Abstract
Heterotrimeric kinesin-2 motors [1; 2] transport intraflagellar transport (IFT)-particles from the base to the tip of the axoneme to assemble and maintain cilia [3; 4; 5; 6; 7; 8; 9; 10]. These motors are distinct in containing two non-identical motor subunits together with an accessory subunit [1; 11; 12; 13; 14; 15]. We evaluated the significance of this organization by comparing purified wild type kinesin-2 holoenzymes with mutant trimers containing only one type of motor domain. In motility assays, wild type kinesin-2 moved microtubules (MTs) at a rate intermediate between the rates supported by the two mutants. Interestingly, one of the mutants, but not the other mutant or the wild-type protein, was observed to drive a persistent counter-clockwise rotation of the gliding MTs. Thus one of the two motor domains of heterotrimeric kinesin-2 exerts torque as well as axial force as it moves along a MT, which may allow kinesin-2 to control its circumferential position around a MT doublet within the cilium.
Keywords: Heterotrimeric kinesin-2, torque generation
1. Introduction
Intraflagellar transport (IFT), the motor-dependent movement of IFT particles along the axoneme, is critical for the assembly, maintenance, and function of motile and sensory cilia, and, consequently, this process underlies ciliary motility, cilium-based signaling, and ciliopathies [3; 4]. A large body of evidence supports the hypothesis that members of the kinesin-2 family of microtubule (MT)-based motors play critical roles in IFT by driving the anterograde transport of IFT particles, together with associated cargo, from the base of the axoneme to the distal tip [1; 2; 5; 6; 7; 8; 9; 10; 16; 17]. It has been proposed that heterotrimeric kinesin-2 serves as the "core" anterograde IFT motor that builds the axoneme foundation in many cell-types, and that this motor's activity can be augmented by "accessory" kinesins that function to confer cilia-specific properties [18].
For example, the simple nervous system of the nematode Caenorhabditis elegans contains 60 sensory neurons whose dendritic endings use cilia of distinct morphology and molecular composition to detect a variety of sensory inputs [19]. In the head of the animal, bundles of amphid channel cilia on chemosensory neurons detect hydrophilic molecules in the environment, whereas the adjacent "wing" cilia detect volatile, hydrophobic odorant molecules [20; 21]. Based on the use of time-lapse fluorescent microscopy-based transport assays in living animals, combined with in vitro motility assays with purified motor proteins, it has been proposed that two members of the kinesin-2 family, heterotrimeric kinesin-II and homodimeric OSM-3 cooperate in a partially redundant fashion to assemble the channel cilia [9; 16; 20; 21; 22; 23; 24; 25; 26; 27; 28; 29]. Specifically, the "middle" or "initial" segment of the axoneme, which serves as the cilium foundation consisting of 9 doublet MTs, is built by the kinesin-II and OSM-3-directed movement of IFT particles, with either motor being dispensable for this process. OSM-3 alone assembles 9 singlet MTs which are required for cilium-based signaling on the distal endings of the axoneme foundation [9; 16]. These pathways of IFT appear to be modulated in a cilia-specific fashion to produce distinct types of cilia in C. elegans itself [20; 24] although there is evidence that the extension of distal singlets on the endings of sensory cilia by OSM-3 homologs may be a general phenomenon [30].
In previous work we studied cooperative motility between purified heterotrimeric kinesin-II and homodimeric OSM-3 and we replicated, in vitro, the rates of IFT observed along the sensory cilia of living animals. We observed that kinesin-II moves MTs at a slow rate of 0.5 µm/s, OSM-3 moves MTs at a fast rate of 1.3 µm/s, and mixtures of the two motors move MTs at intermediate rates. The results support a simple mechanical competition model in which the two motors move the same IFT particle at an intermediate rate of 0.7 µm/s along the axoneme middle segment [16; 18]. Thus, this work supported the hypothesis that these two distinct kinesin-2 holoenzymes cooperate in a partially redundant manner to drive two sequential IFT pathways, but did not address the functional significance of the presence of two non-identical motor subunits within the kinesin-II holoenzyme itself [28].
