Abstract
Steroid receptor coactivators (SRCs) are important transcriptional modulators that regulate nuclear receptor and transcription factor activity to adjust transcriptional output to cellular demands. Highlighting their pleiotropic effects, dysfunction of the SRCs has been found in numerous pathologies including cancer, inflammation, and metabolic disorders. The SRC family is expressed strongly in the brain including the hippocampus, cortex, and hypothalamus. Studies focusing on the effect of SRC loss using congenic SRC knockout mice (SRC−/−) are limited in number, yet strongly indicate that the SRCs play important roles in regulating reproductive behavior, development, and motor coordination. To better understand the unique functions of the SRCs, we performed a neurobehavioral test battery focusing on anxiety and exploratory behaviors, motor coordination, sensorimotor gating, and nociception in both male and female null mice and compared them with their wild-type (WT) littermates. Results from the test battery reveal a role for SRC1 in motor coordination. Additionally, we found that SRC1 regulates anxiety responses in SRC1−/− male and female mice, and nociception sensitivity in SRC1−/− male but not female mice. By comparison, SRC2 regulates anxiety response with SRC2−/− females showing decreased anxiety in novel environments, as well as increased exploratory behavior in the open field compared with WT littermates. Additionally, SRC2−/− males were shown to have deficits in sensorimotor gating. Loss of SRC3 also shows sex differences in anxiety and exploratory behaviors. In particular, SRC3−/− female mice have increased anxiety and reduced exploratory activity and impairments in prepulse inhibition, whereas SRC3−/− male mice show no significant behavioral differences. In both genders, ablation of SRC3 decreases nocifensive behaviors. Collectively, these resource data suggest that loss of the SRCs results in behavioral phenotypes, underscoring the importance of understanding both the general and gender-based activity of SRCs in the brain.
The p160 steroid receptor coactivator (SRC) family comprises 3 homologous genes: SRC1 (NCoA1), SRC2 (NCoA2/GRIP1/TIF2), and SRC3 (NCoA3/AIB1/ACTR/RAC3/Tram-1). Members of the SRC family share several conserved domains: an N-terminal basic helix-loop-helix-Per/ARNT/Sim (bHLH-PAS) domain (1), 3 LXXLL (where L = Leucine and X = any amino acid) motifs forming nuclear receptor interacting α-helices (2, 3), and 2 transcriptional activation domains in the C terminus facilitating the binding of other coregulators (1, 4). SRCs traditionally serve as mediators of ligand-bound nuclear receptor (NR)-activated transcription, but are also known to work with other transcription factors (2, 5). The multifaceted nature of the SRCs allows for complex formation with many other proteins and involvement in various signaling networks necessary to fulfill manifold cellular demands.
As multifarious regulators, SRCs are essential in maintaining homeostatic conditions within different tissues, and dysregulation of the SRCs has been implicated in several diseases (1, 6). Although structurally similar in many respects, each SRC is known to perform unique functions highlighted by the numerous divergent phenotypes found in the SRC−/− mouse models. Ablation of SRC1 in mice impairs gluconeogenesis and increases sensitivity to diet-induced obesity (7, 8). SRC1 is also overexpressed in subpopulations of breast cancers where it promotes cell migration and adhesion (9–12). Highlighting reproductive differences, SRC1−/− mice are fertile, but male and female SRC2−/− mice are infertile: SRC2−/− females display placental hypoplasia, and SRC2−/− males display defects in spermatogenesis (13, 14). Apart from its reproductive functions, SRC2 is also a critical modulator of metabolic homeostasis. SRC2 regulates glucose and fuel availability in the liver and regulates fat accumulation and thermogenesis in white adipose tissue (8, 15, 16). Like SRC1, SRC3 is a pleiotropic oncogene primarily known for its role in breast cancer as the protein amplified in breast cancer 1, strongly mediating the activity of estrogen receptor α (ERα) and regulating several cell cycle and growth-signaling pathways in breast cancer (17). Much like the other SRCs, SRC3 is an important metabolic regulator of skeletal muscle fatty-acid metabolism wherein mice devoid of SRC3 are resistant to diet-induced obesity (6).
Although a number of studies have illuminated the SRCs' role in certain metabolic functions and organ systems, little is known on the role of the SRC family of coactivators in brain and behavior. Nevertheless, the few studies that do focus on the SRC family in the brain indicate their importance in the maintenance of normal behaviors and cognition. SRC1 is by far the most studied SRC family member in the central nervous system. SRC1 is widely expressed throughout the brain with higher levels in the cortex, hippocampus, and olfactory bulbs (18). In many regions of the brain, SRC1 expression is significantly higher in male mice than in female mice (19) and is an important developmental and behavioral regulator of sexually dimorphic regions in the brain (20). Within the hypothalmic-pituitary-adrenal (HPA) axis, SRC1 regulates transcription of CRH and is required for full activation of the CRH promoter (21). SRC1 is also a coregulator for glucocorticoid receptor, a prominent NR in the HPA axis (21, 22). Further, under stressful conditions SRC1 expression was shown to decrease in the male hypothalamus while increasing in the female hypothalamus, suggesting it may be a potential regulator of sexually dimorphic response to stress (23). Examination of the SRC1−/− mouse revealed a motor impairment caused by a developmental defect in Purkinje cells (24). Likewise, SRC1 and SRC2 have prominent roles in regulating female and male reproductive behaviors (25). In reproductive-relevant regions of the hypothalamus, SRC1 colocalizes with SRC2 and ERα, and progesterone receptor (PR) (26, 27). Our laboratory has shown that acute knockdown of SRC1 in the ventromedial nucleus (VMN) of the hypothalamus is sufficient to reduce female receptivity (28). SRC1 has also been shown to regulate body weight through its interactions with ERα in steroidogenic factor-1 positive neurons (29). These studies collectively indicate that SRC1 is likely a prominent mediator of brain development and function.
Like SRC1, SRC2 is widely expressed in the brain. Specific areas include: the amygdala, hippocampus, bed nucleus of stria terminalis, VMN, suprachiasmatic nucleus, and supraoptic nucleus (30). Within reproductive regions of the hypothalamus, SRC2 colocalizes with ligand-bound ERα and PR, and upon testosterone stimulation SRC2 expression decreases (27, 30, 31). Our laboratory has shown that SRC2 plays large compensatory roles in the VMN in the absence of SRC1 (28). Both acute and chronic ablation of SRC2 is sufficient to abolish female reproductive behaviors, indicating that SRC1 is unable to compensate for the loss of SRC2 (28). SRC2−/− mice also have an adrenal gland defect resulting in decreased levels of circulating corticosterone and decreased glucocorticoid receptor and CRH in the paraventricular nucleus (PVN). Further, the increased arginine vasopression in the PVN of SRC2−/− mice implies that loss of SRC2 results in HPA axis dysregulation (32).
