Abstract
Sterile inflammation occurs in acute conditions such as ischemia reperfusion injury and crystal-induced arthritis, and also with chronic diseases such as particle-induced lung diseases and atherosclerosis. The triggers of sterile inflammation are still being identified and the pathways that transduce sterile inflammatory signals are not completely clear. Currently, most of the innate immune pathways that sense infection have been implicated in sterile inflammation, although distinct signaling pathways of sterile inflammation exist. Whether immune pathology ensues after sterile inflammation depends on the balance of induced inflammatory and resolution pathways. Further identification of the molecular mechanisms of sterile inflammation will lead to novel therapeutics to treat a wide range of diseases.
Over the last two decades our understanding of innate immunity has greatly increased. Although much of what has been learnt has come from examining how pathogens induce inflammation, it has become evident that non-microbial activators trigger inflammation. In certain conditions such as trauma, non-microbial and microbial activators both contribute to inflammation. In this Brief Review, we will discuss the conditions that result from sterile inflammation, the triggers of these conditions, signaling pathways of sterile inflammation, the implications of sterile inflammation and potential avenues for future investigation.
Conditions that result from sterile inflammation
The acute conditions that result from sterile inflammation include ischemia reperfusion injury (IRI), trauma, crystal-induced inflammation, and toxin exposure. IRI is a condition in which the blood supply to an organ is abruptly halted and then re-instated (1). Not only does injury to the tissues result from the initial hypoxia but also from the restoration of blood flow and re-oxygenation, which exacerbates inflammation. Acute myocardial infarctions, cerebral infarctions, acute kidney injury and solid organ transplantation are all conditions in which IRI occurs. Some of these disorders (e.g., myocardial and cerebral infarctions, and acute kidney injury) may share common disease pathologies such as atherosclerosis in which acute atherosclerotic plaque rupture or thromboembolism leads to cessation of blood flow. In the case of organ transplantation, the harvest and procurement of the organ leads to tissue ischemia injury and is followed by further inflammation after implantation into the recipient when blood supply to the organ is restored.
Crystal deposition within joints leads to gouty arthritis and elicits the classic clinical signs of inflammation including redness, pain, heat, swelling and loss of function. Toxins, such as acetaminophen or cobra venom, induce hepatic and muscle injury, respectively. Trauma, including crush injury, triggers an abrupt inflammatory response, and endogenous and microbial triggers (from bacterial exposure) may contribute to inflammation in this context. How sterile inflammation is triggered is discussed in the next section.
Chronic conditions that trigger sterile inflammation include particle-induced lung diseases such as asbestosis and silicosis, cardiovascular diseases such as atherosclerosis, some causes of chronic heart failure, and certain cases of tumors (2, 3). Studies in germ free mice provide evidence that microbes are not essential for the development of experimental atherosclerosis (4). With atherosclerosis, the chronic deposition of cholesterol within the arterial wall induces an injury response characterized by the recruitment of immune cells including macrophages, dendritic cells (DCs), T and B cells, leading to the development of the atherosclerotic plaque, which is the hallmark of the disease.
Triggers of sterile inflammation
Cellular necrosis that results from either acute or chronic inflammation leads to the release of immune triggers that are typically “hidden” in quiescent states. This contrasts with apoptosis that is the result of an orchestrated cell death program and is typically immunologically silent. Indeed, necrotic cells induce neutrophil infiltration when injected into the peritoneum of mice (5), indicating that the rupture of plasma membranes releases intracellular contents that activate inflammation.
Conceptually, a putative trigger of sterile inflammation would meet the following criteria. First, the levels of the trigger should correlate with immune activation in an experimental model of sterile inflammation or in relevant clinical samples. Second, the trigger should elicit a proinflammatory response in cultured cells, such as macrophages or DCs. Third, the trigger should provoke an inflammatory response after injection into disease free hosts. Evidence that the trigger is not contaminated by microbial motifs (e.g. LPS) should also be demonstrated. These last two criteria require modification with two-step activators, e.g., those which stimulate the inflammasome (see next section) after priming with another trigger such as TNF-α (6). Lastly, the inflammatory response should be abrogated with blockade of the receptor or inhibition of the signaling pathway of the trigger. This last criterion depends upon whether the receptor and the downstream signaling pathways of the trigger are known.
