Abstract
Background
Medicare Part D and the Department of Veterans Affairs (VA) use different approaches to manage prescription drug benefits, with implications for spending. Medicare relies on private plans with distinct formularies, whereas VA administers its own benefit using a national formulary.
Objective
To compare overall and regional rates of brand-name drug use among older adults with diabetes in Medicare and VA.
Design
Retrospective cohort
Setting
Medicare and VA
Patients
National sample in 2008 of 1,061,095 Part D beneficiaries and 510,485 Veterans age 65+ with diabetes.
Measurements
Percent of patients on oral hypoglycemics, statins, and angiotensin-converting-enzyme inhibitors/angiotensin-receptor-blockers who filled brand-name drugs and percent of patients on long-acting insulin who filled analogues. We compared sociodemographic and health-status adjusted hospital referral region (HRR) brand-name use to examine local practice patterns, and calculated changes in spending if each system’s brand-name use mirrored the other.
Results
Brand-name use in Medicare was 2–3 times that of VA: 35.3% vs. 12.7% for oral hypoglycemics, 50.7% vs. 18.2% for statins, 42.5% vs. 20.8% for angiotensin-converting-enzyme inhibitors/angiotensin-receptor-blockers, and 75.1% vs. 27.0% for insulin analogues. Adjusted HRR brand-name statin use ranged (5th to 95th percentile) from 41.0%–58.3% in Medicare and 6.2%–38.2% in VA. For each drug group, the HRR at the 95th percentile in VA had lower brand-name use than the 5th percentile HRR in Medicare. Medicare spending in this population would have been $1.4 billion less if brand-name use matched the VA for these medications.
Limitation
This analysis cannot fully describe the factors underlying differences in brand-name use.
Conclusions
Medicare beneficiaries with diabetes use 2–3 times more brand-name drugs than a comparable group within VA, at substantial excess cost.
Primary Funding Sources
VA; NIH; RWJF
Introduction
Medicare’s Part D drug benefit provides drug coverage to nearly 30 million beneficiaries, at an annual cost of almost $60 billion.(1) Although Part D has lowered out-of-pocket costs,(2) and improved treatment adherence(3–7) and health outcomes,(8, 9) there is evidence of inefficiency. For example, per capita prescription drug spending in Part D varies more than twofold across hospital referral regions, with 75% of the difference due to variation in use of more expensive drugs.(8) In principle, greater reliance on generic drugs in Medicare could save taxpayers substantial sums without compromising care. However, the mechanisms for achieving these savings, and their potential magnitude, are unknown. Looking to other systems that have achieved greater generic use may provide insight.
Medicare contracts with over 1,000 private plans to administer drug benefits, each using a distinct formulary and cost-sharing arrangement.(9) Other public payers, such as the Department of Veterans Affairs (VA), have taken a different approach. All veterans face the same low cost-sharing, and benefits are managed by a central pharmacy benefits manager (PBM) with a single formulary. This national formulary has substantially lowered pharmacy spending for the VA,(10) although studies suggest that facility-level variation persists in use of certain brand-name drugs.(11, 12)
Comparing medication use and regional variation across these two national payers could shed light on ways to improve efficiency in Medicare Part D, at a time when the federal government is facing substantial budget pressures and seeking ways to reduce costs without undermining quality.(13–15) Prior studies have focused on comparing medication prices between VA and Medicare,(16–18) but not medication choice, which can play just as large a role in determining spending. We constructed two national cohorts of older adults receiving drug benefits in either Medicare Part D or the VA with diabetes, a common chronic condition with high medication use and a wide range of available therapies.(19) We compared use of brand- name medications among patients overall and by geographic region, and estimated how spending would change if use of brand-name drugs in one system mirrored the other.
Methods
Data Sources and Sample
The Medicare cohort was defined using Medicare Denominator, Parts A, B, and Prescription Drug Event (PDE) files for a 40% random sample. We included beneficiaries who (1) were alive and continuously enrolled in fee-for-service Medicare and a stand-alone prescription drug plan from January 1 to December 31, 2008, (2) were ≥age 65, and (3) had ≥2 inpatient or outpatient diagnoses for type 2 diabetes (ICD-9 250.x0, 250.x2) or filled an oral diabetes medication in 2008.(20) We excluded individuals in Medicare Advantage plans because our data did not include all of their claims. We created an identically-defined national cohort of Veterans using 2008 national Medical SAS Datasets, VA data on outpatient prescriptions, and enrollment data. From both cohorts, we excluded individuals whose home address could not be linked by ZIP code to a Dartmouth Atlas of Healthcare Hospital Referral Region(HRR)(21) and individuals with evidence of lengthy institutionalization (either ≥90-day stay in a VA nursing home or receipt of ≥25% of Part D prescriptions from a long-term care pharmacy).
Study Outcomes
We focused on four medication groups commonly used by patients with diabetes: oral hypoglycemics, long-acting insulins, HMG-CoA reductase inhibitors (statins), and angiotensin-converting-enzyme inhibitors (ACE)/angiotensin receptor blockers (ARB). Oral prescriptions were categorized as brand-name or generic using LexiComp Multum.(22) Insulin used as basal coverage was deemed ‘long-acting’ and categorized as “analogue” (e.g. glargine and detemir) or “non-analogue” (e.g., NPH insulin) to parallel cost differences for brand-name and generic oral medications (Online Appendix Table 1).(23) In VA, during our study period, all brand-name drugs among these oral medications were non-formulary, available only with prior authorization;(12) long-acting insulin analogues, however, were on the VA formulary with few restrictions.
For both cohorts, we measured the proportion of patients with ≥1 fill for a brand-name medication (or analogue insulin) for each medication group. We also calculated the percent of standardized 30-day prescriptions dispensed as brand for oral products and the percent of units dispensed as analogues for insulin.