Whereas much is known about SRC3 as a coactivator in other tissues, little is known about the function of SRC3 in the brain. Like the other SRC family members, SRC3 is ubiquitously expressed in the brain, but is concentrated primarily in the hippocampus, olfactory bulb, and cortex (33). Unlike SRC1 and SRC2, SRC3 has not been shown to regulate reproductive behaviors (28). Analysis of circulating estrogen and IGF-1 in SRC3-ablated female mice reveals reduced levels of these hormones, resulting in decreased growth and delayed development (28, 33). Within the pituitary gland, SRC3 was found to interact with PR in response to GnRH and to increase FSHβ expression (14, 34). SRC3 has been demonstrated to interact with estrogen receptor β (ERβ) in the brain (35). In embryonic neurons, SRC3 expression, phosphorylation, and ultimate degradation increase in response to all-trans retinoic acid treatment and may be an essential regulator in developmental programs, but the developmental impact of SRC3 in the central nervous system (CNS) has not been fully characterized (36).
Recurrent studies underscore the importance of the SRC family as fundamental coregulators in many physiologic processes including the CNS. Although the SRCs are broadly expressed in the brain, few studies focus on the consequences of SRC loss in regulating neurobehavioral responses. To better understand the individual functions of the SRC family members, we have performed a comprehensive neurobehavioral assessment of each SRC−/− male and female mouse to screen for possible behavioral phenotypes. This study provides the first comprehensive and comparable examination of the individual and gender-specific behavioral consequences resulting from SRC ablation in mice.
Materials and Methods
Animals and housing
The generation of the SRC1−/−, SRC2−/−, and SRC3−/− mice has been described previously (13, 33, 37). The SRC1−/− colony was maintained on a pure C57BL6/J genetic background. The SRC2−/− and SRC3−/− colonies were maintained on a mixed 129SV/C57BL6/J genetic background due to reported breeding issues associated with these lines being maintained on a pure C57BL6/J genetic background (38). Congenic WT and SRC−/− male and female littermate mice 10–20 weeks of age were used for all studies. All mice were housed under strict temperature control with ad libitum access to food (normal chow, 2920X Teklad Global) and water under 12 hour light/dark conditions. The Animal Care and Research Committee at Baylor College of Medicine approved all of the neurobehavioral studies.
Nonradioactive in situ hybridization
Brain tissue from WT male mice was serially sectioned in coronal orientation at 25 μm (n = 3–4). Nonradioactive in situ hybridization was performed and quantified as previously described with the SRC1, SRC2, and SRC3 probes (39, 40).
Elevated plus maze (EPM)
Prior to testing, mice were habituated in the testing room within their home cage for 30 minutes at 150 lux with 60 dB of white noise. Each mouse was then placed in the center of the maze, which is approximately a 1-meter-diameter plus-shape platform elevated 0.5–1 m off the ground. The activity of each mouse was recorded and measured for 10 minutes using the ANY-maze video tracking system (Stoelting Co.) as previously described, and the total distance traveled between EPM arms and the number of entries between arms of the maze were used as indices of anxiety-related behavior (41, 42).
Open field activity (OFA)
The activity of each mouse was measured for 30 minutes in a Plexiglass chamber (40 × 40 × 30 cm) and recorded with the Versamax animal activity monitoring system equipped with photo beams as horizontal X-Y sensors and/or Z sensors (Accuscan Electronics). Throughout testing a white background noise was present (55 dB). Total distance traveled and the ratio of center distance traveled to total distance traveled were calculated as indicators for anxiety-like and exploratory behaviors (41, 42). For all measurements, the researcher remained out of sight from the mice at all times during the experiment.
Fine motor skills
Wire hang.
Mice grasped a single wire 1–3 mm by their forepaws and their latency to fall was recorded with a maximum of 60 seconds.
Grip strength.
Grip strength was measured exactly as previously described (43). Mice grasped a triangular bar with both forepaws. Once grasped, the mouse was gently pulled from the bar until it released its forepaws from the bar. The maximum force generated was recorded for 3 individual trials.
Rotarod.
Motor coordination learning was recorded by the time each animal was able to walk on the accelerating rotarod (Ugo Basile, Comerio) at 4–40 rpm. Each mouse was tested for 2 days with 4 trials per day, and the time spent on the rotarod was a maximum of 5 minutes per trial with a rest period of at least 10 minutes between trials (41, 42).
Prepulse inhibition (PPI)
The acoustic startle responses and sensorimotor gating of mice were measured using the SR-Lab System (San Diego Instruments). After 30 minutes of habituation, mice were placed in a Plexiglas cylinder and left undisturbed with white noise (70 dB) for 5 minutes prior to the beginning of the 15-minute test, which consists of 7 trial types: one no-stimulus trial for baseline movement in the cylinder; one 40 milliseconds with 120 dB startle alone trial for the startle stimulus; and 5 prepulse plus startle trials in which 5 different prepulse sounds (74, 78, 82, 86, or 90 dB) are presented before the startle stimulus at 120 dB as previously described (41, 42).
Nociception
The response to thermal stimulus was measured using a hot plate set at 55°C (Columbus Instruments). Mice were placed individually on the hotplate, and the latency to either lift the hind limb response, paw shake, licking, or jumping was measured for a maximum of 45 seconds as described previously (41, 42).
Statistics
Results are displayed as the mean ± SEM. Using Graphpad Prism 6 statistical comparisons between 2 different genotype groups (SRC WT and SRC−/−) were performed using individual 2-tailed unpaired Student's t test with differences of P ≤ .05 considered statistically significant.
Database availability
The entire raw data sets used in this Research Resource will be made publicly available through the Nuclear Receptor Signaling Atlas (NURSA) data repository at http://www.nursa.org/.
Supplemental data
Supplemental data include 1 supplemental figure published on The Endocrine Society's Journals Online website at http://mend.endojournals.org.
