttering, a male-to-female ratio of approximately 2:1 has been reported; for persistent stuttering the ratio shifts dramatically, to the range of 4:1–6:1 (Ambrose, Cox, & Yairi, 1997). Kidd (1980, 1984) hypothesized that this gender difference might point to a lower threshold for males, indicating that either more genetic loading or more environmental pressures or both were necessary for persistent stuttering to be expressed in females. This idea suggests that environmental variables, such as breastfeeding, might have different effects in boys than in girls.
Howie (1981) reported a 63% concordance rate for stuttering in monozygotic twins, and a 19% concordance rate in dizygotic twins. This relatively high discordance rate demonstrates the importance of the interaction between genetic and environmental variables in determining stuttering outcomes, since more than a third of the twins with identical genetic material had divergent stuttering outcomes. More recently, Dworzynski and colleagues (2007) reported stuttering concordance rates for children in the Twins Early Development Project and estimated heritability in 4-year-olds at .65, with most of the variability in stuttering outcomes arising from nonshared environmental variables.
The environmental factors that contribute to stuttering outcomes, however, are poorly understood. To our knowledge, diet has never been considered as a potentially relevant environmental influence. Most prior research on environmental variables has emphasized factors related to communication or emotional regulation (see, as examples, Kloth, Janssen, Kraaimaat, & Brutten (1998), and Johnson, Walden, Conture, & Karrass (2010); see also Cox, Seider, and Kidd, whose 1984 study found no prenatal or medical factors that predisposed children to stutter). Our focus in the present study is an environmental variable with potential neurobiological effects; it thus addresses a gap in the existing literature.
A genetic predisposition toward stuttering is an important element of developmental stuttering, but genotype alone offers no certainty that an individual will begin to stutter. For the phenotype to appear, there must be an underlying neurological difference, the specifics of which are still speculative. Alm, for instance, suggested that in fluent speech the basal ganglia provide internal timing cues, while stuttering is a product of inadequate cues (2004). Other research has suggested that developmental stuttering may be associated with atypical myelination during the first year of life (Cykowski, Fox, Ingham, Ingham, & Robin, 2010), reduced white matter integrity (Watkins, Smith, Davis, & Howell, 2008; Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008), or other factors contributing to anomalies in speech timing (Sommer, Koch, Paulus, Weiller, & Büchel, 2002).
Other researchers have proposed theories less tethered to specific neural structures. Bosshardt (2006) summarized two potential models. In one group of theories, it is hypothesized that an underlying instability exists in the speech motor control system. Smith and Kleinow (2000), for instance, found subtle differences in the kinematic parameters of the fluent speech of stuttering adults; Peters, Hulstijn, and van Lieshout (2000) described people who stutter as falling on “the weak end of the speech motor skill continuum” (p. 113). A second group of theories posits a difference that is more cognitive or linguistic than motoric. First articulated by Perkins, Kent, and Curlee in 1991, these theories propose that the linguistic and paralinguistic components of speech are handled by different neural systems, and that stuttering results when they are not synchronized. Bosshardt suggested that adults who stutter are slower to code phonological and semantic data, and concluded that speech planning and production are less modularized in individuals who stutter than they are in normally fluent individuals.
The purpose of this paper is not to argue for or against any of these proposed etiologies. Rather, we propose that they could all be associated with the subtle alterations in the structure and function of neural tissue observed in children without a dietary source of long-chain n-3 fatty acids like DHA in early infancy.
This study was designed to test the hypothesis that breastfeeding would provide dose-related protection against persistent stuttering, i.e., that shorter breastfeeding duration would be associated with higher rates of persistent stuttering. We hypothesized (1) that longer duration of breastfeeding would be associated with higher rates of recovery, and (2) that the observed effect would be more pronounced among the male participants, based on the findings of Broad (1972, 1975, 1983) and boys’ increased vulnerability to persistent stuttering.
2. METHOD
2.1 Participants
The University of Illinois Stuttering Research Project (SRP) has collected longitudinal data on stuttering preschoolers since 1989. Participants entered the program between the ages of 2 and 6 (mean age at entry = 39.84 months, SD = 8.61), typically within 12 months of the onset of stuttering. They were assessed at 6-month intervals for the first 2 years, and annually for two further years, with a final follow-up visit 5–8 years afterward. Participants provided basic demographic data and a medical history at their initial visit, and they were asked for their consent to be contacted for future studies. For further details about the children and their families, see Yairi and Ambrose, 2005.