Patient Covariates
We used residential ZIP codes to assign patients to one of 306 HRRs, as others have done in prior analyses of geographic variation.(8, 24, 25) To adjust estimates of brand-name use for patient differences across HRRs in each system, we identically constructed variables for age, gender, race (black, white, Hispanic, other), a count of chronic conditions (excluding diabetes),(26) count of diabetes complications (diabetic retinopathy, nephropathy, neuropathy, and diabetes-associated peripheral vascular disorder),(27) count of unique oral diabetes medications, and presence of serious mental illness (schizophrenia/schizoaffective and bipolar disorders, delusion and paranoid disorders, other psychoses). We assigned household incomes based on ZIP code-level median incomes from a 2006 extrapolation of 2000 Census data.(21, 28) To account for within-system differences in cost-sharing, we created an indicator in Medicare for Part D low-income subsidy status and in VA for individuals with no prescription copay.
Analysis
Analyses were performed identically for Medicare and VA cohorts. We calculated the proportion of patients using any brand-name drug of interest (or analogue insulin) nationally. We obtained crude HRR-level rates of brand and analogue use, and then estimated adjusted rates, or probabilities, for each HRR using multivariable logistic regression models specifying weights for the coefficients across the classification effects proportional to those in the Medicare or VA population (Proc Genmod, lsmeans/om). The model was performed at the patient level, including the covariates described above and indicators for each HRR. We compared adjusted HRR-level brand-name use between Medicare and VA using distributional dot plots. Because unadjusted and adjusted rates were nearly identical within each cohort (correlation r>0.93), we only present adjusted rates.
We quantified the effect of differences in brand-name use on drug spending by comparing actual spending to estimated spending if Medicare and VA were to adopt the other system’s rate of brand/analogue use. To calculate actual spending, we first calculated the mean amount paid (‘cost’) in 2008 for 30-day supplies of medications for brand and generic drugs separately. Medicare cost data include total reimbursements to the pharmacy, (i.e., plan payment, consumer copayment and dispensing fee) whereas the VA pharmacy data include only ingredient costs; we added patient copayments and an average dispensing fee obtained from the VA PBM to the cost of each VA prescription. These additions to VA costs serve to make Medicare and VA costs more comparable for descriptive purposes, but ultimately we did not focus on these price difference in our analysis. Our focus was on the within system change in spending if utilization of brand-name drugs changed while prices stayed the same. To remove the effect of price outliers, we set any cost per prescription less than the 1st percentile equal to the 1st percentile, and any cost greater than the 99th percentile equal to the 99th percentile.
We calculated total spending for each oral medication group as = [(total prescriptions*percent brand-name*mean cost per brand-name prescription) + (total prescriptions*percent generic*mean cost per generic prescription)]. For insulins, we used a similar approach but calculated spending based on units dispensed and the mean cost for analogues and non-analogues. To estimate the effect of only changing rates of brand-name use in Medicare and VA, we held constant each system’s volume and cost per prescription (or unit) while adopting the other system’s rate of brand/analogue use. Total expenditures in Medicare were projected onto the entire stand-alone Part D population by multiplying spending for the 40% sample by 2.5.
We performed two sensitivity analyses. First, we repeated analyses limiting both samples to men. Second, to limit the possibility of overlap in prescription use in VA and Medicare for those with dual coverage, we excluded Veterans also enrolled in Medicare Part D (29%) and repeated our analysis; we also completed an analysis excluding Veterans enrolled in Part D who also had a Medicare physician visit in 2008 (11.2%), assuming these individuals would be more likely to fill a prescription through Part D. Finally, as an additional analysis, we examined separately the subgroup of prescriptions that are multisource drugs, which are available in both brand-name and generic forms (in contrast to single-source drugs, which have no direct generic substitute). All analyses were carried out using SAS version 9.2 (SAS Institute Inc, Cary, NC) and STATA 11 (College Station, TX).
Role of the Funding Source
Funding for this study was provided by The Department of Veterans Affairs, NIH, and Robert Wood Johnson Foundation, who had no role in the study design, conduct and analysis, nor in the decision to submit the manuscript.
Results
Our sample included 1,061,095 Medicare Part D beneficiaries and 510,485 Veterans with diabetes. Mean patient age was 74.6 in Medicare and 75.0 in VA, and VA patients were predominately (98.6%) male (Table 1). Compared to VA, the Medicare cohort had a slightly higher proportion with no comorbid conditions (52.4% vs. 48.9%) and no diabetes complications (82.1% vs. 75.8%) but also a higher proportion with 3 or more comorbidities (10.6% vs. 6.7%). While the proportion of each cohort using oral hypoglycemics and long-acting insulin was nearly identical, Medicare patients were less likely to use statins (63.0% vs. 75.5%) or ACE/ARBs (69.1% vs. 73.1%), compared to VA patients (Table 1).
Table 1.
