Abstract
Recent evidence indicates that Kingella kingae produces a polysaccharide capsule. In an effort to determine the composition and structure of this polysaccharide capsule, in the current study we purified capsular material from the surface of K. kingae strain 269–492 variant KK01 using acidic conditions to release the capsule and a series of steps to remove DNA, RNA, and protein. Analysis of the resulting material by gas chromatography and mass spectrometry revealed N-acetyl galactosamine (GalNAc), 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo), and galactose (Gal). Further analysis by NMR demonstrated two distinct polysaccharides, one consisting of GalNAc and Kdo with the structure →3)-β-GalpNAc-(1→5)-β-Kdop-(2→ and the other containing galactose alone with the structure →5)-β-Galf-(1→. Disruption of the ctrA gene required for surface localization of the K. kingae polysaccharide capsule resulted in elimination of GalNAc and Kdo but had no effect on the presence of Gal in bacterial surface extracts. In contrast, deletion of the pamABCDE locus involved in production of a reported galactan exopolysaccharide eliminated Gal but had no effect on the presence of GalNAc and Kdo in surface extracts. Disruption of ctrA and deletion of pamABCDE resulted in a loss of all carbohydrates in surface extracts. These results establish that K. kingae strain KK01 produces a polysaccharide capsule with the structure →3)-β-GalpNAc-(1→5)-β-Kdop-(2→ and a separate exopolysaccharide with the structure →5)-β-Galf-(1→. The polysaccharide capsule and the exopolysaccharide require distinct genetic loci for surface localization.
Introduction
Kingella kingae is a gram-negative bacterium that has emerged as a common cause of septic arthritis, osteomyelitis, and bacteremia in children 6–36 months of age [1]. Studies using culture-based and molecular-based detection strategies have established that K. kingae is a common commensal in the upper respiratory tract in young children [1]–[4]. In one report, K. kingae colonization of the pharynx was found in approximately 70% of children at some point during the first two years of life [5]. The pathogenesis of K. kingae disease is believed to involve bacterial translocation across the pharyngeal epithelial barrier, entry into the bloodstream, and dissemination to the joints, bones, or endocardium [4]. This pathogenic model is supported by data demonstrating genotypically identical isolates of K. kingae from the upper respiratory tract and either the bloodstream or joint fluid from patients with invasive disease [6], [7].
Extracellular polysaccharides are produced by many pathogenic bacteria and function as important virulence factors. These polysaccharides can be categorized generally as capsular polysaccharides or exopolysaccharides. Capsular polysaccharides are lipid-anchored outer membrane-associated carbohydrates and are involved in protection from host immune mechanisms, including phagocytosis and complement-mediated killing [8], [9]. These polysaccharides often exhibit a large degree of variation within a single bacterial species, with examples including Escherichia coli and Streptococcus pneumoniae [10]–[12]. In select cases, a specific capsule type can be associated with increased virulence potential, as highlighted by Haemophilus influenzae type b [13]. Exopolysaccharides are secreted carbohydrate polymers that are not anchored to the bacterial surface and that play a variety of roles, including modulation of adherence and modulation of biofilm formation [14], [15].
In recent work, we identified a polysaccharide capsule in K. kingae strain 269–492 that influences adhesive interactions with host cells and is dependent upon the ctrABCD ABC-type capsule export operon for surface localization [16]. In the current study, we set out to characterize the composition and structure of this polysaccharide capsule. Glycosyl analysis of bacterial surface extracts by gas chromatography/mass spectrometry and NMR revealed two distinct extracellular polysaccharides, including the polysaccharide capsule and a separate exopolysaccharide. The polysaccharide capsule contains N-acetyl galactosamine (GalNAc) and 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) and has the structure 3→)-β-GalpNAc-(1→5)-β-Kdop-(2→. In contrast, the exopolysaccharide contains only galactose and has the structure →5)-β-Galf-(1→. Targeted mutagenesis established that the capsular polysaccharide and the exopolysaccharide require separate genetic loci for surface localization.
Materials and Methods
Bacterial Strains
The strains used in this study are listed in Table 1. K. kingae strain 269–492 was originally recovered from the joint fluid of a child with septic arthritis at St. Louis Children’s Hospital, St. Louis, MO. K. kingae KK01 is a stable sparsely piliated natural variant of strain 269–492 that forms nonspreading, non-corroding colonies and was used in all experiments in this study because of its colony morphology [17]. K. kingae strains were grown on chocolate agar plates at 37°C with 5% CO2 supplemented with 50 µg/ml kanamycin or 2 µg/ml erythromycin as appropriate. K. kingae strains were stored at −80°C in brain heart infusion with 30% glycerol. E. coli strain DH5α was used for construction of gene disruption plasmids. E. coli strains were routinely grown at 37°C on Luria-Bertani (LB) agar or in LB broth with 100 µg/ml ampicillin, 50 µg/ml kanamycin, or 500 µg/ml erythromycin as appropriate. E. coli strains were stored at −80°C in LB broth with 15% glycerol.
