Abstract
The discovery of Toll in Drosophila and of the remarkable conservation in pathway composition and organization catalyzed a transformation in our understanding of innate immune recognition and response. At the center of that picture is a cascade of interactions in which specific microbial cues activate Toll receptors, which then transmit signals driving transcription factor nuclear localization and activity. Experiments gave substance to the vision of pattern recognition receptors, linked phenomena in development, gene regulation, and immunity into a coherent whole, and revealed a rich set of variations for identifying non-self and responding effectively. More recently, research in Drosophila has illuminated the positive and negative regulation of Toll activation, the organization of signaling events at and beneath membranes, the sorting of information flow, and the existence of non-conventional signaling via Toll-related receptors. Here, we provide an overview of the Toll pathway of flies and highlight these ongoing realms of research.
Keywords: Innate immunity, NF-κB, Toll, Drosophila, non-conventional pathway
1. Toll pathway conservation and divergence
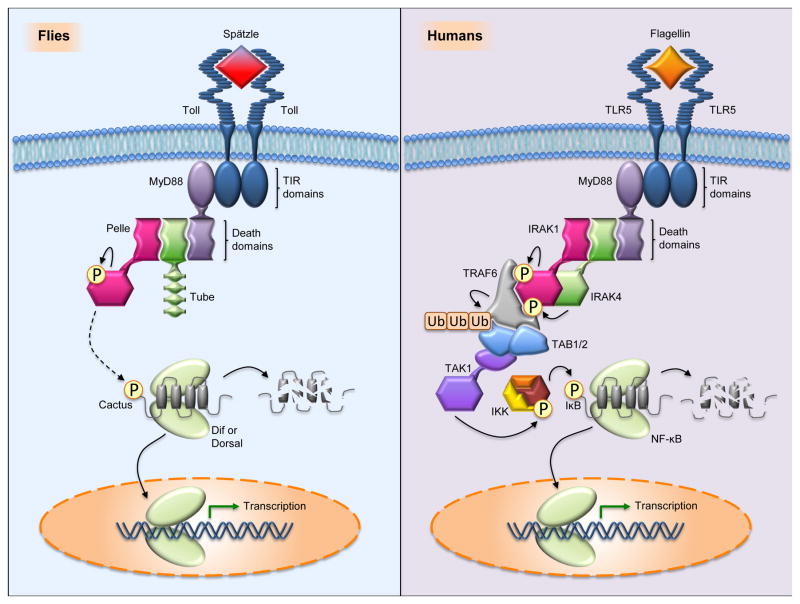
In the face of assault from bacteria, viruses, fungi, and parasites, multicellular organisms defend themselves. Receptors that respond to conserved microbial molecules trigger signaling cascades that direct expression of a battery of antimicrobial peptides, cytokines, and other immune mediators. In both vertebrates and invertebrates, signaling by transmembrane Toll receptors has a central role in these responses (Brennan and Anderson, 2004; Imler et al., 2004; Kawai and Akira, 2011; Kim and Kim, 2005; Pasare and Medzhitov, 2005). Indeed, the pathways mediated by Toll and Toll-like receptors (TLRs) exhibit striking evolutionary conservation, as shown in Fig. 1.
Fig. 1.
Evolutionary conservation of the Drosophila Toll and human TLR signaling pathways. (left) In flies, Toll signaling is activated when a processed form of Spätzle binds the Toll ectodomain. Toll activation triggers dimerization of the intracytoplasmic TIR domains, which promotes binding of the adaptor protein MyD88 through its own TIR domain. MyD88 binds the adaptor protein Tube, which in turn recruits the protein kinase Pelle, each interaction occurring via pairwise interaction of death domains. Although only one signaling module is shown, each TIR domain of the Toll dimer is capable of binding one molecule of MyD88 and there are thus two signaling modules per Toll dimer. Recruitment of Pelle induces its autophosphorylation, triggering phosphorylation and destruction of the inhibitor Cactus. The transcription factor, either Dif or Dorsal depending on the context, is then freed for nuclear translocation. (right) In humans, there are numerous TLR pathways involving often distinct but sometimes overlapping sets of PAMPs, signaling components, and transcription factors. In the example illustrated, TLR5 signaling is activated by Flagellin, a principal component of bacterial flagella. In a manner analogous to Drosophila Toll signaling, human MyD88 builds a signaling complex with the Tube ortholog, IRAK4, and the Pelle ortholog, IRAK1. The complex is much bigger than the Drosophila counterpart, comprising 6 MyD88, 4 IRAK4, and 4 IRAK1 molecules in a complete signaling unit. IRAK4 phosphorylates IRAK1, triggering IRAK1 autophosphorylation and dissociation from the complex. Activated IRAK1 binds TRAF6, which then autoubiquitinates and binds the TAB/TAK1 proteins. TAK1 becomes activated and phosphorylates the IKK complex, which then phosphorylates the inhibitor IκB, leading to its degradation and the nuclear translocation of NF-κB.
The Toll pathway of Drosophila melanogaster was first described in the context of the syncytial blastoderm embryo. There it establishes the dorsoventral axis by regulating nuclear localization of the transcription factor Dorsal (Anderson and Nusslein, 1984; Nüsslein-Volhard et al., 1987; Steward, 1987). Prior to Toll activation, the inhibitor protein Cactus retains Dorsal in the cytoplasm by masking its nuclear localization signal (NLS). Toll signaling, which relieves this inhibition, is triggered by a proteolytically activated form of the Spätzle (Spz) protein (Schneider et al., 1994). Cleaved Spätzle binds to Toll, triggering a conformational change that generates an active Toll dimer. The dimerized cytoplasmic domain of Toll interacts with an adaptor, MyD88, which recruits a second adaptor Tube and the protein kinase Pelle. This signaling complex initiates phosphorylation and degradation of Cactus, freeing Dorsal to enter nuclei (Belvin and Anderson, 1996; Drier and Steward, 1997; Wasserman, 2000). Because Toll signaling is spatially graded across the syncytial embryo, the result is a nuclear concentration gradient of Dorsal that elicits broad stripes of dorsal, lateral, and ventral gene expression (Ip et al., 1991; Kanodia et al., 2009; Stathopoulos et al., 2002; Stein and Stevens, 1991).
The Toll pathway mediating Drosophila innate immunity has the same architecture as that directing embryonic axis formation. Innate immune function, however, involves an alternative transcriptional factor, the Dorsal-related immunity factor (Dif) (Ip et al., 1993). Dorsal and Dif overlap in function in larvae, with either being sufficient for immune function (Ip et al., 1993; Lemaitre et al., 1995b; Manfruelli et al., 1999). In adults, only Dif is required (Meng et al., 1999).
Beginning with Toll, each component of the Drosophila pathway has a mammalian ortholog (see Fig. 1). Thus, for example, the fly proteins MyD88, Tube, and Pelle have direct counterparts in mammalian MyD88, IRAK4, and IRAK1. Each of these proteins contains a death domain, a protein interaction motif first described in apoptotic pathways (Tartaglia et al., 1993). Similarly, the fly proteins Dorsal and Dif are similar in sequence and function to the mammalian NF-κB proteins. Each contains a Rel homology region, a conserved protein domain that has sites for DNA binding, for dimerization, and for interaction with an inhibitor. That inhibitor – Cactus or its ortholog IκB – has N-terminal sites for signal responsiveness, ankyrin repeats that bind Rel proteins, and a destabilizing C-terminal PEST domain.