The triggers of sterile inflammation can be broadly divided into those that are intracellular and those that originate from the extracellular matrix (Table 1 and Figure 1A). Intracellular triggers include nuclear proteins (e.g. High-mobility group box 1 [HMGB1]), mitochondrial DNA and peptides, uric acid and cellular chaperones (e.g., heat shock proteins). Studies have provided evidence that HMGB1 meets several of the above criteria for a trigger of sterile inflammation. Specifically, HMGB1 is elevated in murine renal IRI models (7, 8). Furthermore, HMGB1 translocation from the nucleus to the cytoplasm is abrogated with treatment with ethyl pyruvate and this treatment significantly reduces inflammation in the renal IRI model (7). Importantly, administration of recombinant HMGB1 to disease free mice elicits a systemic inflammatory response (7). Another study also found that HMGB1 levels are increased after renal IRI and that administration of an anti-HMGB1 antibody reduces inflammation, whereas administration of recombinant HMGB1 exacerbates inflammation during renal IRI (8). Additionally, this latter study demonstrated that TLR4−/− mice are protected from renal IRI and are insensitive to the effects of HMGB1 manipulation. Recent studies have also implicated HMGB1 in hepatic IRI (9). Specifically, TLR4 expression in hepatocytes is important for HMGB1 release to promote inflammation within the liver (10).
Table 1. Inflammatory Triggers, Signaling and Associated Diseases.
| Trigger | Senor + signaling pathway |
Associated diseases | References |
|---|---|---|---|
Nuclear proteins
|
|
|
(7-10, 72) |
| Mitochondrial components
|
|
|
(10-12, 73) |
| Monosodium uric acid crystals |
|
|
(19, 20, 22, 74) |
| Components of extracellular matrix
|
|
|
(17, 18, 75) |
| Cholesterol |
|
|
(44) |
Particles
|
|
|
(49) |
| IL-1α |
|
|
(54) |
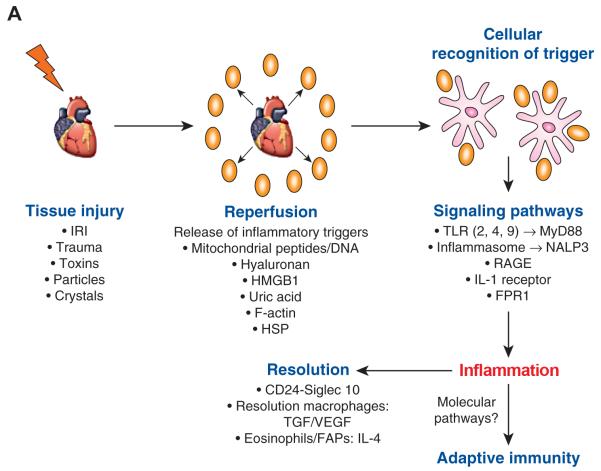
Figure 1A. Pathways of Sterile Inflammation.
A variety of insults induce sterile inflammation. This leads to the release of inflammatory triggers that are sensed by immune (e.g., dendritic cells or macrophages) or non-hematopoietic cells (e.g., endothelial or epithelial cells). These cells respond to the trigger via a variety of pathways, some of which are shared with pathways that respond to microbes and others that are distinct to sterile inflammation (e.g., RAGE and IL-1α). The induction of sterile inflammation leads to repair mechanisms that are mediated by macrophages that clear necrotic or apoptotic neutrophils and produce factors that enhance resolution (e.g., resolvins, TGF-β) or growth of blood vessels into the area (e.g., VEGF). There is evidence that IL-4 produced by eosinophils is sensed by fibro/adipogenic progenitors (FAPs) to mediate repair after acute skeletal muscle necrosis. Activation of sterile inflammation may also enhance adaptive immune responses as in the case of organ transplantation. The molecular pathways by which sterile inflammation enhance adaptive immunity remain to be fully elucidated. HSP=heat shock protein
Mitochondrial components, including mitochondrial peptides or DNA, trigger sterile inflammation. Mitochondria are organelles that may have originated from bacteria that existed in a symbiotic relationship with the host cell. Hence, the release of mitochondrial components during necrosis may lead to activation of inflammatory pathways that are typically stimulated by microbes. One study found that mitochondrial DNA was released into the plasma of patients that had sustained traumatic injury (11). Mitochondrial components stimulate inflammatory responses in neutrophils and transfer of mitochondrial components to rodents induces a systemic inflammatory response. Furthermore, the activity of mitochondrial components is reduced by either inhibition of formyl peptide receptor one (FPR1) or TLR9, indicating that the mitochondrial components that trigger inflammation are either formylated peptides or DNA. Other mitochondrial components, e.g., ATP may also enhance inflammatory responses. Evidence exists that ATP signals via a purinergic receptor PX27, in combination with TLR activation, to induce inflammation via the inflammasome (see next section) (12). This indicates that ATP enhances inflammation, by priming the inflammatory response, rather than being both necessary and sufficient to induce sterile inflammation. ATP may also be released from the cytosol of neutrophils and platelets in addition to mitochondria (reviewed in (13)). Overall, mitochondrial formylated peptides and DNA meet criteria as sterile inflammatory triggers.
Two reports indicate that F-actin, a component of the cytoskeleton, acts as a ligand for the c-type lectin receptor CLEC9A (14, 15), which was previously shown to be expressed by DCs and activated by dead cells (16). However, it remains unknown whether F-actin induces sterile inflammation in relevant in vivo models.