Table 1. Strains used in this study ?.
| Strain | Description | Workcited |
| K. kingae strains | ||
| 269–492 (KK01) | Clinical isolate Spontaneous nonspreading, non-corroding colony variant of 269–492 | [16] |
| KK01pamABCDE. | Deletion of pamABCDE | This work |
| KK01ctrA | Polar insertional mutation resulting in disruption of ctrABCD operon | [16] |
| KK01ctrApamABCDE. | Polar insertional mutation in ctrA and deletion of pamABCDE | This work |
| E. coli strains | ||
| DH5α | E. coli F− ?80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK − mK +) phoA supE441 thi-1 gyrA96 relA1 | [32] |
Strain Construction
Targeted gene disruptions in K. kingae were generated as previously described [16], [18]. Briefly, plasmid-based gene disruption constructs were created in E. coli, linearized, and introduced into K. kingae strain KK01 via natural transformation. Transformants were recovered by plating on chocolate agar plates containing the appropriate antibiotic. Correct localization of gene disruptions was confirmed by PCR. The ctrA disruption was generated as described previously [16] but with the aphA3 kanamycin resistance cassette as the marker. This disruption had a polar effect on the downstream genes in the ctrABCD operon. To delete the pamABCDE locus, fragments corresponding to the surrounding 5′ and 3′ regions of the locus were PCR amplified using the primers pam 5′for (GCGAATTCGGCGTTGGTGGAATATCCTG), pam 5′rev (GCGGATCCACCTTCTGGTCGCTGAAATG), pam 3′for (GCGGATCCTCAAAGGCTGGTATAAACAC), and pam 3′rev (GCAAGCTTCCATATCGCTTTGGCTTTGC), respectively. The resulting fragments were ligated into pUC19, creating puC19pam 5′+3′::BamHI. The ermC erythromycin cassette from pIDN4 was PCR amplified with flanking BamHI sites and ligated into pUC19pam 5′+3′, generating pUC19pam::ermC.
Polysaccharide Extraction and Purification
For small scale capsule extractions, bacteria were washed and resuspended in either Tris acetate pH 5 (acid treatment for one hour) or PBS (heat treatment at 55°C for 1 hour). Cells were removed by centrifugation, and extracts were treated with proteinase K for one hour, and then concentrated as previously described [16].
To prepare strains for extraction of extracellular polysaccharide for purification, lawns of bacteria were grown overnight on chocolate agar plates. Subsequently, bacterial growth was swabbed from plates and resuspended in 50 ml of 1% formaldehyde in PBS to fix cells and allowed to stand for 15 minutes at room temperature. Bacteria were centrifuged at 4,355×g for 10 minutes and then resuspended in 40 ml of 50 mM Tris acetate pH 5. Following vigorous shaking for 1 hour at room temperature, bacteria were pelleted by centrifugation at 12,096×g for 20 minutes. The supernatant was recovered and was then filtered using a 0.22 µm filter. The filtered material was adjusted to pH 7 with 1 M Tris pH 9. To remove contaminating DNA, RNA, and protein, the filtered material was treated with 10 units of DNase (Fermentas) and 0.1 mg of RNase (Fermentas) at 37°C for 5 hours and then with 0.18 mg of proteinase K (Roche) at 55°C overnight. Samples were concentrated to 500 µl using 100 kDa MWCO filters and extracted once with Tris-saturated phenol pH 7.4 and twice with 100% chloroform. Extracted material was dialyzed extensively overnight in deionized water, flash frozen, and lyophilized. To purify exopolysaccharide galactan, we resuspended bacteria in PBS without formaldehyde, shook for one hour, pelleted the bacteria, and subjected the supernatant to the purification described above.
Staining of Polysaccharide
Aliquots of the purified polysaccharide from K. kingae derivatives were separated on 10% SDS-PAGE gels. For Alcian blue staining, gels were stained with 0.125% Alcian blue as previously described [16]. For silver staining, gels were treated as previously described [19].
Chemical Analysis
Monosaccharide composition analysis was performed by methanolysis and trimethylsilyl derivatization as previously described [20].
NMR Spectroscopy
The isolated polysaccharide was deuterium-exchanged by lyophilization from D2O (99.9%D, Aldrich), dissolved in 270 µl D2O (99.96%D, Cambridge Isotope) containing 0.3 µl acetone, and placed in a 5-mm NMR tube with D2O-matched magnetic susceptibility plugs (Shigemi Inc.). One-dimensional proton and 2-D TOCSY and NOESY NMR spectra with solvent presaturation and gradient-enhanced COSY, HSQC, and HMBC NMR spectra were acquired on a Varian Inova 600 spectrometer at 50°C, equipped with a cryogenic triple-resonance probe. Spectral width was 2841 Hz in the proton and 18096 Hz in the carbon dimension. Mixing times were 150 ms for TOCSY and 300 ms for NOESY. The number of increments and scans, respectively were 512 and 4 for COSY, 200 and 8 for TOCSY, 200 and 16 for NOESY, 128 and 64 for HSQC, and 200 and 88 for HMBC. Chemical shifts were measured relative to acetone (δH = 2.218 ppm, δC = 33.0 ppm) [21].