Characteristics of diabetes patients age 65+ in Medicare Part D and the VA, overall and within medication group, 2008.*
| Overall | Oral Hypoglycemics | Long-Acting Insulin | Statin | ACE/ARB | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cohort Characteristics | Medicare | VA | Medicare | VA | Medicare | VA | Medicare | VA | Medicare | VA |
| N (%) | 1,061,095 (100.0) | 510,485 (100) | 780,738 (73.6) | 377,435 (73.9) | 231,869 (21.9) | 113,736 (22.3) | 668,989 (63.0) | 385,196 (75.5) | 733,176 (69.1) | 373,321 (73.1) |
| Mean age (SD) | 74.6 (6.9) | 75.0 (6.4) | 74.4 (6.8) | 74.8 (6.4) | 74.0 (6.7) | 74.0 (6.2) | 74.2 (6.6) | 74.8 (6.3) | 74.4 (6.8) | 74.7 (6.3) |
| Male, % | 38.1 | 98.6 | 38.7 | 98.7 | 36.0 | 98.7 | 38.6 | 98.7 | 37.1 | 98.7 |
| Race/ethnicity, % | ||||||||||
| White | 78.2 | 86.5 | 78.1 | 87.3 | 74.6 | 83.1 | 78.3 | 86.9 | 77.0 | 86.0 |
| Black | 12.1 | 10.3 | 11.5 | 9.6 | 16.1 | 13.5 | 11.5 | 10.0 | 12.9 | 10.8 |
| Hispanic | 3.8 | 0.9 | 4.1 | 0.9 | 4.7 | 1.1 | 3.9 | 0.9 | 4.1 | 1.0 |
| Other | 5.9 | 2.3 | 6.3 | 2.2 | 4.5 | 2.3 | 6.3 | 2.2 | 6.1 | 2.3 |
| Charlson comorbidity count a | ||||||||||
| 0 | 52.4 | 48.9 | 56.0 | 51.3 | 37.9 | 38.4 | 53.0 | 47.8 | 52.8 | 48.3 |
| 1–2 | 37.0 | 44.4 | 35.3 | 43.1 | 43.3 | 49.8 | 36.3 | 45.0 | 36.6 | 44.6 |
| ≥3 | 10.6 | 6.7 | 8.7 | 5.6 | 18.8 | 11.8 | 10.7 | 7.2 | 10.7 | 7.1 |
| Serious mental illness, % b | 2.1 | 2.1 | 1.9 | 2.0 | 3.1 | 2.4 | 1.8 | 2.1 | 1.9 | 2.0 |
| Number of diabetes complications, % | ||||||||||
| 0 | 82.1 | 75.8 | 83.1 | 77.8 | 66.2 | 55.5 | 81.2 | 75.3 | 81.1 | 74.5 |
| 1–2 | 17.3 | 23.4 | 16.5 | 21.7 | 31.8 | 42.0 | 18.1 | 23.9 | 18.3 | 24.7 |
| 3+ | 0.6 | 0.7 | 0.5 | 0.5 | 2.0 | 2.4 | 0.7 | 0.8 | 0.7 | 0.8 |
| Number of oral diabetes medications, % | ||||||||||
| 0 | 26.4 | 26.1 | 0.0 | 0.0 | 41.9 | 40.7 | 22.8 | 11.0 | 23.1 | 11.1 |
| 1 | 43.9 | 45.4 | 59.7 | 61.5 | 34.4 | 37.3 | 44.1 | 53.3 | 44.2 | 52.4 |
| 2 | 22.9 | 24.5 | 31.2 | 33.1 | 18.0 | 19.0 | 25.1 | 30.4 | 25.0 | 31.1 |
| 3 | 6.0 | 3.8 | 8.2 | 5.2 | 4.9 | 2.8 | 7.1 | 5.1 | 6.8 | 5.1 |
| 4+ | 0.7 | 0.2 | 1.0 | 0.3 | 0.8 | 0.1 | 0.9 | 0.3 | 0.9 | 0.3 |
| Estimated household income, $c | 50,863 | 50,007 | 50,821 | 49,826 | 49,062 | 48,762 | 51,425 | 50,122 | 50,708 | 49,790 |
| No or limited copay, %d | 42.5 | 31.5 | 42.5 | 30.8 | 53.1 | 35.2 | 42.7 | 30.5 | 43.7 | 31.9 |
Notes
Medicare refers to patients enrolled in fee-for-service Parts A, B and stand-alone Part D. Statin denotes HMG-CoA reductase inhibitors, ACE denotes angiotensin-converting enzyme inhibitors, and ARB denotes angiotensin receptor blocker.
The count of Charlson comorbidities excludes diabetes.
Serious mental illness is defined based on presence of an ICD-9 code for schizophrenia/schizoaffective disorder, bipolar, delusion and paranoid disorders, and other nonorganic psychoses.
Household income estimates are based on ZIP code median incomes from a 2006 extrapolation of 2000 Census data.
In Part D we measure the percent with a full year of low-income subsidy, and in VA we measure the percent without any copay for the entire year.
Variation in Brand-Name Use
In unadjusted analyses, Medicare beneficiaries taking oral hypoglycemics were nearly three times more likely to use a brand-name drug than VA patients (35.3% vs. 12.7%). Similarly, among those using long-acting insulins, 75.1% in Medicare used analogues compared to only 27.0% in VA. Between-system differences in use of brand-name statins (50.7% vs. 18.2%) and ACE/ARBs (42.5% vs. 20.8%) were of similar magnitude. These differences in brand-name use were almost identical in sensitivity analyses focusing on men only and in analyses that excluded from the VA cohort Veterans dually enrolled in Part D (Online Appendix Tables 2–4).
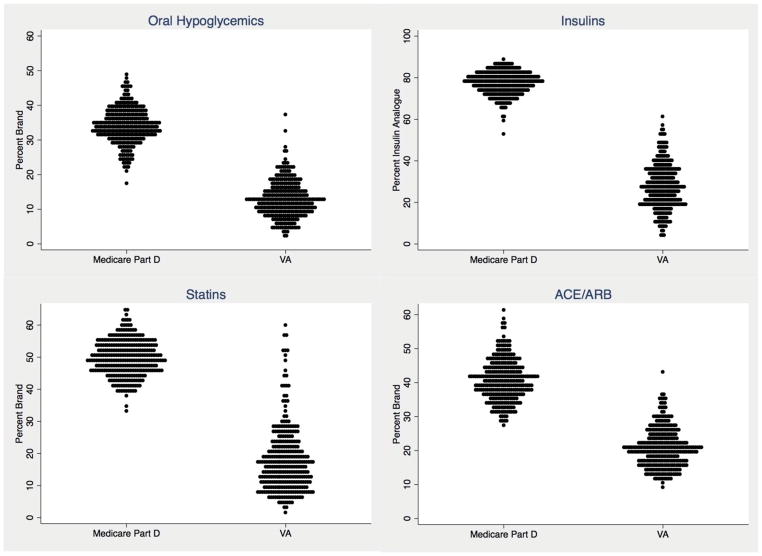
After adjustment for patient characteristics, there was substantial regional variation in brand-name use within each of the four drug groups in both systems (Figure 1). In Medicare, the percent using any brand-name oral hypoglycemics ranged from 25.1% in the 5th percentile to 42.4% in the 95th percentile of HRRs; in VA, the range from 5th to 95th percentile was 5.1%–21.9%. Similarly, in Medicare, HRR-level use of any insulin analogues ranged from 68.3%–85.4%, compared to a range of 10.6%–46.9% in the VA. Similar regional variation was evident for statins (range 41.0%–58.3% for Medicare and 6.2%–38.2% for VA) and ACE/ARBs (range 31.1–51.1 for Medicare and 12.7%–31.0% for VA) (Figure 1). In each drug group, the HRR at the 95th percentile of brand-name use in VA had lower rates of brand-name use than the HRR at the 5th percentile in Medicare.