Although each pathway component in flies has a counterpart in mammals, the converse is not true. TLR signaling to NF-κB and IκB requires a number of components not found in the Drosophila Toll pathway. These include the TRAF6, TAB, and TAK1 proteins, as well as the proteins that make up the IκB kinase (IKK) complex (Chen and Chen, 2013; Karin and Delhase, 2000). Many of these signaling proteins are, however, found in Drosophila and function in a second innate immune pathway termed Imd and discussed below.
The amenability of Drosophila to genetic, molecular, biochemical, and physiological studies has fueled a steady stream of contributions to the Toll field over the last two decades (Dionne and Schneider, 2002; Ganesan et al., 2011; Govind, 2008; Lemaitre and Hoffmann, 2007; Valanne et al., 2011). In this review we provide an overview of Toll signaling in innate immunity and highlight a few of the most exciting recent developments in this area.
2. Fly Toll signaling mechanism
The initiating event for Toll signaling is cleavage of Spätzle and the binding of the C-terminal fragment to the leucine-rich repeats (LRR) of Toll (Weber et al., 2007). Binding of the Spz fragment to the Toll LRRs induces a conformational change, generating an active form of the Toll dimer (Gangloff et al., 2008). Recent studies have demonstrated that endocytosis is required for signaling by the activated Toll receptor in both dorsoventral patterning and in innate immunity (Huang et al., 2010; Lund et al., 2010). Drosophila Toll signaling is therefore now believed to take place at endosomal membranes, as is true for mammalian TLR3, TLR7, TLR8, and TLR9 (Kawai and Akira, 2011).
Once activated, Toll signals via a cytoplasmic TIR domain, which forms a homotypic interaction with the TIR domain of MyD88 (Horng and Medzhitov, 2001; Tauszig-Delamasure et al., 2002). Where is MyD88 prior to signaling? In flies, as in mammals, MyD88 appears to be localized at the membrane before any interaction with Toll (Kagan and Medzhitov, 2006; Sun et al., 2004). In mammals, the MyD88 adaptor-like protein (Mal) effects MyD88 localization by interacting both with MyD88 and with regions of the membrane enriched for phosphatidylinositol 4,5-bisphosphate (PIP2). In flies, a C-terminal domain of MyD88 itself interacts with phosphoinositides and provides membrane localization essential for wild-type Toll signaling in both immunity and development (Marek and Kagan, 2012).
Tube, Pelle, and MyD88 interact via specific pairwise interactions of their death domains to form a submembranous signaling complex (Moncrieffe et al., 2008; Sun et al., 2002; Sun et al., 2004; Towb et al., 1998). The Tube death domain is bivalent, interacting with the death domain of MyD88 on one surface and with that of Pelle on the other. With the demonstration that Tube is the fly IRAK4 ortholog, it was hypothesized that the architecture of the fly death domain oligomer was likely to be conserved in the mammalian pathway (Towb et al., 2009). Biophysical studies confirmed this prediction, with the physical arrangement of IRAK4 and IRAK2 in the mammalian structure matching that of co-crystallized Tube and Pelle in a previous X-ray study (Lin et al., 2010; Motshwene et al., 2009; Xiao et al., 1999). Whereas the fly death domains appear to form a simple ternary complex, those in the mammalian pathway form a helical tower involving a total of 14 polypeptides (Lin et al., 2010; Wasserman, 2010).
As highlighted in Fig. 1, the parallels between fly and mammalian Toll signalling end abruptly downstream of the death domain complex. The mammalian pathway is rich with additional adaptors and kinases, culminating in phosphorylation of IκB by the IKK complex. In contrast, the Drosophila pathway appears to skip directly from Pelle to Cactus phosphorylation. Might there be additional kinases that were missed in the original genetic screens defining the pathway? Perhaps. However, recent large-scale RNAi screens in three separate laboratories have failed to identify any kinase other than Pelle that functionally links Toll to Cactus (Huang et al., 2010; Kuttenkeuler et al., 2010; Valanne et al., 2010). The simplest explanation is that Pelle is in fact the Cactus kinase, a hypothesis currently under active investigation.
3. Toll activation in Drosophila immunity
3.1 PAMPs
Once microbes cross chemical and physical barriers to infection, host defense relies on non-self recognition. As first hypothesized by Janeway, pathogen recognition receptors (PRRs) detect conserved portions of microbes termed pathogen-associated molecular patterns (PAMPs) (Janeway, 1992). In humans, the ten TLRs function as PRRs, binding specifically to PAMPs of bacteria, fungi, and viruses. These PAMPs include bacterial flagellin, the lipopolysaccharide (LPS) of Gram-negative bacteria, and yeast zymosan (Kawai and Akira, 2011).
Fungi and bacteria activate the Toll pathway of flies. However, unlike mammalian TLRs, Toll is not a pattern recognition receptor. Rather, secreted immune factors act as PRRs and initiate proteolytic cascades that activate the Toll ligand Spätzle (Gobert et al., 2003; Leulier et al., 2003; Ligoxygakis et al., 2002b).
For fungi, it is the β-1,3-glucans (polymers of D-glucose) of cell walls that serve as PAMPs (Fig. 2). The corresponding PRR is GNBP3, a member of the Glucan-binding protein (GNBP) family. Circulating GNBP3 binds specifically to β-1,3-glucans and triggers Toll activation (Gottar et al., 2006). Inactivating the GNBP3 locus dramatically decreases resistance to infection by yeasts such as Candida albicans and Beauveria bassiana, but has no effect on resistance to bacterial infection. In functioning as a PRR for fungi, GNBP3 also has Toll independent functions, including activation of the defensive enzyme phenoloxidase and initiation of attack complexes that target invading microbes (Matskevich et al., 2010).
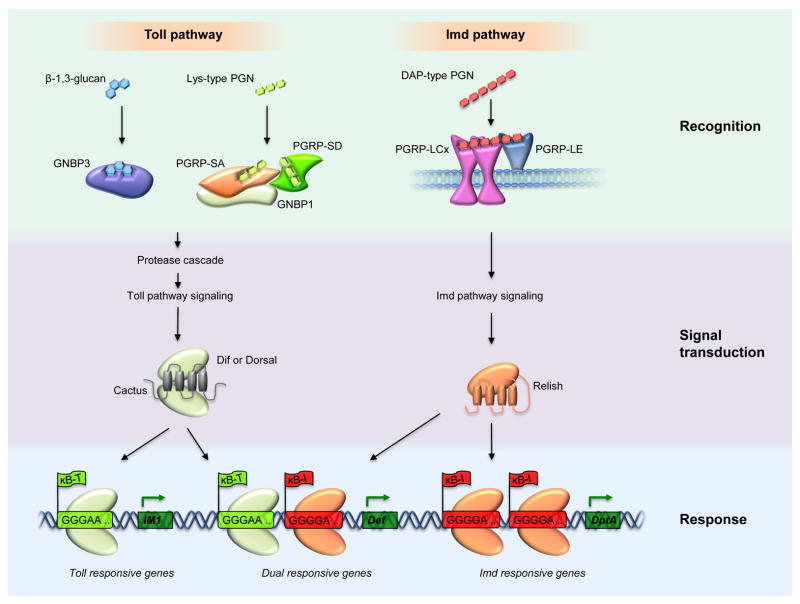
Fig. 2.