Results
Polysaccharide can be Extracted and Purified from the Surface of K. kingae Strain 269–492 Variant KK01
In order to extract and purify the polysaccharide capsule, we incubated a bacterial suspension of K. kingae strain 269–492 variant KK01 in Tris acetate pH 5, aiming to dissociate the polysaccharide capsule from the bacterial surface by the acidic pH. To confirm that our extraction procedure yielded pure polysaccharide material, we examined the material by Alcian blue straining and silver staining (Figure 1). Alcian blue is a cationic dye and has been used previously to demonstrate the presence of the K. kingae capsule [16]. As shown in Figure 1, the extracted and purified material from the surface of K. kingae KK01 stained prominently with Alcian blue and silver reagents (lane 3), similar to control small scale heat and acid extracts from K. kingae KK01 (lanes 1–2). The Tris acetate extract from KK01ctrA (lacking a functional capsule export locus) failed to stain with either Alcian blue or silver reagents (lane 5), while the PBS surface extract from KK01ctrA stained well with Alcian blue and silver reagents (lane 6).
Figure 1. Staining profile and purity of polysaccharide material used for analysis.
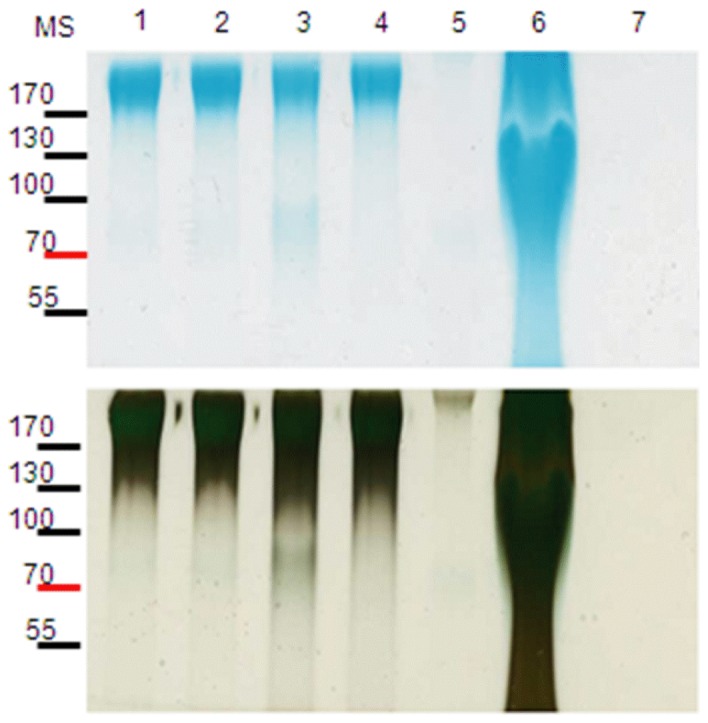
Alcian blue staining is shown on the top, and silver staining is on the bottom. Lane 1, extract from KK01 using heat; lane 2, extract from KK01 using Tris acetate; lane 3, purified material from KK01 using Tris acetate; lane 4, purified material from KK01pamABCDE using Tris acetate; lane 5, purified material from KK01ctrA using Tris acetate; lane 6, purified material from KK01ctrA using surface PBS extraction; lane 7, purified material from KK01ctrA pamABCDE using Tris acetate. MS = molecular size (in kDa).
Kingella kingae KK01 Extracellular Polysaccharide Contains GalNAc, Kdo, and Galactose
As shown in Table 2, composition analysis of the polysaccharide extracted from K. kingae KK01 revealed that the dominant components were GalNAc, Kdo, and galactose in roughly equimolar quantities. In addition, there were small quantities of xylose, glucose, N-acetyl glucosamine, and heptose, all common contaminants in this kind of preparation.
Table 2. Glycosyl composition of extracellular preparations of K. kingae strains used in this study.
| Glycosyl residue | KK01 Trisacetate prep | KK01pamABCDETris acetate prep | KK01ctrA Trisacetate prep | KK01ctrA surfacePBS extraction | KK01ctrA pamABCDETris acetate prep |
| Xylose | 0.3 (4.6) | 1.0 (14.5) | 9.0 (91.1) | 0.1 (1.6) | 10 (100) |
| Galactose | 2.3 (27.1) | – | 0.9 (7.5) | 6.2 (64.5) | – |
| Glucose | 0.2 (1.9) | – | 0.1 (0.8) | 1.8 (18.4) | – |
| N-Acetyl Galactosamine | 3.0 (28.5) | 4.2 (41.3) | – | 0.1 (1.2) | – |
| N-Acetyl Glucosamine | 0.4 (3.4) | – | – | 0.4 (3.4) | – |
| Kdo* | 3.4 (28.8) | 4.8 (43.9) | – | 0.8 (6.0) | – |
| Heptose | 0.6 (5.6) | – | – | 0.5 (4.8) | – |
Mass in µg is listed first, and percentage is shown in parentheses. Mass and percentage are per 10 µg polysaccharide material analyzed.
3-Deoxy-D-Manno-oct-2-ulosonic acid.