Figure 1.
Distribution of adjusted HRR-level percent of patients with diabetes in Medicare Part D and the VA using brand-name drugs (and insulin analogues), among users of four groups of medications, 2008.
Notes
Each dot is one HRR (hospital referral region), and all HRR percents are adjusted for sociodemographic and health status variables.
Abbreviations: Statins, HMG-CoA reductase inhibitors; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
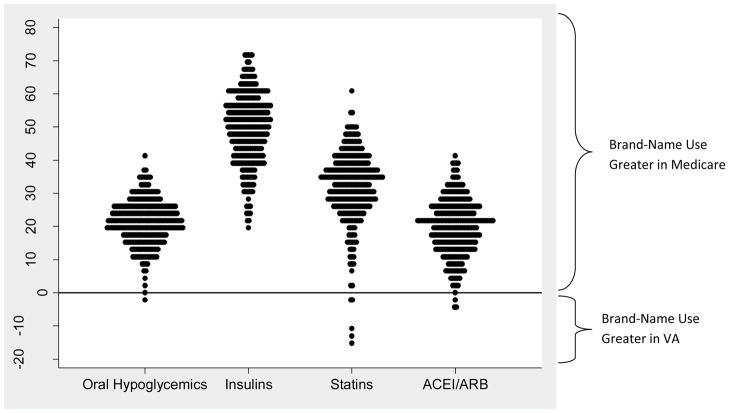
Use of brand-name drugs was greater in Medicare than VA in 298 of 306 HRRs, but there was substantial variation in these differences (Figure 2). Most notably, in 3 HRRs the proportion of patients using brand-name statins was >10 percentage points higher in VA than in Medicare. These outlier HRRs were geographically clustered in a small area of the country within one VA regional network.
Figure 2.
Absolute difference, within each HRR, in adjusted percent of diabetes patients age 65+ in Medicare Part D and the VA using brand-name drugs, among users of four groups of medications, 2008.
Notes
Each dot is one HRR (hospital referral region), and all HRR percents are adjusted for sociodemographic and health status variables. More positive differences indicate higher rates of brand-name use in Medicare compared to VA in a given HRR.
Abbreviations: Statins, HMG-CoA reductase inhibitors; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Multisource Drugs
Use of multisource drugs (medications available in both brand and generic forms) was substantially higher in VA than in Medicare (92.2% vs. 77.4% of all prescriptions for oral hypoglycemics, 89.8% vs. 54.7% for statins, and 80.2% vs. 64.9% for ACE/ARBs) (Table 2). However, for the most common multisource drugs, prescriptions were dispensed primarily as generics in both Medicare and VA.
Table 2.
Number of multisource prescriptions dispensed in three classes and percent dispensed as brand-name among diabetes patients age 65+ in Medicare Part D and the VA, 2008.
| Number (%) of Prescriptions Filled for Multisource Medications | Percent of Multisource Medications Filled as Brand-Name | |||
|---|---|---|---|---|
|
| ||||
| Drug Classes and Select Multisource Drugs* | Medicare | VA | Medicare | VA |
| Oral Hypoglycemics (overall) | N=10,385,905 | N=5,098,778 | - | - |
|
| ||||
| Multisource Hypoglycemics | 8,039,310 (77.4%) | 4,701,283 (92.2%) | 1.1% | 0.2% |
| Metformin | 3,858,287 (37.1) | 2,178,778 (42.7) | 1.3 | 0.1 |
| Glipizide | 1,761,020 (17.0) | 1,341,495 (26.3) | 0.8 | 0.1 |
| Glyburide | 1,005,586 (9.7) | 1,105,382 (21.7) | 0.4 | 0.3 |
|
| ||||
| Statins (Overall) | N=6,095,802 | N=3,880,351 | - | - |
|
| ||||
| Multisource Statins | 3,335,724 (54.7%) | 3,483,255 (89.8%) | 0.5 | 2.3 |
| Simvastatin | 2,391,498 (39.2) | 3,152,515 (81.2) | 0.4 | 2.5 |
| Lovastatin | 526,221 (8.6) | 183,289 (4.7) | 0.9 | 0.02 |
| Pravastatin | 418,005 (6.9) | 147,451 (3.8) | 0.8 | 0.6 |
|
| ||||
| ACE/ARBS (Overall) | N=7,274,793 | N=3,907,993 | - | - |
|
| ||||
| Multisource ACE/ARBS | 4,724,142 (64.9%) | 3,136,052 (80.2%) | 4.2 | 0.3 |
| Lisinopril | 2,224,565 (30.6) | 2,493,730 (63.8) | 0.2 | 0.01 |
| Enalapril | 531,402 (7.3) | 145,615 (3.7) | 0.5 | 0.08 |
| Lisinopril/HCTZ | 354,640 (4.9) | 237,225 (6.1) | 0.4 | 0.1 |
Notes
The top 3 multisource drugs within Medicare are included for each class of oral medications. For statins, there were only three multisource drugs, which are listed. For ACE/ARBs, benazepril/amlodipine was the 3rd most commonly dispensed multisource drug but was not included for comparison because only 535 prescriptions were dispensed within VA. Medicare refers to patients enrolled in fee-for-service Parts A, B and stand-alone Part D.