A distinct property of all heterotrimeric kinesin-2 motors is the presence of two non-identical motor subunits, together with an accessory KAP subunit. Heterodimerization of the two motor subunits appears to be favored over homodimerization but the functional significance of this organization is unknown [31; 32; 33; 34]. This problem was previously addressed in vertebrates using comparative in vitro motility assays in which engineered chimeric motor subunit heterodimers versus homodimers were compared in terms of their motility rates; significant differences were observed in one study but not in the other [34; 35]. In the current study, we addressed this by investigating possible functional differences between the two nonidentical motor subunits present in the C.elegans heterotrimeric kinesin-2 motor, kinesin-II [9; 16].
2. Materials and methods
2.1. Cloning, expression, and purification of recombinant kinesin-II mutants
The KLP-20 motor/KLP-11 stalk-tail and the KLP-11 motor/KLP-20 stalk- tail genes, containing stop codons in the pDONR-221 vectors, were inserted into pDEST8 by Gateway LR recombination (Invitrogen) and were subsequently cloned into Bacmid, transfected into Sf9 cells for expression and purification as described previously [16]. The only exception is that the kinesin-II(homo-20), unlike kinesin-II and kinesin-II(homo-11), could not be purified by Talon column affinity chromatography using a C-terminal 6xHis-tagged KAP-1 together with untagged KLP-20 motor/KLP-11 stalk-tail and KLP-20 (the reason for this is unknown). Instead, we cloned and expressed C-terminal 6xHis-tagged KLP-20 and untagged KAP-1 using the same baculovirus system and successively purified the kinesin-II(homo-20) by co-infection of the viruses containing the two genes mentioned above together with untagged KLP-2011.
2.2. Hydrodynamic analysis and motility assays
The in vivo motility assays and in vitro microtubule gliding assays were performed as previously described [16], details of the assay can be found in [40]. A Superose 6 10/300 (GE Healthcare) was used for gel filtration analyses. The assay buffer contained 80 mM PIPES, pH 6.9, 1 mM MgSO4, 1 mM EGTA, 200 mM NaCl.
2.3. Strains
The wild type strain was N2 Bristol. The following mutant alleles were used in this study: kap-1(ok676), klp-11(tm324), osm-3 (p802), dpy-20(e1282), osm-6(p811), mnIs17 [osm-6::GFP, unc-36(+)]. All strains were grown at 20°C.
2.4. Expression vectors and construction of transgenic worms
The expression vector pCJF8 containing an osm-5 promoter region, followed by polycloning sites and CFP coding sequences was generously provided by [41]. A Gateway cassette (Invitrogen) was inserted into the SmaI site. KLP-2011 containing the head domain of KLP-20 and stalk-tail region of KLP-11, was generated using a PCR fusion strategy, and the same strategy was used to create the KLP-11 motor/KLP-20 stalk tail [42]. The PCR products were confirmed by sequencing after cloning them into plasmid pDONR221 (Invitrogen). The stop codons between target genes were removed by site-directed mutagenesis using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene). The target genes were inserted into the Gateway cassette in modified pCJF8 by Gateway recombination (Invitrogen). Worms were injected with plasmid constructs at 2–10 ng/µl along with markers containing 100 ng/ul of rol-6 and/or dpy-20. Transgenic worms carrying the injected plasmids were identified by their right-handed roller or dumpy phenotype [43].
2.5. Fluorescence microscopy
IFT was assayed as described previously [9; 25; 26]. For an extended description of the methods used please see Hao et al [40].
