Abstract
In both perceptual and motor learning, numerous studies have shown specificity of learning to the trained eye or hand and to the physical features of the task. However, generalization of learning is possible in both perceptual and motor domains. Here, I review evidence for perceptual and motor learning generalization, suggesting that generalization patterns are affected by the way in which the original memory is encoded and consolidated. Generalization may be facilitated during fast learning, with possible engagement of higher-order brain areas recurrently interacting with the primary visual or motor cortices encoding the stimuli or movements memories. Such generalization may be supported by sleep, involving functional interactions between low and higher-order brain areas. Repeated exposure to the task may alter generalization patterns of learning and overall offline learning. Development of unifying frameworks across learning modalities and better understanding of the conditions under which learning can generalize may enable to gain insight regarding the neural mechanisms underlying procedural learning and have useful clinical implications.
Introduction
Procedural learning enables sustainable long-term improvements in skill performance following repeated practice. Performance improvements in perceptual (visual) and motor tasks, the focus of this review, can occur within the training session (online learning) or following termination of the training session as evident in subsequent test sessions (between-session offline learning) (for a review see Censor et al., 2012). It was suggested that these offline gains in performance are supported by consolidation of the acquired memory (Karni and Sagi, 1991), which classically refers to the process by which a memory stabilizes and becomes resistant to interference by competing stimuli or tasks. Interestingly, it has been shown that already consolidated memories may be modified following their reactivation, possibly through reconsolidation mechanisms, resulting in their degradation, maintenance, or further strengthening (Nader and Hardt, 2009; Walker et al., 2003; Censor et al., 2010). Different stages of between-session sleep have been associated with facilitation of offline procedural learning (for a review see Diekelmann and Born, 2010).
A crucial aspect regarding procedural learning, is the extent to which learning generalizes (transfers) to the untrained eye or hand, to an untrained stimulus or movement, or to other contexts. The notion of generalization is critical for daily life: obviously, it may be beneficial to use what has been learned in specific settings and apply it to novel conditions without the need to invest time and energy in a new learning process. Such ability to generalize learning may also be valuable in disease conditions, in which for example there is impairment in dominant hand function and a critical need to re-learn to perform skills with the functional non-dominant hand. On the other hand, generalization of skills is not necessarily beneficial, since it may result in merging of distinct memory traces. For example it is possible that in perceptual learning, gradual changes in faces stimuli over multiple days which resulted in merging of the memories of the faces with novel faces being identified as already familiar (Blumenfeld et al., 2006; Preminger et al., 2009), is a consequence of generalization of the perceived stimuli into the same class of representations. However, evidence is still required to elucidate whether indeed in some instances generalization may imply decrease in the ability to discriminate between different stimuli. Finally, understanding the conditions under which learning may generalize is not only important from a behavioral point of view, but may also enable to gain insight regarding the neural mechanisms underlying procedural learning. Here, I will review and discuss generalization of procedural perceptual and motor learning, suggesting frameworks which may explain the underlying mechanisms involved.
General framework
Generalization may be affected by the way in which the memory is encoded and consolidated. Efficient encoding of the memory, in part during the initial phase of learning (fast learning) may set the network interactions between the primary visual or motor cortices and higher-order brain regions (Censor et al., 2012) enabling generalization of learning. On the other hand, over-exposure to the trained stimuli or task may alter generalization patterns of learning, possibly as a result of incorporation of noise to the encoded memory trace, thus over-fitting of a specific neural representation. Then, when a small variation is presented, generalization may be reduced (Sagi, 2011). Sleep may support generalization by improving the signal to noise ratio and also by facilitating the engagement of and interactions with higher-order brain areas which may improve generalization (Diekelmann and Born, 2010). In addition, there are specific similarities across domains which may further account for improved generalization, for example the link between goal and movement based learning in the motor domain (Clark and Ivry, 2010; Robertson, 2009) and the link between task and stimulus based learning in the visual domain (Seitz and Watanabe, 2009; Xiao et al., 2008; Zhang et al., 2010), as discussed below.