Components of the extracellular matrix, including hyaluronan (HA), a glycosaminoglycan produced by mesenchymal cells, have been implicated in sterile inflammation. In quiescent states, HA is in a high weight form, but during inflammation HA is degraded into smaller fragments (135 KDa), which have been shown to activate macrophages via TLR2 and 4 in vitro (17). In a lung model of sterile inflammation in which bleomycin is administered to mice, HA levels are induced and inflammation is reduced in mice that are deficient in TLRs 2 and 4 (17). However, HA is important for resolving sterile inflammation induced by bleomycin as mice in which high weight molecular HA is overexpressed on lung epithelial cells exhibit protection from lung injury (17). However, the role that HA plays in inflammation resolution may depend on its context as overexpressing HA in myofibroblasts within the lung enhances severe lung fibrosis after bleomycin administration (18). Overall, there is evidence that HA promotes sterile inflammation within the lung, but it may also aid in inflammation resolution. Differential effects of HA may reflect the cellular source and the molecular weight of HA.
Uric acid is produced by all cells and is a product of purine metabolism. Upon cell necrosis, the increased uric acid concentration allows for formation of monosodium urate crystals (MSU) when exposed to extracellular sodium. Although not formally proven experimentally, this may explain why physiological circulating uric acid levels are not associated with inflammation. MSU activates DCs in vitro by upregulating costimulatory molecules and producing proinflammatory cytokines such as IL-1β and TNFα (19). Direct injection of monosodium MSU into the peritoneum of mice induces neutrophil recruitment in vivo, an inflammatory response that is abrogated in IL-1R −/− and MyD88-/- mice (20). IL-1R expression on non-bone marrow derived cells is required for inflammation in this model. By employing transgenic mice, which overexpress uricase, it has been demonstrated that uric acid is required for inflammation induced by injection of necrotic cells (21). Uric acid also activates the NLPR3 inflammasome (see next section) to induce IL-1β production (22). Overall, there is clear evidence that uric acid is a trigger of sterile inflammation.
Cells that have not yet undergone necrosis but are damaged send signals that are sensed by the immune system. For example, DNA damage from tumorigenesis leads to the upregulation NKG2D ligands, which are sensed by NKG2D receptors on NK cells and initiate anti-tumor immunity (23, 24). Although this pathway is not necessarily the result of tissue necrosis, it is a mechanism by which cellular damage alerts the immune system.
In sum, several triggers of sterile inflammation exist. However, it is likely that many more are yet to be identified. Most studies have focused on examination of one trigger when it is likely that several may contribute and synergize to induce inflammation. Un-biased approaches (e.g., proteomics or genomics) may be required to identify novel inflammatory triggers in sterile inflammation. Such approaches have been employed to identify triggers of inflammation in experimental models of organ transplantation (25).
Signaling Pathways of Sterile Inflammation
The evolution of the immune system was likely shaped by the pressure to defend against pathogens. Although some sterile inflammatory conditions have been evident for many centuries (e.g., trauma and gout), some entities have arisen due to modernization of our society (e.g., organ transplantation and atherosclerosis). Evidence indicates that pathways that have evolved to respond to infection are also employed to respond to non-microbial triggers, although some distinct pathways exist.
TLRs are one of the principle innate immune receptors that have been implicated as mediators of certain sterile inflammatory conditions. These innate immune receptors respond to a broad range of microbial motifs and are expressed within the cell or on the cell surface on both hematopoietic and non-hematopoeitic cells (reviewed in (26)). TLRs 2 and 4 have been implicated in IRI, specifically in coronary ligation models and models of acute kidney injury (27-30). In both models, TLR 2 and 4 have been shown to signal via the signal adaptor MyD88 to contribute to inflammation. In the kidney IRI model, TLR4 on epithelial cells contributes to inflammation (27). Furthermore, inhibition of TLR2 can reduce renal IRI in mice (31). Within the heart, cardiomyocytes and vascular cells express TLRs, and these cells have been shown to transduce inflammatory responses at least in vitro (32, 33). Not only are TLRs involved in acute sterile inflammatory conditions, but also chronic sterile vascular diseases as TLR 2, 4 and MyD88 enhance the development of atherosclerosis in mice (34-36). In hepatic IRI models, TLR 4 is critical for inflammation, yet this effect is independent of MyD88 and dependent on IRF-3 (37). As stated above, HMGB1 has been implicated in hepatic injury via TLR4 signaling on hepatocytes (38, 39). Although hepatic IRI models are likely sterile, it is possible that microbial motifs from commensal bacteria, such as LPS, enter the hepatic circulation with induction of ischemia. Acetaminophen-induced hepatic injury requires TLR 9, a TLR that is expressed within endosomes, by signaling in sinusoidal endothelial cells. Finally, TLR3, another TLR expressed within endosomes that typically responds to double stranded viral RNA, is required for macrophage inflammation after stimulation by necrotic neutrophils, indicating that TLR3 may respond to endogenous RNA (40).However, the in vivo implications of this finding has so far been tested in experimental models of septic peritonitis, which are not sterile inflammatory models (40).