Abbreviations: Statins, HMG-CoA reductase inhibitors; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, HCTZ, Hydrochlorothiazide
Spending Calculations with Changes in Brand-Name Use
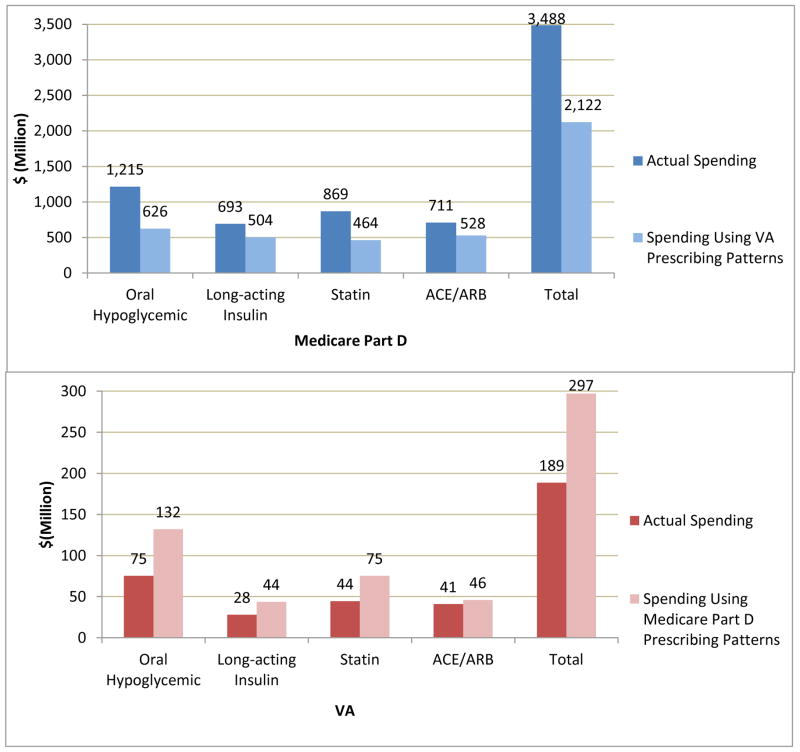
Table 3 shows the per-capita volume of prescriptions filled among users of each medication group, which was slightly lower in Medicare than in VA, whereas per capita drug costs were substantially higher. Across the four drug groups, drug spending in stand-alone Part D would have been $1.4 billion less (39%) in 2008 if brand-name use had resembled that of VA, keeping volume and price unchanged (savings of $589 million for oral hypoglycemics, $189 million for insulins, $404 million for statins, and $183 million for ACE/ARBs) (Figure 3). Conversely, spending in VA (where prices are substantially lower) would have increased by $108 million (57%) if patients used brand-name drugs at the same rate as in Medicare.
Table 3.
Total number of dispensed prescriptions, percent of prescriptions dispensed as brand-name, and mean cost per prescription, in each of four drug groups among diabetes patients age 65+ in Medicare Part D and the VA, 2008.
| Oral Hypoglycemics | Long-acting Insulina | Statin | ACEI/ARB | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Medicare | VA | Medicare | VA | Medicare | VA | Medicare | VA | |
| Total number of 30-day Prescriptions (or units)a | 10,385,905b | 5,098,778 | 2,875,278,349a,b | 1,978,168,710a | 6,095,802b | 3,880,351 | 7,274,793b | 3,907,993 |
| Mean prescriptions per patient per yeara | 13.3 | 13.5 | 12,400a | 17,393a | 9.1 | 10.1 | 9.9 | 10.5 |
| Percent of prescriptions for branda | 23.3 | 7.4 | 60.6a | 16.8a | 45.5 | 12.3 | 37.8 | 20.0 |
| Mean cost ($) per 30-day supply for brand druga,c | 156.2 | 79.6 | 0.12a | 0.03a | 100.6 | 32.5 | 74.3 | 16.2 |
| Mean cost ($) per 30-day supply for generic druga,c | 13.6 | 9.6 | 0.06a | 0.01a | 20.7 | 8.5 | 17.7 | 9.1 |
Notes
Medicare refers to patients enrolled in fee-for-service Parts A, B and stand-alone Part D.
Values for long-acting insulin are based on number of units dispensed rather than number of prescriptions. Mean costs for long-acting insulin are costs per unit for analogue (‘brand’) and non-analogue (‘generic’) insulins.
The number of prescriptions (insulin units) dispensed are for a 40% random sample Medicare denominator, and for our spending calculations we multiplied by 2.5 to represent potential savings if applied to the entire fee-for-service Medicare Part D program.
Because VA costs typically include only ingredient costs and Medicare costs include total reimbursements to the pharmacy, (i.e., plan payment, consumer copayment and dispensing fee), we added patient copayments and an average dispensing fee to the cost of each VA prescription. Abbreviations: Statins, HMG-CoA reductase inhibitors; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Figure 3.
Prescription spending and projected spending if use of brand-name drugs would change, in each of four drug groups among diabetes patients age 65+ in Medicare Part D (top) and the VA (bottom), 2008.
Notes
Medicare refers to patients enrolled in fee-for-service Parts A, B and stand-alone Part D.
Abbreviations: Statins, HMG-CoA reductase inhibitors; ACE/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Discussion
Our analysis of over 1 million Medicare Part D beneficiaries with diabetes and an identically-defined cohort of older VA patients reveals stark differences in rates of brand-name medication use. Medicare beneficiaries are more than twice as likely to use brand-name drugs across four groups of commonly used medications. Had Medicare’s medication use patterns mirrored those of VA for these medications in those with diabetes alone, the program could have saved over $1 billion in 2008. Yet, we observed similarly wide regional variation in brand-name use in both systems, suggesting that non-health system factors play a major role in such variation.