Additional frameworks may explain generalization of learning. For example, it is possible that generalization of learning is facilitated by attentional mechanisms, guiding top-down learning processes. This is in line with hierarchal models of learning, in which under easy task conditions learning involves higher-order brain regions and generalizes, and when task-difficulty increases lower-level regions play a more dominant role in the learning process, which then therefore becomes specific (Ahissar and Hochstein, 1997). Studies in the visual domain showing perceptual learning of stimuli that are not relevant to the task may challenge this view (Seitz and Watanabe, 2009; see below). In the motor domain, differential mechanisms for implicit and explicit learning have also been suggested (Robertson et al., 2004; Censor et al., 2012), and their relation to learning generalization remains to be further determined. Nonetheless, a common notion which may persist across different theories, is that generalization of learning is affected by the way in which the memory is encoded, thus driven by the characteristics of the presented stimuli or task, as discussed below. An issue to be further explored is the notion of consolidation in relation to generalization of learning. Overall, the suggestion that offline processes such as sleep may facilitate generalization and that numerous generalization effects are long-lasting, support the consolidation account.
Common forms of generalization in perceptual and motor learning
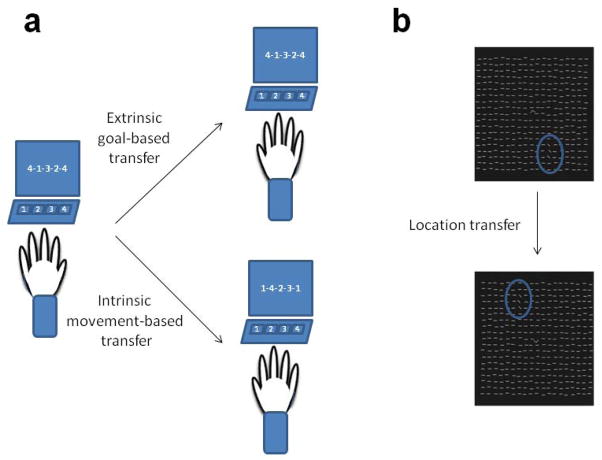
In perceptual and motor learning, generalization may be looked at as a two dimensional process. First, learning can generalize within the same movement goal or perceptual task. For example in motor learning, one of the most common forms of generalization is intermanual transfer, in which learning of a motor task with one hand may result in performance improvements in the other, non-practicing hand (for example, Perez et al., 2007). In goal-based intermanual transfer, there is generalization of the learned skill preserving the same goal, but with a different set of motor movements (for review see Clark and Ivry, 2010; Robertson, 2009). A common example is transfer of learning to perform a sequence of finger movements (Figure 1a). In goal-based intermanual transfer, the sequence to be tapped is preserved but the individual finger movements are now different when performed with the opposite hand. Thus, the sequential movements are transferred into an extrinsic, spatial coordinate frame (allocentric) and are therefore effector-independent (Witt et al., 2010; Grafton et al., 2002). In the visual domain, learning can also generalize within the same task, as shown for example in some instances of the texture discrimination task (Figure 1a; Censor and Sagi, 2009; Harris and Sagi, 2012). Here, learning may generalize to untrained locations in the visual field, or to the untrained eye or orientation (Karni and Sagi, 1993).
Figure 1.
Illustration of representative generalization tasks in motor and perceptual learning. (a) In the sequential finger-tapping task, generalization can be measured when subjects are trained to tap a sequence (4-1-3-2-4) with their left hand as quickly and accurately as they can during limited 30 seconds trials (Karni et al., 1995). Performance is measured for example as the average number of correct sequences per trial at the end of training. Then, subjects are tested with the untrained hand. In extrinsic goal-based transfer, the tapped sequence is maintained, however the movements are now different since they are performed with the right hand. In intrinsic movement-based transfer, the movements are performed by the same fingers, only now with the right instead of the left hand, therefore the sequence tapped is different. (b) In the texture discrimination task (Karni and Sagi, 1991), a target is defined as an array of three diagonal bars embedded in a background of horizontal bars. The presentation is briefly displayed on the screen, and subjects are required to discriminate whether the target array was in a global vertical or horizontal orientation. To enforce fixation, a letter discrimination task (between a ‘T’ and an ‘L’) is given at the center of the display. A patterned mask is displayed briefly after the target presentation, in order to limit processing time and enable to measure performance: The time interval between target and mask is gradually decreased within the session, increasing the difficulty of the task. The performance measure is usually the time interval (in milliseconds) at which approximately 80% of the discrimination responses are correct. One way of generalization assessment here is when subjects are tested on a target at a different, untrained location in the visual field.
