Abstract
In this study we recorded activity from afferent fibers innervating the mouse plantar skin, the same region evaluated in pain behavior experiments. We compared responses of afferents from incised and unincised hind paw skin. The plantar skin together with attached medial and lateral plantar nerves were dissected until they could be completely removed intact and placed in an organ bath chamber continuously perfused with oxygenated Kreb’s solution and temperature maintained at 32°C. Afferent nerve activities to feedback controlled mechanical and heat stimuli and cooling were recorded. Eighty-five single Aδ and C-fiber afferents were recorded, 42 from control and the remainder from incised animals. A greater proportion of C-fibers (11/34) from incised skin had spontaneous activity than the unincised preparation (2/32). The mechanical thresholds of both Aδ and C-fiber units were not different between control and incised groups but the responses to suprathreshold mechanical stimulation were increased in low threshold Aδ and C-fibers. The greatest change in heat sensitivity was apparent when multi-fiber total activity was measured; threshold was reduced, total spikes were greater and the peak discharge frequency was increased. In summary, feedback-controlled stimulation identified mechanical sensitization after incision in an in vitro preparation. Few fibers were excited by cooling. Heat sensitization of primary afferents was more prominent when activities of unclassified afferents are included. The preparation allows us to study afferent function of the same tissue that is examined for in vivo pain behavior assays in mice.
Introduction
Mice are increasingly important in experimental pharmacologic and physiologic studies. In particular, transgenic animals have enabled discovery of mechanisms and pathways of disease for which pharmacologic agents have not yet been discovered. In addition, given the wide variety of inbred stains, differences in responsiveness among strains have been evaluated and comparative genotyping identifies potential therapeutic targets based on strain differences[23].
Pain research has taken advantage of these molecular discoveries through the development of mouse models for persistent pain states[6,18,24,32]. The resulting pain related behaviors produced in these models, as expected, have improved our understanding of the mechanisms of nociception and hyperalgesia, exaggerated pain responses. In these studies, a variety of tests have been utilized. Perhaps, the most often-studied body region is the hind paw where stimulation is applied at the glabrous skin. Examples of such responses are withdrawal to heat or mechanical stimuli, and spontaneous paw lifting, licking and guarding responses.
To a large extent, primary afferent sensitization is the physiologic basis for exaggerated pain responses produced in these behavioral studies[22]. However mouse neurophysiologic studies examining sensory fiber function, have focused mainly on hind paw hairy skin[9,16,21,26,27] with few exceptions[7,8]. We undertook this study to develop a mouse skin nerve preparation to characterize nociceptor function of the same tissue that is examined for in vivo pain behavior assays. The preparation is adapted from the rat glabrous skin nerve preparation[2,11]. In this study, we characterized response properties of Aδ and C-fiber nociceptors to computer-controlled heat and mechanical stimuli. Sensitization of nociceptors to heat generally occurs during many experimental conditions; however, only a few studies showed mechanical sensitization [1,12,30] but most did not identify mechanical sensitization after experimental injury[3,7,15,22,27,28,31]. We evaluated nociceptor responses one day after plantar incision to both heat and mechanical stimuli.
Methods
Mice
A total of 42 C57Bl/6 mice were used in this study. Male mice, 20-30 grams and 6-12 weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME). The Animal Care and Use Committee at The University of Iowa, Iowa City, Iowa approved experiments, and the animals were treated in accordance with the Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals.
Plantar Incision
Anesthesia was induced by placing animals in a plastic box that contained 4% isoflurane in air. After loss of the righting reflex, anesthesia was maintained by administrating 2-3% isoflurane in air through a tightly fitting mask. An 8-mm longitudinal incision was made with a number 11 blade through the skin, fascia, and muscle of the right hind paw as described previously [4] and modified from Pogatzki and Raja (2003). The skin was apposed with two single sutures of 6-0 nylon, and the wound site was covered with triple antibiotic ointment. After surgery, anesthesia was discontinued, and the animals were allowed to recover in their cages. The electrophysiologic recordings were performed one day after incision.
Mouse in vitro glabrous skin nerve preparation
Preparation
Animals were killed using CO2 inhalation and the glabrous skin of the mouse hind paw and its intact plantar nerves were dissected free from the gastrocnemius muscle and tendon (Figure 1a). The underlying flexor muscle and connective tissue of the hind paw were also removed. We dissected plantar nerves up into the sciatic nerve at mid-thigh. The length of the nerve after dissection was in the range of 18 to 25 mm.
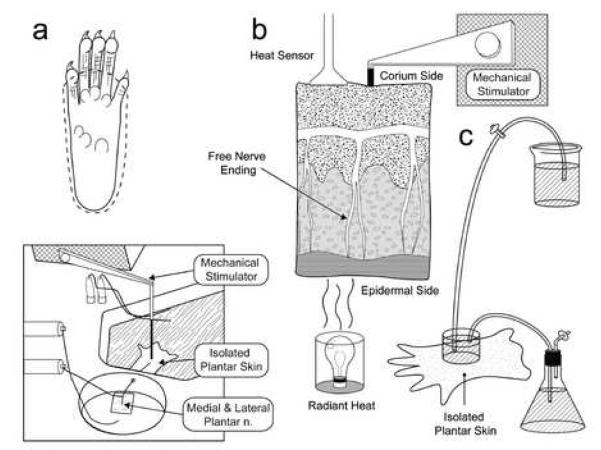
Figure 1. Experimental set up.