Parents and researchers independently evaluated the fluency of participating children on a 0–7 scale. The assessments combined objective ratings, including the number of disfluencies per hundred syllables, with perceptual evaluations of fluency. Criteria for natural recovery included a mutual rating of stuttering severity as <1, fewer than 3 units of stuttering-like disfluency per 100 syllables uttered, and normally fluent speech over at least a 12-month period as judged by both parents and clinicians (Yairi & Ambrose, 1999). Children in the natural recovery group did not receive speech therapy. A child’s stuttering was labeled persistent if it continued for at least 4 years past onset, as judged by either parents or evaluators.
2.2 Procedures
We sent a 10-item questionnaire designed to obtain information on initiation, exclusivity, and duration of breastfeeding to the mothers of 145 current and past SRP participants (see Appendix). All mothers were offered the option of completing the questionnaire electronically, either as an email attachment or at the website http://www.surveymonkey.com, or receiving a paper copy via postal mail. Thirty-two of the questionnaires sent via postal mail were returned as undeliverable, along with six of those sent via email. For those participants whose stuttering persisted, we used phone directories to search for valid postal addresses. We were able to obtain current addresses for nine families. Follow-up efforts were concentrated on participants with persistent stuttering in hopes of creating more symmetrically-sized groups for analysis.
Although retrospective studies are subject to recall bias, previous research has shown that mothers' recollections of breastfeeding duration are reasonably accurate, even years after weaning (Promislow, Gladen, & Sandler, 2005). For this reason, although we collected information on exclusivity, our analyses focused on initiation (was the child ever breastfed?) and total duration (for how long was the child breastfed?). Readers will note that while the questionnaire emphasizes direct breastfeeding, two of the questions ask about breastmilk intake, allowing for responses from mothers who expressed milk for their babies to be given via bottle. The comments sections provided space for mothers to describe their particular situation, whether it involved direct breastfeeding, bottle-feeding expressed breastmilk, bottle-feeding formula, or a blend of the three. Since our interest centered on the constituents of human milk rather than the action of direct breastfeeding, mothers who bottle-fed their expressed milk were grouped together with those who breastfed directly.
Participants' histories were reviewed to rule out potential confounding factors. Length of gestation was documented as a possible confounding variable, because preterm babies have lower reserves of essential fatty acids and are at higher risk of premature weaning. All participants were born prior to 2001, when sales of EFA-supplemented formula began in the US, eliminating the need to consider formula type as a potential confounding variable.
3. RESULTS
To the 145 questionnaires we sent out, we received 54 responses with information about feeding in infancy. Seven of these questionnaires could not be used in the present study because the children’s stuttering had not yet been labeled persistent or recovered, leaving a total of 47 usable responses. Of these 47, there were 17 responses from mothers of children with persistent stuttering (13 boys, 4 girls), and 30 responses from mothers of children who recovered (22 boys, 8 girls). The 36.2% prevalence of persistent stuttering in the present sample is higher than the 24% rate in the Stuttering Research Program as a whole, probably because follow-up efforts were focused on children with persistent stuttering.
Within this sample, one important potential confounding factor was reverse causality. It might be the case that children who stutter are more vulnerable to subtle oral motor sequencing deficits than normally fluent children. Difficulties with coordinating the suck-swallow-breathe sequence can spell an early end to breastfeeding, since problems in this domain may be associated with painful breastfeeding and slow weight gain (Page-Goertz & Riordan, 2005). In other words, we hypothesized that early formula-feeding might contribute to persistent stuttering, but it was crucial to rule out the possibility that a tendency toward persistent stuttering might contribute to early formula-feeding. To this end, all mothers who initiated breastfeeding were asked about problems with latch-on and effective suck.
Only 2 of the 14 breastfed children with persistent stuttering were described as having any difficulties with latch-on or sucking (14.3%), as compared to 7 of the 27 breastfed children who recovered (25.9%). Both of these values fall within the typical range for proportions of babies with early breastfeeding difficulties (Dewey, Nommsen-Rivers, Heinig, & Cohen, 2003). These results do not support the idea that a tendency toward persistent stuttering might manifest itself as poor oral motor coordination in infancy, predisposing those children toward early weaning.
Prematurity was also considered as a potential confounding variable; two children in the sample were born before 37 weeks' gestation, one at 34 weeks and one at 36 weeks. Both were breastfed, and both recovered. Since the concern was that prematurity might predispose children to both persistent stuttering and to breastfeeding difficulties, these results did not require further investigation.