Second, learning can generalize to a different goal or task. In movement-based intermanual transfer, there is generalization of the learned skill preserving the same set of movements and thus achieving a different movement-goal since these same movements are now performed with the opposite hand. Therefore the movements are now mirror-reversed, however the spatial sequence becomes different (Figure 1a). Thus, the intrinsic, body-related frame is preserved (egocentric) and therefore this transfer has been termed effector-dependent (Soechting and Flanders, 1989). The distinction between goal and movement based learning in the motor domain may resemble some similarity to the distinction between task and stimulus training in the visual domain. For example, it has been shown in the visual domain that stimulus features that are irrelevant to the useful performance of the task can be learned when consistently presented with the task. This has been termed task-irrelevant perceptual learning (TIPL, Seitz and Watanabe, 2009).
Intermanual generalization of learning has also been documented in motor adaptation paradigms such as performing reaching movements in force fields (Criscimagna-Hemminger et al., 2003; Malfait and Ostry, 2004) and with visuomotor perturbations (Taylor et al., 2011). In regards to learning generalization of reaching movements in motor adaptation paradigms, it was suggested that different generalization patterns depend on the information gained from the history of prior training (Krakauer et al., 2006; Taylor et al., 2011), and on the spatial characteristics of the visual workspace (Woolley et al., 2011; Wang and Müsseler, 2012).
Engagement of higher-order brain areas
Top-down mechanisms that engage attentional and executive resources have been suggested to play an important role in learning in both motor and visual domains (Censor et al., 2012; Dayan and Cohen, 2011; Karni and Sagi, 1993; Honda et al., 1998; Floyer-Lea and Matthews, 2005; Karni and Sagi, 1991; Ahissar and Hochstein, 1997). These mechanisms may support transfer of learning from the trained to the untrained eye, documented for example in the visual texture discrimination task (Karni and Sagi, 1993), and intermanual transfer of motor sequence learning (Grafton et al., 2002) which may be supported by involvement of frontoparietal-associative striatum-cerebellar circuits associated with top-down processing (Dayan and Cohen, 2011; Honda et al., 1998; Floyer-Lea and Matthews, 2005; Grafton et al., 2002; Sun et al., 2007; Hikosaka et al., 2002; Doyon et al., 2005), the mediotemporal lobe (MTL) supporting higher-order associations (Schendan et al., 2003), and the supplementary motor area (SMA, Perez et al., 2007, 2008).
While goal-based learning was shown to engage parietal and prefrontal cortices (Grafton et al., 1998; Hikosaka et al., 2002), intrinsic movement-based encoding and learning has suggested strong involvement of the primary motor cortex (M1) (Scott and Kalaska, 1997; Arce et al., 2010; Hikosaka et al., 2002; Grafton et al., 1998; Orban de Xivry et al., 2011). For example, when transcranial direct current stimulation (tDCS) was applied to M1, it increased generalization of force-field adaptation learning in intrinsic but not in extrinsic coordinates. It was therefore suggested that this effect could be driven by a larger recruitment of M1 cells or an increase in modulation of M1 neural activity (Orban de Xivry et al., 2011).
We have mentioned before that the distinction between goal and movement based learning in the motor domain may resemble some similarity to the distinction between task and stimulus training in the visual domain. Interestingly, it has been shown that when the two forms of task and stimulus training are combined into double-training paradigms, generalization of learning can be achieved (Xiao et al., 2008; Zhang et al., 2010). First, subjects were trained at a certain location in the visual field to successfully discriminate between a given feature of the visual stimulus (such as contrast or orientation). Then they were trained at a different location with a different task. When subjects were tested with the first task at the second location, they showed transfer of learning. Thus, they were able to successfully perform the first task at the new location in which they were never trained with that task before. These results may therefore also suggest that generalization of learning is enabled by interaction between early visual networks and higher order brain regions, such as anterior cingulate cortex (ACC) and the lateral intraparietal area (LIP) (Law and Gold, 2008; Kahnt et al., 2011). Similarly in the motor domain, interactions between primary cortical processing (M1) and frontal regions such as premotor cortex (PMC) and the supplementary motor area (SMA) (Grafton et al., 2002; Perez et al., 2007, 2008), as well as the striatum and hippocampus (Albouy ey al., 2008; Debas et al., 2010), may enable learning generalization (Censor et al., 2012).