A. above, dissection of rat plantar skin is done along the dotted line with medial and lateral plantar nerves. Below is a schematic of organ bath chamber and nerve-skin preparation. B. Computer controlled heat stimulation is given on the epidermal side skin, temperature was recorded by sensor in the corium side (dermis). Computer controlled mechanical stimulation is given in the corium side of skin. C. rapid exchange of ice cold Kreb’s solution onto receptive field produced cooling to 12 to 16°C.
Organ bath
The organ bath consists of two chambers separated by inert acrylic-based wall. There are two small holes in the bottom of the wall; one for free flow of perfusate between the chambers and another for the passage of the tibial nerve. The larger chamber is the perfusion chamber which is continuously superfused with a modified Krebs-Hensleit solution (in mM: 110.9 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2So4, 24.4 NaHCO3, and 20 glucose), which was saturated with a gas mixture of 95% O2 and 5% CO2. The temperature of the bath solution was maintained at 32 ± 1°C. After dissection, the preparation was placed with ‘epidermal side down’. The nerves attached to the skin were drawn through one small hole to the second chamber, which was filled with liquid paraffin. The nerves were placed on a fixed mirror, their sheaths removed and nerve filaments repeatedly teased to allow single fiber recording to be made by using double-platinum electrodes (one for recording and another for reference). Single nociceptive afferent fibers were recorded extracellularly with a differential amplifier (DAM50, Harvard Apparatus, Holliston, MA). Neural activity was amplified and filtered using standard techniques. Amplified signals were led to a digital oscilloscope and an audio monitor and fed into PC computer via a data acquisition system (spike2/CED1401 program). Action potentials collected on a computer were analyzed off-line with a template matching function of spike 2 software.
Identification of afferents
The search strategy was mechanical stimulation by a fire-polished glass rod; thus, mechanosensitive afferents were recorded. Only units with a clearly distinguished signal to noise ratio were further studied. Rapidly adapting, low threshold A-β and D-hair fibers were not studied. After the initial assessment, fibers were evaluated for their responsiveness to controlled heat and mechanical stimuli.
Feedback controlled mechanical stimulation
Servo-controlled mechanical stimulation (Series 300B dual mode servo system, Aurora Scientific, Canada) was used to measure mechanosensitivity. The flat and cylindrical metal probe (tip size 0.7 mm) attached to the tip of stimulator arm was placed just close to the receptive field so that no force was generated (Fig 1a,b). The computer controlled ascending series of ramp-shaped force stimuli was applied to the most sensitive spot of the receptive field at 60-s intervals. Each force stimulus was 5s in duration and started from zero to 5, 10, 20, 40, 80, 120, 160 and 200mN. When an afferent produced a response to a particular force controlled ramp, it received three more ascending series of stimuli (as shown in Fig. 2, 3). To avoid fatigue, in no cases, more than three suprathreshold stimuli were applied; for example, if an afferent produced a response during the 20mN ramp, it received the 40, 80 and 120mN ramp. To quantify the fibers’ mechanical response, the total number of spikes per stimulus was assessed and peri-stimulus time histograms were averaged (see Fig. 2, 3).
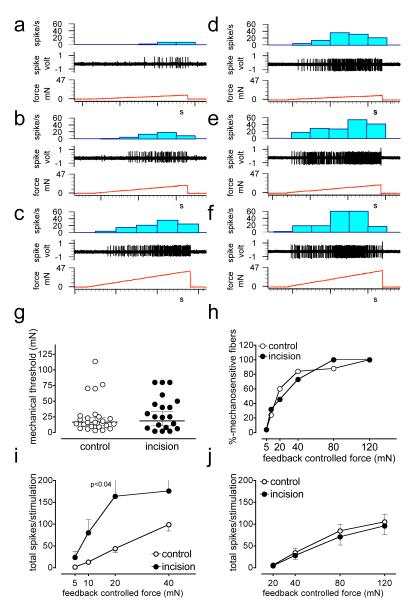
Figure 2. A-δ fiber responses to mechanical stimuli.
a-c, digitized oscilloscope tracings of an A-δ fiber responsive to mechanical stimuli. Single action potentials were recorded from fine filaments teased from medial or lateral plantar nerves of control mice while the receptive field was stimulated by a feedback controlled force stimulator. Three consecutive recordings show increasing responses to the ascending series of ramp shaped force stimuli starting from zero to 10 (a), 20 (b), and 40 (c) mN. The stimulus duration of each pulse was 5 sec and they were delivered at 60s intervals. d-f, digitized oscilloscope tracing of an A-δ fiber responsive to mechanical stimuli recorded from mice incised one day before recording (d, 10 mN, e, 20mN and f, 40mN). g-h, comparison of mechanical response thresholds between control and incised mice. The afferents are separated into two groups based on their mechanical response thresholds. Low threshold afferents produced responses by less than 10mN stimuli (i) while the threshold of high threshold afferents were above 10mN. Total number of spikes generated during ascending series of force pulses is compared in low (i) and high threshold (>10 mN) (j) afferents recorded from incised and control mice. P<0.04 vs control, Bonferroni’s post-hoc test.