Protease cascades leading to Toll activation. The active form of the Toll ligand Spätzle results from a specific cleavage triggered by any of four serine protease cascades. In these illustrations, horizontal red arrows denote proteolytic conversion of the zymogens to their active forms and a reddish glow denotes the active form of a protease. (left) In early embryogenesis, positional cues laid out during oogenesis establish the dorsoventral axis through the localized activation of Toll on the ventral side of the embryo. The protease cascade that triggers this Toll activation involves Nudel, Gastrulation defective (gd), Snake, and Easter. Nudel, directly or indirectly activates the Gastrulation defective protease, which then activates Snake. With the involvement of the sulfotransferase Pipe, activated Snake cleaves and activates Easter. Activated Easter processes Spätzle, completing generation of a functional ligand for Toll. (middle, right) A similar mechanism operates in innate immunity, where three protease cascades converge at the activation step for the Spätzle processing enzyme (SPE). In the case of fungi and Gram-positive bacteria, the cell wall components β-1,3-glucan and Lys-type peptidoglycan, respectively, are recognized by circulating pathogen recognition receptors and trigger separate, but related protease cascades. The serine protease ModSP integrates signals from these recognition molecules and activates the protease Grass, which activates SPE. Other immune factors, such as the serine proteases Spirit, Sphinx, and Spheroide may function between Grass and SPE. In addition to recognizing PAMPs, the innate immune system is capable of sensing fungi and bacteria via the zymogen Persephone. Virulence factors (proteases) secreted from microbes cleave Persephone, resulting in activation of SPE. At several points in the pathways shown, serpins are known to provide negative regulation of these immune protease cascades. Necrotic inhibits Persephone and Spn1 inhibits upstream of Grass, with ModSP a likely target.
For bacteria, peptidoglycan (PGN) acts as the PAMP in Drosophila. PGN, a major bacterial cell wall component, is a polymer assembled from alternating N-acetylmuramic acid and N-acetylglucosamine subunits. Short stem peptides cross-link the sugar chains and exhibit sequence variation characteristic of broad groups of bacteria. The Toll pathway is activated specifically by PGN in which lysine occupies the third position of the stem peptide. This Lys-type PGN is found in most, but not all, Gram-positive bacteria. Recognition involves the combined activity of two peptidoglycan recognition proteins (PGRP-SA and PGRP-SD) and a GNBP family member, GNBP1 (Bischoff et al., 2004; Buchon et al., 2009; Gobert et al., 2003; Michel et al., 2001).
Downstream of recognition, the signaling pathways that respond to β-1,3-glucans and Lys-type PGN merge. Binding of recognition proteins to either class of PAMP triggers activation of the modular serine protease (ModSP) (Buchon et al., 2009). ModSP in turn activates another serine protease, Grass (El Chamy et al., 2008; Kambris et al., 2006). The cascade continues with activation of Spätzle processing enzyme (SPE), which cleaves Spz, generating a functional Toll ligand (Jang et al., 2006). RNAi-based experiments indicate that the pathway linking Grass to SPE likely involves additional serine protease family members, including Spirit (serine protease immune response integrator), Spheroide, and Sphinx (the Sphinx1 and Sphinx2 proteins) (Kambris et al., 2006).
As diagrammed in Fig. 2, there is a marked parallel between innate immune responses and dorsoventral axis formation in the proteolytic processing of Spätzle to activate Toll signaling. From this perspective, SPE occupies the same position in the hierarchy as Easter, the terminal serine protease in the extracellular cascade in dorsoventral patterning (Chasan and Anderson, 1989; DeLotto and DeLotto, 1998). SPE has 44% overall amino acid identity with Easter and the two enzymes act at the identical site in Spätzle – 106 residues from the C-terminus (Jang et al., 2006).
Conservation between Toll activation in immunity and development is not limited to processing of Spätzle. Both pathways require proteases that contain a clip domain. This motif, first identified in a pro-clotting enzyme of the horseshoe crab, plays a central role in cascades of sequential protease activation (Muta et al., 1990; Piao et al., 2005). The immune factors Grass, Spirit, and SPE each contain a clip domain, as do the developmental factors Easter and Snake (see Fig. 2).
3.2 Danger signals
PAMP-based recognition has a shortcoming: it is ill suited for distinguishing between commensal and pathogenic microbes within body fluids. One evolutionary solution to this problem is the detection of danger signals such as virulence factors and, in some cases, their sequelae – molecules released upon the disruption of host epithelial or cellular integrity.
The Toll pathway is now known to recognize danger signals in addition to PAMPs. The particular danger signals that activate the fly Toll pathway are secreted proteases of fungi and bacteria (El Chamy et al., 2008; Gottar et al., 2006). Microbes use such proteases to degrade adherence junctions, enabling penetration of the epithelial barrier. Proteases are also key to the virulent attack of Drosophila and other insects by entomopathogenic fungi, which invade the insect body by boring through the insect cuticle. The protease Pr1, for example, has a crucial role in this invasive process by the fungi B. bassiana and Metarhizium anisopliae.
Detection of protease danger signals in Drosophila relies on Persephone (Psh), itself a serine protease (Ligoxygakis et al., 2002b). The interaction of the zymogen form of Persephone with microbial proteases that have subtilisin-like activity leads to cleavage and activation of Persephone. Once activated, Persephone directly cleaves and activates SPE, as shown in Fig. 2.
Some Gram-negative bacteria, including those belonging to the genera Pseudomonas and Serratia, secrete proteolytic enzymes. It may be that Persephone also detects these proteases and that this detection underlies the limited, but detectable, responsiveness of the Toll pathway to particular Gram-negative species (El Chamy et al., 2008).
Persephone-mediated detection of danger signals and the recognition and response to PAMPs involving ModSP and Grass each have a significant in vivo role in regulating Toll responses. Whereas Toll signaling remains readily observable in mutants lacking either psh or grass function, knocking out both systems eliminates Toll pathway activity (El Chamy et al., 2008).
Initiation of innate immune Toll signaling is subject to negative regulation at several points. In particular, serpins (serine protease inhibitors) inhibit the activity of a number of key immune response proteases. For example, the serpin Necrotic (nec or Spn43Ac) helps maintain Persephone in an inactive state in the absence of infection. This inhibitory control by Necrotic is essential to normal immune homeostasis: Toll activation occurs in response to either loss of Necrotic function (Levashina et al., 1999) or overexpression of Persephone (Ligoxygakis et al., 2002b).
Serpin activity is also important in regulating the response to fungal PAMPs. In particular, the serpin Spn1 (Spn42Dd) has a significant antagonistic role in the response to fungal cell wall components. Inactivation of the Spn1 increases induction of Toll regulated response loci, whereas overexpression of Spn1 inhibits signaling in response to fungal infection (Fullaondo et al., 2011). Epistasis analysis places Spn1 upstream of Grass and downstream of GNBP3, making ModSP a likely target (see Fig. 2).