Fast learning and the effect of repeated exposure to the task on generalization
Fast learning during initial exposure to visual or motor tasks has been suggested to engage higher-order brain areas and top-down processing (for a review see Censor et al., 2012). For example in the visual domain, it has been shown that fast learning in the texture discrimination task transfers from the trained to the untrained eye (Karni and Sagi, 1993). In the motor domain, evidence for successful intermanual transfer can be documented after one short practice session (Perez et al., 2007), yet interestingly, little or no transfer has been reported following longer term (5 weeks) training of an explicitly known sequence of finger movements (Karni et al., 1995). These results are consistent with data from non-human primates, suggesting that in the early stage of learning, memory of the correct performance of a sequential procedure is not specific to the hand originally used to perform the sequence, unlike the well-learned stage, where the transfer was incomplete (Rand et al., 1998). Models of motor skill memory have therefore suggested that fast learning occurring with short practice of even a single session can produce a memory with a large goal-based component and more generalization, whereas slow learning with prolonged practice may produce a memory with a large movement-based component (Hikosaka et al., 2002; Robertson, 2009). Clark and Ivry (2010) have suggested that extended practice may tie a skill to a particular mode of execution, preventing development of more abstract levels of representation and limiting predominantly goal-based generalization. In line with this view, motor adaptation paradigms enable fast adjustments to the new environment, leading to generalization (Clark and Ivry, 2010). The notion that extensive practice may alter generalization patterns of learning may apply not only to prolonged practice over multiple sessions, but also to extended practice within a single session. In perceptual learning of the visual discrimination task (Figure 1b), it was shown that shorter sessions result in generalization of learning to the untrained visual field (Censor et al., 2009). These results have challenged the specificity of perceptual learning observed in various previous studies (Karni and Sagi, 1991, 1993; Adini et al., 2002; Fahle et al., 2004).
Using the same texture discrimination task, it was further shown that location specificity is a consequence of sensory adaptation, resulting from selective reduced sensitivity due to repeated stimulation (Harris et al., 2012). When adaptation was removed, learning fully generalized to the untrained location in the visual field. It was therefore suggested that these results may explain extended generalization with shorter training sessions (Karni and Sagi, 1993; Aberg et al., 2009), easy tasks (Ahissar and Hochstein, 1997), or coarse discrimination (Jeter et al., 2009) – since in reduced amount of stimulation or variation of the visual stimuli used during training, the adaptation effect does not build up, enabling generalization of learning (Harris et al., 2012). According to this notion, generalization is achieved by a readout mechanism (classifier, Lu and Dosher, 2009; Mollon and Danilova, 1996) in higher-order brain areas which learns to perform the task based on the input it receives from low-level local visual networks encoding the stimuli (Censor and Sagi, 2009; Harris et al., 2012; Censor et al., 2012). This classifier consolidates and enables efficient readout of untrained local visual networks, resulting in generalization of learning. However, when the visual networks are adapted, the readout is impaired and no generalization is observed. Going back to the motor domain, a similar framework may explain alteration of goal-based generalization with repeated exposure to the task, as discussed above.
The concept of over-fitting may provide a computational foundation to explain the link between over-exposure to the task and alteration of generalization, for example in perceptual learning. According to this notion, extended practice may cause the neural networks encoding the stimulus to model spurious properties of the stimulus, possibly incorporating noise in addition to the informative signal. This over-fitted model of the stimulus then fails when the stimulus is presented with even a small variation, resulting in reduced generalization (Sagi, 2011). Interestingly, the concept of over-fitting may fit well with Clark and Ivry’s (2010) account in the motor domain described above, according to which extended practice may tie a skill to a specific mode of execution, preventing abstract levels of representation and limiting goal-based generalization.