Our study is the first to demonstrate the magnitude of difference in brand versus generic prescribing between Medicare and VA among a comparable population. These findings point to opportunities for improving efficiency without harming quality of care or access to effective medicines. Although we cannot determine the optimal rate of brand-name use in either system, there is no evidence to suggest the differences we report reflect underuse of brand-name drugs in VA.(29–32) In fact, VA provides a reasonable benchmark for use of generic drugs in Medicare because it performs as well or better than commercial health plans and Medicare on several measures of quality for diabetes and related conditions.(29–32)
Furthermore, there is strong evidence of comparable effectiveness of generic versus brand-name drugs in the classes we studied.(33–36) For example, generic and brand-name statins have been shown to be equally effective for clinical endpoints.(34, 36) Any indication for a more potent, brand-name statin (e.g., insufficient lipid lowering with generic statin) is unlikely to be substantially more prevalent in Medicare than in VA. Similarly, although some clinicians recommend insulin analogues to individual patients over NPH insulin because of lower rates of symptomatic nocturnal hypoglycemia, their overall effectiveness is comparable,(33) and differences in prevalence of nocturnal hypoglycemia alone are unlikely to explain why three of every four Medicare patients on insulin use an analogue vs. one of four VA patients.
One structural factor that likely explains much of the between-system difference in brand-name use is the VA’s ability to promote ‘therapeutic substitution’ by prescribers using a national formulary. Therapeutic substitution is the interchange by clinicians of generic drugs in the same class as, but not identical to, single-source brand-name drugs (e.g., generic simvastatin instead of brand atorvastatin (Lipitor). This practice is distinct from mere ‘generic substitution,’ in which brand and generic versions of the same drug are substituted (e.g., switching simvastatin for Zocor). Our analysis of multisource drugs suggests that the source of the different rates of brand-name drug use in VA and Medicare is a difference in the use of single-source drugs, and not a difference in generic substitution among multisource drugs; generic substitution is, in fact, comparable in the two systems. In addition to its formulary, the existence of a national electronic medical record with e-prescribing, limits on access by pharmaceutical sales representatives, and a salaried physician workforce may explain these lower rates of brand-name use in VA.
Part D plans also have tools at their disposal for encouraging use of less costly drugs (e.g., placing brand-name drugs on high cost-sharing tiers, applying utilization management, or excluding drugs from the formulary), but they have applied them less extensively than VA. For example, only 8%, 12%, and 61% of Part D enrollees faced step therapy requirements for atorvastatin, valsartan, and pioglitazone in 2011, respectively, and none faced prior authorization.(37) In contrast, all VA enrollees faced step therapy and prior authorization requirements for these drugs. Part D plans may lack the incentives to apply these tools. In the current system, private Part D plans, which compete with each other to enroll members, may lose market share if they restrict the use of widely used drugs and also may lose rebates on these drugs from pharmaceutical manufacturers.(38)
Although a change in legislation to make Part D function like VA may be neither politically feasible nor warranted, policy levers for increasing appropriate use of generic medications in Part D are currently available. The Center for Medicare and Medicaid Innovation is currently experimenting with pilot projects to increase efficiency in the Medicare program. Part D plans are currently rated and rewarded based on measures of customer service, patient safety and medication adherence.(39) These existing pilot project and incentive mechanisms could be used to reward greater efficiency as well.
Our analyses also demonstrate similarly wide regional variation in the use of brand-name drugs in both systems, even after adjusting for important differences in patient demographics and health status. That the magnitude of the variation was similar between the two systems indicates that while VA’s central formulary has reduced the average rate of brand-name use, it has not eliminated geographic variation. This may be due, in part, to VA’s prior authorization policy, through which patients and their providers can request off-formulary brand-name medications, a process that is adjudicated locally, not centrally. Local physician practice patterns, determined by a complex yet poorly understood set of factors including the supply of specialists, academic affiliations, social and professional physician networks, and prescription drug marketing, may also contribute to geographic variation in brand-name use seen in both systems.(40, 41)
Our study has several limitations. First, there may be unmeasured differences in the populations being compared. However, potential unmeasured differences across Medicare and VA are unlikely to explain these large differences in brand-name drug use. Second, while some individuals filled prescriptions in both Medicare and VA, when we excluded dually enrolled Veterans with Part D from our analysis, our results were unchanged. Third, we cannot estimate the effect of discount pharmacies offering $4 generics on our findings, because in both VA and Medicare some individuals could purchase $4 generics for cash without generating an insurance claim.(42, 43) Fourth, we may overestimate potential savings as some of the brand-name drugs in our analysis have lost patent protection since our study year (2008); however, similar patterns of brand-name use likely exist among other drug groups. Fifth, we are unable to account for rebates negotiated with manufacturers by Medicare plans, which are not publicly available. Incorporating rebates in our analyses could reduce Medicare brand-name drug spending by up to approximately 19% based on one analysis;(38) nonetheless, the magnitude of our savings estimate would still be over $1 billion. Sixth, we estimate savings associated with changing only use of brand-name drugs, holding prices constant in each system. Estimates of cost-savings for Medicare would be larger were it feasible to obtain VA prices, although the government is specifically forbidden from negotiating drug prices on behalf of the Medicare program.(17, 18) Finally, our analyses also assume no behavioral response by the pharmaceutical industry, which when faced with lower sales in Medicare might change its pricing or other marketing strategies, nor do we account for any potential indirect costs associated with brand-name use (e.g. lower adherence).(44–46)
In conclusion, we find large differences in rates of brand-name drug use among patients with diabetes in Medicare Part D and VA, with substantial economic implications. These differences likely reflect structural differences in formulary management between the two systems. For four medication groups alone, we estimate over a billion dollars of potentially avoidable spending on brand-name drugs in 2008 in Medicare Part D. Importantly, our findings draw on actual rates of generic use in an existing high-performing, high-quality health system and demonstrate what should be attainable in Medicare. These potential savings could be realized through policies that promote Part D plan efficiency and by encouraging physicians to consider costs and value in their prescribing.(14, 47–51)
Acknowledgments
We thank Hal Sox, MD for his comments on a prior draft of the manuscript.