Figure 3. C-fiber responses to mechanical stimuli.
a-c, digitized oscilloscope tracings of a C-fiber responsive to mechanical stimuli. Three consecutive recording show increasing responses to the ascending series of ramp shaped force stimuli (a, 10 mN, b, 20mN and C, 40mN). d-f, digitized oscilloscope tracing of a C-fiber responsive to mechanical stimuli recorded from mice incised in the plantar hind paw one day before recording (d, 10 mN, e, 20mN and f, 40mN). g-h, comparison of mechanical response thresholds between control and incised mice. Comparison of total evoked spikes in the low (i) and high (j) threshold afferents to suprathreshold mechanical stimuli. . P<0.01 vs control, Bonferroni’s post-hoc test.
Feedback controlled heat stimulation
After mechanical stimulation, a standard feedback controlled, heat ramp was delivered by a customized heat stimulator (Bioengineering, University of Iowa, Iowa City, IA). The mechanical receptive field of each unit was isolated with a small metal ring, which could seal by its own weight. In some cases silicone grease was added to prevent leakage from the bath into the receptive field within ring. The solution was removed and a thermocouple was gently placed to measure the subcutaneous temperature. A radiant lamp was placed in the translucent area underneath the organ bath and the light beam was focused onto the epidermal side of the skin (Fig 1b). A computer controlled standard heat ramp was delivered starting from 33° to 49°C over 15 seconds. We determined that the temperature of the epidermal side was about 1 degree centigrade higher than the corium side where free nerve ending are located. Fibers having a receptive field in a previously heat stimulated area were avoided for subsequent recordings.
Cooling
Cooling was tested in some fibers. The receptive field was isolated from the perfusion chamber as described above. The receptive field temperature was continuously monitored by a thermocouple lightly touching the corium side of skin. Then rapid exchange of ice-cold Kreb’s solution (temperature approximately 1 to 2 0C) onto the receptive field decreased the receptive field temperature to 12 to 16°C (Fig 1C). The duration of stimulus was two minutes and despite heat conduction from the surrounding perfusate, the cooling was maintained during the stimulation period.
Conduction velocity and fiber categorization
To avoid damage to the receptive field or alteration of fiber properties, the conduction velocity was always measured at the end of protocol. The conduction velocity of the axon was determined by monopolar electrical stimulation through an epoxy-coated electrode. The electrical stimulation (1-20 V at 0.2-1 Hz for 0.5-2 ms) was delivered at the sensitive spot of a receptive field. The intensity of the stimulus started from 0.1V and gradually increased until the similar shape spike appeared. The distance between receptive field and the recording electrode (conduction distance) was divided by the latency of the action potential (stimulus artifact to the appearance to spike).
The fibers were classified using criteria from Koltzenburg et al. [16] on mouse hairy skin in vitro. We set up a cutoff of 1.2 m/s to distinguish between myelinated A-fibers and unmyelinated C-fibers. Fibers conducting faster than 10.0 m/s were considered to be large myelinated (Aβ) axons, whereas units below this value were considered Aδ myelinated axons. In this study, the CV for Aδ fibers ranged from 1.5 to 8 m/s. It should be noted that the conduction velocities of afferent fibers are generally lower in vitro due to the bath temperature slightly slowing conduction velocity. In this study, we have reported properties of Aδ and C-fibers.
Data analyses
Single fiber analyses were performed in all experiments to evaluate mechanical, heat and cooling responses. The spike shapes and amplitude of single units were distinct and could be easily discriminated other smaller units. If the unit discharged at a rate of 0.1 impulses per second or more without any intentional stimuli, it was categorized as spontaneously active. Activity was counted in one-second bins. Threshold was determined as that force or temperature which elicited the first action potential in a response. If background activity was present, threshold was determined by the force or heat that increased background activity by at least 2 standard deviations greater than the background average for 10 sec (1 sec bins). Background activity was subtracted from any evoked responses; thus, assuming background activity was sustained during the stimulus period.
In the experiments for heat responses, previously unnoticed, small amplitude units often were excited by heat. The discrimination of these units into individual units was not always possible. To include responses of these unclassified units, a separate multi-unit analysis was performed exclusively for heat responses (Fig. 5). For this analysis, we counted ‘total afferent activity’ from the multi-fiber activity during heat stimulation. Again, if one of these small, unclassified fibers had spontaneous activity, the average total activity in 30-second pre-stimulus period was subtracted from the responses. These additional fibers responding in the multi-fiber analyses are presumably C-fibers since Aδ fibers are almost always unresponsive to the heat ramp used in this study (See below).