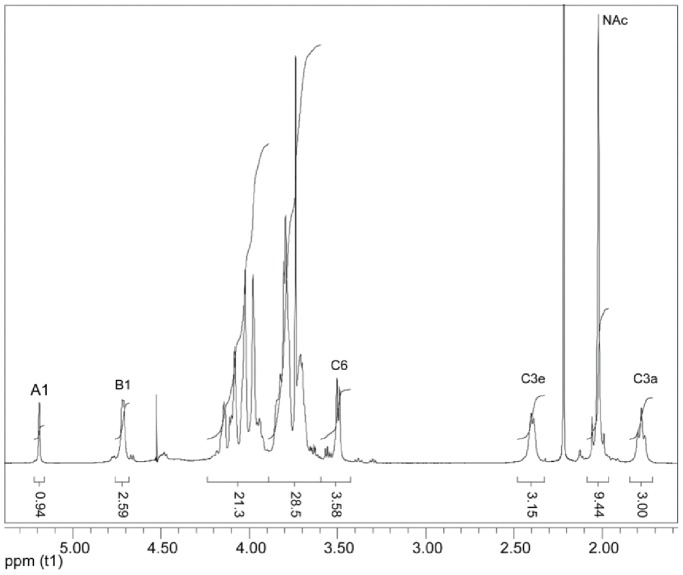
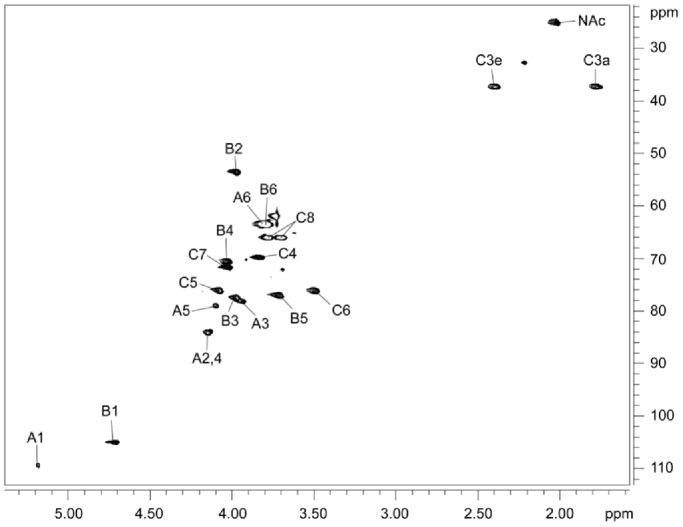
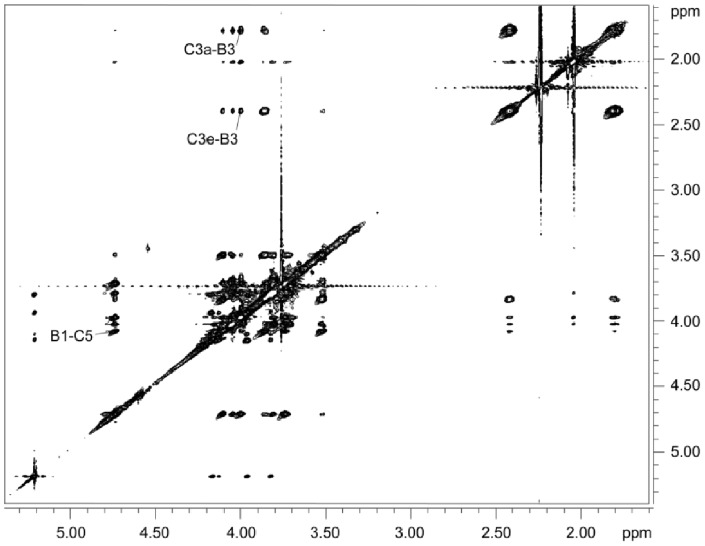
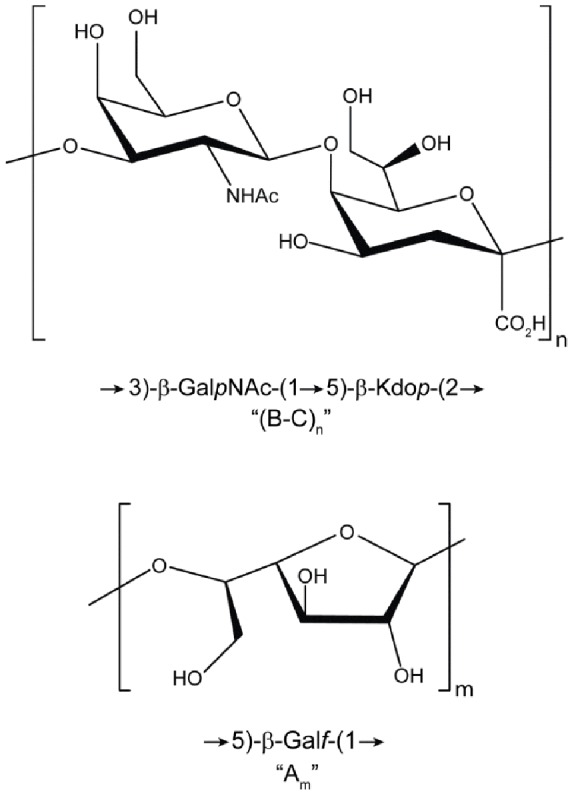
The 1-D proton NMR spectrum of the purified polysaccharide (Figure 2) showed two anomeric signals at 5.18 and 4.70 ppm, with an intensity ratio of 1∶3, and two upfield methylene signals at 2.40 and 1.77 ppm, each of equal intensity with the larger anomeric peak. The HSQC spectrum (Figure 3) showed that the signal at 5.18 ppm was associated with a carbon chemical shift of 109.4 ppm, implying that this residue was a furanose. Chemical shift assignment of the remaining nuclei (Table 3) demonstrated that the furanose had a galacto-configuration, and a 5.9-ppm downfield displacement of its C-5 chemical shift indicated that it was glycosylated in the 5-position. The further chemical shift assignments, based on 2-D COSY, TOCSY, and HSQC spectra, showed that the anomeric signal at 4.70 ppm belonged to a β-GalNAc residue and the methylene signals at 2.40 and 1.77 ppm belonged to a β-Kdo residue. Downfield shifts of the carbon nuclei involved in glycosidic linkages indicated that GalNAc was 3-linked and Kdo was 5-linked (see Table 3). Beside these major residues, small amounts (∼5%) of non-reducing end GalNAc and reducing end 5-α-Kdo were also detected and are likely the result of cleavage of some of the very acid labile Kdo glycosidic bonds during isolation. The NOESY spectrum (Figure 4) revealed NOE contacts between H-1 of GalNAc and H-5 of Kdo and between both H-3 s of Kdo and H-3 of GalNAc. The Galf anomeric signal gave NOE contacts only to its own protons. HMBC showed a correlation between H-1 of GalNAc and C-5 of Kdo.