4. Drosophila humoral immunity
A hallmark of insect innate immunity is the rapid and massive induction of antimicrobial peptide (AMP) genes (Steiner et al., 1981). Released into insect hemolymph, which acts as both circulatory and interstitial fluid, AMPs kill microbes or block their growth by disrupting membrane integrity. In Drosophila, there are roughly 20 AMP loci; most encode small (<100 residues), secreted, cationic peptides. Induction is remarkably robust, with the concentration of the anti-fungal AMP Drosomycin reaching 100 μM in the hemolymph (Fehlbaum et al., 1994).
Two Drosophila recognition and response pathways trigger induction of AMP genes and other immune loci upon systemic infection. One is the Toll pathway, the other is the Imd (immune deficiency) pathway (Lemaitre et al., 1995a; Rutschmann et al., 2000; Silverman et al., 2000). The Imd pathway is specifically activated by bacterial peptidoglycan that contains meso-diaminopimelic acid (DAP) at the third position of the stem peptide (Choe et al., 2002; Kaneko and Silverman, 2005; Kaneko et al., 2006). This DAP-type PGN structure is characteristic of all Gram-negative bacteria and a few Gram-positive genera, including Bacillus and Clostridium. The Imd pathway thus detects a group of bacteria complementary to those detected by the Toll pathway.
In composition and in mechanism, the Imd pathway bears similarity to the mammalian TNFR1 pathway. Upon binding DAP-type PGN, the transmembrane receptor PGRP-LC alone, or in coordination with the related protein PGRP-LE, recruits Imd, a homolog of the mammalian Receptor Interacting Protein (RIP) (Choe et al., 2005; Ferrandon et al., 2007; Georgel et al., 2001). Activated Imd then signals through a branched pathway that includes orthologs of the mammalian TAK protein (a MAP kinase kinase kinase) and IKK (IκB kinase) proteins (Lu et al., 2001; Silverman et al., 2003). The target of signaling is the transcription factor Relish, which, like mammalian p105, contains an N-terminal Rel domain and a C-terminal IκB-like autoinhibitory domain. Acting in part via a caspase that cleaves a target site between these domains, the pathway effects Relish activation (Erturk-Hasdemir et al., 2009; Meinander et al., 2012; Stoven et al., 2003).
Flies deficient for both the Imd and Toll pathways fail to induce any of the known antimicrobial peptides and succumb readily to infection (Tzou et al., 2002). Moreover, activation of either pathway by, for example, overexpressing a pathway component, is sufficient to trigger AMP expression in the absence of infection (Georgel et al., 2001; Tauszig-Delamasure et al., 2002). Toll and Imd are thus necessary and sufficient for the major humoral response of Drosophila.
In response to infection, the Toll and Imd pathways each direct expression of a set of immune response loci. Some loci are pathway specific, whereas others can be induced by either the Toll pathway or the Imd pathway (De Gregorio et al., 2001; De Gregorio et al., 2002; Hedengren-Olcott et al., 2004; Imler et al., 2004; Irving et al., 2001; Lemaitre et al., 1997). Are the responses matched to the distinct inducers? Yes, at least in some cases. For example, fungi activate Toll, but not Imd, signaling and it is Toll that directs expression of the AMPs with the best-characterized antifungal activity in vitro – Metchnikowin and Drosomycin (Fehlbaum et al., 1994; Levashina et al., 1995).
Do Drosomycin and Metchnikowin in fact have a major role in the fly’s antifungal defense? Answering such a question about the in vivo function of particular AMP loci is not trivial. Genetic screens focused on immunity have not yielded loss-of-function mutations in individual AMP genes. The failure of AMP loci to be identified in this way likely reflects a combination of the screening strategies employed, the small target size of individual AMP genes, and the expected functional redundancy among sets of related AMPs. There has been, however, one elegant and informative study that circumvented this problem. In it, individual AMP loci were heterologously expressed in flies defective for both Toll and Imd signaling. By itself, this immunodeficient background rendered lethal an infection by many bacteria and fungi to which wild-type flies are resistant (Tzou et al., 2002). In this background, expression of Drosomycin alone restored wild-type resistance to the fungus Neurospora crassa, demonstrating that Drosomycin contributes significantly to anti-fungal defenses induced by the Toll pathway.
How do Toll and Imd direct expression of distinct but overlapping gene sets? Each pathway regulates a Rel family transcription factor: Dif (or Dorsal) alone is sufficient to mediate Toll responses and Relish alone is sufficient to mediate Imd responses. Each binds to sequence motifs in the DNA termed κB sites. It turns out that a regulatory code based on site number and sequence governs regulation of AMP genes and other innate immune loci by Toll and Imd (Busse et al., 2007). Loci regulated only by the Toll pathway have a single κB site characterized by a GGGAA consensus half-site. In contrast, loci under only Imd control have multiple κB sites with a consensus half-site sequence of GGGGA. Bioinformatic analyses, in vitro binding studies, and experiments with transfected cultured cells confirm the validity of this code (Busse et al., 2007).
Some innate immune loci are regulated by both Toll and Imd signaling (De Gregorio et al., 2001; De Gregorio et al., 2002). How is their expression regulated? There are two competing hypotheses. One is that Dif (or Dorsal) induced by Toll heterodimerizes with Relish induced by Imd, as observed when expression of both factors is driven in the same cells (Han and Ip, 1999; Tanji et al., 2007; Tanji et al., 2010). The resulting heterodimer would be analogous in structure to NF-κB, which is comprised of p65 and p50, and would have a DNA binding specificity distinct from either subunit. Dif and Dorsal closely resemble p65 in structure and sequence, and the cleaved form of Relish is comparable to the p50 cleavage product of p105. Furthermore, a tethered chimera of Dif and Relish is active in vivo (Tanji et al., 2010). It is likely, however, that the individual domains of the chimera can form intermolecular homodimers, which could be responsible for the observed activity. Moreover, heterodimers of Dif and Relish appear to be scarce in fly cells (Han and Ip, 1999), unlike the near ubiquity of p50/p65 heterodimers in mammalian cells expressing both proteins.
The alternative hypothesis for co-regulation of loci by Toll and Imd is the presence of κB sites that separately recruit Dif and Relish to the same promoter proximal region (Busse et al., 2007). This model, illustrated in Fig. 3, is consistent with site analysis of loci subject to dual regulation. Assuming κB sites act cooperatively, this model, like the alternative, can readily explain the synergistic effect on some loci of activating both pathways (Busse et al., 2007) (Tanji et al., 2010).
Fig. 3.
Specificity and synergy in Toll and Imd signaling. The Toll and Imd pathways recognize distinct PAMPs and generate distinct responses, with either separate or coordinate regulation of transcriptional outputs. In the case of fungi and most Gram-positive bacteria, cell wall components (β-1,3,-glucan from fungi; Lys-type peptidoglycan from bacteria) are recognized by extracellular pathogen recognition receptors and the signal is transduced through protease cascades to activate Toll pathway signaling. A pure Toll response involves binding of a homodimer of the transcription factor Dif or Dorsal, typically at a single Toll-specific κB site (κB-T) upstream of Toll-responsive genes. In the case of Gram-negative bacteria, and select Gram-positive species, polymeric DAP-type peptidoglycan (PGN) is recognized by a dimer of PGRP-LCx and an extracellular version of PGRP-LE (containing only the PGRP domain) to activate Imd signaling. A pure Imd response involves two or more homodimers of the transcription factor Relish binding at neighboring Imd-specific κB sites (κB-I) upstream of Imd-responsive genes. In the event that both pathways are stimulated, Toll- and Imd-regulated Rel proteins can cooperatively regulate a third set of genes. The promoters of such dual-responsive genes contain neighboring Toll-specific and Imd-specific κB sites, where homodimers of Dif or Dorsal and of Relish, respectively, can bind to effect transcription. IM1: Immune-induced molecule 1. Def: Defensin. DptA: Diptericin A.