Results and Discussion
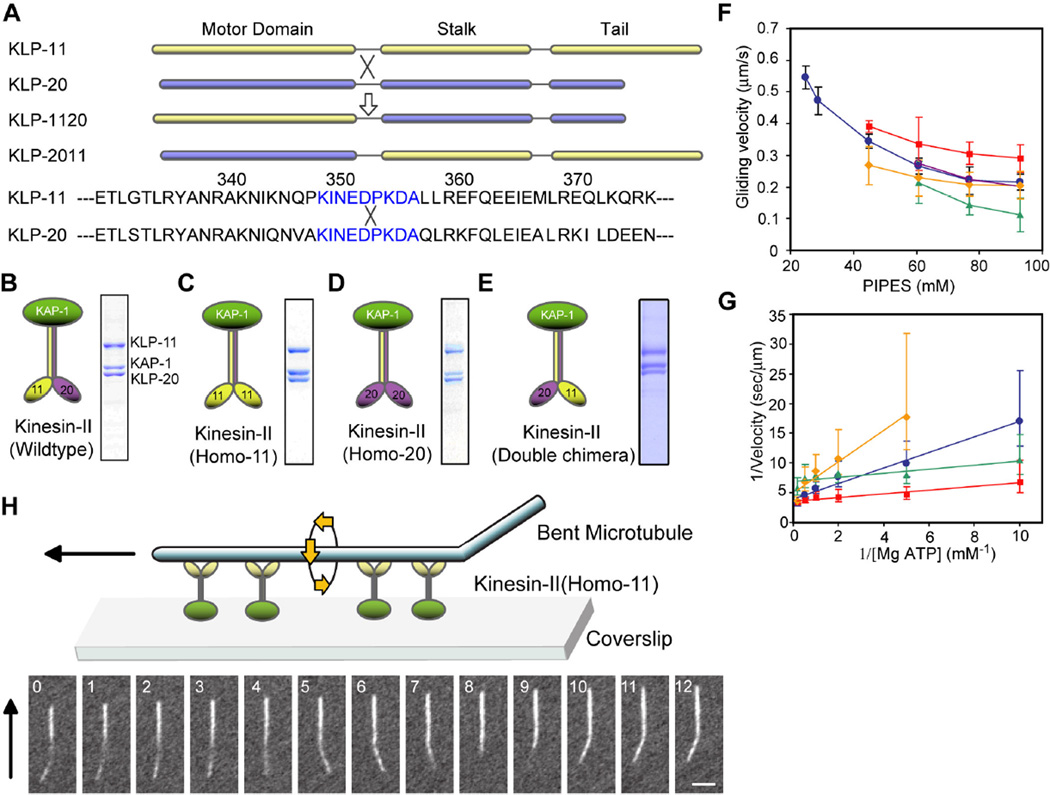
C. elegans kinesin-II consists of the two motor subunits, KLP-20 and KLP-11, and the accessory subunit, KAP-1 (former names CeKRP85, CeKRP95, CeKAP) [16; 28]. Wild type subunits co-assemble into active, heterotrimeric complexes that can be purified using the baculovirus system (Table 1 and Fig. 1B–E) [16] and they can complement loss-of-function mutants in vivo (Table 2). To compare the properties of native kinesin-II containing the two non-identical motor domains with kinesin-II complexes containing two KLP-20 motor domains or two KLP-11 motor domains, we adapted the strategy of [34] as modified by [35], involving the co-expression of chimeric and wild type motor subunits, but we also included the KAP subunit to permit testing of heterotrimer formation (Fig. 1A). Chimeric motor subunits expressed in ciliated chemosensory neurons fail to complement loss of function mutants (Table 2), but they do co-assemble into active heterotrimeric complexes in vitro (Fig 1B–E: Table 1). For example, we used the baculovirus system to express and purify (i) “kinesin-II(homo-20)”, a complex of KLP-20: KLP-20 motor/KLP-11 stalk-tail: KAP-1; (ii) “kinesin-II(homo-11)”, a complex of KLP-11: KLP11 motor/KLP-20 stalk-tail: KAP-1; and (iii) “kinesin-II(double chimera)” a complex of KLP-11 motor/KLP-20 stalk-tail: KLP-20 motor/KLP-11 stalk-tail:KAP-1. Based on hydrodynamic analysis and gel densitometry, kinesin-II(homo-20), kinesin-II(homo-11) and kinesin-II(double chimera) all behave as heterotrimeric complexes of two motor subunits and a KAP subunit, like "wild type" kinesin-II (Fig. 1B–E; Table 1). This reveals that heterotrimeric kinesin-2 motors do not require two non-identical motor domains in order to co-assemble with the KAP subunit.