Results
SRC phenotypes and expression
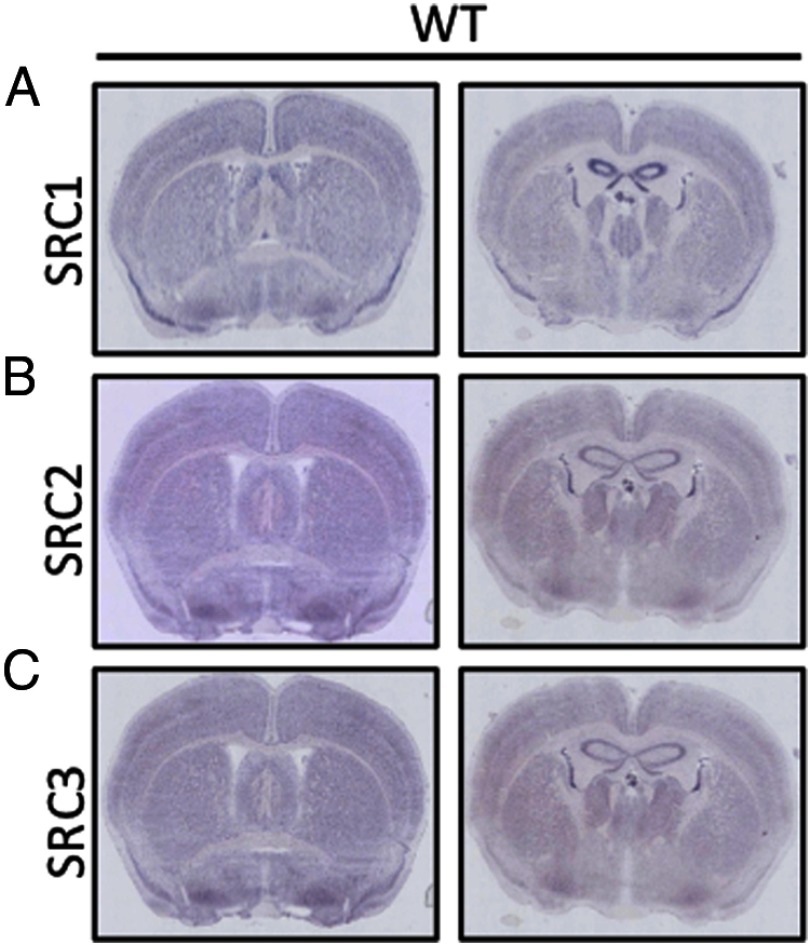
The SRC family of coactivators is undoubtedly important in the CNS, with most studies focusing on the role of SRCs as coactivators for NRs in mediating steroid-mediated actions in the brain (Table 1). With ubiquitous expression throughout the brain, it is difficult to ascribe any one role for the different SRCs based exclusively on localized expression (Figure 1, A–C). A more comprehensive overlap of the SRCs expression patterns with that of NR and other transcription factors can be found at the Allen Brain Atlas (www.brain-map.org) or in published resources (44). To this end, we performed an unbiased and comprehensive neurobehavioral test battery including anxiety and exploratory tests, locomotor coordination, strength tests, sensorimotor gating, acoustic startle response, and nociception tests to elucidate the phenotypes associated with individual genetic ablation of each SRC (Table 2) (41, 42).
Table 1.
Summary of Previously Reported Phenotypes of SRCs in the CNS
| Coactivator | Physiologic Function | References |
|---|---|---|
| SRC1 | Expression higher in males than females | (18, 19) |
| Important in HPA axis regulating CRH for glucocorticoid receptor | (21, 22) | |
| Hypothalamic expression in males decreases during stress | (23) | |
| Loss increases corticosterone in response to stress | (21, 22) | |
| Loss impairs motor coordination through developmental Purkinje cell defect | (24) | |
| Colocalizes with ER and PR in reproductive relevant regions of brain | (26, 27) | |
| Regulates reproductive behaviors | (28) | |
| In SF-1 neurons interacts with ERα to regulate feeding behaviors | (29) | |
| SRC2 | Colocalizes with ER and PR | (27, 30) |
| Expression decreases upon testosterone administration | (30) | |
| Compensates for loss of SRC1 in the VMN | (28) | |
| Loss abolishes female reproductive behaviors | (28) | |
| Ablation decreases corticosterone in males from an adrenal gland insufficiency altering HPA axis | (32) | |
| SRC3 | Loss decreases circulating estrogen and IGF-1 | (28, 33) |
| In the pituitary interacts with PR and increase FSHβ | (14, 34) | |
| Expression and activity in embryonic neurons changes in response to all-trans retinoic acid | (36) | |
| Interacts with ERβ | (35) | |
| In the pituitary activates PR in response to GnRH | (14, 34) | |
| Decreased endurance from CACT deficiency | (6) |
SF-1, steroidogenic factor 1; CACT, carnitine-acylcarnitine translocase.
Figure 1.
SRC Expression throughout the Brain. A–C, SRC expression in WT brain as measured by in situ hybridization (ISH) in coronal sections from WT mice for SRC1 (A), SRC2 (B), and SRC3 (C), respectively.
Table 2.
Summary of Phenotypes Observed in the Neurobehavioral Test Battery.
| Behavior | Paradigm | SRC1 |
SRC2 |
SRC3 |
|||
|---|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ||
| Locomotor/anxiety | OFA | N.C. | N.C. | ↓ | N.C. | ↑ | N.C. |
| Anxiety | EPM | N.C. | ↓ | ↓ | N.C. | ↑ | N.C. |
| Motor coordination | Grip strength/wire hang/dowel walking | N.C. | N.C. | ↓ | N.C. | N.C. | N.C. |
| Motor learning | Rotarod | N.C. | ↓a | N.C. | N.C. | N.C. | N.C. |
| Sensorimotor gating | PPI | N.C. | N.C. | N.C. | ↓ | ↓ | N.C. |
| Nociception | Hotplate | N.C. | ↓ | ↑ | N.C. | ↑ | ↑ |
↑/↓ = P < 0.05. N.C., no statistical change.