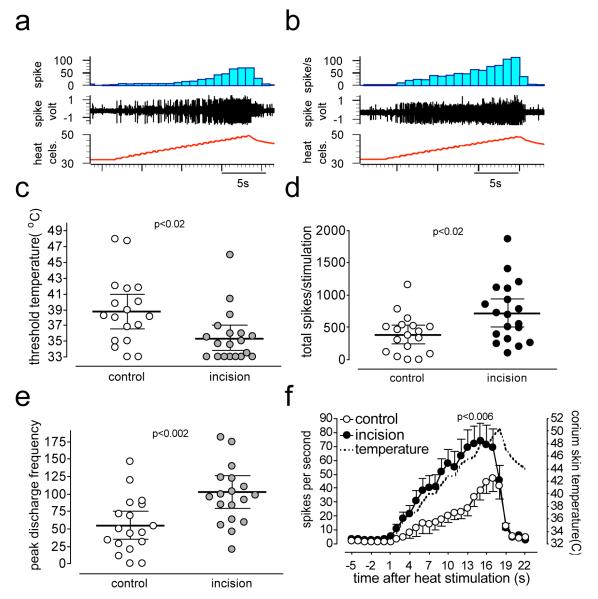
Figure 5. Heat responses after incision in multi-fiber analyses.
Digitized oscilloscope tracings of multi-fiber responses to heat stimuli recorded from control (a) and incised (b) mice. In some recordings, in addition to mechanically identified single C-fiber, previously unidentified, heat responsive units were present. c shows the threshold temperature for activation of afferents in the multi-unit analyses and d is the total spikes counted during heat stimulation. e is the peak discharge frequency of afferents during heat response. The stimulus-response curve was drawn by averaging responses in one-second bins and by counting the total activity of fiber/(s) in that bin (f).
Statistics
A Fishers’ exact test was used to compare the percentage of spontaneously discharging fibers and the percentage of heat sensitive fibers between groups. The response thresholds for heat and mechanical stimuli were compared by unpaired t-test. The stimulus–response relationship for the mechanical and heat-evoked responses were compared using 2 way ANOVA and post-hoc t-tests. The graded increase in the response due to increasing force stimuli is evaluated by 1 way ANOVA and post-hoc Neuman-Keuls test. The total evoked action potentials and peak discharge frequency during heat stimulation were compared by a nonparametric Mann Whitney test P<0.05 was considered statistically significant. Data are presented as mean ±SD.
Results
Mouse glabrous skin nerve preparation
In 90% of the preparations, stable recordings were obtained. The preparation was usually not viable for more than 6 hours. Care must be taken to dissect the plantar nerves proximally from the skin and muscle. The sealing of the receptive field was not always possible due to the small size of the glabrous skin-nerve preparation. Controlled mechanical stimulation was applied in all afferents studied, but heat and cold stimulation could not be tested in all units, as they required sealing of the receptive field from the surrounding bath solution. The lowest and highest number of units we recorded from one preparation was one and five, respectively. Heat stimulation was not given more than 3 times in one preparation to avoid effects of repeated heating. Fibers having receptive fields in the heat-stimulated area were avoided in the subsequent recording. Otherwise, recording of more units is possible.
General properties of mouse glabrous skin afferents in vitro
Eighty-five single mechanosensitive afferents are recorded: 42 from unincised, control mice and the remainder from incised mice. The conduction velocities of the Aδ-fibers ranged from 1.5 to 8.0 m/s (3.1±1.6 m/s), and C-fibers were between 0.2 and 1.3 m/s (0.7±0.04 m/s). No difference was seen in the conduction velocity of afferents between the incised and unincised groups (Aδ-fibers, 2.9±1.1 m/s vs. 3.2±1.7 m/s; C-fibers, 0.6±0.2 m/s vs. 0.5±0.2 m/s). No Aδ fiber from either preparations was spontaneously active. In contrast, 30% of C-fibers (11/34) from incised skin showed spontaneous activities, which was significantly higher than C-fibers from unincised control mice (6%, 2/32; p<0.02). The mean discharge rate of the two control C-fibers was 0.8 spike/s and that of incised C-fibers was 0.9±0.2 spike/s.
Responses to controlled mechanical stimulation
The distribution and summary of mechanical response thresholds of Aδ and C-fibers is shown in Figs. 2 and 3. The response threshold of control (unincised) C and Aδ-fibers ranged from 3 to 139 mN and 3 to 114mN, respectively. In the incised preparation, the response threshold of C and Aδ fibers ranged 3 to 114 mN and 2 to 80 mN, respectively. No difference was seen when mechanical thresholds were compared between control and incised groups (Fig 2g,h and Fig 3g,h).