The inflammasome is a multi-protein intracellular complex that upon activation of its receptors, the NOD like receptors (NLRs), leads to recruitment of apoptosis-associated speck-like protein contains a caspase recruitment domain (ASC). This leads to activation of caspase-1 and production of mature IL-1β. Patients who died of acute myocardial infarction display ASC expression, implying that the inflammasome may have contributed to the pathology (41). Furthermore, ASC−/− mice exhibit increased heart function after cardiac IRI via coronary ligation as compared to wild type mice (WT) (41). Although infarct sizes were similar between the groups, the degree of fibrosis was smaller in the ASC−/− mice as compared to the WT, indicating that the inflammasome is important for efficient inflammation resolution (41). This study also found evidence that inflammasome signaling within bone marrow cells and non-hematopoietic, cardiac resident cells (e.g., cardiomyocytes) contribute to sterile inflammation after cardiac IRI. In a complementary study, ASC formation occurred in mice subjected to permanent ligation of the coronary artery with subsequent acute myocardial infarction (42). Caspase 1 is activated in cardiomyocytes that are exposed to ischemia or to the combined effects of LPS activation and ATP stimulation in vitro (42). Furthermore, in vivo inhibition of the P2X7 receptor, a purinergic receptor that responds to extracellular ATP by increasing K+ influx, reduces infarct size in the acute myocardial infarction model in mice (42). Hence, these two studies indicate that the inflammasome is essential for sterile inflammation in acute models of cardiac ischemia.
NOD-, LRR- and pyrin domain-containing 3 (NLRP3), is one of the best-studied NLRs in sterile inflammation. In a chronic murine model of cardiac inflammation induced by overexpression of calcineurin within the myocardium, increased IL-1β and NLRP3 activation was noted (43). Importantly, genetic ablation of NLRP3 decreases inflammation and improved cardiac function in this model (43). NLRP3 has also been implicated in atherosclerosis. Specifically, cholesterol crystals activate NLRP3 in vitro and LDLr−/− (atherosclerotic prone) mice, when reconstituted with NLRP3−/− bone marrow exhibit reduced atherosclerosis as compared to mice reconstituted with WT bone marrow (44). NLRP3 has been implicated in other sterile inflammatory disorders, including mechanical stretch-induced lung inflammation. In this latter instance, a study found that ventilator-induced lung injury in mice leads to increased reactive oxidative stress release from mitochondria that in combination with TLR4 signaling, activates NLRP3 to produce IL-1β and lung inflammation (45) in agreement with other recent reports in both humans and mice (46, 47). Another study found that mitochondria released from necrotic cells activate NLRP3 in macrophages in vitro and NLRP3 is required for maximal inflammation after renal IRI (12). Lastly, NLRP3 also contributes to inflammation in acute hepatic inflammation induced after acetaminophen (48) exposure and there is evidence that NLRP3 is important for particle-induced inflammation (49). In sum, NLRP3 has been implicated in several sterile inflammatory conditions. Whether other inflammasomes such as the Absent in Melanoma 2 (AIM2), which responds to DNA, contributes to sterile inflammation is yet to be determined.
Not all sterile inflammatory responses engage receptors that are also known to respond to pathogens. The receptor for advanced glycation end products (RAGE) is such a receptor. This receptor is a type 1 transmembrane protein that is expressed at low levels in vascular endothelial cells, cardiomyocytes and immune cells (e.g., neutrophils, DCs, and lymphocytes). It responds to a variety of ligands, e.g., advanced glycated end products, DNA, RNA, HMGB1, S100 proteins and β-fibril sheets. RAGE has been implicated in cardiac IRI (50) and in the development of atherosclerosis (51).
The release of bioactive cytokines from necrotic cells occurs with sterile inflammation and enhances the inflammatory response. In particular, IL-1α is released from necrotic cells and sensed by the IL-1 receptor to induce the production of neutrophil attracting chemokines (e.g.,CXCL1) in mesothelial cells (52). In a murine model in which inflammation was induced by injection of MSU crystals into the peritoneum, IL-1 inhibition via an inhibitory antibody or injection of MSU in IL-1 receptor−/− mice reduces neutrophil recruitment into the peritoneum (53). The same group of investigators subsequently found that injection of necrotic T cell lymphoma cells into peritoneum of mice induces neutrophil recruitment via an IL-1α-dependent (but IL-1β independent) mechanism (20). IL-1α also enhances adaptive immunity after sterile inflammation as shown in an experimental model, in which a human coronary artery is implanted into an immunodeficient mouse, which is reconstituted with allogeneic human peripheral blood mononuclear cells (54). In this model, IL-1α is present in endothelial cells lining the human artery and inhibiting IL-1α reduces subsequent T cell graft infiltration (54). This study also indicates that sterile inflammatory pathways may enhance subsequent adaptive immunity. A recent study has provided evidence that intracellular pro-IL-1α is not active after release from necrotic cells unless converted to an active form (55). This study also found that IL-1α release is controlled by intracellular IL-1R2, which prevents cleavage of IL-1α. Furthermore, cells found to induce inflammation after necrosis (e.g., vascular smooth muscle cells) either do not express IL-1R2 or have evidence of caspase 1 activation, which abrogates the effects of IL-1R2 (55).