Phenotype previously reported (24)
Anxiety and exploration tests
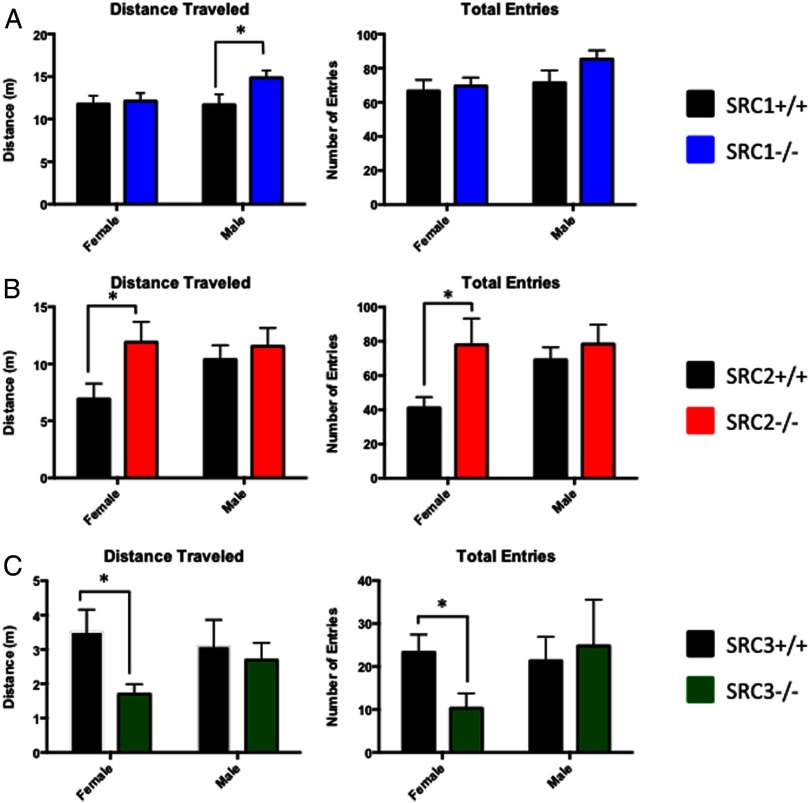
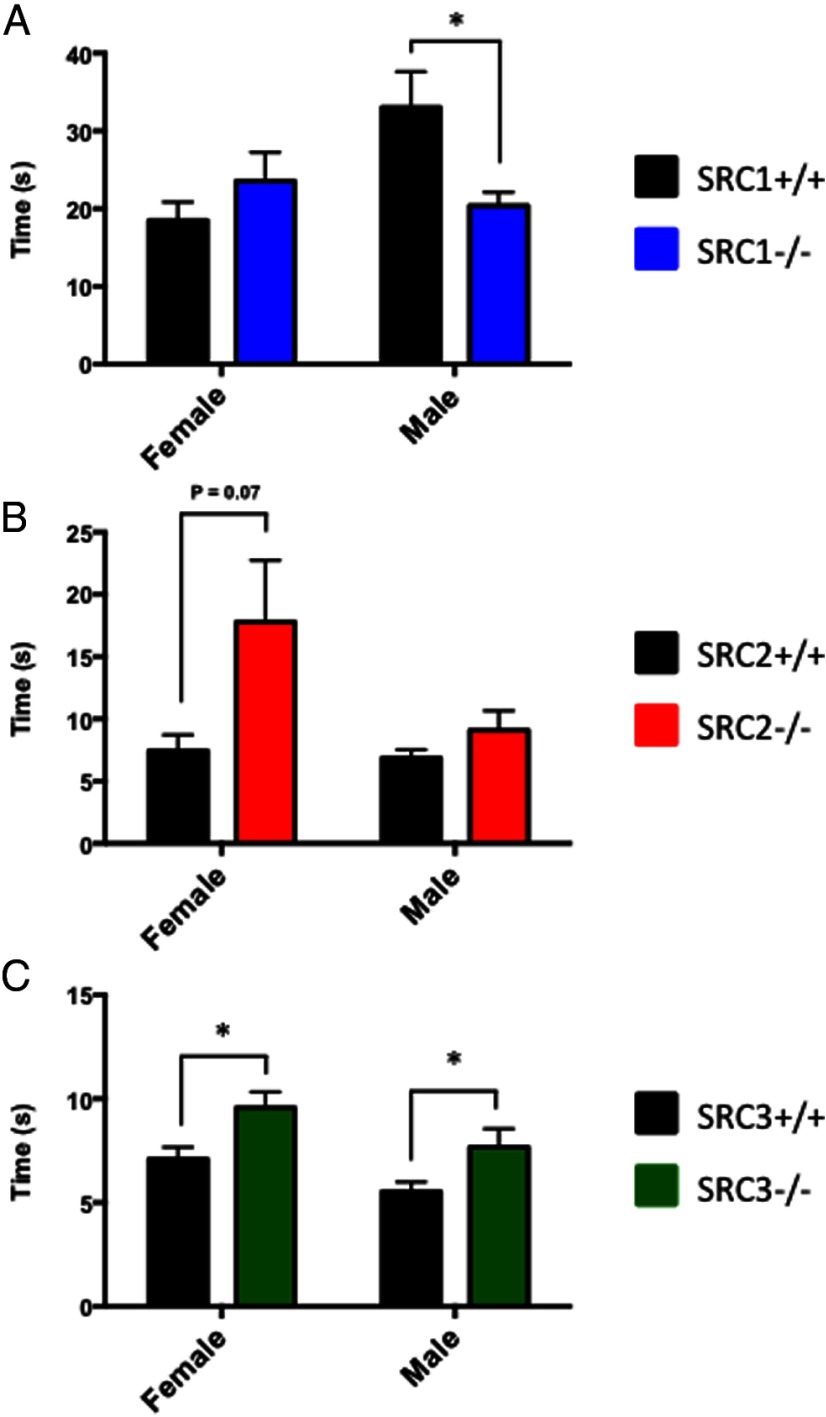
In an attempt to understand the role(s) of the SRCs in regulating anxiety and stress response behaviors, male and female WT and SRC−/− littermate mice were placed in the EPM. No differences were found between SRC1−/− and WT female mice; however, we found that SRC1−/− male mice traveled significantly more in the EPM, t(22) = 2.13, P < .05, (Figure 2A). In the SRC2 mice, we found that female SRC2−/− mice displayed decreased anxiety-like behaviors with an increase in distance traveled in the EPM t(17) = 2.28, P < .05, and an increase in total EPM entries, t(17) = 2.12, P < .05 (Figure 2B). No significant differences between WT and SRC2−/− male mice in the EPM were observed. By comparison, SRC3−/− female mice exhibited increased anxiety behaviors with a decrease in the total distance traveled in the EPM, t(17) = 2.63, P < .05, and SRC3−/− female mice crossed less than half of the EPM entries of their WT female littermates, t(17) = 2.45, P < .05 (Figure 2C). No discernable differences were observed between WT and SRC3−/− male mice (Figure 2C). Taken together, these results suggest that the SRCs differentially influence anxiety, and this regulation is potentially complex because sex differences were observed in all 3 SRC genotypes. SRC1−/− male and SRC2−/− female mice displayed decreased anxiety-related behaviors, whereas SRC3−/− female mice displayed increased anxiety-related behaviors in the EPM respective to their WT littermates.
Figure 2.
Analysis of SRC Ablation in the EPM. A–C, Total distance traveled and total entries made in the EPM in WT and SRC−/− male and female littermate mice. A, SRC1−/− male mice travel greater distances and have increased entries in the EPM. B, SRC2−/− female mice have increased total distance traveled than WT female mice. C, SRC3−/− females travel less distance and cross fewer entries than WT female mice. Mean ± SEM; *, P < .05.