Figure 2. 1-D proton NMR spectrum of the polysaccharide.
The integration values show the relative molar ratio of the two polysaccharides, Am and (B–C)n of about 1∶3. For numbering, see Table 3 (C3e and C3a designate the equatorial and axial proton, respectively, of the C-3 methylene group).
Figure 3. 2-D HSQC NMR spectrum of the polysaccharide with assignments of all the signals of the three major residues.
For numbering, see Table 3.
Table 3. Complete chemical shift assignment of the isolated polysaccharides.
| No. | Residue | Chemical shift | NOE | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | HMBC | ||
| A | 5-β-Galf | 5.18 | 4.15 | 3.94 | 4.15 | 4.1 | 3.82/3.82 | |||
| 109.4 | 84.1 | 78.5 | 84.1 | 79.2 | 62.1 | A-5 | ||||
| B | 3-β-GalpNAc | 4.7 | 3.97 | 3.97 | 4.03 | 3.7 | 3.82/3.80 | C-5 | ||
| 105.2 | 53.6 | 77.5 | 70.5 | 77.1 | 63.5 | C-5 | ||||
| C | 5-β-Kdop | – | – | 1.77/2.40 | 3.83 | 4.08 | 3.5 | 4.03 | 3.79/3.70 | B-3 |
| 174.3 | 104.5 | 37.2 | 70 | 76.1 | 76.1 | 71.9 | 65.8 | |||
N-acetyl signal: 2.04/25.0 ppm.
Carbon chemical shifts are in italics. Carbon chemical shifts with characteristic downfield displacement due to glycosylation are in bold.
Figure 4. 2-D NOESY NMR spectrum of the polysaccharide.
The sequence-determining correlations are labeled. For numbering, see Table 3.
Taken together, these results demonstrated that the sample contained two polysaccharides, one composed of a disaccharide repeating unit with the structure →3)-β-GalpNAc-(1→5)-β-Kdop-(2→ and the other a galactofuranosyl homopolymer (galactan) with the structure →5)-β-Galf-(1→ (Figure 5). The galactan homopolymer accounted for the galactose in the composition and methylation analyses and was present at about one-third the molar concentration of the GalNAc-Kdo polymer.
Figure 5. Structure of capsule polysaccharide repeating unit (top) and galactan exopolysaccharide repeating unit (bottom).