Table 1.
Biochemical properties of recombinant kinesin-II and its mutants
| Wildtype | Homo-20 | Homo-11 | Double chimera | |
|---|---|---|---|---|
| S Value | 7.6 | 7.2 | 7.6 | 7.8 |
| Stokes Radius (nM) | 7.4 | 9.0 | 9.0 | 8.2 |
| Molecular Weight (Da) | 2.3 × 105 | 2.7 × 105 | 2.8 × 105 | 2.6 × 105 |
| Velocity (µm/s)* | 0.55 ± 0.04 | 0.39 ± 0.02 | 0.21 ± 0.07 | 0.27 ± 0.06 |
| Vmax (µm/s) | 0.26 | 0.28 | 0.15 | 0.2 |
| Km (mM) | 0.28 | 0.084 | 0.051 | 0.54 |
| Rotation Pitch (µm) | None | None | 1.31 ± 0.51 | None |
Note:
these velocities were measured under optimized conditions (PIPES and MgATP) for each molecule.
Figure 1.
Recombinant kinesin-II chimeras form heterotrimeric complexes. (A) Diagram showing the sequences of KLP-11, KLP-20, and the regions of exchange for chimeric KLP-20 motor/KLP-11stalk-tail (KLP-2011) and KLP-11 motor/KLP-20stalk-tail (KLP-1120). (B – E) The components and SDS gels of purified recombinant kinesin-II (B), kinesin-II(homo-11) (C), kinesin-II(homo-20) (D), and kinesin-II(double chimera) (E). (F–H) In vitro motility assay results of recombinant kinesin-II chimeras. (F) Velocities of MT gliding driven by kinesin-II (blue circle), kinesin-II(homo-20) (red), kinesin-II(homo-11) (green), and kinesin-II(double chimera) (yellow) in 5mM [Mg-ATP] and different PIPES concentrations. The kinesin-II chimeras did not bind to microtubules at PIPES concentrations below 40 mM for kinesin-II(homo-20) and kinesin-II(double chimera) or 60 mM for kinesin-II(homo-11). The velocities of gliding driven by kinesin-II were close to the average of the velocities of the kinesin-II(homo-11) and kinesin-II(homo20) motors. (G) Double reciprocal plots of the rates of MT gliding driven by kinesin-II (blue), kinesin-II(homo-20) (red), kinesin-II(homo-11) (green), and kinesin-II(double chimera) (yellow) versus [Mg-ATP]. (H) Rotation of gliding microtubules by kinesin-II(homo-11). The numbers show the time in seconds and the black arrows indicate the direction of translocation of the microtubules. Bar: 2.5 µm.
Table 2.