Due to the wide distribution of response thresholds and to compare responses between groups to weak mechanical stimuli, Aδ and C-fibers were further classified into two groups: a low threshold groups were those fibers with thresholds less than 10mN and the high threshold responded to forces greater than 10mN. The cut-off 10-mN was chosen from our previous behavioral investigation [4]. In this study, the withdrawal responses to forces from 3 to 25 mN filaments were greatly exaggerated after incision. For example, mice did not withdraw to a 6 mN filament before incision but withdrew approximately 80% of the time on postoperative day 1. The behavioral response to this filament must be mediated by fibers responding to less than 10 mN. The current study recorded from the same tissue that was examined in the previous behavioral study and therefore, behavioral data and the mechanical response threshold of fibers could be integrated. Furthermore, a previous study that was aimed at identifying mechanical sensitization also separated afferents based on nearly similar response thresholds [31].
Twenty-four low threshold fibers in control (n=11) and incised (n=13) hind paws were tested with complete stimulus response functions of 5, 10, 20 and 40 mN. Forty-one high threshold fibers from control (n=24) and incised (n=17) mice were tested with complete stimulus response functions of 20, 40, 80, and 120 mN.
In the low threshold Aδ groups, the thresholds of fibers from the control (n=5) and incised (n=7) preparations ranged respectively from 3 to 9.1 mN (6.3±2.7 mN, mean ±SD) and from 2.0 to 8.3 mN (5.0±2.8 mN). The force-response curves showed increases in responses to greater mechanical forces (p< 0.008) and control groups and an effect of incision (P< 0.018). When compared with control fibers, the responses of the Aδ fibers from incised skin (164.0±124.1) were significantly greater than control (43.6±20.6) at the 20 mN stimulus (Fig. 2i; p<0.05, Bonferroni’s post-hoc test).
The thresholds of C-fibers, which responded to forces less than 10mN, ranged from 3.0 to 8.2 mN (5.4±1.2mN) in the control group (n=6) and from 1.3 to 9.1 mN (5.1±3.2mN) in the incised group (n=6). The force-response curves showed increases in responses to greater mechanical forces (P< 0.0001) and control groups and an effect of incision (P< 0.003). When compared with control fibers, the responses of the C-fibers from incised skin were significantly greater than control at the 40 mN stimulus (Fig. 2i; p<0.01, Bonferroni’s post-hoc test). Overall, low threshold C-fibers from control mice produced much smaller responses to the same mechanical stimuli compared to a similar group of low threshold Aδ fibers (5mN, 1.2±2.7 vs. 0.5± 1.2; 10mN, 13.7±7.7 vs. 6.5±1.8; 20mN, 43.6±20.6 vs. 17.3±6.8; 40mN, 98.6±32.0 vs. 45±33.8 spikes/stimulation; p<0.02, 2-way ANOVA).
Twenty-five Aδ fibers had response thresholds of greater than 10mN, were classified as high threshold, and were tested with 20, 40, 80, and 120 mN forces. Among the Aδ fibers, thresholds ranged from 12.9 to 113.9 mN (32.6±29.9 mN) in the control (n=16) and from 12.0 to 79.0 mN (39.7±39.6mN) in the incised (n=9) preparation. Sixteen C fibers had response thresholds of fibers that were greater than 10mN, were classified as high threshold and were tested with 20, 40, 80, and 120 mN forces. The thresholds of 16 C-fibers ranged 12.9 to 139.0 mN (39.6±39.6 mN) and 10.3 to 91.0 mN (30.7±23.8 mN) in the control (n= 8) and incised (n=8) preparations, respectfully. In high threshold Aδ and C-fibers, the force-response curves showed a graded increase in the responses up to 80 mN forces in both control and incised skin (p<0.05, 1 way ANOVA followed by Neuman-Keuls test). Moreover, unlike low threshold fibers, high threshold fibers from the control and incised preparations had no differences in the force-response curves (Fig 2j and 3j).
Single fiber responses to heat stimulation
Using a mechanical search stimulus, few Aδ fibers were heat responsive. Eighteen out of 35 Aδ units were tested in the control preparation and none responded to heat (up to 49°C). Similarly, in the incised preparation, 12 of 24 Aδ units are tested and only two units were responsive to heat (16.7%). Responses to heat were tested in the 20 C-fiber units in the control group and 15 fibers were responsive (75%); 29 units were tested for heat stimulation in the incised group and 19 fibers showed responses (65%). There was no difference in the incidence of heat sensitivity of either Aδ or C-fibers between the control and incised groups. The threshold temperatures to excite single mechanosensitive C-fibers were similar in the control and incised group (Fig 4c). The total spikes and peak discharge frequency in the responses of single, mechanosensitive C-fibers were similar between groups (Fig. 4d). Only the peak discharge frequency was greater in the incised group (Fig. 4e).
Figure 4. C-fiber responses to heat stimuli.
Digitized oscilloscope tracings of C-fibers responsive to heat stimuli recorded from control (a) and incised (b) mice. c shows the threshold temperature for activation of C-fibers in single fiber analyses and d is the total spikes counted during heat stimulation. e shows peak discharge frequency during heat responses. P<0.03, unpaired t-test.