Finally, because of the frequent concerns about confounding with maternal education and SES in the literature on the effects of breastfeeding, we compared levels of maternal education between the recovered and persistent groups. Maternal education was recorded on a 10-point scale; 10 meant a mother had no formal education, 5 meant she had completed high school, and 1 meant she had completed a graduate degree. Results were available for 37 of the 47 cases, with 4 values missing in the persistent group and 6 missing in the recovered group. For the sample as a whole, values ranged from 1 to 5. The mean level of education was 2.57 (SD = 1.28), with 2 representing a completed bachelor’s degree and 3 indicating at least one year of college. A t test revealed no significant differences between mothers in the persistent (M = 3.00, SD = 1.29) and recovered (M = 2.33, SD = 1.24) groups, t(23.9) = 1.52, p = 0.14. Furthermore, a logistic regression model revealed no relationship between maternal education and stuttering persistence (β = −0.42, p = 0.14). We found a marginally significant relationship between maternal education and breastfeeding initiation (β = −0.71, p = 0.07), a finding in keeping with the trend observed in larger populations toward higher breastfeeding prevalence among more highly educated women (Coates and Riordan, 2005). Taken together, these results do not support the idea that any differences we might attribute to breastfeeding actually reflect differences in maternal education.
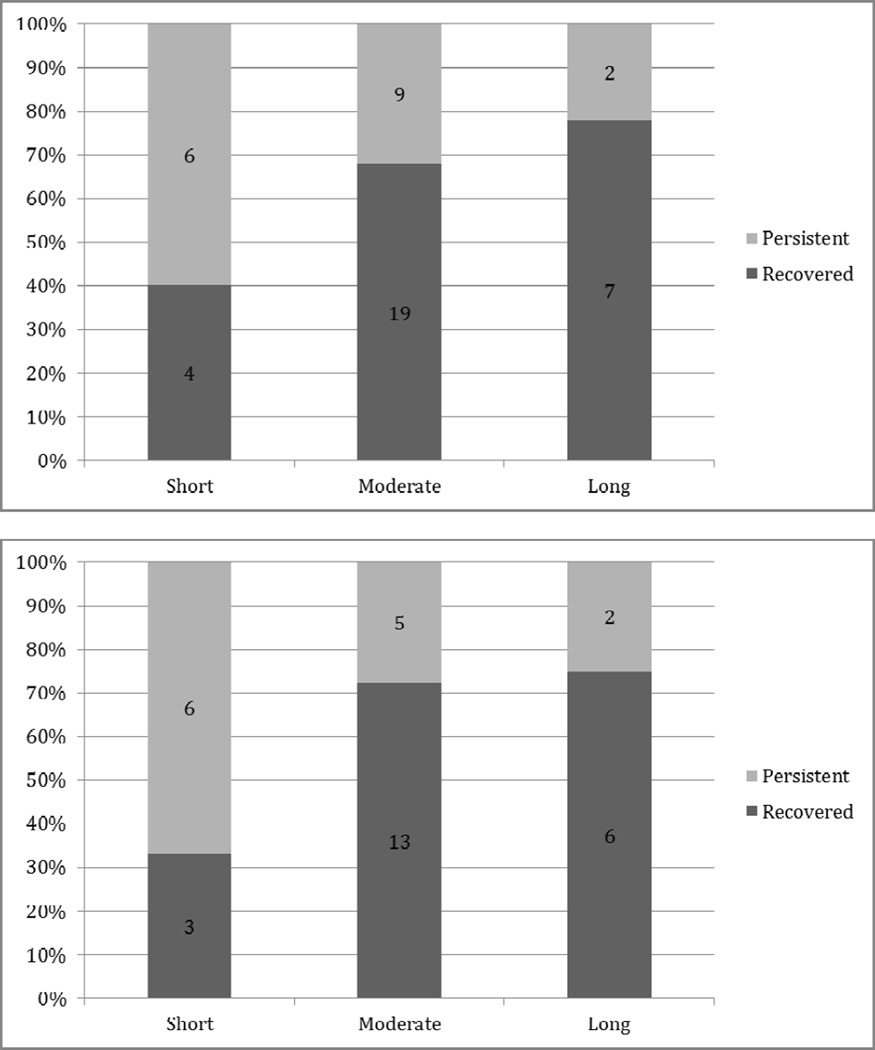
Of the 47 children in the current study, 41 received at least some human milk; the remaining 6 (3 with persistent stuttering, and 3 who recovered) were exclusively formula-fed. Breastfeeding duration was recorded in months; it ranged from 0 (for the 6 exclusively formula-fed children) to 30, with a mean of 8.4 (SD = 7.9) and a median of 6.0. To test our hypothesis that the rate of natural recovery would rise in association with total breastfeeding duration, we divided the sample into three groups, with duration classed as short (< 3 months), moderate (3 months ≤ x ≤ 12 months), or long (> 12 months). These classifications were based on existing findings in the literature (specifically, reports of a threshold effect, or a “dose” at which effects are evident, with 3 months of breastfeeding, cf. Dee, Li, Lee, & Grummer-Strawn, 2007) and clinical guidelines, notably the American Academy of Pediatrics recommendation that babies be breastfed for a minimum of one year (2005).