Resolution of Sterile Inflammation
Regardless of whether inflammation is sterile or microbial in origin, immunopathology will ensue without effective repair and resolution (reviewed extensively in (56)). One of the first cellular mediators of sterile inflammation are neutrophils, which home to sites of sterile inflammation within hours of injury and form clusters, as was recently shown by dynamic two photon imaging in murine models of cardiac IRI and burn injury (57, 58). Neutrophils are short-lived cells that propagate the initial inflammatory response. Effective resolution of inflammation requires that these cells be efficiently cleared after they undergo apoptosis or necrosis. Furthermore, it is important that recruitment of more neutrophils is inhibited to the inflammatory site for efficient inflammation resolution. In this regard, apoptotic neutrophils secrete mediators (e.g., annexin A1), which can impair further neutrophil recruitment. Furthermore, neutrophils upregulate signals (e.g., sphingosine 1-phosphage) that enhance uptake by macrophages, a process termed efferocytosis (59). The release of extracellular adenosine that occurs in IRI also dampens inflammation possibly by stabilizing hypoxia-inducible factor, which promotes a switch to glycolytic metabolism (60). Macrophages typically follow neutrophils into sites of inflammation and initially potentiate the inflammatory response. However, macrophages are dynamic cells and they can change their phenotype to one that enhances resolution. Indeed, a transcriptional analysis comparing macrophages isolated at either an inflammatory or resolution phase of murine peritonitis found that resolution-phase macrophages express genes such as TIM4 and TGFβ, which are involved in tissue repair and clearance of inflammatory cells (61). Macrophages also secrete VEGF, which enhances tissue angiogenesis and restoration of oxygen to the hypoxic tissue and immunosuppressive cytokines (e.g., IL-10), which may allow for recruitment of regulatory T cells that further assist in inflammation resolution. Finally, macrophages secrete pro-resolution mediators such as lipoxins, and resolvins that enhance inflammation resolution (reviewed in (62)).
Although macrophages have been implicated in inflammation resolution, a recent study provided evidence that eosinophils, which secreted IL-4, rather than macrophages are required to resolve acute muscle inflammation induced by cobra toxin (63). IL-4 produced by eosinophils is sensed by fibro/adipogenic progenitors (FAPs), a specific stem cell implicated in muscle cell regeneration, which enhances the ability of FAPs to clear necrotic cells, induce muscle cell proliferation and regeneration (63). Whether this pathway of inflammation resolution is relevant to other forms of sterile inflammation has not yet been determined.
There are reports that endogenous innate immune triggers can stimulate specific receptors, not known to respond to pathogens, to dampen sterile inflammation. For example, CD24 is a glycosyl-phosphoinositol-anchored protein that provides costimulation to T cells, but it also signals in conjunction with a lectin Siglec-10, to dampen sterile inflammation (64). Specifically, CD24-Siglec-10 signaling suppresses sterile inflammation after acute hepatic injury induced by acetaminophen, possibly by inhibiting signals mediated by HMGB1 or heat shock proteins (64). Future research is required to determine how broadly applicable this pathway is to other forms of sterile inflammation.
Activation of adaptive immunity by sterile inflammation: the case of organ transplantation
Before the molecular and cellular pathways of innate immunity were heavily investigated, it was postulated that innate immune activation enhances the induction of adaptive immune responses (65). Subsequent work using model antigens indicated that this is the case (66), which has been expanded to infectious models (reviewed in (67)). Although activation of innate immunity during sterile inflammation alerts the host to the presence of a noxious stimulus, which can lead to a repair response, it has been argued whether this enhances the induction of adaptive immunity as compared to inflammation induced by a pathogen. The example of organ transplantation indicates that sterile inflammation may enhance adaptive immunity. In this context, one study provided evidence that the re initiation of IRI led to acute allograft rejection in cardiac allografts that were accepted by the host for < fifty days (68). In another study, human coronary arteries were implanted into immune-deficient murine hosts that were reconstituted with allogeneic human peripheral blood mononuclear cells (69). Increasing hypoxic injury to the artery graft prior to implantation led to increased T cell recruitment to the graft (69). As both these studies employed sterile inflammation models of transplantation, they indicate that the presence or degree of IRI enhances anti-donor, adaptive T cell responses and provide evidence that sterile inflammation enhances adaptive immunity. Clearly, there is clinical evidence that increasing donor ischemic time, and thus IRI, enhances acute and chronic rejection (70). Furthermore, a clinical study revealed that IRI leads to innate immune cell migration into kidney allografts that precedes the recruitment of T cells (71). Thus, in the case of organ transplantation there is evidence that sterile inflammation enhances adaptive immunity. Whether this occurs in other forms of sterile inflammation and the molecular links between sterile inflammation and induction of adaptive immunity are yet to be determined.