When the afferents were grouped by mechanical response threshold and heat responses were compared, the low and high threshold groups had similar prevalence of heat responsive fibers. In the incised skin, 3/6 (50%) and 4/7 (57%) C-fibers from the low and high threshold groups, respectively, were heat-responsive. In the unincised animals, 4/6 (66%) and 7/9 (77%) heat responsive C-fibers were recorded from the low and high threshold groups, respectively.
Multi-fiber responses to heat stimulation
When total evoked multi-fiber activity from the heat stimulus was measured, significant differences were evident (p<0.02, unpaired t-test) in the threshold for heat activation (Fig. 5c) and the total number of spikes generated (Fig 5d) during the heat stimulus. Also, differences in total peak multi-fiber activity between control and incision were present (Fig 5e-f, p<0.02). The stimulus response curves for the heat stimuli generated by counting total multi-fiber activity showed that heat responses increased in parallel to the temperature increase in the receptive field (Fig 5f). However, differences were present in the stimulus response curves for fibers from incised skin, which responded to a greater extent than the control preparation when the temperature was increased (Fig 5f, p<0.006, two way ANOVA).
Responses to cooling
In the control group, five of 35 Aδ units were tested for cooling and only one was activated (20%) with an activation threshold of 22°C. Similarly, 10 out of 24 Aδ units were tested in the incised group and only one unit (10%) was excited. The threshold temperature was 31°C (Fig. 6a). Nine control C-fibers were tested for cooling and all were unresponsive (0%). Twenty-two C-fibers tested in the incised group and only 2 showed excitation (9%). The response thresholds of these two C-fibers were 28°C and 32°C (Fig. 6b).
Figure 6. Cooling induced excitation.
Digitized oscilloscope tracings of single fiber responses to cooling from incised mice. A is a recording from an A-δ fiber and b is the recording from a C-fiber. Two different response patterns are noted.
Discussion
The present study successfully describes the first in vitro recording from mouse glabrous skin afferents. Two new findings are evident. First, mechanical sensitization of low threshold A-δ and C-fibers was present after incision. The 10 to 20 mN forces needed to excite these sensitized fibers correspond to the force evoking paw withdrawal in 80 to 90% of mice incised one day after incision[4]. Before incision, these mice were largely unresponsive to these forces. Second, heat sensitization of mechanosensitive afferents is limited, only peak activity was greater after incision. Heat sensitization, however, is most prominent when the total activity of unclassified afferents was included. As previously noted in rats [2], more C-fibers have spontaneous activity in incised skin.
The mouse glabrous hind paw skin is most commonly used in the pain behavior assays. Because of increased development and use of transgenic and knockout mice for the study of pain, we need a comprehensive understanding of afferents that innervate this area used for behavioral testing. Cain and colleagues [7] first characterized mouse glabrous skin afferents using in vivo methods. However, the introduction of an in vitro preparation by the current study offers many advantages. In an in vitro study, monitoring the animal condition is not required, and stimuli can be easily controlled. Because of these advantages, the preparation can be more productive when compared to an in vivo recording. A most important advantage of this preparation is the pharmacologic manipulation of receptive fields. Stimuli can be directly applied to the receptive fields of afferents without concern for variables like interactions with blood borne mediators, bioavailability, and pharmacokinetics. With this method a more precise method for activation of an afferent can be utilized. A limitation of this approach, however, is the general concern for an in vitro method and the influence of surgery. Using the rat preparation, it has been suggested that the properties of afferent fibers in vitro are essentially the same as in vivo [17]. In our experiments, we sacrificed animals before surgery, thus, the influence of mediators released by an incision is less likely.
Because in vitro preparations can be more advantageous, previous studies [9, 21,26,27] on gene-altered mice, extensively used in vitro rather than in vivo recording methods. The unique aspect of the current study is the ability to record from afferents affected by incision and mediating the pathologic pain behavior we have studied.
In other species, the types of afferents present in hairy skin are different from that of glabrous skin [13,34]. It has been shown that slowly conducting unmyelinated C-fiber afferents, that responds to innocuous stimuli, innervate several anatomical sites including hairy skin, but are lacking in the glabrous skin [13]. Although glabrous skin areas have lower heat detection threshold, interestingly, they have higher heat pain threshold [33]. Treede and his colleagues provided a possible explanation for such differences by showing that glabrous skin lacks a distinct class of lower threshold heat nociceptors [34]. The pain ratings for stimulation of glabrous skin have been shown to begin later than the hairy skin, but increased at a faster rate to reach equal and often higher maximum pain ratings during maintained noxious cold stimulation [14]. The skin thickness appears to be a likely explanation for why pain perception is delayed, but it has also been shown that the conduction velocity of A-mechano-heat nociceptors is faster in the glabrous skin compared to hairy skin [34]. Therefore, it is important to study glabrous skin afferents to provide correlative neurophysiologic evidence for in vivo pain behavior assays.