The proportions of persistent and recovered stuttering in each group are illustrated in the upper portion of Figure 1. A chi-squared test for linear trend was used to assess for a dose-response relationship within the whole group, with expected frequencies of 10 in each cell for the recovered group, and 5.67 in each cell for the persistent group. The results of this two-tailed test narrowly missed statistical significance, with a p value of exactly .05, χ2(1, n = 47) = 3.83, p = .05049. Although the results were only marginally significant, the odds ratio associated with this test was 0.19, suggesting that the children breastfed for >12 months had approximately one-fifth the odds of developing persistent stuttering that children breastfed for <3 months did. Because boys are considerably more vulnerable to persistent stuttering, chi-squared testing for linear trend was repeated for the boys alone to evaluate our third hypothesis. Here the expected frequencies for each cell were 7.33 for the recovered boys and 4 for the persistent boys. This test yielded a significant result (χ2[1, n = 35] = 4.18, p = .04) with an associated odds ratio of 0.17, indicating that boys breastfed for more than a year had approximately one-sixth the odds of developing persistent stuttering. The proportions of persistence and recovery for the boys in the sample are shown in the lower portion of Figure 1.
Figure 1.
Proportion of persistent vs. recovered stuttering as a function of breastfeeding duration for the full sample (above) and for the boys alone (below)
4. DISCUSSION
Our results provide qualified support for our hypothesis that longer breastfeeding duration would be associated with a decrease in persistent stuttering. Our tests of association yielded significant results for the boys in the sample and missed statistical significance by the smallest of margins for the full sample. In this sample, in other words, children breastfed for longer were less likely to continue to stutter, with a six-fold increase in the odds of persistent stuttering observed among the boys. In the section that follows we discuss possible mechanisms underlying our finding, review its ramifications, and discuss the strengths and limitations of the study.
We propose two mechanisms for the association between longer breastfeeding duration and decreased odds of persistent stuttering. First, the differences observed in the brains of formula-fed children may have functional ramifications (Farquharson, Jamieson, Logan, Cockburn, & Ainslie Patrick, 1992). In populations with the genetic vulnerability to stuttering, differences in the makeup of neural tissues including myelin, specifically an increase in n-6 fatty acids and a corresponding decrease in n-3 fatty acids, could contribute to differences in cellular communication and subtle impairments in neurotransmission (Oddy, 2006; Lauritzen, Hansen, Jørgensen, & Michaelsen, 2001). These changes could trigger a related breakdown in speech fluency.
The second hypothesized mechanism arises from the role of fatty acids in regulating gene expression. Fatty acids alter the availability of transcription factors, binding to the factors, in some cases, and thereby playing a role in gene transcription (Jump, 2004). In this way, fatty acid intake might also affect expression of the genes responsible for stuttering. Individuals with very similar genotypes could present with markedly different stuttering phenotypes as a result of differences in gene transcription and expression.
This association between breastfeeding duration and decreasing odds of persistent stuttering has ramifications for families concerned about stuttering and for stuttering researchers. Pending corroboration of these results, there may be ramifications for stuttering prevention in families with a genetic history of stuttering and for those who work with the families of stuttering preschoolers. Specifically, it could be valuable for both families and service providers to know that increased breastfeeding duration reduces the odds that a child’s stuttering will persist.
Second, this study may prove useful to researchers investigating the interplay of genetic and environmental variables related to stuttering. A recent doctoral dissertation has suggested that the FADS2 gene plays a role in stuttering (Kraft, 2010); this gene is also associated with fatty acid metabolism. As researchers continue to elucidate the role of early fatty acid intake in the subsequent structure and function of neural tissue, the causes of stuttering may be more clearly understood. These results might also cast some light on earlier findings, such as the 1999 Yairi and Ambrose paper that suggested persistence and recovery may run in families. Since child-rearing practices are often influenced by decisions made in one’s extended family, including ideas about how it is best to feed one’s children (Sussner, Lindsay, & Peterson, 2008) it is possible that this variability among families may be related to family child-rearing trends as well as family genotypes.