Velocity of IFT particles and kinesin-II in kinesin-2 mutant animals
| Anterograde motility of |
Strain | Average velocities (µm s−1) | |||
|---|---|---|---|---|---|
| Middle segment |
n | Distal segment |
n | ||
| IFT Particle OSM-6::GFP | klp-11 | 1.23 ± 0.17 | 101 | 1.17± 0.18 | 146 |
| klp-11; KLP-11(wt) | 0.76 ± 0.13 | 185 | 1.27± 0.23 | 109 | |
| klp-11; KLP-2011 | 1.18 ± 0.13 | 137 | 1.23± 0.14 | 91 | |
| kap-1 | 1.15 ± 0.14 | 202 | 1.14 ± 0.18 | 173 | |
| kap-1; KAP-1(wt) | 0.77 ± 0.14 | 142 | 1.17 ± 0.17 | 106 | |
| Kinesin-II subunit KAP-1::GFP | klp-11 | None | None | ||
| klp-11; KLP-11(wt) | 0.65 ± 0.09 | 125 | None | ||
| klp-11; KLP-2011 | None | None | |||
| kap-1; osm-3; KAP-1(wt) | 0.58 ± 0.11 | 105 | None | ||
| klp-11; osm-3; KLP-11(wt) | 0.45 ± 0.10 | 97 | None | ||
| klp-11;osm-3; KLP-2011 | None | None | |||
C. elegans loss-of-function klp-11 and KAP-1 mutant animals have full length amphid channel cilia which are built by OSM-3 in the absence of kinesin-II function, and they display abnormally fast OSM-3 driven transport of IFT-particles at 1.1 – 1.3 µm/s along the ciliary middle segments[9]. The slower, wild type rate of transport of the IFT-particle protein, OSM-6::GFP (0.7 µm/s) along ciliary middle segments was restored by the wild type KLP-11 and KAP-1 subunits but not by the chimeric KLP-2011 subunit. Double mutants lacking both KLP-11 and OSM-3 function lack the entire ciliary axoneme. Wild type KLP-11 and KAP-1 subunits restored the assembly of axoneme middle, but not distal, segments and slow IFT at rates characteristic of kinesin-II alone, but the chimeric subunit did not. (wt: wild type, n: number of fluorescent particles).
Purified kinesin-II, kinesin-II(homo-20), kinesin-II (homo-11) and kinesin-II(double chimera) were all active in MT motility assays under standard assay conditions (Table 1; Fig 1F, G). We previously noted that, in order to replicate the rate of motility displayed by kinesin-II along cilia, it was necessary to vary the PIPES buffer concentration slightly [16]. Using this strategy we optimized conditions for each motor and observed slight differences in the maximal rates of MT gliding driven by these motors (Fig 1F). The different maximal rates observed for kinesin-II, kinesin-II(homo-20), kinesin-II(homo-11) and kinesin-II(double chimera) under these conditions (i.e. 0.55 µm/s; 0.4 µm/s; 0.2 µm/s, and 0.3 µm/s, respectively) were somewhat smaller than those published for the vertebrate KIF3 heterodimers, KIF3A/3B, KIF3A/3A and KIF3B/3B (i.e. 0.2 µm/s; 0.04 µm/s; and 0.4 µm/s, respectively [35]), but it is unknown if this is due to the influence of the KAP subunit, which is present in our preparations (Fig. 1B–E). We also noted that, under standard assay conditions, all the motors drove motility according to simple Michaelis-Menten kinetics with respect to MgATP, displaying linear double reciprocal plots (Fig. 1G). However, both kinesin-II(homo-20) and kinesin-II(homo-11) displayed significantly lower Km values than the wildtype and double chimera, indicating a higher apparent affinity for MgATP (Fig. 1G; Table 1). Thus heterotrimeric kinesin-II motors containing identical versus non-identical motor domains are able to drive MT-based motility, but display subtle differences in this activity.
Unlike wild type kinesin-II, the three mutant complexes were inactive at low PIPES concentrations, but within the range of PIPES concentrations which supported motility (using 5mM [Mg-ATP] as the fuel), the rate of MT gliding driven by kinesin-II was consistently found to be the average of the faster rate driven by kinesin-II(homo-20) and the slower rate driven by kinesin-II(homo-11) (Fig 1F). The kinesin-II(double chimera) displayed very similar motility to the wild type protein at 80 or 100mM PIPES, but became more similar to the kinesin-II(homo-11) at lower PIPES concentrations (Fig. 1F). The results are generally consistent with the idea that the rate of motility of kinesin-2 complexes represents a composite of the activity of its two constituent motor subunits.