Generalization supported by sleep
Generalization of visual or motor procedural learning may be supported by sleep, possibly due to reactivated functional interactions (Diekelmann and Born, 2010) between low and higher level brain regions during sleep, interactions which may be important for generalization as mentioned above. This notion may be supported by evidence of coordinated interactions between V1 and the hippocampus in the visual domain (Ji and Wilson, 2007), and involvement of the hippocampus and striatum in the motor domain (Albouy et al., 2008; Debas et al., 2010).
Studies in motor sequence learning have suggested that offline consolidation during wakefulness supports movement-based intrinsic generalization of sequence learning, whereas between-session sleep facilitates goal-based extrinsic generalization of learning (Cohen et al., 2005; Witt et al., 2010). This was shown both in the serial reaction time task (SRTT, Cohen et al., 2005) and the explicit sequential finger-tapping task (Witt et al., 2010). These findings are consistent with the notion that M1, involved in movement-based learning, contributes to motor learning during wakefulness (Muellbacher et al., 2002; Robertson et al., 2005), whereas fronto-parietal regions, involved in goal-based learning, are modified overnight (Braun et al., 1997; Huber et al., 2004; Maquet et al., 2003).
Considering the notion mentioned above that extensive training may alter generalization of learning, it is conceivable that sleep supports generalization by downscaling of synaptic strength, eliminating noise and therefore improving signal to noise ratio (Tononi and Cirelli, 2006). This in turn may therefore support consolidation of a more global memory trace which can be applied to the different task conditions, resulting in more efficient generalization.
Interestingly, beyond generalization, extensive practice may impair learning itself. In the visual domain, intensive practice with the texture discrimination task results in short-term deterioration in performance as well as prevention of offline gains in performance (Mednick et al., 2005; Censor et al., 2006; Ofen et al., 2007). Both deterioration and blockade of offline gains can be counteracted by between-session sleep, or by short initial training sessions (Mednick et al., 2002; Censor et al., 2006; Censor and Sagi, 2008). A study in motor sequence learning may point to similar effects in the motor domain (Brawn et al., 2010). In this study, the authors show that when training begins in the morning, motor-sequence performance deteriorates across wakefulness and recovers after sleep, whereas performance remains stable across both sleep and subsequent waking with evening training. Thus, sleep restored motor sequence performance after it had deteriorated during a period of wakefulness before sleep, and sleep stabilized the motor memory against degradation during a subsequent day of wakefulness (Brawn et al., 2010).
Memory reconsolidation
Following their retrieval or reactivation, already existing memories can be modified, possibly through reconsolidation mechanism. Such modification may result in degradation, maintenance, or further strengthening of the memory (Nader and Hardt, 2009). In the motor domain, reactivation of a consolidated motor sequence followed by introduction of a competing sequence was shown to degrade the original consolidated motor sequence memory (Walker et al., 2003). A study using the same task showed that inhibitory 1 Hz rTMS applied to M1 during memory reactivation prevented strengthening of the consolidated motor memory, pointing to the role of M1 processing in motor memory modification (Censor et al., 2010).
As mentioned above in the visual domain, repeated presentation of gradually changing faces stimuli resulted in merging of the memories of the faces, with novel faces being identified as already familiar (Blumenfeld et al., 2006; Preminger et al., 2009). Future studies in both visual and motor domains may reveal whether memory reactivation may serve as a time window not only to modify memories, but also to enable better generalization of the acquired skill.
Summary and future directions
Generalization of learning in perceptual or motor domains may depend on the way by which the original memory is encoded and consolidated. Generalization of learning may be facilitated during fast learning, with the engagement of higher-order brain areas which recurrently interact with the primary cortices (visual or motor) encoding the stimuli or movements memories. Such generalization may be supported by sleep, involving functional interactions between low and high level brain regions. On the other hand, generalization patterns are altered by repeated exposure to the task, inducing suppressive effects such as sensory adaptation. The concept of over-fitting may provide a theoretical tool to explain the link between over-exposure to the task and alteration of generalization in both perceptual and motor learning.
The data reviewed here in perceptual learning may suggest the existence of a possible continuum – over-exposure to the stimuli and task may alter generalization of learning, whereas further increasing that exposure may alter overall offline learning (Censor et al., 2006; Brawn et al., 2010). Future research may enable to directly test this hypothesis, and possibly extend it to different learning paradigms in the motor domain. It also remains to be tested whether memory reconsolidation can serve as an opportunity to generalize existing memories.