Two limitations of this study should be mentioned here, the first being its retrospective data collection. Mothers reported breastfeeding duration long after their children had weaned, and we did not corroborate the information they provided. This limitation is unlikely to affect the validity of our results for two reasons: first, research has established that mothers’ recall of breastfeeding duration is reasonably accurate even years after the fact (Kark, Troya, Friedlander, Slater, & Stein, 1984), and second, there is no reason to suppose that any recall bias would affect mothers in the two groups differently. Errors, in other words, are likely to be distributed similarly across both the recovered and the persistent groups.
The second limitation of our study is its necessarily correlational nature: while we have hypothesized a causal relationship, caution is warranted in interpreting our results. It is possible, for instance, that breastfeeding serves as a proxy variable within this sample for another variable that is actually responsible for the observed effects. Our results indicate, however, that the common confounding variable of maternal education is not a concern in this sample.
These limitations aside, our study offers some important strengths. First, since stuttering is an equal-opportunity disorder, which can affect individuals regardless of their SES or level of education, our study is less vulnerable to the confounding variables that plague research into the link between, for instance, breastfeeding and cognition. Moreover, our assessment of between-groups differences in maternal education levels indicates that this variable is not driving the results observed in this sample. In addition, the lengthy follow-up that characterizes the Illinois project strengthens judgments of persistence and recovery.
Our study corroborates and extends the existing body of work indicating that breastfeeding plays a part in neurodevelopment for young children. Children who have heavy genetic loading predisposing them to stutter, or those whose environments contribute in as-yet-unspecified ways to their stuttering, may stutter persistently regardless of their early feeding history. For a subset of children who begin stuttering, however, breastfeeding in infancy appears to confer a measure of protection against what might otherwise be a frustrating lifelong disability. Our findings accord with other research into the impact of breastfeeding on neurodevelopment, where it is commonplace to find that breastfeeding diminishes the odds that a child will develop a condition and equally commonplace to find that it is not a cure-all: some children remain vulnerable to certain disorders regardless of their infant feeding histories.
The finding that increased duration of breastfeeding is associated with decreased rates of stuttering persistence raises other important questions for stuttering researchers. Might a similar association exist between breastfeeding duration and stuttering incidence? Similarly, there might be a relationship between feeding history and stuttering severity in cases of persistent stuttering. For children who stutter it is possible that fatty acid supplementation might serve as a useful adjunct to traditional intervention, as researchers have proposed for disorders including dyspraxia, dyslexia, ADHD, and autism (Richardson, 2004; Richardson & Montgomery, 2005). We encourage other researchers to consider these questions in hopes of shedding additional light on the interplay between genetic and environmental factors in stuttering, and with the goal of providing families and SLPs with evidence-based recommendations for the prevention of persistent stuttering.
Highlights.
Breastfeeding may play a role in neurodevelopment and gene expression.
We investigated the feeding history of 47 children with developmental stuttering.
For boys, longer breastfeeding duration was tied to improved odds of recovery.
This effect was not related to early problems with oral motor sequencing.
These findings may be significant for stuttering prevention and research.
Learning outcomes.
The reader will be able to give at least one reason why human milk may make a difference in neurodevelopment generally and with regard to stuttering outcomes specifically. Additionally, the reader will be able to describe the relationship between breastfeeding duration and stuttering recovery observed in this sample.
Appendix: Questionnaire
-
How close to your due date was your child born? If you don't remember the precise difference, check one of the boxes below.
__≥3 wks early __6–20 days early __within 5 days of due date __6–14 days late __≥2 wks late
How sure are you about your response? __very sure __moderately sure __not sure
Was your child ever breastfed? If not, proceed to item 9.
-
Was there a time when your child received only breastmilk, with no formula or solid food?
If so, how long did it last? Check more than one if exclusive breastfeeding was briefly interrupted and then resumed.
__1–7 days __8–14 days __15–30 days __31–60 days __61–90 days __91–120 days __>4 months
How sure are you about your response? __very sure __moderately sure __not sure
-
If you breastfed your child, did he or she seem to have any difficulty with latching on or sucking effectively?
If so, please describe the nature of the problem and how long it lasted.
-
How old was your child when you first introduced solid food?
How sure are you about your response? __very sure __moderately sure __not sure
-
How old was your child when he or she stopped breastfeeding?
How sure are you about your response? __very sure __moderately sure __not sure
Why did you stop breastfeeding?
-
If you partially breastfed for a time, please estimate your child's breastmilk intake as a percentage of his or her overall intake during that time.