Funding/Support: Dr. Gellad was supported by VA Health Services Research & Development (HSR&D) grants CDA 09-207 and LIP 72-057, and by the VA VISN 4 Competitive Pilot Project Fund (XVA 72-156). Drs. Gellad and Donohue were jointly supported by a RAND-University of Pittsburgh Health Institute grant. Dr. Donohue was supported by AHRQ R01HS017695. Dr. Morden and Mr. Smith were supported by NIH/NIA P01 AG019783 “Causes and Consequences of Health Care Efficiency” as well as the Robert Wood Johnson Foundation Dartmouth Atlas Project 059491.
Footnotes
Financial Disclosures: The Authors have no conflicts of interest to report.
Disclaimer: This work represents the opinions of the authors alone and does not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
Reproducible Research Statement: Study Protocol available from Dr. Gellad (walid.gellad@va.gov). Statistical code: not available. Data set: available from the Department of Veterans Affairs and Center for Medicare and Medicaid Services with the proper agreements.
“This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
References
- 1.Congressional Budget Office. [Accessed April 1, 2012];Effects of Using Generic Drugs on Medicare’s Prescription Drug Spending. 2010 Sep; http://www.cbo.gov/sites/default/files/cbofiles/ftpdocs/118xx/doc11838/09-15-prescriptiondrugs.pdf.
- 2.Polinski JM, Kilabuk E, Schneeweiss S, Brennan T, Shrank WH. Changes in drug use and out-of-pocket costs associated with Medicare Part D implementation: a systematic review. J Am Geriatr Soc. 2010;58(9):1764–1779. doi: 10.1111/j.1532-5415.2010.03025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donohue JM, Zhang Y, Lave JR, et al. The Medicare drug benefit (Part D) and treatment of heart failure in older adults. Am Heart J. 2010;160(1):159–165. doi: 10.1016/j.ahj.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Donohue JM, Lave JR, Gellad WF. The impact of Medicare Part D on medication treatment of hypertension. Health Serv Res. 2011;46(1 Pt 1):185–198. doi: 10.1111/j.1475-6773.2010.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madden JM, Graves AJ, Zhang F, et al. Cost-related medication nonadherence and spending on basic needs following implementation of Medicare Part D. JAMA. 2008;299(16):1922–1928. doi: 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue JM, Zhang Y, Aiju M, et al. Impact of Medicare Part D on antidepressant treatment, medication choice, and adherence among older adults with depression. Am J Geriatr Psychiatry. 2011;19(12):989–997. doi: 10.1097/JGP.0b013e3182051a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polinski JM, Donohue JM, Kilabuk E, Shrank WH. Medicare Part D’s effect on the under- and overuse of medications: a systematic review. J Am Geriatr Soc. 2011;59(10):1922–1933. doi: 10.1111/j.1532-5415.2011.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donohue JM, Morden NE, Gellad WF, et al. Sources of regional variation in Medicare Part D drug spending. N Engl J Med. 2012;366(6):530–538. doi: 10.1056/NEJMsa1104816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser Family Foundation. [Accessed April 1, 2012];Fact Sheet: The Medicare Prescription Drug Benefit. 2009 Mar; http://www.kff.org/medicare/upload/7044-09.pdf.
- 10.Huskamp HA, Epstein AM, Blumenthal D. The impact of a national prescription drug formulary on prices, market share, and spending: lessons for Medicare? Health Aff (Millwood) 2003;22(3):149–158. doi: 10.1377/hlthaff.22.3.149. [DOI] [PubMed] [Google Scholar]
- 11.Gellad W, Mor M, Zhao X, Donohue J, Good C. Variation in Use of High-Cost Diabetes Mellitus Medications in the VA Healthcare System. Arch Intern Med. 2012;172(20):1608–9. doi: 10.1001/archinternmed.2012.4482. [DOI] [PubMed] [Google Scholar]
- 12.Gellad WF, Good CB, Lowe JC, Donohue JM. Variation in prescription use and spending for lipid-lowering and diabetes medications in the Veterans Affairs Healthcare System. Am J Manag Care. 2010;16(10):741–750. [PMC free article] [PubMed] [Google Scholar]
- 13.Antos JR, Pauly MV, Wilensky GR. Bending the Cost Curve through Market-Based Incentives. N Engl J Med. 2012 Aug 1; doi: 10.1056/NEJMsb1207996. [DOI] [PubMed] [Google Scholar]
- 14.Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel E, Tanden N, Altman S, et al. A Systemic Approach to Containing Health Care Spending. N Engl J Med. 2012;367 (10):949–54. doi: 10.1056/NEJMsb1205901. [DOI] [PubMed] [Google Scholar]
- 16.Congressional Research Service. Pharmaceutical Costs: A Comparison of Department of Veterans Affairs (VA) [Accessed May 1, 2012];Medicaid, and Medicare Policies. 2007 Jan 17; http://assets.opencrs.com/rpts/RL33802_20070117.pdf.
- 17.Frakt AB, Pizer SD, Feldman R. Should Medicare adopt the Veterans Health Administration formulary? Health Econ. 2012;21(5):485–495. doi: 10.1002/hec.1733. [DOI] [PubMed] [Google Scholar]
- 18.Gellad WF, Schneeweiss S, Brawarsky P, Lipsitz S, Haas JS. What if the federal government negotiated pharmaceutical prices for seniors? An estimate of national savings. J Gen Intern Med. 2008;23(9):1435–1440. doi: 10.1007/s11606-008-0689-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med. 2008;168(19):2088–2094. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27 (Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 21.The Dartmouth Atlas of Health Care Working Group. [Accessed April 1, 2012];The Dartmouth Atlas of Health Care. 2008 http://www.dartmouthatlas.org/atlases/atlas_series.shtm.