Overall, it may be a desirable outcome to be able to generalize learning to novel conditions or contexts. However on the other hand, over-generalization may result in a low-resolution representation which does not encompass the fine-tuned details of the acquired information. Future research is needed to investigate this potential tradeoff. Nevertheless, better understanding of the conditions in which learning generalizes is important for uncovering the underlying mechanisms of learning, and in addition may have valuable clinical implications. For example, generalization of learning may be crucially important in neurological conditions such as stroke or traumatic brain injury, which impair daily life functions that need to be relearned and generalized from previous knowledge.
Highlights.
Evidence for perceptual and motor learning generalization is reviewed.
Generalization can be affected by the encoding and consolidation of the memory.
Generalization may be facilitated by engagement of higher-order brain areas.
Repeated task exposure may alter generalization and overall offline learning.
Generalization may be supported by sleep.
Acknowledgments
I thank the reviewers for their useful comments. This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS), US National Institutes of Health (NIH), and an NINDS Ruth L. Kirschstein National Research Service Award (NRSA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg KC, Tartaglia EM, Herzog MH. Perceptual learning with Chevrons requires a minimal number of trials, transfers to untrained directions, but does not require sleep. Vision Res. 2009;49:2087–2094. doi: 10.1016/j.visres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Adini Y, Sagi D, Tsodyks M. Context-enabled learning in the human visual system. Nature. 2002;415:790–793. doi: 10.1038/415790a. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Albouy G, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Arce F, Novick I, Mandelblat-Cerf Y, Israel Z, Ghez C, Vaadia E. Combined adaptiveness of specific motor cortical ensembles underlies learning. J Neurosci. 2010;30:5415–5425. doi: 10.1523/JNEUROSCI.0076-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld B, Preminger S, Sagi D, Tsodyks M. Dynamics of memory representations in networks with novelty-facilitated synaptic plasticity. Neuron. 2006;52:383–394. doi: 10.1016/j.neuron.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Braun AR, et al. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- Brawn TP, Fenn KM, Nusbaum HC, Margoliash D. Consolidating the effects of waking and sleep on motor-sequence learning. J Neurosci. 2010;30:13977–13982. doi: 10.1523/JNEUROSCI.3295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Modification of existing human motor memories is enabled by primary cortical processing during memory reactivation. Curr Biol. 2010;20:1545–1549. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, Karni A, Sagi D. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46:4071–4074. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D. Benefits of efficient consolidation: short training enables long-term resistance to perceptual adaptation induced by intensive testing. Vision Res. 2008;48:970–977. doi: 10.1016/j.visres.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D. Global resistance to local perceptual adaptation in texture discrimination. Vision Res. 2009;49:2550–2556. doi: 10.1016/j.visres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D, Cohen LG. Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci. 2012;13:658–664. doi: 10.1038/nrn3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Ivry RB. Multiple systems for motor skill learning. Wiley Interdisciplinary Reviews: Cognitive Science. 2010;1:461–467. doi: 10.1002/wcs.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol. 2003;89:168–176. doi: 10.1152/jn.00622.2002. [DOI] [PubMed] [Google Scholar]
- Debas K, et al. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: a case for early selection. J Vis. 2004;4:879–890. doi: 10.1167/4.10.4. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci. 1998;18:9420–9428. doi: 10.1523/JNEUROSCI.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp Brain Res. 2002;146:369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Harris H, Gliksberg M, Sagi D. Generalized perceptual learning in the absence of sensory adaptation. Curr Biol. 2012;22:1813–1817. doi: 10.1016/j.cub.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121:2159–2173. doi: 10.1093/brain/121.11.2159. [DOI] [PubMed] [Google Scholar]