__0–25% __26–50% __51–75% __76–99%
How sure are you about your response? __very sure __moderately sure __not sure
Please add any additional comments below.
CEU QUESTIONS
- Which of the following explains why human milk may play a role in neurodevelopment?
- Human milk contains long-chain omega-3 fatty acids which are constituents of the growing brain.
- Human milk constituents may affect gene transcription and expression, modifying the phenotype associated with a given genotype.
- Infant formula has a different nutrient profile from human milk.
- All of the above.
- Which of the following nutrients is found in human milk although it is frequently absent from infant formula?
- a. iron b. magnesium c. docosohexaenoic acid d. taurine
- Which of the following statements is true of the findings reported in this article?
- The children in this sample were significantly less likely to stutter persistently if they were breastfed for 3 months.
- The children in this sample were significantly more likely to stutter persistently if they were breastfed for 3 months.
- A significant inverse relationship was observed between breastfeeding duration and stuttering persistence for the boys in this sample: boys breastfed for longer were less likely to stutter.
- A significant positive relationship was observed between breastfeeding duration and stuttering persistence for the boys in this sample: boys breastfed for longer were more likely to stutter.
- Which of the following variables was ruled out as a potential confounder in this sample?
- Early breastfeeding difficulties
- Maternal employment
- Maternal-child interaction
- All of the above
- Which of the following statements is most accurate regarding the results of this study?
- All of the children breastfed for at least a year recovered from stuttering.
- The boys breastfed for at least a year were significantly more likely to recover naturally.
- The boys breastfed for at least a year were significantly less likely to recover naturally.
- Longer breastfeeding duration may be associated with adverse speech-language outcomes.
Answer key: 1 d. 2 c. 3 c. 4 a. 5 b.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alm P. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders. 2004;37:325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ambrose NG, Cox N, Yairi E. The genetic basis of persistence and recovery in stuttering. Journal of Speech, Language, and Hearing Research. 1997;40:567–580. doi: 10.1044/jslhr.4003.567. [DOI] [PubMed] [Google Scholar]
- Bosshardt H-G. Cognitive processing load as a determinant of stuttering: summary of a research programme. Clinical Linguistics and Phonetics. 2006;20(5):371–385. doi: 10.1080/02699200500074321. [DOI] [PubMed] [Google Scholar]
- Broad FE. The effects of infant feeding on speech quality. New Zealand Medical Journal. 1972;76:28–31. [PubMed] [Google Scholar]
- Broad FE. Further studies on the effects of infant feeding on speech quality. New Zealand Medical Journal. 1975;82:373–376. [PubMed] [Google Scholar]
- Broad FE, Duganzich DM. The effects of infant feeding, birth order, occupation, and socio-economic status on speech in six-year-old children. New Zealand Medical Journal. 1983;96:483–486. [PubMed] [Google Scholar]
- Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates MM, Riordan J. Tides in breastfeeding practice. In: Riordan J, editor. Breastfeeding and Human Lactation. 3rd. Sudbury, MA: Jones and Bartlett; 2005. pp. 3–29. [Google Scholar]
- Cox NJ, Seider RA, Kidd KK. Some environmental factors and hypotheses for stuttering in families with several stutterers. Journal of Speech, Language, and Hearing Research. 1984;27(4):543–548. doi: 10.1044/jshr.2704.543. [DOI] [PubMed] [Google Scholar]
- Cykowski MD, Fox PT, Ingham RJ, Ingham JC, Robin DA. A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. Neuroimage. 2010;52(4):1495–1504. doi: 10.1016/j.neuroimage.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Motor speech disorders. Philadelphia: W.B.: Saunders; 1975. [Google Scholar]
- de Jong C, Kikkert HK, Fidler V, Hadders-Algra M. The Groningen LCPUFA study: No effect of postnatal long-chain polyunsaturated fatty acids in healthy term infants on neurological condition at 9 years. British Journal of Nutrition. 2010;104(4):566–572. doi: 10.1017/S0007114510000863. [DOI] [PubMed] [Google Scholar]