In behavioral studies, we characterized the withdrawal responses to mechanical stimuli in the mouse [4]. In the unincised mouse, the withdrawal frequency to 3.1 and 6.0 mN filaments were less than 10% and 14.0 and 26.4 mN filaments elicited withdrawal frequencies less than 25-30% of the time in normal skin. One day after incision, the responses to 3.1 and 6.0mN were in the 60-80% range; the stronger filaments, 14.0 and 26.4mN, increased withdrawal into the 80-90% range [4]. Thus, behavioral evidence indicates sensitization to weak mechanical stimuli one day after incision and the nearly maximum withdrawal frequency can be evoked by forces in the 14 to 26mN range. It is likely that the low threshold nociceptors contribute to these withdrawal responses and those fibers responding to forces greater than 10mN and eliciting minimal responses to 20 mN forces (Figs 2j and 3j) are less likely to be involved. Furthermore, because the prevalence of low threshold fibers (less than 10 mN) was not different between incision and control groups (Fig 2h and 3h), it is unlikely incision converted high threshold afferents to low threshold afferents. We recognize that we may have excluded some large myelinated A-β high threshold afferent fibers that may transmit nociceptive input and could be sensitized by incision[10]. This is a topic we have not addressed.
Many previous studies failed to identify mechanical sensitization after experimental pathological conditions [3,15,22,28,31]. This led to the hypothesis that sensitization to mechanical stimuli is purely a central phenomenon or sensitization occurring due to recruitment of mechano-insensitive units, which develop novel mechanosensitivity after injury [31]. A recent study that used mechanical stimulation in vitro showed mechanical sensitization of rat glabrous skin afferents after Complete Freund’s adjuvant[12].
Threshold was decreased and activity was greater in the Complete Freund’s adjuvant treated skin. Our results show that incision increases nociceptor responses to suprathreshold stimuli but threshold is not altered. The difference is perhaps due to incisional pain mechanisms having differences from a purely inflammatory pain state [5,25,35]. Species differences may also contribute.
In the present study differences in the threshold and total spikes of single mechanosensitive units to heat did not reach statistically significance, but the peak discharge frequencies were different. Unlike mechanical stimulation, heat stimulation often caused other unclassified fibers to be activated. Thus, they were not identified mechanically before heat stimulation. In the previous study in rats, these fibers were called ‘unclassifed’ units [2]. Here, in addition to mechanically identified single fiber responses, we also analyzed activities of all afferents including these unclassified afferents as total multi-fiber activity during heat stimulation. The multi-fiber responses in the control versus incised preparations were significantly different in threshold temperature, total spikes, and peak discharge frequency. Moreover, temperature response curves were significantly increased in the incised preparation. In our behavioral experiments, heat withdrawal latency decreased from 25 to 5 seconds one day after incision[4,25]. Although we can not state that the exaggerated response to heat is exclusively due to peripheral sensitization, the limited heat sensitization of mechanosenitive fibers indicates that heat sensitive afferents, other than CMH fibers, may contribute to heat the robust hyperalgesia observed by us and others [24].
We previously identified the existence of a distinct class of afferents, which were not readily identified by mechanical stimulation and appear during heat stimulation [2]. In this study, we showed that in mouse this group of afferents profoundly sensitized by incision in comparison with mechanosensitive afferents. From this observation, we hypothesize that perhaps mechanical and heat sensitization occurred in the two distinct classes of fibers. In other words, the mechanosensitive afferents do not participate in heat sensitization and vice versa. Supporting this, it has been[19] showed that a distinct class of afferents is present in a mouse ex-vivo preparation, which are responsive to heat but mechanically insensitive. These fibers are categorized as C-heat afferents. The C-heat and C-mechano-heat fibers have similar thresholds for heat response, but C-heat fibers had greater maximal firing frequencies to heat and slower conduction velocities (Dr. Koerber, personal communication). Thus, the afferent we labeled as ‘unclassified’ may represent C-heat fibers that contribute to profound heat hyperalgesia in the incised mice.
The methodology used in the present study to detect excitation to cooling may not be adequate to detect noxious cold. None of 35 control C-fibers tested were responsive to 12-16°C cold temperature and only one of 5 Aδ showed responsiveness. As expected, incision did not influence excitation to cooling. Although previous studies used different temperatures to detect cold sensitivity, Leem et al. [20] found that only 8% of C-fiber nociceptors in the rat glabrous skin were excited by cold stimuli using a maximal stimulating intensity of 12°C. This is in agreement with the present data in mice.
The mouse hind paw is extensively used in the pain behavior assay; the goal of this study was, therefore, to develop a mouse glabrous skin nerve preparation, which will allow studies of afferent function of the same anatomic site as in pain behavior studies. This study demonstrates that more mechanosensitive C-fibers have spontaneous activity in the incised preparation. Mechanical sensitization can be identified by a force-controlled stimulator. Heat sensitization is present in the mechanosensitive C-fibers but more prominent in the unclassified afferents.