5. A non-conventional pathway for Toll-related receptors
The Toll gene family has multiple members in the majority of animal species. To date, the sea urchin (Strongylocentrotus purpuratus), which has 253 Toll loci, represents the zenith for documented duplication and divergence (Buckley and Rast, 2012; Buckley and Smith, 2007). Humans have ten TLRs, each functioning as a PRR. TLR4, for example, recognizes LPS, whereas TLR-3, -7,-8, and-9 recognize viral nucleic acids (Kawai and Akira, 2011; Poltorak et al., 1998).
Flies encode eight Toll-related receptors (Toll-2 to Toll-9) in addition to Toll itself. Furthermore flies have five genes encoding homologs of Spätzle. It might therefore seem reasonable to hypothesize that distinct pairs of Spz and Toll related proteins mediate particular immune responses to particular pathogens. Yet until recently there had been little evidence to support such a hypothesis. A mutation mapping to Toll-2 (18-wheeler) impaired immune induced AMP expression, but turned out to perturb development of immune tissues and perhaps affect a neighboring gene rather than Toll-2 (Ligoxygakis et al., 2002a; Williams et al., 1997). The Toll-related receptor Toll-9 was suggested to activate a constitutive innate immune defense (Ooi et al., 2002), but loss of Toll-9 function does not lead to an antibacterial response defect (Narbonne-Reveau et al., 2011). Furthermore, experiments reveal an effect on immune responses of inactivating Toll, but little effect of inactivating nearly all of the Toll-related receptors (Bilak et al., 2003; Luo et al., 2001; Tauszig et al., 2000; Yagi et al., 2010).
One insightful way to address the function of the fly Toll-related receptors comes from considering downstream adaptors. Mammals have four adaptors required for signaling by one or more TLRs: MyD88, TRIF, TRAM, and Mal (also known as TIRAP). Of the four, only MyD88 is found in flies. Significantly, an inactivating mutation in MyD88 has the same phenotypes in a range of immune function assays as a loss-of-function mutation in Toll. Mutations in Tube and Pelle similarly resemble Toll mutations. It thus appears that the immune function of the conventional Toll pathway is provided exclusively by Toll, and not the fly Toll-related receptors.
Could fly Toll-related receptors signal through a non-conventional pathway? A potential breakthrough in this regard came from a study on the immune response in the fly trachea. It had been shown that the Imd pathway controls AMP production in tracheae, as in many other epithelial tissues (Ferrandon et al., 1998; Wagner et al., 2008). However, recent experiments provide evidence that one of the fly Toll-related receptors, Toll-8, negatively regulates this function of Imd (Akhouayri et al., 2011). Inactivation of Toll-8 significantly increases the strength of the immune response in trachea and, in particular, the transcription of AMP loci induced by the Imd pathway. Furthermore, providing Toll-8 activity constitutively inhibits Imd-mediated AMP expression.
Although Toll-8 was found to inhibit Imd signaling in trachea, mutating MyD88 had no effect. Furthermore, neither Tube nor Pelle is expressed in this tissue (Wagner et al., 2008). How then does Toll-8 carries out its inhibitory role? The answer apparently lies in the fly homolog of SARM, the broadly conserved Sterile-Alpha and Armadillo Motif protein (Kenny and O’Neill, 2008; Mink et al., 2001). In mammals, SARM does not promote TLR signaling or other immune responses, but instead exerts an inhibitory effect on TLR3 and TLR4 pathways (Peng et al., 2010; Yuan et al., 2010). When SARM (Ect4) was inactivated in Drosophila trachea, the effect was the same as blocking Toll-8 function (Akhouayri et al., 2011).
Filling out the story further, inactivating mutations in spz2 (the fly neurotrophin 1 gene) behave identically to mutations in Toll-8 or SARM, i.e., they enhance the strength of the Imd response (Akhouayri et al., 2011). Furthermore, constitutive overexpression of Spz2 or of SARM suppressed Imd-mediated AMP expression. Thus, for the first time there is evidence for the function of a fly Toll-related receptor in a non-conventional signaling pathway.
How might the Spz2/Toll-8/SARM pathway function? Although the exact activity of SARM in the mammalian TLR3 and TLR4 pathways is not known, experiments demonstrate that it binds to the TLR adaptor TRIF and prevents signaling from TRIF to RIP (Carty et al., 2006). Given that PGRP-LC, like TRIF, contains a RHIM (RIP homotypic interaction motif) domain and that Imd is a RIP homolog, it seems reasonable to speculate that SARM similarly interferes with the recruitment and activation of Imd by PGRP-LC. This interaction might help maintain the Imd pathway in an inactive state in the absence of infection or provide a route for down-regulation after an initial induction.
Is the non-conventional pathway mediated by Toll-8 dedicated to immunity? Probably not. Toll-8 was previously implicated in neuron-specific glycosylation and neural patterning during embryogenesis (Ayyar et al., 2007; Seppo et al., 2003). Furthermore, Drosophila SARM was identified in a screen for mutations that inhibit Wallerian degeneration – the disintegration and death of an axon that has been severed from the neuron cell body (Osterloh et al., 2012). There is also good evidence that SARM functions in neuronal tissue in other organisms, including Caenorhabditis elegans, which lacks canonical Toll signaling in immunity (Chuang and Bargmann, 2005; Couillault et al., 2004).
What of the remaining Drosphila Toll-related receptors? At least one, Toll-7, appears to function in antiviral defenses. Major antiviral defenses in flies include the Dicer-2 mediated response to dsRNA (RNAi) and activation of the JAK-STAT pathway (Deddouche et al., 2008; Dostert et al., 2005; Wang et al., 2010). One of the additional antiviral defenses involves autophagy in response to infection by particular viruses, such as vesicular stomatitis virus (VSV) (Shelly et al., 2009). A recent study indicates that Toll-7 activates antiviral autophagy and that inactivating Toll-7 in VSV-infected flies increases viral RNA production (Nakamoto et al., 2012). Whether SARM and a Spz family member also participate has not yet been established.
Conclusions
Work in the last few years has clarified a number of questions regarding the composition and organization of the canonical Toll pathway. There remain, however, underexplored areas. Toll signaling in embryos and larvae contributes to hematopoiesis and blood cell survival, as well as to patterning at the neuromuscular junction, but the nature of these contributions is as yet ill defined (Halfon and Keshishian, 1998; Heckscher et al., 2007; Matova and Anderson, 2010; Qiu et al., 1998). Furthermore, RNAseq data reveal robust Dorsal expression in adult males, suggesting that Dorsal’s function extends beyond maternally directed embryonic patterning and larval innate immunity.
Why evolution resulted in PAMP binding by mammalian TLRs but an endogenous ligand for Drosophila Toll remains a subject of lively debate. One idea is that the presence of protease cascades upstream of fly Toll might indicate a past fusion of the Toll pathway with a more ancient defense cascade, such as that triggering melanization (Cerenius et al., 2010). It is worth noting in this regard that recent studies of wound healing in flies have demonstrated an interrelationship of the wound response pathways both with protease cascades and with Toll signaling (Juarez et al., 2011; Markus et al., 2005).