- Dee DL, Ruowei L, Lee L, Grummer-Strawn LM. Associations between breastfeeding practices and young children's language and motor development. Pediatrics. 2007;119(Suppl. 1):S92–S98. doi: 10.1542/peds.2006-2089N. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Nommsen-Rivers LA, Heinig J, Cohen RJ. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics. 2003;112(3):607–619. doi: 10.1542/peds.112.3.607. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Remington A, Rijsdijk F, Howell P, Plomin R. Genetic etiology in cases of recovered and persistent stuttering in an unselected, longitudinal sample of young twins. American Journal of Speech-Language Pathology. 2007;16(2):169–178. doi: 10.1044/1058-0360(2007/021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson Jamieson, Logan Cockburn, Patrick Ainslie, et al. Infant cerebral cortex phospholipid fatty-acid composition and diet. Lancet. 1992;340:810–813. doi: 10.1016/0140-6736(92)92684-8. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Beautrais AL, Silva PA. Breast-feeding and cognitive development in the first seven years of life. Social Science & Medicine. 1982;16:1705–1708. doi: 10.1016/0277-9536(82)90096-x. [DOI] [PubMed] [Google Scholar]
- Gibson-Davis CM, Brooks-Gunn J. Breastfeeding and verbal ability of 3-year-olds in a multicity sample. Pediatrics. 2006;118:1444–1451. doi: 10.1542/peds.2006-0072. [DOI] [PubMed] [Google Scholar]
- Howie PM. Concordance for stuttering in monozygotic and dizygotic twin pairs. Journal of Speech and Hearing Research. 1981;24:317–321. doi: 10.1044/jshr.2403.317. [DOI] [PubMed] [Google Scholar]
- Innis SM. Dietary (n-3) fatty acids and brain development. Journal of Nutrition. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- Johnson KN, Walden TA, Conture EG, Karrass J. Spontaneous regulation of emotions in preschool children who stutter: Preliminary findings. Journal of Speech, Language, and Hearing Research. 2010;53:1478–1495. doi: 10.1044/1092-4388(2010/08-0150). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark JD, Troya G, Friedlander Y, Slater PE, Stein Y. Validity of maternal reporting of breast feeding history and the association with blood lipids in 17 year olds in Jerusalem. Journal of Epidemiology and Community Health. 1984;38:218–225. doi: 10.1136/jech.38.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd K. Genetic models of stuttering. Journal of Fluency Disorders. 1980;5:187–201. [Google Scholar]
- Kidd K. Stuttering as a genetic disorder. In: Curlee R, Perkins W, editors. Nature and treatment of stuttering. San Diego: College-Hill; 1984. pp. 149–169. [Google Scholar]
- Kloth S, Janssen P, Kraaimaat F, Brutten GJ. Child and mother variables in the development of stuttering among high-risk children: A longitudinal study. Journal of Fluency Disorders. 1998;23(4):217–230. [Google Scholar]
- Koo WWK. Efficacy and safety of docosahexaenoic acid and arachidonic acid addition to infant formulas: Can one buy better vision and intelligence? Journal of the American College of Nutrition. 2003;22(2):101–107. doi: 10.1080/07315724.2003.10719282. [DOI] [PubMed] [Google Scholar]
- Kraft SJ. Genome-wide association study of persistent developmental stuttering. (Doctoral dissertation) 2010. Retrieved from https://www.ideals.illinois.edu/handle/2142/17054. [Google Scholar]
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Archives of General Psychiatry. 2008;65(5):578–585. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Fombonne E, Igumnov S, Vanilovich I, Matush L, Mironova E, …Platt RW, et al. Effects of prolonged and exclusive breastfeeding on child behavior and maternal adjustment: Evidence from a large, randomized trial. Pediatrics. 2008;121(3):e435–e440. doi: 10.1542/peds.2007-1248. [DOI] [PubMed] [Google Scholar]
- Lauritzen L, Hansen HS, Jørgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Progress in Lipid Research. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- Lawrence RA, Lawrence RM. Breastfeeding: a guide for the medical profession. 6th ed. Philadelphia: Elsevier Mosby; 2005. [Google Scholar]
- Léon-Cava N, Ross J, Lutter C, Martin L. Quantifying the benefits of breastfeeding: a summary of the evidence. Washington, DC: Pan American Health Organization; 2002. [Google Scholar]
- Lim SY, Hoshiba J, Salem J. An extraordinary degree of structural specificity is required in neural phospholipids for optimal brain function: n-6 docosapentaenoic acid substitution for docosahexaenoic acid leads to a loss in spatial task performance. Journal of Neurochemistry. 2005;95:848–857. doi: 10.1111/j.1471-4159.2005.03427.x. [DOI] [PubMed] [Google Scholar]
- Nettleton JA. Omega-three Fatty Acids and Health. New York: Springer; 1995. [Google Scholar]