The GalNAc-Kdo Polymer is the Polysaccharide Capsule and the Galactan Homopolymer is an Exopolysaccharide
In a recent report, K. kingae strain PYKK181 was found to produce an exopolysaccharide that is a galactan homopolymer and is encoded by the pamABCDE locus [22]. To elucidate the relationship between the K. kingae polysaccharide capsule and the two distinct polysaccharide structures present in our bacterial surface extracts, we began by creating a derivative of KK01 with a deletion of pamABCDE. As shown in Figure 1 (lane 4) and Table 2, the polysaccharide extract from KK01pamABCDE stained with Alcian blue, contained large amounts of GalNAc and Kdo, and had only trace quantities of galactose, suggesting that the →5)-β-Galf-(1→ homopolymer corresponds to the galactan exopolysaccharide present in K. kingae strain PYKK181. As shown in Figure 6, examination of the colony morphology of KK01pamABCDE revealed mucoid colonies, indicating encapsulation and suggesting that the GalNAc-Kdo polysaccharide corresponds to the polysaccharide capsule. To confirm these conclusions, we examined polysaccharide extracts from strain KK01ctrA, recognizing that the ctrA gene is required for export and surface localization of the polysaccharide capsule. Interestingly, glycosyl composition analysis revealed no GalNAc or Kdo and minimal amounts of galactose compared to the quantities of contaminating xylose and glucose (Table 2), raising the possibility that either the absence of the GalNAc-Kdo polysaccharide results in free release of the galactan exopolysaccharide or that ctrA is essential for surface localization of both the GalNAc-Kdo polysaccharide and the galactan homopolymer. To address these possibilities, we modified the extraction procedure to eliminate both the initial fixation step and the Tris acetate treatment, aiming to recover loosely associated polysaccharide from strain KK01ctrA. Using this technique, a large quantity of galactose was detected in the purified material and only minimal amounts of GalNAc and Kdo were present (probably reflecting some bacterial lysis) (Table 2), indicating that strain KK01ctrA localizes the →5)-β-Galf-(1→ polymer but not the →3)-β-GalpNAc-(1→5)-β-Kdop-(2→ polymer on the bacterial surface. Consistent with our previous work, examination of the colony morphology of KK01ctrA revealed non-mucoid colonies (Figure 6), indicating lack of a capsule. Analysis of the KK01ctrA pamABCDE double mutant revealed no Alcian blue staining material, complete loss of GalNAc, Kdo, and galactose, and non-mucoid colonies (Figure 1, Table 2, and Figure 6). Together these results provide strong evidence that the GalNAc-Kdo polysaccharide is the polysaccharide capsule and that the galactan polymer is an exopolysaccharide.
Figure 6. Colony morphology of K. kingae strains used in this study.
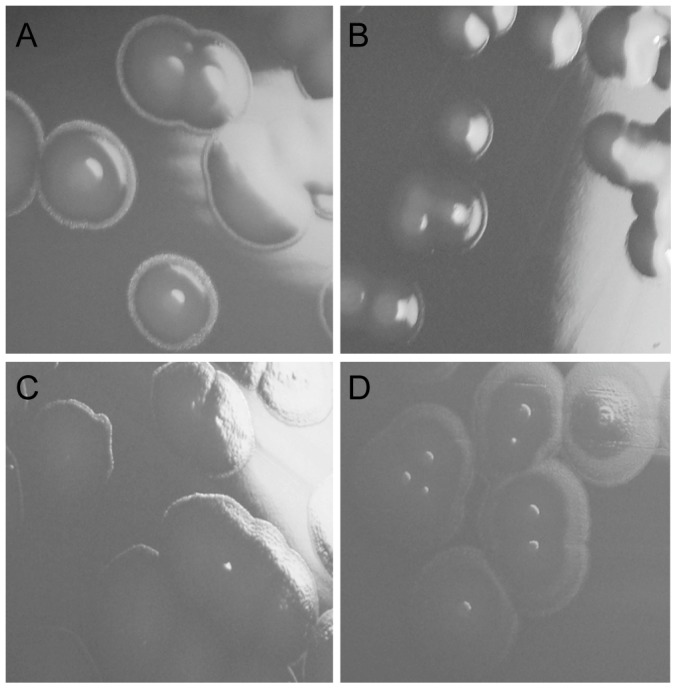
Strain KK01 (A) and KK01pamABCDE (B) form mucoid colonies consistent with encapsulation. KK01ctrA (C) and KK01ctrA pamABCDE (D) form rough, non-mucoid colonies consistent with loss of encapsulation.
Discussion
In previous work we showed that K. kingae produces a polysaccharide capsule and contains a capsule export locus designated ctrABCD with high homology to the capsule export locus in N. meningitidis. In addition, we demonstrated that the K. kingae capsule is associated with a mucoid colony phenotype on chocolate agar and is visible by thin section transmission electron microscopy after staining with cationic ferritin [16]. In the present study we set out to characterize the composition and structure of the K. kingae polysaccharide capsule. As a first step, we developed a capsule extraction and purification procedure that generated material amenable to analysis by GC/MS and NMR. Examination of the extracted material from K. kingae strain KK01 revealed GalNAc, Kdo, and galactose in roughly equimolar amounts. Additional analysis of the extracted material from derivatives of strain KK01 containing targeted mutations in the capsule export locus, the galactan biosynthesis locus, or both loci established that GalNAc and Kdo make up the capsule and that galactose makes up a galactan exopolysaccharide.
In considering our results, it is noteworthy that Bendaoud et al. reported two polysaccharides in the supernatant from K. kingae strain PYKK181, including a polysaccharide with the structure →6)-α-D-GlcpNAc-(1→5)-β-Kdop-(2→ and a galactan homopolymer with the structure →3)-β-Galf-(1→6)-β-Galf-(1→ that was designated poly-DNA-containing anti-adhesive material extract (PAM galactan) and was found to inhibit biofilm formation by a variety of organisms [22]. In the report by Bendaoud and coworkers, the specific relationship between these two polysaccharides and the organism was unclear. Based on the results of our mutational studies, we believe that the GlcNAc-Kdo polymer from strain PYKK181 is a polysaccharide capsule and that the PAM galactan is an exopolysaccharide.