To further examine anxiety and exploratory behaviors, mice were placed in an open field arena, and their open field activity (OFA) was recorded. We observed that SRC1−/− female mice traveled significantly less in the center of the open field, t(19) = 3.06, P < .01, with no difference in the total distance traveled (Figure 3A), suggesting that SRC1−/− female mice are less exploratory and more anxious than their WT littermates. No behavioral differences between WT and SRC1−/− male mice were measured. Similar to the EPM, SRC2−/− female mice traveled significantly more in the open field than WT mice, t(16) = 2.41, P < .05, and trend toward increased center/total distance traveled, t(16) = 1.8, P = .09, indicating that loss of SRC2 in female mice reduces anxiety-like behaviors and increases exploratory behaviors (Figure 3B). To further support these findings, SRC2−/− female mice moved significantly more in the OFA, t(16) = 2.66, P < .05, and showed slightly more rearing activity than WT mice, t(16) = 1.78, P < .09. The locomotor activity of WT and SRC2−/− female mice was measured in their home cage environment for 24 hours, and we found no differences in home cage activity levels (data not shown), indicating that the behavioral differences found in the EPM and the OFA are a result of the novel environments. As before, there were no significant differences between WT and SRC2−/− male behavior, suggesting SRC2 may differentially regulate anxiety responses. Complementing activity in the EPM, the total distance SRC3−/− female mice traveled in the open field was significantly less than WT mice, t(17) = 3.09, P < .01, as was the center/total distance traveled, t(17) = 2.46, P < .05, indicating that SRC3−/− female mice are significantly more anxious and less exploratory than their WT littermates (Figure 3C). Consistent with their locomotor activity, SRC3−/− female mice also moved significantly less in the open field, t(17) = 2.19, P < .05, and presented less rearing activity than WT mice, t(19) = 1.85, P = .08 (Figure 3C). Home-cage monitoring of WT and SRC3−/− female mice showed no differences in locomotor activity over 24 hours (data not shown), indicating that the decrease in activity of the SRC3−/− female mice was from increased anxiety-related behaviors from the novel environments of the EPM and OFA. No behavioral differences were found between WT and SRC3−/− male mice in the OFA.
Figure 3.
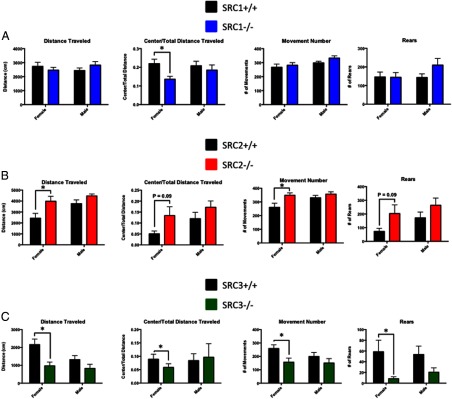
Analysis of SRC Ablation in the OFA. A–C, Distance traveled, center distance traveled to total distance traveled ratio, number of movements, and number of rears measured during the OFA in WT and SRC−/− genotypes in male and female littermate mice. A, SRC1−/− female mice have decreased center/total distance traveled. B, SRC2−/− female mice have increased total distance traveled, increased center/total distance ratio, and increased movements compared with WT littermates in the OFA. C, SRC3−/− female mice have decreased total distance traveled, decreased center to total distance ratio, and decreased movements and rearing behavior. Mean ± SEM; *, P < .05.
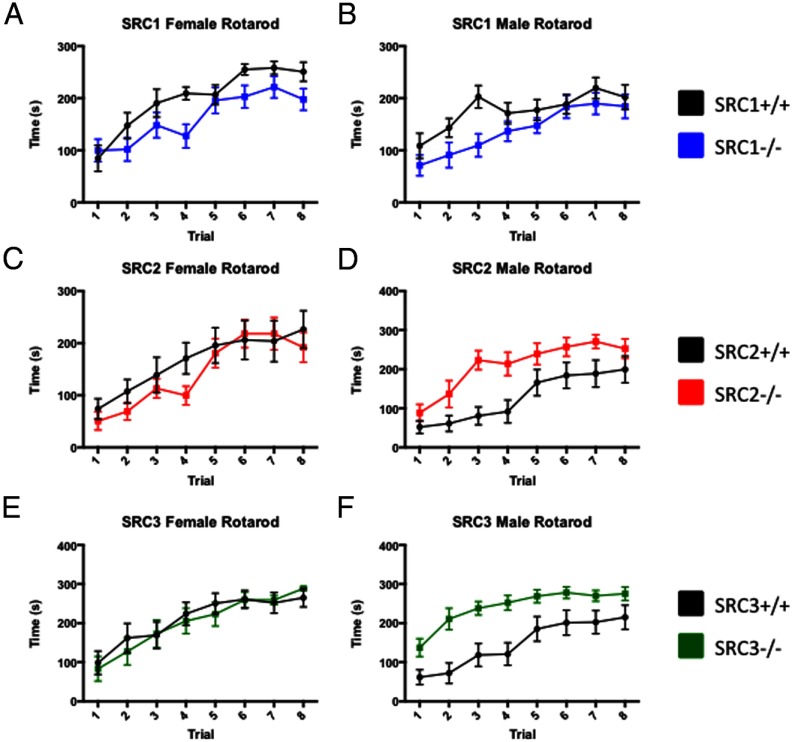
Motor coordination
Previous studies have shown that loss of SRC1 in male mice decreases motor coordination and strength due to a Purkinje cell defect as measured by the rotarod and the wire hang tests, respectively (24). We further tested the fine-motor coordination of the SRC1−/− female mice and found no differences in grip strength, the wire-hang test, or the dowel test (summarized in Table 3 and Supplemental Figure 1). When we tested the SRC1−/− female and SRC1−/− male mice on the rotarod, in line with previous publications, we found that although not significant, the SRC1−/− mice did spend less time walking on the rotarod than the WT mice (Figure 4, A and B). We found that SRC2−/− male and SRC2−/− female mice had decreased grip strength compared with their WT littermates (Table 3). SRC2−/− female mice performed as well on the rotarod as WT female mice (Figure 4C), and SRC2−/− males performed better on the rotarod, indicating that unlike SRC1, there is no impairment of motor coordination with loss of SRC2 (Figure 4D). Increased performance may be the result of weight limitation and effect on the rotarod (Table 3). In SRC3−/− female mice we measured a significant decrease in grip strength and an increased latency to fall on the wire-hang test, but SRC3−/− female mice had no differences in performance on the rotarod compared with WT littermates (Table 3 and Figure 4E). Male SRC3−/− showed improved motor coordination compared with WT mice on the rotarod with decreased latency to fall, but the difference may also be due to size effects on the rotarod (Figure 4F and Table 3). Interestingly, published studies from our laboratory have shown that loss of SRC-3 leads to decreased muscle endurance capacity resulting from an inability to metabolize long-chain fatty acids (6). These data help to further clarify that the skeletal muscle defect observed in the SRC-3−/− is not due to impairment of motor coordination.
Table 3.