Acknowledgments
Support: This work was performed in and supported by the Department of Anesthesia at the University of Iowa and by National Institutes of Health, Bethesda, Maryland grant GM-55831 to T.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All experiments were carried out in this institution
References
- [1].Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999 Nov;82(5):2649–56. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- [2].Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004 Nov;112(1-2):204–13. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- [3].Banik RK, Kozaki Y, Sato J, Gera L, Mizumura K. B2 receptor-mediated enhanced bradykinin sensitivity of rat cutaneous C-fiber nociceptors during persistent inflammation. Journal of neurophysiology. 2001 Dec;86(6):2727–35. doi: 10.1152/jn.2001.86.6.2727. [DOI] [PubMed] [Google Scholar]
- [4].Banik RK, Woo YC, Park SS, Brennan TJ. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006 Dec;105(6):1246–53. doi: 10.1097/00000542-200612000-00025. [DOI] [PubMed] [Google Scholar]
- [5].Brennan TJ, Zahn PK, Pogatzki-Zahn EM. Mechanisms of incisional pain. Anesthesiology clinics of North America. 2005 Mar;23(1):1–20. doi: 10.1016/j.atc.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [6].Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. The Journal of urology. 2003 Sep;170(3):1008–12. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- [7].Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. Journal of neurophysiology. 2001 Apr;85(4):1561–74. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- [8].Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, et al. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001 Dec 1;21(23):9367–76. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000 Apr 14;288(5464):306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [10].Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004 Oct;46(2):131–45. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- [11].Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001 Jan;89(2-3):187–98. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- [12].Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006 Feb;137(3):999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- [13].Georgopoulos AP. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- [14].Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain. 1999 Nov;83(2):123–35. doi: 10.1016/s0304-3959(99)00099-8. [DOI] [PubMed] [Google Scholar]
- [15].Kirchhoff C, Jung S, Reeh PW, Handwerker HO. Carrageenan inflammation increases bradykinin sensitivity of rat cutaneous nociceptors. Neurosci Lett. 1990 Mar 26;111(1-2):206–10. doi: 10.1016/0304-3940(90)90369-k. [DOI] [PubMed] [Google Scholar]
- [16].Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. Journal of neurophysiology. 1997 Oct;78(4):1841–50. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- [17].Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992 Aug;68(2):581–95. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- [18].LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, Deleo JA. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 2005 Aug 15;30(16):1821–7. doi: 10.1097/01.brs.0000174122.63291.38. [DOI] [PubMed] [Google Scholar]
- [19].Lawson JJ, McIlwrath SL, Woodbury CJ, Davies BM, Koerber HR. Society for Neuroscience. Atlanta, Georgia: 2006. Mouse cutaneous C-fibers containing TrpV1 are responsive to heat, but mechanically insensitive. 2006 abstract viewer/itinerary planner; 2006. [Google Scholar]
- [20].Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993 May;69(5):1684–99. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- [21].Leffler A, Monter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006 May 12;139(2):699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- [22].Mizumura K. Peripheral mechanism of hyperalgesia--sensitization of nociceptors. Nagoya journal of medical science. 1997 Nov;60(3-4):69–87. [PubMed] [Google Scholar]
- [23].Mogil JS, Yu L, Basbaum AI. Pain genes?: natural variation and transgenic mutants. Annu Rev Neurosci. 2000;23:777–811. doi: 10.1146/annurev.neuro.23.1.777. [DOI] [PubMed] [Google Scholar]
- [24].Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003 Oct;99(4):1023–7. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- [25].Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005 Jun;115(3):296–307. doi: 10.1016/j.pain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [26].Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, et al. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000 Oct 26;407(6807):1007–11. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- [27].Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, et al. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001 Dec 20;32(6):1071–83. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- [28].Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neuroscience letters. 1986 May 15;66(2):141–6. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- [29].Reeh PW. Sensory receptors in a mammalian skin-nerve in vitro preparation. Progress in brain research. 1988;74:271–6. doi: 10.1016/s0079-6123(08)63024-1. [DOI] [PubMed] [Google Scholar]
- [30].Schaible HG, Schmidt RF. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. J Neurophysiol. 1985 Nov;54(5):1109–22. doi: 10.1152/jn.1985.54.5.1109. [DOI] [PubMed] [Google Scholar]
- [31].Schlegel T, Sauer SK, Handwerker HO, Reeh PW. Responsiveness of C-fiber nociceptors to punctate force-controlled stimuli in isolated rat skin: lack of modulation by inflammatory mediators and flurbiprofen. Neurosci Lett. 2004 May 6;361(1-3):163–7. doi: 10.1016/j.neulet.2003.12.073. [DOI] [PubMed] [Google Scholar]
- [32].Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life sciences. 2004 Apr 9;74(21):2593–604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [33].Taylor DJ, McGillis SL, Greenspan JD. Body site variation of heat pain sensitivity. Somatosens Mot Res. 1993;10(4):455–65. doi: 10.3109/08990229309028850. [DOI] [PubMed] [Google Scholar]
- [34].Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995 Mar 15;483(Pt 3):747–58. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, et al. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol. 2004 Jan;141(1):85–91. doi: 10.1038/sj.bjp.0705568. [DOI] [PMC free article] [PubMed] [Google Scholar]