Conclusions
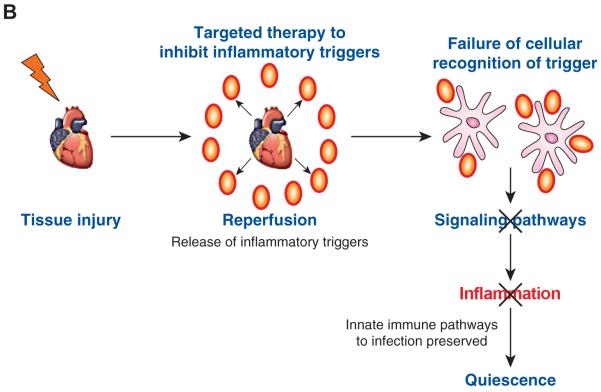
Sterile inflammation occurs in several broad medical conditions. Therefore, dissecting out the mechanisms by which sterile inflammation induces pathology will have a large biomedical impact. It is likely that there are many yet to be identified triggers of sterile inflammation, and exploratory approaches (e.g., proteomics, genomics or metabolomics) will be required to identify these triggers. Identifying relevant sterile inflammatory triggers may allow for the development of localized therapeutics that will dampen inflammation at the injury site without disabling systemic innate immune pathways that afford protection against infection (Figure 1B). This is particularly relevant in organ transplantation or in the setting of IRI due to acute myocardial infarction or stroke in which the host is either immune suppressed (i.e., organ transplant recipient) or likely to have compromised immune function (i.e., older people, who are at increased risk of vascular disease and exhibit immune senescence). In these susceptible populations, systemically inhibiting innate immune pathways that are employed both to respond to sterile and microbial-induced inflammation could lead to detrimental infectious complications. In conclusion, further investigation into the initiation and resolution of sterile inflammation will lead to novel therapeutics that could impact a wide array of human diseases.
Figure 1B. Therapeutic paradigm: localized inhibition of inflammatory triggers may reduce inflammation without compromising systemic innate pathways required for protection from infection.
As several pathways are shared between sterile inflammation and inflammation sensed by microbes, targeted localized therapy that is aimed at the triggers could reduce inflammation without impairing host defense to infection. Such a strategy may be beneficial for localized sterile inflammation that occurs with organ procurement and implantation. In this instance, the organ could be pretreated prior to implantation.
Acknowledgments
DRG is supported by NIH grants AG028082, AG033049, AI098108, AI101918 and an Established Investigator Award (0940006N) from the American Heart Association. DK is supported by NIH grants HL113931 and HL094601.
References
- 1.Eltzschig HK, E T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G. Cancer and Inflammation: An Old Intuition with Rapidly Evolving New Concepts. Ann Rev Immunol. 2012;30:677–706. doi: 10.1146/annurev-immunol-020711-075008. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Gardner SE, Clarke MCH. Cell Death, Damage-Associated Molecular Patterns, and Sterile Inflammation in Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–2786. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 4.Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, Sparrow CP, Detmers PA, Chao Y-S. Infectious Agents Are Not Necessary for Murine Atherogenesis. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 6.Franchi L, Eigenbrod T, Núñez G. Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB. HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol. 2012;303:F873–F885. doi: 10.1152/ajprenal.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 Contributes to Kidney Ischemia Reperfusion Injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamo N, Ke B, Ghaffari AA, Shen X.-d., Busuttil RW, Cheng G, Kupiec-Weglinski JW. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology. 2013;58:351–362. doi: 10.1002/hep.26320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nace GW, Huang H, Klune JR, Eid RE, Rosborough BR, Korff S, Li S, Shapiro RA, Stolz DB, Sodhi CP, Hackam DJ, Geller DA, Billiar TR, Tsung A. Cellular-specific role of toll-like receptor 4 in hepatic ischemia-reperfusion injury in mice. Hepatology. 2013;58:374–387. doi: 10.1002/hep.26346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser C. Circulating mitochondrial DAMPs cause inflammatory responses to injur. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eltzschig HK, MacManus CF, Colgan SP. Neutrophils as Sources of Extracellular Nucleotides: Functional Consequences at the Vascular Interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahrens S, Zelenay S, Sancho D, P HanČ, Kjær S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, Batista F, Thompson B, Way M, Reis e Sousa C, Schulz O. F-Actin Is an Evolutionarily Conserved Damage-Associated Molecular Pattern Recognized by DNGR-1, a Receptor for Dead Cells. Immunity. 2012;36:635–645. doi: 10.1016/j.immuni.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J-G, Czabotar Peter E., Policheni Antonia N., Caminschi I, Wan S. San, Kitsoulis S, Tullett Kirsteen M., Robin Adeline Y., Brammananth R, van Delft Mark F., Lu J, O’Reilly Lorraine A., Josefsson Emma C., Kile Benjamin T., Chin Wei J., Mintern Justine D., Olshina Maya A., Wong W, Baum J, Wright Mark D., Huang David C. S., Mohandas N, Coppel Ross L., Colman Peter M., Nicola Nicos A., Shortman K, Lahoud Mireille H. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity. 2012;36:646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, e.Sousa CR. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, N PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011 doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Evans J, Rock K. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 20.Chen C-J, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 21.Kono H, Chen C-J, Ontiveros F, Rock KL. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J Clin Invest. 2010;120:1939–1949. doi: 10.1172/JCI40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Goutassociated uric acid crystals activate the NALP3 inflammasome. Nature. 2006:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 23.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasser S, Raulet DH. The DNA Damage Response Arouses the Immune System. Cancer Research. 2006;66:3959–3962. doi: 10.1158/0008-5472.CAN-05-4603. [DOI] [PubMed] [Google Scholar]
- 25.Shen H, Song Y, Colangelo CM, Wu T, Bruce C, Scabia G, Galan A, Maffei M, Goldstein DR. Haptoglobin activates innate immunity to enhance acute transplant rejection in mice. J Clin Invest. 2012;122:383–387. doi: 10.1172/JCI58344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 27.Shishido T, Nozaki N, Yamaguchi S, Shibata Y, Nitobe J, Miyamoto T, Takahashi H, Arimoto T, Maeda K, Yamakawa M, Takeuchi O, Akira S, Takeishi Y, Kubota I. Toll-Like Receptor-2 Modulates Ventricular Remodeling After Myocardial Infarction. Circulation. 2003;108:2905–2910. doi: 10.1161/01.CIR.0000101921.93016.1C. [DOI] [PubMed] [Google Scholar]
- 28.Oyama J, Blais C, Jr., Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation. 2004;109:784–789. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 29.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. Clin. Invest. J. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activa ischemia/reperfusion injury. J. Clin. Invest. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrar CA, Keogh B, McCormack W, O’Shaughnessy A, Parker A, Reilly M, Sacks SH. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB. 2012;26:799–807. doi: 10.1096/fj.11-195396. [DOI] [PubMed] [Google Scholar]
- 32.Favre J, Musette P, Douin-Echinard V, Laude K, Henry J-P, Arnal J-F, Thuillez C, Richard V. Toll-Like Receptors 2-Deficient Mice Are Protected Against Postischemic Coronary Endothelial Dysfunction. Arterioscler Thromb Vasc Biol. 2007;27:1064–1071. doi: 10.1161/ATVBAHA.107.140723. [DOI] [PubMed] [Google Scholar]
- 33.Boyd JH, Mathur S, Wang Y, Bateman RM, Walley KR. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-κB dependent inflammatory response. Research Cardiovascular. 2006;72:384–393. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, F MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10 doi: 10.1038/nm1008. Epub 2004. [DOI] [PubMed] [Google Scholar]
- 35.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai Y, Shen X.-d., Connell R, Gao F, Lassman C, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Cutting Edge: TLR4 Activation Mediates Liver Ischemia/Reperfusion Inflammatory Response via IFN Regulatory Factor 3-Dependent MyD88-Independent Pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 38.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, Stolz DB, Geller DA, Rosengart MR, Billiar TR. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. 2005;201:1135–1143. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, i. Taniguchi S, Ikeda U. Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 42.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR, MacDonald JA, Lees-Miller JP, Roach D, Semeniuk LM, Duff HJ. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1β. Exp Physiol. 2013;98:462–472. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 44.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Yan Z, Schwartz DE, Yu J, Malik AB, Hu G. Activation of NLRP3 Inflammasome in Alveolar Macrophages Contributes to Mechanical Stretch-Induced Lung Inflammation and Injury. J Immunol. 2013;190:3590–3599. doi: 10.4049/jimmunol.1200860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuipers MT, Aslami H, Janczy JR, van der Sluijs KF, Vlaar APJ, Wolthuis EK, Choi G, Roelofs JJTH, Flavell RA, Sutterwala FS, Bresser P, Leemans JC, van der Poll T, Schultz MJ, Wieland CW. Ventilator-induced Lung Injury Is Mediated by the NLRP3 Inflammasome. Anesthesiology. 2012;116:1104–1115. doi: 10.1097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]