Work on the Toll-related receptors and their alternative signaling in flies is in its early stages. Is Spz2 a Toll-8 ligand? It seems likely, although this pairing was not anticipated by structural considerations of the Toll-related receptors (Gangloff et al., 2013). Does SARM act in conjunction with other fly Toll-related receptors? The idea is certainly appealing and is consistent with the fact that Drosophila SARM, unlike Toll, is an essential gene. Are there additional components to the non-conventional pathway? One expects so, but only time, and more experiments, will tell.
Conservation in Toll signaling extends to the death domain oligomeric interactions
Bacteria and fungi induce Drosophila Toll activity via both PAMPs and danger signals
The Toll and Imd pathways exhibit specificity in activation as well as response
Drosophila Spz2, Toll-8, and SARM form a non-conventional inhibitory Toll pathway
Acknowledgments
Authors are supported by NIH grant RO1 GM05054516 (to S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhouayri I, Turc C, Royet J, Charroux B. Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium. PLoS Pathog. 2011;7:e1002319. doi: 10.1371/journal.ppat.1002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV, Nusslein VC. Information for the dorsal-ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984;311:223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- Ayyar S, Pistillo D, Calleja M, Brookfield A, Gittins K, Goldstone C, Simpson P. NF-kappaB/Rel-mediated regulation of the neural fate in Drosophila. PLoS One. 2007;2:e1178. doi: 10.1371/journal.pone.0001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Ann Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Bilak H, Tauszig-Delamasure S, Imler JL. Toll and Toll-like receptors in Drosophila. Biochem Soc Trans. 2003;31:648–651. doi: 10.1042/bst0310648. [DOI] [PubMed] [Google Scholar]
- Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, Royet J. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. doi: 10.1038/ni1123. [DOI] [PubMed] [Google Scholar]
- Brennan CA, Anderson KV. Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol. 2004;22:457–483. doi: 10.1146/annurev.immunol.22.012703.104626. [DOI] [PubMed] [Google Scholar]
- Buchon N, Poidevin M, Kwon HM, Guillou A, Sottas V, Lee BL, Lemaitre B. A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A. 2009;106:12442–12447. doi: 10.1073/pnas.0901924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Rast JP. Dynamic evolution of toll-like receptor multigene families in echinoderms. Front Immunol. 2012;3:136. doi: 10.3389/fimmu.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley KM, Smith LC. Extraordinary diversity among members of the large gene family, 185/333, from the purple sea urchin, Strongylocentrotus purpuratus. BMC Mol Biol. 2007;8:68. doi: 10.1186/1471-2199-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse MS, Arnold CP, Towb P, Katrivesis J, Wasserman SA. A kappaB sequence code for pathway-specific innate immune responses. The EMBO journal. 2007;26:3826–3835. doi: 10.1038/sj.emboj.7601798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- Cerenius L, Kawabata S, Lee BL, Nonaka M, Soderhall K. Proteolytic cascades and their involvement in invertebrate immunity. Trends in biochemical sciences. 2010;35:575–583. doi: 10.1016/j.tibs.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Chasan R, Anderson KV. The role of easter, an apparent serine protease, in organizing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1989;56:391–400. doi: 10.1016/0092-8674(89)90242-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen ZJ. Regulation of NF-kappaB by ubiquitination. Curr Opin Immunol. 2013;25:4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Lee H, Anderson KV. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1122–1126. doi: 10.1073/pnas.0404952102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:359–362. doi: 10.1126/science.1070216. [DOI] [PubMed] [Google Scholar]
- Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes & development. 2005;19:270–281. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci U S A. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deddouche S, Matt N, Budd A, Mueller S, Kemp C, Galiana-Arnoux D, Dostert C, Antoniewski C, Hoffmann JA, Imler JL. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9:1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- DeLotto Y, DeLotto R. Proteolytic processing of the Drosophila Spatzle protein by easter generates a dimeric NGF-like molecule with ventralising activity. Mechanisms of development. 1998;72:141–148. doi: 10.1016/s0925-4773(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Dionne MS, Schneider DS. Screening the fruitfly immune system. Genome biology. 2002;3 doi: 10.1186/gb-2002-3-4-reviews1010. REVIEWS1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostert C, Jouanguy E, Irving P, Troxler L, Galiana-Arnoux D, Hetru C, Hoffmann JA, Imler JL. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- Drier EA, Steward R. The dorsoventral signal transduction pathway and the Rel-like transcription factors in Drosophila. Seminars in Cancer Biology. 1997;8:83–92. doi: 10.1006/scbi.1997.0059. [DOI] [PubMed] [Google Scholar]
- El Chamy L, Leclerc V, Caldelari I, Reichhart JM. Sensing of ‘danger signals’ and pathogen-associated molecular patterns defines binary signaling pathways ‘upstream’ of Toll. Nat Immunol. 2008;9:1165–1170. doi: 10.1038/ni.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erturk-Hasdemir D, Broemer M, Leulier F, Lane WS, Paquette N, Hwang D, Kim CH, Stoven S, Meier P, Silverman N. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9779–9784. doi: 10.1073/pnas.0812022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, Hoffmann JA. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nature reviews Immunology. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffmann JA. A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. Embo J. 1998;17:1217–1227. doi: 10.1093/emboj/17.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullaondo A, Garcia-Sanchez S, Sanz-Parra A, Recio E, Lee SY, Gubb D. Spn1 regulates the GNBP3-dependent Toll signaling pathway in Drosophila melanogaster. Mol Cell Biol. 2011;31:2960–2972. doi: 10.1128/MCB.01397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Current topics in microbiology and immunology. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff M, Arnot CJ, Lewis M, Gay NJ. Functional insights from the crystal structure of the N-terminal domain of the prototypical toll receptor. Structure. 2013;21:143–153. doi: 10.1016/j.str.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff M, Murali A, Xiong J, Arnot CJ, Weber AN, Sandercock AM, Robinson CV, Sarisky R, Holzenburg A, Kao C, Gay NJ. Structural insight into the mechanism of activation of the Toll receptor by the dimeric ligand Spatzle. J Biol Chem. 2008;283:14629–14635. doi: 10.1074/jbc.M800112200. [DOI] [PubMed] [Google Scholar]
- Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- Gobert V, Gottar M, Matskevich AA, Rutschmann S, Royet J, Belvin M, Hoffmann JA, Ferrandon D. Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science. 2003;302:2126–2130. doi: 10.1126/science.1085432. [DOI] [PubMed] [Google Scholar]
- Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind S. Innate immunity in Drosophila: Pathogens and pathways. Insect Sci. 2008;15:29–43. doi: 10.1111/j.1744-7917.2008.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfon MS, Keshishian H. The Toll pathway is required in the epidermis for muscle development in the Drosophila embryo. Dev Biol. 1998;199:164–174. doi: 10.1006/dbio.1998.8915. [DOI] [PubMed] [Google Scholar]