Summary of the Fine Motor Skills Tested in the SRC−/− Mice
| Paradigm | SRC1 |
SRC2 |
SRC3 |
|||
|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| Grip strength | N.C. | N.C. | ↓ | ↓ | ↓ | N.C. |
| Wire hang | N.C. | ↓a | N.C. | N.C. | ↓ | N.C. |
| Dowel test | N.C. | N.C. | N.C. | N.C. | N.C. | N.C. |
| Weight | N.C. | N.C. | ↓ | N.C. | ↓ | ↓ |
↓ = P < 0.05. N.C., no statistical change.
Phenotype previously reported (24)
Figure 4.
Analysis of SRC−/− Mice Performance in Motor Coordination. A–C, The latency to fall during 8 trials on the rotarod for SRC−/− genotypes and respective WT male and female littermates were measured. A, WT and SRC1−/− female mice showed a slight decrease in the time spent on the rotarod. B, WT and SRC1−/− male mice had a modest increase in latency to fall from the rotarod. C, WT and SRC2−/− female mice show no differences on the rotarod. D, SRC2−/− male mice show increased ability to perform on the rotarod compared with WT mice. E, WT and SRC3−/− female mice show no differences on the rotarod. F, SRC3−/− male mice show a decrease in latency to fall from the rotarod. Mean ± SEM; *, P < .05.
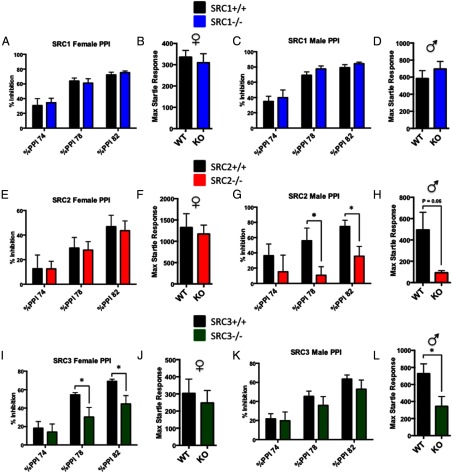
Sensorimotor gating and acoustic startle response
Sensorimotor gating was assessed by measuring the acoustic startle reflex of the SRC−/− and WT mice in response to an acoustic PPI. The acoustic paradigms exploited assess normal cognitive function of sensory information processing and detect defects or impairments in the acoustic startle response or sensorimotor gating, which have been linked to several psychiatric disorders including schizophrenia (45). We found no differences in sensorimotor gating or acoustic startle response between male and female SRC1−/− and WT mice (Figure 5, A–D). Similarly, no significant differences between WT and SRC2−/− female mice were found in PPI or acoustic startle response (Figure 5, E and F). In SRC2−/− male mice, however, significant impairments were found in percent inhibition with PPI at 78 dB, t(10) = 2.28, P < .05, and PPI at 82 dB, t(10) = 2.57, P < .05 (Figure 5G). In the acoustic startle response, SRC2−/− males showed significantly less startle response than WT male mice, t(10) = 2.42, P < .05 (Figure 5H). Significant PPI disruptions were found in SRC3−/− female mice at 78 dB, t(17) = 2.16, P < .05, and at 82 dB, t(17) = 2,51, P < .05 (Figure 5I), but no differences in startle amplitude were found in SRC3−/− females (Figure 5J). No differences were found between WT and SRC3−/− male mice in PPI (Figure 5K); however, SRC3−/− male mice did show significantly less startle response than WT mice, t(21) = 2.26, P < .05 (Figure 5L).
Figure 5.
Sensorimotor Gating and Acoustic Startle Response in the SRC−/− Mice. A–F, Sensorimotor gating and acoustic startle response tested through PPI in WT and SRC−/− male and female littermates. A–D, PPI and acoustic startle response in WT and SRC1−/− female (A and B) and male mice (C and D) show no differences. E and F, PPI and acoustic startle response in WT and SRC2−/− female mice show no differences. G and H, Decreased PPI and acoustic startle response were observed in SRC2−/− male mice compared with WT. I and J, Decreased PPI with no change in acoustic startle response in SRC3−/− female mice compared with WT mice. K and L, No changes observed between WT and SRC3−/− male mice in PPI (K), whereas SRC3−/− male mice showed a decrease in the acoustic startle response (L). Mean ± SEM; *, P < .05.
Nociception
The hot-plate test was used to test nociceptive behaviors with the pain threshold of mice measured by the latency to lift, shake, or lick the hind paw. SRC1−/− male mice were significantly more sensitive to the hot-plate test than WT animals, t(21) = 2.67, P < .05, and no differences were observed in SRC1−/− female mice (Figure 6A). SRC2−/− females trend toward a higher tolerance to the hot-plate test than WT mice t(17) = 1.92, P = .07, but male SRC2−/− mice showed no differences in sensitivity to the hot plate (Figure 6B). SRC3−/− female mice were significantly less sensitive to the hot plate than WT mice, t(22) = 2.59, P < .05; likewise, SRC3−/− males were significantly less sensitive to the hot plate than WT mice t(20) = 2.11, P < .05 (Figure 6C). Collectively, the SRCs appear to differentially regulate sensorimotor skills, in particular nociceptive behaviors, by either increasing or decreasing pain responsiveness.
Figure 6.
Nociception test in SRC−/− mice. A–C, WT and SRC−/− male and female mice were placed on a hot plate at 55°C, and the latency to lift, shake, or lick their hind paws was measured. A, SRC1−/− male mice show an increased sensitivity to the hot plate with no changes observed between WT and SRC1−/− female mice. B, SRC2−/− female mice have decreased sensitivity to the hot plate, with no changes observed for SRC2−/− males. C, SRC3−/− male and SRC3−/− female mice display decreased latency to the hot plate. Mean ± SEM; *, P < .05.
Discussion
Studies focusing on the family of SRCs as transcriptional coregulators have revealed many related functions, including regulation of central processes like steroid-mediated regulation of growth and metabolism (1, 4). However, increasingly we find that each of the SRCs possesses unique functions and autonomously regulates a selective variety of physiologic processes. Previous reports of SRC regulation in the brain are no different. SRCs are widely recognized to regulate reproductive behaviors in both male and female mice, and the SRCs have been shown to regulate the HPA axis and stress response (21, 28, 32). Here, we have attempted to describe the neurobehavioral phenotypes associated with male and female mice within the 3 different SRC genotypes. Unsurprisingly, each SRC shows distinct phenotypes in the behavioral test battery, and further, previously unidentified sex differences were evident within the SRC−/− genotypes.