- 47.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, Haspel JA, Landazury R, Eppanapally S, Christie JD, Meyer NJ, Ware LB, Christiani DC, Ryter SW, Baron RM, Choi AMK. Inflammasome-regulated Cytokines Are Critical Mediators of Acute Lung Injury. Am J Resp Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, Song F, Qu W, Gomez T, Zou YS, Yan SF, Schmidt AM, Ramasamy R. Receptor for Advanced-Glycation End Products: Key Modulator of Myocardial Ischemic Injury. Circulation. 2006;113:1226–1234. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 51.Ueno H, Koyama H, Shoji T, Monden M, Fukumoto S, Tanaka S, Otsuka Y, Mima Y, Morioka T, Mori K, Shioi A, Yamamoto H, Inaba M, Nishizawa Y. Receptor for advanced glycation end-products (RAGE) regulation of adiposity and adiponectin is associated with atherogenesis in apoE-deficient mouse. Atherosclerosis. 2010;211:431–436. doi: 10.1016/j.atherosclerosis.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Eigenbrod T, Park J-H, Harder J, Iwakura Y, Núñez G. Cutting Edge: Critical Role for Mesothelial Cells in Necrosis-Induced Inflammation through the Recognition of IL-1α Released from Dying Cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C-J, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock KL. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J Clin Invest. 2006;116:2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y, Humphry M, Maguire Janet J., Bennett Martin R., Clarke Murray C. H. Intracellular Interleukin-1 Receptor 2 Binding Prevents Cleavage and Activity of Interleukin-1α, Controlling Necrosis-Induced Sterile Inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Molecular Medicine. 2013;5:661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W, Nava RG, Bribriesco AC, Zinselmeyer BH, Spahn JH, Gelman AE, Krupnick AS, Miller MJ, Kreisel D. Intravital 2-photon imaging of leukocyte trafficking in beating heart. J Clin Invest. 2012;122:2499–2508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013 doi: 10.1038/nature12175. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. Eur J Immunol. 2011;41:2515–2518. doi: 10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic Signaling during Inflammation. N Eng J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Progress in Lipid Research. 2011;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Jose E Heredia, Mukundan L, Chen Francis M., Mueller Alisa A., Deo Rahul C., Locksley Richard M., Rando Thomas A., Chawla A. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate MuscleRegeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.GY C, J T, P Z, Y L. CD24 and Siglec-10 Selectively Repress Tissue Damage-Induced Immune Responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janeway CA., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 67.Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chalasani G, Li Q, Konieczny BT, Smith-Diggs L, Wrobel B, Dai Z, Perkins DL, Baddoura FK, Lakkis FG. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J Immunol. 2004;172:7813–7820. doi: 10.4049/jimmunol.172.12.7813. [DOI] [PubMed] [Google Scholar]
- 69.Yi T, Fogal B, Hao Z, Tobiasova Z, Wang C, Rao DA, Al-Lamki RS, Kirkiles-Smith NC, Kulkarni S, Bradley JR, Bothwell AL, Sessa WC, Tellides G, Pober JS. Reperfusion injury intensifies the adaptive human T cell alloresponse in a human-mouse chimeric artery model. Arterioscler Thromb Vasc Biol. 2012;32:353–360. doi: 10.1161/ATVBAHA.111.239285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gjertson D. Impact of delayed graft function and acute rejection on kidney graft survival. Clin Transp. 2000:467–480. [PubMed] [Google Scholar]
- 71.Hoffmann SC, Kampen RL, Amur S, Sharaf MA, Kleiner DE, Hunter K, John Swanson S, Hale DA, Mannon RB, Blair PJ, Kirk AD. Molecular and immunohistochemical characterization of the onset and resolution of human renal allograft ischemia-reperfusion injury. Transplantation. 2002;74:916–923. doi: 10.1097/00007890-200210150-00003. [DOI] [PubMed] [Google Scholar]
- 72.Huang Y, Yin H, Han J, Huang B, Xu J, Zheng F, Tan Z, Fang M, Rui L, Chen D, Wang S, Zheng X, Wang CY, Gong F. Extracellular Hmgb1 Functions as an Innate Immune-Mediator Implicated in Murine Cardiac Allograft Acute Rejection. Am J Trans. 2007;7:799–808. doi: 10.1111/j.1600-6143.2007.01734.x. [DOI] [PubMed] [Google Scholar]
- 73.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CCM, Beck PL, Muruve DA, Kubes P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 74.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VFJ, Ryffel B, Couillin I. Uric Acid Is a Danger Signal Activating NALP3 Inflammasome in Lung Injury Inflammation and Fibrosis. Am J Resp Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 75.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of Lung Inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]