- Oddy WH. Fatty acid nutrition, immune and mental health development from infancy through childhood. In: Huang JD, editor. Frontiers in Nutrition Research. New York: Nova Science; 2006. pp. 177–211. [Google Scholar]
- Page-Goertz S, Riordan J. The ill child: Breastfeeding implications. In: Riordan J, editor. Breastfeeding and Human Lactation. 3rd ed. Sudbury, MA: Jones and Bartlett; 2005. pp. 541–589. [Google Scholar]
- Perkins WH, Kent RD, Curlee RF. A theory of neuropsycholinguistic function in stuttering. Journal of Speech and Hearing Research. 1991;34:734–752. doi: 10.1044/jshr.3404.734. [DOI] [PubMed] [Google Scholar]
- Peters HFM, Hulstijn W, van Lieshout PHHM. Recent developments in speech motor research into stuttering. Folia Phoniatrica et Logopaedica. 2000;52:103–119. doi: 10.1159/000021518. [DOI] [PubMed] [Google Scholar]
- Promislow JHE, Gladen BC, Sandler DP. Maternal recall of breastfeeding duration by elderly women. American Journal of Epidemiology. 2005;161:289–296. doi: 10.1093/aje/kwi044. [DOI] [PubMed] [Google Scholar]
- Richardson AJ. Clinical trials of fatty acid treatment in ADHD, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;70(4):383–390. doi: 10.1016/j.plefa.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Montgomery P. The Oxford-Durham study: A randomized controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115(5):1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- Riordan J. The biological specificity of breastmilk. In: Riordan J, editor. Breastfeeding and Human Lactation. 3rd ed. Sudbury, MA: Jones and Bartlett; 2005. pp. 97–135. [Google Scholar]
- Ryan AS, Zhou W, Arensberg MB. The effect of employment status on breastfeeding in the United States. Women’s Health Issues. 2006;16:243–251. doi: 10.1016/j.whi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Jäncke L, Witte OW, Freund H-J. Functional organization of the auditory cortex is different in stutterers and fluent speakers. NeuroReport. 1998;9(10):2225–2229. doi: 10.1097/00001756-199807130-00014. [DOI] [PubMed] [Google Scholar]
- Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M, et al. Breastfeeding, infant formula supplementation, and Autistic Disorder: The results of a parent survey. International Breastfeeding Journal. 2006;1:16. doi: 10.1186/1746-4358-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Kleinow J. Kinematic correlates of speaking rate changes in stuttering and normally fluent adults. Journal of Speech, Language, and Hearing Research. 2000;43:521–536. doi: 10.1044/jslhr.4302.521. [DOI] [PubMed] [Google Scholar]
- Smith VL, Gerber SE. Infant feeding and phonologic development. International Journal of Pediatric Otorhinolaryngology. 1993;28:41–49. doi: 10.1016/0165-5876(93)90145-s. [DOI] [PubMed] [Google Scholar]
- Smithers LG, Collins CT, Simmonds LA, Gibson RA, McPhee A, Makrides M. Feeding preterm infants milk with a higher dose of docosahexaenoic acid than that used in current practice does not influence language or behavior in early childhood: a follow-up study of a randomized controlled trial. American Journal of Clinical Nutrition. 2010;91:628–634. doi: 10.3945/ajcn.2009.28603. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Sussner KM, Lindsay AC, Peterson KE. The influence of acculturation on breastfeeding initiation and duration in low-income women in the US. Journal of Biosocial Science. 2008;40:673–696. doi: 10.1017/S0021932007002593. [DOI] [PubMed] [Google Scholar]
- Tanoue Y, Oda S. Weaning time of children with infantile autism. Journal of Autism and Developmental Disorders. 1989;19(3):425–434. doi: 10.1007/BF02212940. [DOI] [PubMed] [Google Scholar]
- Taylor B, Wadsworth J. Breast feeding and child development at five years. Developmental Medicine and Child Neurology. 1984;26(1):73–80. doi: 10.1111/j.1469-8749.1984.tb04409.x. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Smith E, Zhang X. Epidemiology of specific language impairment: prenatal and perinatal risk factors. Journal of Communication Disorders. 1997;30(4):325–344. doi: 10.1016/s0021-9924(97)00015-4. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Obel C, Henriksen TB, Sørensen HT, Skajaa E, Østergaard J. Duration of breastfeeding and developmental milestones during the latter half of infancy. Acta Paediatrica. 1999;88(12):1327–1332. doi: 10.1080/080352599750030022. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering I: Persistency and recovery rates. Journal of Speech, Language, and Hearing Research. 1999;42(5):1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering. Austin, TX: Pro-Ed; 2005. [Google Scholar]