- Han ZS, Ip YT. Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. Journal of Biological Chemistry. 1999;274:21355–21361. doi: 10.1074/jbc.274.30.21355. [DOI] [PubMed] [Google Scholar]
- Heckscher ES, Fetter RD, Marek KW, Albin SD, Davis GW. NF-kappaB, IkappaB, and IRAK control glutamate receptor density at the Drosophila NMJ. Neuron. 2007;55:859–873. doi: 10.1016/j.neuron.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedengren-Olcott M, Olcott MC, Mooney DT, Ekengren S, Geller BL, Taylor BJ. Differential activation of the NF-kappaB-like factors relish and Dif in drosophila melanogaster by fungi and gram-positive bacteria. J Biol Chem. 2004 doi: 10.1074/jbc.M313856200. In press. [DOI] [PubMed] [Google Scholar]
- Horng T, Medzhitov R. Drosophila MyD88 is an adapter in the Toll signaling pathway. Proc Natl Acad Sci U S A. 2001;98:12654–12658. doi: 10.1073/pnas.231471798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HR, Chen ZJ, Kunes S, Chang GD, Maniatis T. Endocytic pathway is required for Drosophila Toll innate immune signaling. Proc Natl Acad Sci U S A. 2010;107:8322–8327. doi: 10.1073/pnas.1004031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler JL, Ferrandon D, Royet J, Reichhart JM, Hetru C, Hoffmann JA. Toll-dependent and Toll-independent immune responses in Drosophila. J Endotoxin Res. 2004;10:241–246. doi: 10.1179/096805104225005887. [DOI] [PubMed] [Google Scholar]
- Ip YT, Kraut R, Levine M, Rushlow CA. The dorsal morphogen is a sequence-specific DNA-binding protein that interacts with a long-range repression element in Drosophila. Cell. 1991;64:439–446. doi: 10.1016/0092-8674(91)90651-e. [DOI] [PubMed] [Google Scholar]
- Ip YT, Reach M, Engstrom Y, Kadalayil L, Cai H, Gonzalez-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, Hoffmann JA, Hetru C. A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci U S A. 2001;98:15119–15124. doi: 10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, Ochiai M, Kambris Z, Brun S, Hashimoto C, Ashida M, Brey PT, Lee WJ. A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Juarez MT, Patterson RA, Sandoval-Guillen E, McGinnis W. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet. 2011;7:e1002424. doi: 10.1371/journal.pgen.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125:943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kambris Z, Brun S, Jang IH, Nam HJ, Romeo Y, Takahashi K, Lee WJ, Ueda R, Lemaitre B. Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol. 2006;16:808–813. doi: 10.1016/j.cub.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol. 2005;7:461–469. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- Kanodia JS, Rikhy R, Kim Y, Lund VK, DeLotto R, Lippincott-Schwartz J, Shvartsman SY. Dynamics of the Dorsal morphogen gradient. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21707–21712. doi: 10.1073/pnas.0912395106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Delhase M. The IκB kinase (IKK) and NF-κB: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Kenny EF, O’Neill LA. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Kim T, Kim YJ. Overview of innate immunity in Drosophila. J Biochem Mol Biol. 2005;38:121–127. doi: 10.5483/bmbrep.2005.38.2.121. [DOI] [PubMed] [Google Scholar]
- Kuttenkeuler D, Pelte N, Ragab A, Gesellchen V, Schneider L, Blass C, Axelsson E, Huber W, Boutros M. A large-scale RNAi screen identifies Deaf1 as a regulator of innate immune responses in Drosophila. J Innate Immun. 2010;2:181–194. doi: 10.1159/000248649. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proceedings of the National Academy of Sciences of the United States of America. 1995a;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Meister M, Govind S, Georgel P, Steward R, Reichhart JM, Hoffmann JA. Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO Journal. 1995b;14:536–545. doi: 10.1002/j.1460-2075.1995.tb07029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Reichhart JM, Hoffmann JA. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc Natl Acad Sci U S A. 1997;94:14614–14619. doi: 10.1073/pnas.94.26.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Parquet C, Pili-Floury S, Ryu JH, Caroff M, Lee WJ, Mengin-Lecreulx D, Lemaitre B. The Drosophila immune system detects bacteria through specific peptidoglycan recognition. Nat Immunol. 2003;4:478–484. doi: 10.1038/ni922. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Ohresser S, Bulet P, Reichhart JM, Hetru C, Hoffmann JA. Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal properties. Eur J Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Bulet P, Reichhart JM. Critical evaluation of the role of the Toll-like receptor 18-Wheeler in the host defense of Drosophila. EMBO Rep. 2002a;3:666–673. doi: 10.1093/embo-reports/kvf130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002b;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu LP, Anderson KV. The antibacterial arm of the drosophila innate immune response requires an IkappaB kinase. Genes Dev. 2001;15:104–110. doi: 10.1101/gad.856901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund VK, DeLotto Y, DeLotto R. Endocytosis is required for Toll signaling and shaping of the Dorsal/NF-kappaB morphogen gradient during Drosophila embryogenesis. Proc Natl Acad Sci U S A. 2010;107:18028–18033. doi: 10.1073/pnas.1009157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Shen B, Manley JL, Zheng L. Tehao functions in the Toll pathway in Drosophila melanogaster: possible roles in development and innate immunity. Insect Mol Biol. 2001;10:457–464. doi: 10.1046/j.0962-1075.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Reichhart JM, Steward R, Hoffmann JA, Lemaitre B. A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J. 1999;18:3380–3391. doi: 10.1093/emboj/18.12.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek LR, Kagan JC. Phosphoinositide binding by the Toll adaptor dMyD88 controls antibacterial responses in Drosophila. Immunity. 2012;36:612–622. doi: 10.1016/j.immuni.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101:108–111. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Matova N, Anderson KV. Drosophila Rel proteins are central regulators of a robust, multi-organ immune network. Journal of cell science. 2010;123:627–633. doi: 10.1242/jcs.060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matskevich AA, Quintin J, Ferrandon D. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur J Immunol. 2010;40:1244–1254. doi: 10.1002/eji.200940164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinander A, Runchel C, Tenev T, Chen L, Kim CH, Ribeiro PS, Broemer M, Leulier F, Zvelebil M, Silverman N, Meier P. Ubiquitylation of the initiator caspase DREDD is required for innate immune signalling. The EMBO journal. 2012;31:2770–2783. doi: 10.1038/emboj.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Khanuja BS, Ip YT. Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev. 1999;13:792–797. doi: 10.1101/gad.13.7.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. doi: 10.1038/414756a. [DOI] [PubMed] [Google Scholar]
- Mink M, Fogelgren B, Olszewski K, Maroy P, Csiszar K. A novel human gene (SARM) at chromosome 17q11 encodes a protein with a SAM motif and structural similarity to Armadillo/beta-catenin that is conserved in mouse, Drosophila, and Caenorhabditis elegans. Genomics. 2001;74:234–244. doi: 10.1006/geno.2001.6548. [DOI] [PubMed] [Google Scholar]