- 22.Lexi-Data Basic database (Lexicomp) Denver: Cerner Multum; ( http://www.lexi.com/businesses/solutions/clinical-decision-support) [Google Scholar]
- 23.Treatment Guidelines: Drugs for Type 2 Diabetes. 108. Vol. 9. New Rochelle, NY: The Medical Letter, Inc; 2011. [PubMed] [Google Scholar]
- 24.Zhang Y, Baicker K, Newhouse JP. Geographic variation in the quality of prescribing. N Engl J Med. 2010;363(21):1985–1988. doi: 10.1056/NEJMp1010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Steinman MA, Kaplan CM. Geographic variation in outpatient antibiotic prescribing among older adults. Arch Intern Med. 2012;172(19):1465–1471. doi: 10.1001/archinternmed.2012.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol Jun. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 28.Census 2000, summary file: American fact finder. Washington, DC: Census Bureau; ( http://factfinder.census.gov/servlet/DTGeoSearchByListServlet?ds_name=DEC_2000_SF3U&_lang=en&_ts=283785721496) [Google Scholar]
- 29.Trivedi AN, Matula S, Miake-Lye I, Glassman PA, Shekelle P, Asch S. Systematic review: comparison of the quality of medical care in Veterans Affairs and non-Veterans Affairs settings. Med Care. 2011;49(1):76–88. doi: 10.1097/MLR.0b013e3181f53575. [DOI] [PubMed] [Google Scholar]
- 30.Kerr EA, Gerzoff RB, Krein SL, et al. Diabetes care quality in the Veterans Affairs Health Care System and commercial managed care: the TRIAD study. Ann Intern Med. 2004;141(4):272–281. doi: 10.7326/0003-4819-141-4-200408170-00007. [DOI] [PubMed] [Google Scholar]
- 31.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348(22):2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 32.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 33.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613. doi: 10.1002/14651858.CD005613.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300(21):2514–2526. doi: 10.1001/jama.2008.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 36.Green JB, Ross JS, Jackevicius CA, Shah ND, Krumholz HM. When choosing statin therapy: the case for generics. JAMA Intern Med. 2012;173(3):229–232. doi: 10.1001/jamainternmed.2013.1529. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser Family Foundation. [Accessed April 1, 2012];Analysis of Medicare Prescription Drug Plans in 2011 and Key Trends Since 2006. 2011 Sep; http://www.kff.org/medicare/upload/8237.pdf.
- 38.Department of Health & Human Services. [Accessed May 1, 2012];Office of the Inspector General. Higher Rebates for Brand-Name Drugs Result in Lower Costs for Medicaid Compared to Medicare Part D. 2011 Aug; https://oig.hhs.gov/oei/reports/oei-03-10-00320.pdf.
- 39.Hargrave E, Hoadley J, Summer L, Merrell K. Toward Meaningful Quality and Performance Measures in Part D. [Accessed May 1, 2012];Report to the Medicare Payment Advisory Commission. 2010 (10–11) http://www.medpac.gov/documents/Oct10_PartDQualityandPerformanceMeasuresPartD_CONTRACTOR_RS.pdf.
- 40.Barnett ML, Christakis NA, O’Malley J, Onnela JP, Keating NL, Landon BE. Physician patient-sharing networks and the cost and intensity of care in US hospitals. Med Care. 2012;50(2):152–160. doi: 10.1097/MLR.0b013e31822dcef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kronick R, Gilmer TP. Medicare and Medicaid spending variations are strongly linked within hospital regions but not at overall state level. Health Aff (Millwood) 2012;31(5):948–955. doi: 10.1377/hlthaff.2009.1065. [DOI] [PubMed] [Google Scholar]
- 42.Choudhry NK, Shrank WH. Four-dollar generics--increased accessibility, impaired quality assurance. N Engl J Med. 2010;363(20):1885–1887. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y, Gellad WF, Zhou L, Lin YJ, Lave JR. Access to and use of $4 generic programs in medicare. J Gen Intern Med. 2012;27(10):1251–1257. doi: 10.1007/s11606-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006;166(3):332–337. doi: 10.1001/archinte.166.3.332. [DOI] [PubMed] [Google Scholar]
- 46.Frank RG, Newhouse JP. Should drug prices be negotiated under part D of Medicare? And if so, how? Health Aff (Millwood) 2008;27(1):33–43. doi: 10.1377/hlthaff.27.1.33. [DOI] [PubMed] [Google Scholar]
- 47.Gabow P, Halvorson G, Kaplan G. Marshaling leadership for high-value health care: an Institute of Medicine discussion paper. JAMA. 2012;308(3):239–240. doi: 10.1001/jama.2012.7081. [DOI] [PubMed] [Google Scholar]
- 48.Laine C. High-value testing begins with a few simple questions. Ann Intern Med. 2012;156(2):162–163. doi: 10.7326/0003-4819-156-2-201201170-00016. [DOI] [PubMed] [Google Scholar]
- 49.Smith CD. Teaching high-value, cost-conscious care to residents: the Alliance for Academic Internal Medicine-American College of Physicians Curriculum. Ann Intern Med. 2012;157(4):284–286. doi: 10.7326/0003-4819-157-4-201208210-00496. [DOI] [PubMed] [Google Scholar]
- 50.Wilt TJ, Qaseem A. Implementing High-Value, Cost-Conscious Diabetes Mellitus Care Through the Use of Low-Cost Medications and Less Intensive Glycemic Control Target: Comment on “Variation in Use of High-Cost Diabetes Mellitus Medications in the VA Healthcare System”. Arch Intern Med. 2012;172(20):1610–1. doi: 10.1001/2013.jamainternmed.203. [DOI] [PubMed] [Google Scholar]
- 51.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307(17):1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]