The most striking difference observed in these motility assays was the induction of MT rotation by one of the two homo-motor complexes (Fig. 1H). Specifically, kinesin-II(homo-11) drives the rotation of sliding MTs in motility assays, exerting torque that rotates the MTs counter-clockwise with pitch 1.3 µm as they are translocated axially with their minus ends leading (Table 1). In contrast, wild type kinesin-II, kinesin-II(double chimera) and kinesin-II(homo-20) did not drive rotation of translocating MTs. A similar rotation of gliding MTs has been observed in assays of the mitotic kinesin-14, Ncd [36] and of monomeric kinesin-1 constructs [37] and is proposed to be a general property of non-processive motors. The counterclockwise rotation mediated by kinesin-II(homo-11) is of the same handedness as that driven by the kinesin-1 constructs [37] whereas the minus-end-directed kinesin-14 mediates clock-wise rotation of MTs [36]. Our observation that the KLP-11 motor domain rotates a moving MT whereas the KLP-20 motor domain does not, suggests functional differentiation between the two non-identical motor domains within the wild type kinesin-II heterotrimer. Thus it is possible that the KLP-11 head may be a low processivity motor that exerts torque as well as axial force on a MT whereas the more processive KLP-20 motor domain may counteract the torque component to prevent rotation of kinesin-II relative to the MT track, possibly facilitating processive motility of IFT-particles in vivo. Interestingly, optical trap studies done independently by Okten and colleagues [38] support the idea that KLP-11 is non-processive, whereas KLP-20 can drive persistent processive motility. Conversely it is possible that a KLP-11 homodimer can actually move processively along a MT, but in the optical trap, its rotational motion around the circumference of the immobilized MT may hinder its translational movement, giving rise to low processivity.
Thus, chimeric motor subunits are capable of translocating MTs and forming heterotrimers in vitro, but appear to be non-functional in vivo (Table 2). Based on fluorescence microscopy, the KLP-2011 chimera is expressed at similar levels to the wild type subunit in ciliated chemosensory neurons (not shown), and based on the in vitro data, it is plausible to think that KLP-2011 can co-assemble with KLP-20 and KAP-1 in transgenic animals. However, this would create heterotrimeric kinesin-II(homo-20) complexes with different motility and transport properties from those of wild type kinesin-II, which could account for the failure of kinesin-II(homo-20) to function properly. To contribute to IFT, kinesin-II must execute a complex sequence of events, including entry into the cilium, docking onto an IFT-particle, then cooperating with the homodimeric kinesin-2, OSM-3 to move an IFT-particle to the distal tip of the axoneme [9]. For faithful execution of these processes, the rates and other motility properties of the two motor domains of kinesin-II may need to be optimal, with one motor domain, but not the other, exerting torque on the MT track. In this context it is tempting to speculate that KLP-11 exerts torque in order allow kinesin-2 to adjust its circumferential position around MT doublets within the axoneme and maintain its association with the B-tubule [39]. For example, in C. elegans sensory cilia, kinesin-II and OSM-3 motors cooperate to move IFT particles along the axoneme, yet there is evidence that kinesin-II moves along B-tubules of the doublets whereas OSM-3 moves on the A-tubules [9]. Perhaps an IFT particle being moved by both motors must be confined to protofilaments located at the junction of the A and B tubules, with KLP-11 generating torque in order to resist kinesin-II being pulled too far around the doublet from the B tubule onto the A tubule by OSM-3.
In summary, our study shows while chimeric motor subunits are capable of translocating MTs and forming heterotrimers in vitro, they appear to be non-functional in vivo (Table 2). Why? To move IFT-particles along the axoneme may require that one motor domain of kinesin-II, but not the other, must exert torque on a MT track to maintain its trajectory along the B-tubule of a doublet [39]. This could pose a particular problem in C. elegans sensory cilia, where a single IFT particle is moved by both heterotrimeric kinesin-II and homodimeric OSM-3, which appear to move along the B-and A-tubules, respectively [9]. Perhaps such a particle moves along the junction of the A and B tubules, with KLP-11 generating torque in order to resist being pulled off the B-tubule by OSM-3. Thus our finding that one of the two motor subuits of heterotrimeric kinesin-2 can exert both torque and axial force on a MT raises fascinating questions about its role in IFT and ciliogenesis.
Research Higlights.