- Moncrieffe MC, Grossmann JG, Gay NJ. Assembly of oligomeric death domain complexes during Toll receptor signaling. The Journal of biological chemistry. 2008;283:33447–33454. doi: 10.1074/jbc.M805427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem. 2009;284:25404–25411. doi: 10.1074/jbc.M109.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muta T, Hashimoto R, Miyata T, Nishimura H, Toh Y, Iwanaga S. Proclotting enzyme from horseshoe crab hemocytes. cDNA cloning, disulfide locations, and subcellular localization. The Journal of biological chemistry. 1990;265:22426–22433. [PubMed] [Google Scholar]
- Nakamoto M, Moy RH, Xu J, Bambina S, Yasunaga A, Shelly SS, Gold B, Cherry S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity. 2012;36:658–667. doi: 10.1016/j.immuni.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne-Reveau K, Charroux B, Royet J. Lack of an antibacterial response defect in Drosophila Toll-9 mutant. PLoS One. 2011;6:e17470. doi: 10.1371/journal.pone.0017470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Frohnhofer HG, Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987;238:1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Ooi JY, Yagi Y, Hu X, Ip YT. The Drosophila Toll-9 activates a constitutive antimicrobial defense. EMBO Rep. 2002;3:82–87. doi: 10.1093/embo-reports/kvf004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv Exp Med Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- Peng J, Yuan Q, Lin B, Panneerselvam P, Wang X, Luan XL, Lim SK, Leung BP, Ho B, Ding JL. SARM inhibits both TRIF- and MyD88-mediated AP-1 activation. European journal of immunology. 2010;40:1738–1747. doi: 10.1002/eji.200940034. [DOI] [PubMed] [Google Scholar]
- Piao S, Song YL, Kim JH, Park SY, Park JW, Lee BL, Oh BH, Ha NC. Crystal structure of a clip-domain serine protease and functional roles of the clip domains. EMBO J. 2005;24:4404–4414. doi: 10.1038/sj.emboj.7600891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. doi: 10.1242/dev.125.10.1909. [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Zhou R, Silverman N, Hoffmann JA, Ferrandon D. Role of Drosophila IKK gamma in a toll-independent antibacterial immune response. Nat Immunol. 2000;1:342–347. doi: 10.1038/79801. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- Seppo A, Matani P, Sharrow M, Tiemeyer M. Induction of neuron-specific glycosylation by Tollo/Toll-8, a Drosophila Toll-like receptor expressed in non-neural cells. Development. 2003;130:1439–1448. doi: 10.1242/dev.00347. [DOI] [PubMed] [Google Scholar]
- Shelly S, Lukinova N, Bambina S, Berman A, Cherry S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity. 2009;30:588–598. doi: 10.1016/j.immuni.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Erlich RL, Hunter M, Bernstein E, Schneider D, Maniatis T. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–48934. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Stoven S, Pandey N, Hultmark D, Maniatis T. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos A, Van Drenth M, Erives A, Markstein M, Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell. 2002;111:687–701. doi: 10.1016/s0092-8674(02)01087-5. [DOI] [PubMed] [Google Scholar]
- Stein DS, Stevens LM. Establishment of dorsal-ventral and terminal pattern in the Drosophila embryo. Current Opinion in Genetics & Development. 1991;1:247–254. doi: 10.1016/s0959-437x(05)80078-4. [DOI] [PubMed] [Google Scholar]
- Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987;238:692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Stoven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engstrom Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci U S A. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Bristow BN, Qu G, Wasserman SA. A heterotrimeric death domain complex in Toll signaling. Proc Natl Acad Sci U S A. 2002;99:12871–12876. doi: 10.1073/pnas.202396399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Towb P, Chiem DN, Foster BA, Wasserman SA. Regulated assembly of the Toll signaling complex drives Drosophila dorsoventral patterning. The EMBO journal. 2004;23:100–110. doi: 10.1038/sj.emboj.7600033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Hu X, Weber AN, Ip YT. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol. 2007;27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Yun EY, Ip YT. Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A. 2010;107:14715–14720. doi: 10.1073/pnas.1009473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Tauszig S, Jouanguy E, Hoffmann JA, Imler JL. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc Natl Acad Sci U S A. 2000;97:10520–10525. doi: 10.1073/pnas.180130797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauszig-Delamasure S, Bilak H, Capovilla M, Hoffmann JA, Imler JL. Drosophila MyD88 is required for the response to fungal and Gram- positive bacterial infections. Nat Immunol. 2002;3:91–97. doi: 10.1038/ni747. [DOI] [PubMed] [Google Scholar]
- Towb P, Galindo RL, Wasserman SA. Recruitment of Tube and Pelle to signaling sites at the surface of the Drosophila embryo. Development. 1998;125:2443–2450. doi: 10.1242/dev.125.13.2443. [DOI] [PubMed] [Google Scholar]
- Towb P, Sun H, Wasserman SA. Tube Is an IRAK-4 homolog in a Toll pathway adapted for development and immunity. J Innate Immun. 2009;1:309–321. doi: 10.1159/000200773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002;99:2152–2157. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valanne S, Myllymaki H, Kallio J, Schmid MR, Kleino A, Murumagi A, Airaksinen L, Kotipelto T, Kaustio M, Ulvila J, Esfahani SS, Engstrom Y, Silvennoinen O, Hultmark D, Parikka M, Ramet M. Genome-wide RNA interference in Drosophila cells identifies G protein-coupled receptor kinase 2 as a conserved regulator of NF-kappaB signaling. J Immunol. 2010;184:6188–6198. doi: 10.4049/jimmunol.1000261. [DOI] [PubMed] [Google Scholar]
- Valanne S, Wang JH, Ramet M. The Drosophila Toll signaling pathway. Journal of immunology. 2011;186:649–656. doi: 10.4049/jimmunol.1002302. [DOI] [PubMed] [Google Scholar]
- Wagner C, Isermann K, Fehrenbach H, Roeder T. Molecular architecture of the fruit fly’s airway epithelial immune system. BMC Genomics. 2008;9:446. doi: 10.1186/1471-2164-9-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Valanne S, Ramet M. Drosophila as a model for antiviral immunity. World J Biol Chem. 2010;1:151–159. doi: 10.4331/wjbc.v1.i5.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman SA. Toll signaling: the enigma variations. Curr Opin Gen Dev. 2000;10:497–502. doi: 10.1016/s0959-437x(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Wasserman SA. Structural biology: Immunity takes a heavy Toll. Nature. 2010;465:882–883. doi: 10.1038/465882a. [DOI] [PubMed] [Google Scholar]
- Weber AN, Gangloff M, Moncrieffe MC, Hyvert Y, Imler JL, Gay NJ. Role of the Spatzle Pro-domain in the generation of an active toll receptor ligand. J Biol Chem. 2007;282:13522–13531. doi: 10.1074/jbc.M700068200. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Rodriguez A, Kimbrell DA, Eldon ED. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. Embo Journal. 1997;16:6120–6130. doi: 10.1093/emboj/16.20.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Towb P, Wasserman SA, Sprang SR. Three-dimensional structure of a complex between the death domains of Pelle and Tube. Cell. 1999;99:545–555. doi: 10.1016/s0092-8674(00)81542-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Nishida Y, Ip YT. Functional analysis of Toll-related genes in Drosophila. Dev Growth Differ. 2010;52:771–783. doi: 10.1111/j.1440-169X.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Wu K, Yang M, Xu L, Huang L, Liu H, Tao X, Huang S, Xu A. Amphioxus SARM involved in neural development may function as a suppressor of TLR signaling. Journal of immunology. 2010;184:6874–6881. doi: 10.4049/jimmunol.0903675. [DOI] [PubMed] [Google Scholar]