Interestingly, Patchev et al. (32) reported an adrenocortical defect in SRC2−/− male mice, causing decreased corticosterone levels leading to dysregulation of the HPA axis (32), which suggests that SRC2−/− male mice may have impairments in stress responses. Our studies indicate that SRC2−/− female mice, but not SRC2−/− male mice, demonstrate decreased exploratory and anxiety-like behaviors in the OFA and the EPM, respectively. Sex differences in the stress response may be due to altered HPA axis regulation arising from the adrenal gland defect; however, when taken collectively, these data suggest that SRC2 may be an important regulatory component that dynamically controls the HPA axis in a gender-specific manner. In comparison, we have observed another previously unidentified sex difference in anxiety-related behaviors between the SRC3−/− male and SRC3−/− female mice with SRC3−/− female mice appearing to be more anxious in both the EPM and the OFA than female WT mice. Yet, no significant differences were observed between the WT and the SRC3−/− male mice. Interestingly, loss of SRC2 and loss of SRC3 appear to have opposite effects on female mice anxiety responses. We believe that these data highlight the complexity of the SRCs' regulation of anxiety and exploratory-related phenotypes and that they contribute to these phenotypes in a sexually dimorphic manner.
Within motor tests, previous studies have shown that ablation of SRC1 results in a Purkinje cell defect (24), and we too found that SRC1−/− mice were somewhat impaired in motor coordination on the rotarod. Similarly, both SRC2−/− male and female mice were observed to have decreased grip strength compared with WT mice, but their equal (or even superior) performance on the rotarod suggests that loss of SRC2, unlike loss of SRC1, does not affect the overall fine motor or coordination skills of the mice. Likewise, SRC3−/− female mice had decreased grip strength and an increased latency to fall from the wire hang, but both male and female performance on the rotarod indicates that loss of SRC3 does not significantly affect motor coordination. These findings are of particular importance when considered within the context of skeletal muscle performance. Two independent studies from our laboratory have highlighted that SRC3 has clear metabolic functions in skeletal muscle (6, 46). The results from the motor coordination studies presented here on the SRC3−/− mice help to solidify the conclusions drawn from previous findings by eliminating a potential motor coordination defect as a contributing factor to the observed skeletal muscle phenotypes. From a collective perspective, although published studies and data presented here support that SRC1 is an important coactivator in the development of the cerebellar Purkinje cells, it does not appear that loss of SRC2 or SRC3 regulate similar developmental programs related to motor coordination.
We observed no changes with loss of SRC1 in male or female mice in the sensorimotor gating tests. Conversely, loss of SRC2 in male mice did significantly impair PPI and the acoustic startle response. Interestingly, no phenotype was observed in female mice, further suggesting that in addition to anxiety-related behaviors, loss of SRC2 may differentially regulate the cognitive processes associated with sensorimotor gating. SRC3−/− female mice also showed an impaired inhibition in PPI, but no significant change in the acoustic startle response. Taken together, these data suggest that each of the SRCs play different roles in regulating sensorimotor gating responses. Interestingly, deficits in sensorimotor gating are used as endophenotypes for schizophrenia, and PPI defects are comparable across species, facilitating the use of genetic mouse models to unearth the mechanisms behind schizophrenia. Taken together, our findings raise the possibility that the SRCs may perform a genetic role in the molecular regulation of human neuropsychiatric disorders including schizophrenia (47). These studies highlight a novel role for the SRCs in regulating sensory information and processing and, in the future, may be a useful model to further our understanding of the mechanistic details and sex differences behind sensory motor gating.
Nociceptive tests revealed that SRC1−/− male mice had a decreased pain tolerance on the hot plate compared with WT mice. In contrast, SRC2−/− female and SRC3−/− male and female mice all showed an increased tolerance to the hot-plate test. These findings support a novel role for SRC regulation of pain and the somatosensory system. Studies on the SRC3−/− mice have found that these mice are more susceptible to endotoxic shock resulting from bacterial toxin exposure (48). The findings reported here that SRC-3−/− mice are more tolerant to pain suggests that the endotoxic susceptibility is not complicated by a decreased pain threshold. Moreover, the increased pain tolerance of the SRC3−/− mice also helps to clarify that the observed defect in sustained endurance exercise of the SRC3−/− mice is not simply due to a lowered pain limit (6).
Collectively, the work presented in this neurobehavioral resource largely supports previously published work on the SRCs' regulation of the CNS. The findings of this resource demonstrate that loss of each SRC confers several behavioral defects unique to their respective genotypes. In fact, when taken in the context of a recent research resource on the metabolic functions of the SRCs (49), the neurobehavioral resource presented here adds another layer of support for our long-held contention of nonredundant physiologic roles for the SRCs. Cumulatively, these studies make it more apparent that each of the SRCs has unique tissue- and gender-specific functions such that genetic ablation of a single SRC cannot be overcome simply through compensation by another SRC. Furthermore, this work highlights that the SRCs have even greater regulatory roles for cognitive processing and neurobehaviors than perhaps originally appreciated and that these findings can serve as a foundation to expand and elucidate the mechanisms underlying the described phenotypes.
Supplementary Material
Acknowledgments
We thank C. Ljungberg and staff at the IDDRC RNA In Situ Hybridization Core for performing all in situ hybridization staining. We thank C. Spencer and R. Paylor for the use of Baylor College of Medicine IDDRC core and help in the behavioral studies.
This work was supported by Public Health Service grants F31CA171350 (to E.S.), and NIDDK PO1 HD08818–37, Welch Grant Q1521, and 3U19DK062434–10W1 (to B.W.O) and by Baylor College of Medicine IDDRC (Grant 5P30HD024064–23) from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Eunice Kennedy Shriver National Institute Of Child Health & Human Development.
Author Contributions: E.S., L.W., and S.M. designed the experiments. E.S. and L.W. performed all of the behavior tests and statistical analysis. E.S., L.W., B.Y., and B.W.O wrote the manuscript.
Disclosure Summary: The authors declare no competing interests.
Footnotes
- CNS
- central nervous system
- EPM
- elevated plus maze
- ER
- estrogen receptor
- HPA
- hypothalmic-pituitary-adrenal
- NR
- nuclear receptor
- OFA
- open field activity
- PPI
- prepulse inhibition
- PR
- progesterone receptor
- SRC
- steroid receptor coactivator
- VMN
- ventromedial nucleus
- WT
- wild-type.