Heterotrimeric kinesin-2 motors containing identical versus non-identical motor domains are able to drive MT-based motility, but display subtle differences in this activity
One of the two motor domains of heterotrimeric kinesin-2 exerts torque on microtubules
Exertion of torque on microtubules may allow kinesin-2 to control its circumferential position in vivo
Acknowledgements
This work has been supported by NIH grant GM50718. We thank Dr Guangshou Ou for teaching X.P. the in vivo transport assays. We thank Drs Ou, Gul Civelekoglu-Scholey, Limin Hao, Mel Thein and Frank McNally for critically reading the manuscript and them, together with Dr Rob Cross and the UCD "worm supergroup" for valuable discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cole DG, Chinn SW, Wedaman KP, Hall K, Vuong T, Scholey JM. Novel heterotrimeric kinesin-related protein purified from sea-urchin eggs. Nature. 1993;366:268–270. doi: 10.1038/366268a0. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LSB, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy ASN, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 4.Scholey JM. Intraflagellar transport. Annu. Rev. Cell Dev. Biol. 2003;19:423–443. doi: 10.1146/annurev.cellbio.19.111401.091318. [DOI] [PubMed] [Google Scholar]
- 5.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein Fla10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JM, Marsala C, Kosoy R, Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol. Biol. Cell. 1999;10:3081–3096. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 9.Snow JJ, Ou GS, Gunnarson AL, Walker MRS, Zhou HM, Brust-Mascher I, Scholey JM. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C-elegans neurons. Nat. Cell Biol. 2004;6 doi: 10.1038/ncb1186. 1109-U23. [DOI] [PubMed] [Google Scholar]
- 10.Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J. Cell Biol. 1997;138:1009–1022. doi: 10.1083/jcb.138.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholey JM. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J. Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirokawa N. Stirring up development with the heterotrimeric kinesin KIF3. Traffic. 2000;1:29–34. doi: 10.1034/j.1600-0854.2000.010105.x. [DOI] [PubMed] [Google Scholar]
- 13.Marszalek JR, Goldstein LSB. Understanding the functions of kinesin-II. Biochim. Biophys. Acta-Mol. Cell Res. 2000;1496:142–150. doi: 10.1016/s0167-4889(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 14.Doodhi H, Ghosal D, Krishnamurthy M, Jana SC, Shamala D, Bhaduri A, Sowdhamini R, Ray K. KAP, the Accessory Subunit of Kinesin-2, Binds the Predicted Coiled-Coil Stalk of the Motor Subunits. Biochemistry. 2009;48:2248–2260. doi: 10.1021/bi8018338. [DOI] [PubMed] [Google Scholar]
- 15.Wedaman KP, Meyer DW, Rashid DJ, Cole DG, Scholey JM. Sequence and submolecular localization of the 115-kD accessory subunit of the heterotrimeric kinesin-II (KRP(85/95)) complex. J. Cell Biol. 1996;132:371–380. doi: 10.1083/jcb.132.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan XY, Ou GS, Civelekoglu-Scholey G, Blacque OE, Endres NF, Tao L, Mogilner A, Leroux MR, Vale RD, Scholey JM. Mechanism of transport of IFT particles in C-elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J. Cell Biol. 2006;174:1035–1045. doi: 10.1083/jcb.200606003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walther Z, Vashishtha M, Hall JL. The Chlamydomonas fla10 gene encodes a novel kinesin-homologous protein. J. Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell's antenna. J. Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inglis PN, Ou G, Leroux MR, Scholey JM. The sensory cilia of Caenorhabditis elegans. WormBook. 2007:1–22. doi: 10.1895/wormbook.1.126.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans JE, Snow JJ, Gunnarson AL, Ou GS, Stahlberg H, McDonald KL, Scholey JM. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 2006;172:663–669. doi: 10.1083/jcb.200509115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 22.Burghoorn J, Dekkers MPJ, Rademakers S, de Jong T, Willemsen R, Jansen G. Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2007;104:7157–7162. doi: 10.1073/pnas.0606974104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jauregui AR, Nguyen KCQ, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J. Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukhopadhyay S, Lu Y, Qin H, Lanjuin A, Shaham S, Sengupta P. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C-elegans. Embo J. 2007;26:2966–2980. doi: 10.1038/sj.emboj.7601717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orozco JT, Wedaman KP, Signor D, Brown S, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature. 1999;398:674–674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- 26.Ou GS, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436:583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 27.Shakir MA, Fukushige T, Yasuda H, Miwa J, Siddiqui SS. C. elegans osm-3 gene mediating osmotic avoidance-behavior encodes a kinesin-like protein. Neuroreport. 1993;4:891–894. doi: 10.1097/00001756-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Signor D, Wedaman KP, Rose LS, Scholey JM. Two heteromeric kinesin complexes in chemosensory neurons and sensory cilia of Caenorhabditis elegans. Mol. Biol. Cell. 1999;10:345–360. doi: 10.1091/mbc.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J. Mol. Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- 30.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. The homodimeric kinesin, Kif17, is essential for vertebrate photoreceptor sensory outer segment development. Dev. Biol. 2008;316:160–170. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chana MS, Tripet BP, Mant CT, Hodges R. Stability and specificity of heterodimer formation for the coiled-coil neck regions of the motor proteins Kif(3)A and Kif(3)B: the role of unstructured oppositely charged regions. J. Pept. Res. 2005;65:209–220. doi: 10.1111/j.1399-3011.2005.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Marco V, Burkhard P, Le Bot N, Vernos I, Hoenger A. Analysis of heterodimer formation by Xklp3A/B, a newly cloned kinesin-II from Xenopus laevis. Embo J. 2001;20:3370–3379. doi: 10.1093/emboj/20.13.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashid DJ, Wedaman KP, Scholey JM. Heterodimerization of the 2 motor subunits of the heterotrimeric kinesin, Krp(85/95) J. Mol. Biol. 1995;252:157–162. doi: 10.1006/jmbi.1995.0484. [DOI] [PubMed] [Google Scholar]
- 34.Yamazaki H, Nakata T, Okada Y, Hirokawa N. Kif3a/b - a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J. Cell Biol. 1995;130:1387–1399. doi: 10.1083/jcb.130.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Hancock WO. The two motor domains of KIF3A/B coordinate for processive motility and move at different speeds. Biophys. J. 2004;87:1795–1804. doi: 10.1529/biophysj.104.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker RA, Salmon ED, Endow SA. The Drosophila-claret segregation protein is a minus-end directed motor molecule. Nature. 1990;347:780–782. doi: 10.1038/347780a0. [DOI] [PubMed] [Google Scholar]
- 37.Yajima J, Cross RA. A torque component in the kinesin-1 power stroke. Nat. Chem. Biol. 2005;1:338–341. doi: 10.1038/nchembio740. [DOI] [PubMed] [Google Scholar]
- 38.Brunnbauer M, Mueller-Planitz F, Kosem S, Ho TH, Dombi R, Gebhardt JCM, Rief M, Okten Z. Regulation of a heterodimeric kinesin-2 through an unprocessive motor domain that is turned processive by its partner. Proc. Natl. Acad. Sci. USA. 2010;107:10460–10465. doi: 10.1073/pnas.1005177107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hao LM, Acar S, Evans J, Ou GS, Scholey JM. Analysis of Intraflagellar Transport in C-elegans Sensory Cilia. Cilia: Model Organisms and Intraflagellar Transport. Methods in Cell Biol. 2009;93:235–266. doi: 10.1016/S0091-679X(08)93013-2. [DOI] [PubMed] [Google Scholar]
- 41.Haycraft CJ, Schafer JC, Zhang QH, Taulman PD, Yoder BK. Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp. Cell Res. 2003;284:251–263. doi: 10.1016/s0014-4827(02)00089-7. [DOI] [PubMed] [Google Scholar]
- 42.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C-elegans. Biotechniques. 2002;32:728–730. doi: 10.2144/02324bm01. [DOI] [PubMed] [Google Scholar]
- 43.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene-transfer in C.elegans - extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]