It is interesting that the strain KK01 polysaccharide capsule has the structure →3)-β-GalpNAc-(1→5)-β-Kdop-(2→ and the strain PYKK181 polysaccharide capsule has the structure →6)-α-D-GlcpNAc-(1→5)-β-Kdop-(2→ [22]. The fact that the polysaccharide capsules expressed by these strains have different structures raises the possibility that there may be multiple K. kingae capsule types, similar to the case with E. coli, S. pneumoniae, N. meningitidis, H. influenzae, and a number of other bacterial pathogens [10], [11], [23]. Along these lines, it is notable that Amit et al. observed an association between K. kingae PFGE clonal types and specific clinical presentations [24]. In ongoing work, we are examining whether there is variability in capsule type among different clones and conservation of capsule type within a given clone. It is possible that certain capsule types confer greater virulence than others and are associated with specific disease processes.
Both the strain KK01 polysaccharide capsule and the strain PYKK181 polysaccharide capsule contain Kdo, a molecule that is well recognized as an essential component of lipopolysaccharide and is also present in a small number of bacterial polysaccharide capsules. The capsule produced by strain KK01 has the same structure as the capsule produced by Moraxella nonliquefaciens strain 3828/60 [25] and has the same composition but different glycosyl linkages compared to the capsules produced by E. coli K14 [→6)-β-D-GalpNAc-(1→5)-β-Kdop(2→] [26] and N. meningitidis serogroup 29E [→3)-α-D-GalpNAc-(1→7)-β-Kdop(2→] [27]. Other examples of bacterial polysaccharide capsules that contain Kdo include the capsule produced by Actinobacillus pleuropneumoniae serotype 5a (identical to the capsule produced by K. kingae strain PYKK181) [28], the capsule produced by Sinorhizobium meliloti strain 1021 with the structure →7)-β-Kdop(2→ [29], and the capsule expressed by E. coli K15 with the structure →4)-α-D-GlcpNAc-(1→5)-β-Kdop(2→ [30].
In considering the genetic determinants of K. kingae polysaccharide capsule biosynthesis, it is notable that the ctrABCD capsule export locus is not flanked by a capsule synthesis operon, thus differing from the configuration of capsule genes in most other bacteria that produce a polysaccharide capsule [8], [9], [31]. Based on available K. kingae genome data, there is no apparent capsular polysaccharide synthesis operon. We speculate that the genes required for synthesis of the capsular polysaccharide are not arranged in an operon and instead are located at separate loci throughout the genome.
Sequence analysis of the PAM locus in K. kingae strain KK01 and the PAM locus in K. kingae strain PYKK 181 reveals almost complete identity. Despite this sequence conservation, the structures of the galactan homopolymers in these strains are different, with distinct linkages. One possibility is that there are other genes beyond pamABCDE involved in biosynthesis of the galactan exopolysaccharides. Alternatively, the limited sequence variation may result in significant changes in enzymatic specificity.
To summarize, in this study we have established that K. kingae expresses two extracellular polysaccharides that require distinct genetic loci for surface localization, including a polysaccharide capsule and an exopolysaccharide. In future studies we will examine the contribution of these polysaccharides to the pathogenesis of K. kingae disease.
Funding Statement
This study was supported by the Ubited States Department of Energy, DE-FG02-93ER-20097. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yagupsky P, Porsch E, St Geme III JW (2011) Kingella kingae: An emerging pathogen in young children. Pediatrics 127: 557–565. [DOI] [PubMed] [Google Scholar]
- 2. Yagupsky P, Dagan R, Howard CW, Einhorn M, Kassis I, et al. (1992) High prevalence of Kingella kingae in joint fluid from children with septic arthritis revealed by the BACTEC blood culture system. J Clin Microbiol 30: 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dubnov-Raz G, Scheuerman O, Chodick G, Finkelstein Y, Samra Z, et al. (2008) Invasive Kingella kingae infections in children: Clinical and laboratory characteristics. Pediatrics 122: 1305–1309. [DOI] [PubMed] [Google Scholar]
- 4. Weiss-Salz I, Yagupsky P (2011) Kingella kingae Infections in Children: An Update. Adv Exp Med Biol 719: 67–80. [DOI] [PubMed] [Google Scholar]
- 5. Yagupsky P, Dagan R, Prajgrod F, Merires M (1995) Respiratory carriage of Kingella kingae among healthy children. Pediatr Infect Dis J 14: 673–678. [DOI] [PubMed] [Google Scholar]
- 6. Yagupsky P, Porat N, Pinco E (2009) Pharyngeal colonization by Kingella kingae in children with invasive disease. Pediatr Infect Dis J 28: 155–157. [DOI] [PubMed] [Google Scholar]
- 7.Bidet P, Collin E, Basmaci R, Courroux C, Prisse V, et al. (2013) Investigation of an outbreak of osteoarticular infections caused by Kingella kingae in a childcare center using molecular techniques. Pediatr Infect Dis J doi:10.1097/INF.0b013e3182867f5e [DOI] [PubMed]
- 8. Roberts IS (1996) The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol 50: 285–315. [DOI] [PubMed] [Google Scholar]
- 9.Corbett D, Hudson T, Roberts IS (2010) Bacterial Polysaccharide Capsules. In: Konig C, Varma, editor. Prokaryotic Cell Wall Components: Springer. 111–132.