References
- 1. Xu J, Wu RC, O'Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009; 9:615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darimont BD, Wagner RL, Apriletti JW, et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes, Dev. 1998;12:3343–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736 [DOI] [PubMed] [Google Scholar]
- 4. York B, O'Malley BW. Steroid receptor coactivator (SRC) Family: masters of systems biology. J Biol Chem. 2010;285:38743–38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mussi P, Yu C, O'Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006;20:3105–3119 [DOI] [PubMed] [Google Scholar]
- 6. York B, Reineke EL, Sagen JV, et al. Ablation of steroid receptor coactivator-3 resembles the human CACT metabolic myopathy. Cell Metab. 2012;15:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Louet JF, Chopra AR, Sagen JV, et al. The Coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab. 2010;12:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Picard F, Géhin M, Annicotte J, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941 [DOI] [PubMed] [Google Scholar]
- 9. Qin L, Chen X, Wu Y, et al. Steroid receptor coactivator-1 upregulates integrin α expression to promote breast cancer cell adhesion and migration. Cancer Res. 2011;71:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming FJ. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by β-estradiol and 4-hydroxytamoxifen in human breast cancer. J Clin Endocrinol Metab. 2004;89:375–383 [DOI] [PubMed] [Google Scholar]
- 11. Fleming FJ. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol. 2004;57:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Myers E, Fleming FJ, Crotty TB, et al. Inverse relationship between ER-β and SRC-1 predicts outcome in endocrine-resistant breast cancer. Br J Cancer. 2004;91:1687–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mukherjee A. Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl Recept Signal. 2007;5:e011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chopra AR, Louet JF, Saha P, et al. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science 2008;322:1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chopra AR, Kommagani R, Saha P, et al. Cellular energy depletion resets whole-body energy by promoting coactivator-mediated dietary fuel absorption. Cell Metab. 2011;13:35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oh A, List HJ, Reiter R, et al. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res. 2004;64:8299–8308 [DOI] [PubMed] [Google Scholar]
- 18. Meijer OC, Steenbergen PJ, De Kloet ER. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology. 2000;141:2192–2199 [DOI] [PubMed] [Google Scholar]
- 19. Bian C, Zhang D, Guo Q, Cai W, Zhang J. Localization and sex-difference of steroid receptor coactivator-1 immunoreactivities in the brain of adult female and male mice. Steroids. 2011;76:269–279 [DOI] [PubMed] [Google Scholar]
- 20. Auger AP, Tetel MJ, McCarthy MM. Steroid receptor coactivator-1 (SRC-1) mediates the development of sex-specific brain morphology and behavior. Proc Natl Acad Sci USA 2000;97:7551–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lachize S, Apostolakis EM, van der Laan S, et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci USA. 2009;106:8038–8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winnay JN. Steroid receptor coactivator-1-deficient mice exhibit altered hypothalamic-pituitary-adrenal axis function. Endocrinology. 2006;147:1322–1332 [DOI] [PubMed] [Google Scholar]
- 23. Bousios S, Karandrea D, Kittas C, Kitraki E. Effects of gender and stress on the regulation of steroid receptor coactivator-1 expression in the rat brain and pituitary. J Steroid Biochem Mol Biol. 2001;78:401–407 [DOI] [PubMed] [Google Scholar]
- 24. Nishihara E, Yoshida-Komiya H, Chan CS, et al. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J Neurosci. 2003;23:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charlier TD. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25:906–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tetel MJ, Siegal NK, Murphy SD. Cells in behaviourally relevant brain regions coexpress nuclear receptor coactivators and ovarian steroid receptors. J Neuroendocrinol. 2007;19:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tognoni CM, Chadwick JG, Jr., Ackeifi CA, Tetel MJ. Nuclear receptor coactivators are coexpressed with steroid receptors and regulated by estradiol in mouse brain. Neuroendocrinology. 2011;94:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O'Malley BW. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol. 2002;16:1511–1523 [DOI] [PubMed] [Google Scholar]
- 29. Zhu L, Yang Y, Xu P, et al. Steroid receptor coactivator-1 mediates estrogenic actions to prevent body weight gain in female mice. Endocrinology. 2013;154:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yore MA, Im D, Webb LK, et al. Steroid receptor coactivator-2 expression in brain and physical associations with steroid receptors. Neuroscience. 2010;169:1017–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92:1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patchev AV, Fischer D, Wolf SS, et al. Insidious adrenocortical insufficiency underlies neuroendocrine dysregulation in TIF-2 deficient mice. FASEB J. 2007;21:231–238 [DOI] [PubMed] [Google Scholar]
- 33. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 2000;97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. An BS, Selva DM, Hammond GL, Rivero-Muller A, Rahman N, Leung PC. Steroid receptor coactivator-3 is required for progesterone receptor trans-activation of target genes in response to gonadotropin-releasing hormone treatment of pituitary cells. J Biol Chem. 2006;281:20817–20824 [DOI] [PubMed] [Google Scholar]
- 35. Paramanik V, Thakur MK. AIB1 shows variation in interaction with ERβTAD and expression as a function of age in mouse brain. Biogerontology. 2011;12:321–328 [DOI] [PubMed] [Google Scholar]
- 36. Frigo DE. p38 Mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20:971–983 [DOI] [PubMed] [Google Scholar]
- 37. Xu J. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925 [DOI] [PubMed] [Google Scholar]
- 38. Chen X, Liu Z, Xu J. The cooperative function of nuclear receptor coactivator 1 (NCOA1) and NCOA3 in placental development and embryo survival. Mol Endocrinol. 2010;24:1917–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carson JP, Eichele G, Chiu W. A method for automated detection of gene expression required for the establishment of a digital transcriptome-wide gene expression atlas. J Microsc. 2005;217:275–281 [DOI] [PubMed] [Google Scholar]
- 40. Yaylaoglu MB, Titmus A, Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive expression atlas of fibroblast growth factors and their receptors generated by a novel robotic in situ hybridization platform. Dev Dyn. 2005;234:371–386 [DOI] [PubMed] [Google Scholar]
- 41. McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717 [DOI] [PubMed] [Google Scholar]
- 42. Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: Effect of test interval. Physiol Behav. 2006;87:95–102 [DOI] [PubMed] [Google Scholar]
- 43. Mandillo S, Tucci V, Holter SM, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol Genomics. 2008;34:243–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gofflot F, Chartoire N, Vasseur L, et al. Systematic gene expression mapping clusters nuclear receptors according to their function in the brain. Cell. 2007;131:405–418 [DOI] [PubMed] [Google Scholar]
- 45. Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053 [DOI] [PubMed] [Google Scholar]
- 46. Coste A, Louet JF, Lagouge M, et al. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1{alpha}. Proc Natl Acad Sci. 2008;105:17187–17192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Powell SB, Weber M, Geyer MA. Genetic models of sensorimotor gating: relevance to neuropsychiatric disorders. In: Current Topics in Behavioral Neurosciences. Berlin, Heidelberg, Germany: Springer; 2012;251–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007;25:765–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. York B, Sagen JV, Tsimelzon A, et al. Research resource: Tissue- and pathway-specific metabolomic profiles of the steroid receptor coactivator (SRC) family. Mol Endocrinol. 2013;27:366–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.