- Huber R, Felice Ghilardi M, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Jeter PE, Dosher BA, Petrov A, Lu ZL. Task precision at transfer determines specificity of perceptual learning. J Vis. 2009;9:1–13. doi: 10.1167/9.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neurosci. 2007;10:100–107. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Grueschow M, Speck O, Haynes J. Perceptual learning and decision-making in human medial frontal cortex. Neuron. 2011;70:549–559. doi: 10.1016/j.neuron.2011.02.054. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci USA. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Karni A, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol. 2006;4:e316. doi: 10.1371/journal.pbio.0040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. Mechanisms of Perceptual Learning. Learn Percept. 2009;1:19–36. doi: 10.1556/LP.1.2009.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci. 2004;24:8084–8089. doi: 10.1523/JNEUROSCI.1742-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Schwartz S, Passingham R, Frith C. Sleep-related consolidation of a visuomotor skill: Brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci. 2003;23:1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SC, et al. The restorative effect of naps on perceptual deterioration. Nature Neurosci. 2002;5:677–681. doi: 10.1038/nn864. [DOI] [PubMed] [Google Scholar]
- Mednick SC, Arman AC, Boynton GM. The time course and specificity of perceptual deterioration. Proc Natl Acad Sci USA. 2005;102:3881–3885. doi: 10.1073/pnas.0407866102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollon JD, Danilova MV. Three remarks on perceptual learning. Spat Vis. 1996;10:51–58. doi: 10.1163/156856896x00051. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Ofen N, Moran A, Sagi D. Effects of trial repetition in texture discrimination. Vision Res. 2007;47:1094–1102. doi: 10.1016/j.visres.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, et al. Stimulation of the human motor cortex alters generalization patterns of motor learning. J Neurosci. 2011;31:7102–7210. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, et al. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007;17:1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci. 2008;28:9664–9669. doi: 10.1523/JNEUROSCI.3416-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preminger S, Blumenfeld B, Sagi D, Tsodyks M. Mapping dynamic memories of gradually changing objects. Proc Natl Acad Sci USA. 2009;106:5371–5376. doi: 10.1073/pnas.0802111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand MK, Hikosaka O, Miyachi S, Lu X, Miyashita K. Characteristics of a long-term procedural skill in the monkey. Exp Brain Res. 1998;118:293–297. doi: 10.1007/s002210050284. [DOI] [PubMed] [Google Scholar]
- Robertson EM. From creation to consolidation: a novel framework for memory processing. PLoS Biol. 2009;7:e19. doi: 10.1371/journal.pbio.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A. Off-Line Learning and the Primary Motor Cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D. Perceptual learning in Vision Research. Vision Res. 2011;51:1552–1566. doi: 10.1016/j.visres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Scott SH, Kalaska JF. Reaching movements with similar hand paths but different arm orientations. I. Activity of individual cells in motor cortex. J Neurophysiol. 1997;77:826–852. doi: 10.1152/jn.1997.77.2.826. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. The phenomenon of task-irrelevant perceptual learning. Vision Res. 2009;49:2604–2610. doi: 10.1016/j.visres.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting JF, Flanders M. Errors in pointing are due to approximations in sensorimotor transformations. J Neurophysiol. 1989;62:595–608. doi: 10.1152/jn.1989.62.2.595. [DOI] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA, D’Esposito M. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb Cortex. 2007;17:1227–1234. doi: 10.1093/cercor/bhl033. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Wojaczynski GJ, Ivry RB. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol. 2011;106:3157–3172. doi: 10.1152/jn.01008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Wang L, Müsseler J. Generalization of visuomotor adaptation depends on the spatial characteristic of visual workspace. Exp Brain Res. 2012;223:353–365. doi: 10.1007/s00221-012-3264-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K, Margraf N, Bieber C, Born J, Deuschl G. Sleep consolidates the effector-independent representation of a motor skill. Neuroscience. 2010;171:227–234. doi: 10.1016/j.neuroscience.2010.07.062. [DOI] [PubMed] [Google Scholar]
- Woolley DG, de Rugy A, Carson RG, Riek S. Visual target separation determines the extent of generalisation between opposing visuomotor rotations. Exp Brain Res. 2011;212:213–224. doi: 10.1007/s00221-011-2720-1. [DOI] [PubMed] [Google Scholar]
- Xiao LQ, et al. Complete transfer of perceptual learning across retinal locations enabled by double training. Curr Biol. 2008;18:1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, et al. Rule-based learning explains visual perceptual learning and its specificity and transfer. J Neurosci. 2010;30:12323–12328. doi: 10.1523/JNEUROSCI.0704-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]