- 10. Kalin M (1998) Pneumococcal serotypes and their clinical relevance. Thorax 53: 159–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orskov IF, Orskov FB, Jann B, Jann K (1977) Serology, chemistry, and genetics of O and K antigens of Escherichia coli . Bacteriol Rev 41: 667–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinberger D, Harboe Z, Sanders E, Ndiritu M, Klugman K, et al. (2010) Association of serotype with risk of death due to pneumococcal pneumonia: a meta-analysis. Clin Infect Dis 51: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turk DC (1984) The pathogenicity of Haemophilus influenzae . J Med Microbiol 18: 1–16. [DOI] [PubMed] [Google Scholar]
- 14. Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: Main examples of synthesis, excretion, genetics and extraction. Carbohyd Polym 87: 951–962. [Google Scholar]
- 15. Kumar AS, Mody K, Jha B (2007) Bacterial exopolysaccharides – a perception. J Basic Microb 47: 103–117. [DOI] [PubMed] [Google Scholar]
- 16. Porsch E, Kehl-Fie T, St. Geme III JW (2012) Modulation of Kingella kingae adherence to human epithelial cells by type IV pili, capsule, and a novel trimeric autotransporter. mBio 3: e00372–00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kehl-Fie TE, Porsch EA, Yagupsky P, Grass EA, Obert C, et al. (2010) Examination of type IV pilus expression and pilus-associated phenotypes in Kingella kingae clinical isolates. Infect Immun 78: 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kehl-Fie TE, Miller SE, St. Geme III JW (2008) Kingella kingae expresses type IV pili that mediate adherence to respiratory epithelial and synovial cells. J Bacteriol 190: 7157–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim J, Reuhs B, Rahman MM, Ridley B, Carlson R (1996) Separation of bacterial capsular and lipopolysaccharides by preparative electrophoresis. Glycobiology 6: 433–437. [DOI] [PubMed] [Google Scholar]
- 20. Heiss C, Klutts JS, Wang Z, Doering TL, Azadi P (2009) The structure of Cryptococcus neoformans galactoxylomannan contains beta-D-glucuronic acid. Carbohyd Res 344: 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wishart DS, Bigam CG, Yao J, Abildgaard F, Dyson HJ, et al. (1995) 1H, 13C, and 15N chemical shift referencing in biomolecular NMR. J Biomol NMR 6: 135–140. [DOI] [PubMed] [Google Scholar]
- 22. Bendaoud M, Vinogradov E, Balashova N, Kadouri D, Kachlany S, et al. (2011) Broad-spectrum biofilm inhibition by Kingella kingae exopolysaccharide. J Bacteriol 193: 3879–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Virji M (2009) Pathogenic neisseriae: Surface modulation, pathogenesis and infection control. Nat Rev Microbiol 7: 274–286. [DOI] [PubMed] [Google Scholar]
- 24. Amit U, Porat N, Basmaci R, Bidet P, Bonacorsi S, et al. (2012) Genotyping of invasive Kingella kingae isolates reveals predominant clones and association with specific clinical syndromes. Clin Infect Dis 55: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 25. Reistad R, Zähringer U, Bryn K, Alstad J, Bøvre K, et al. (1993) A polysaccharide produced by a mucoid strain of Moraxella nonliquefaciens with a 2-acetamido-2-deoxy-5-O-(3-deoxy-beta-D-manno-octulopyranosyl)-beta-D-galactopyranosyl repeating unit. Carbohyd Res 245: 129–136. [DOI] [PubMed] [Google Scholar]
- 26. Jann B, Hofmann P, Jann K (1983) Structure of the 3-deoxy-d-manno-octulosonic acid-(KDO)- containing capsular polysaccharide (K14 antigen) from Escherichia coli 06:K14:H31. Carbohyd Res 120: 131–141. [DOI] [PubMed] [Google Scholar]
- 27. Bhattacharjee A, Jennings H, Kenny C (1978) Structural elucidation of the 3-deoxy-D-manno-octulosonic acid containing meningococcal 29e capsular polysaccharide antigen using carbon-13 nuclear magnetic resonance. Biochemistry 17: 645–651. [DOI] [PubMed] [Google Scholar]
- 28. Perry M, Altman E, Brisson J-R, Beynon L, Richards J (1990) Structural characteristics of the antigenic capsular polysaccharides and lipopolysaccharides involved in the serological classification of Actinobacillus (Haemophilus) pleuromoniae strains. Serodiag Immun Inf D 4: 299–308. [Google Scholar]
- 29. Fraysse N, Lindner B, Kaczynski Z, Sharypova L, Holst O, et al. (2005) Sinorhizobium melliloti strain 1021 produces a low-molecular-mass capsular polysaccharide that is a homopolymer of 3-deoxy-D-manno-oct-2-ulosonic acid harboring a phospohlipid anchor. Glycobiology 15: 101–108. [DOI] [PubMed] [Google Scholar]
- 30. Jann K, Jann B (1983) The K antigens of Escherichia coli . Prog Allergy 33: 53–79. [DOI] [PubMed] [Google Scholar]
- 31. Dolan-Livengood JM, Miller YK, Martin LE, Urwin R, Stephens DS (2003) Genetic basis for nongroupable Neisseria meningitidis . J Infect Dis 187: 1616–1628. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.