About 1 in 79 Canadians will have pancreatic cancer in their lifetime, making it the 12th most common malignant disease and the 4th leading cause of death from cancer.1,2 A family physician can expect to encounter 1 to 2 patients with pancreatic cancer each year, with increases in case volumes anticipated as the Canadian population grows and ages.3 When considering all stages, the overall 5-year survival for pancreatic cancer is about 6% (Table 1),4,5 making it one of the most fatal diseases.1
Table 1:
Staging and prognosis in pancreatic cancer
| Stage | Tumour grade | Node status | Distant metastases | 5-yr survival, %* | Median survival, mo* | Characteristics |
|---|---|---|---|---|---|---|
| IA | T1 | N0 | M0 | 14 | 24 | Tumour < 2 cm in pancreas only |
| IB | T2 | N0 | M0 | 12 | 21 | Tumour > 2 cm in pancreas only |
| IIA | T3 | N0 | M0 | 7 | 15 | Tumour extends beyond the pancreas, but with no involvement of the celiac or superior mesenteric artery |
| IIB | T1–3 | N1 | M0 | 5 | 13 | Regional lymph node metastasis |
| III | T4 | N0–1 | M0 | 3 | 11 | Tumour involves the celiac or superior mesenteric artery |
| IV | T1–4 | N0–1 | M1 | 1 | 5 | Distant metastases |
In the clinical setting, and for the purpose of this article, we use the term pancreatic cancer to refer to the ductal adenocarcinoma subtype, which accounts for 90% of cases.6 The aim of this article is to explore our current evidence-based understanding of pancreatic cancer (Box 1), focusing on diagnostic and treatment strategies relevant to the general clinician.
Box 1: Summary of the literature review.
We searched Medline and PubMed databases (from 1947 onward) using the following medical subject headings (MeSH): “pancreas cancer,” “pancreatic cancer,” “PDAC,” “cancer statistics,” “pancreas cancer genetics” and “PDAC diagnosis and management.” We searched the Cochrane database for relevant systematic reviews. We searched Google Scholar for clinical practice guidelines using the same MeSH terms. In addition, we reviewed the reference lists of identified studies. We prioritized queried articles based on clinical and biological relevance, as well as on the basis of citations and journal impact.
When should pancreatic cancer be suspected and how is it diagnosed?
Risk factors
Active smoking remains the most established environmental risk factor for pancreatic cancer (odds ratio [OR] 1.74, 95% confidence interval [CI] 1.61–1.87),7 and cessation is the only recommended disease-specific preventive measure. Other putative associations identified in epidemiologic case–control studies include body mass index (BMI) of more than 35 (OR 1.55, 95% CI 1.16–2.07), consumption of more than 6 alcoholic beverages per day (OR 1.46, 95% CI 1.16–1.83)7 and the presence of non–type O blood antigens (OR 1.42, 95% CI 1.21–1.66),8 which display aberrant expression on pancreatic ductal cells, thus affecting signal transduction and cellular adhesion. Allergies have recently been associated with a lower incidence of pancreatic cancer (OR 0.40, 95% CI 0.26–0.87); however, the mechanism for this association is unknown.9
A family history of pancreatic cancer is seen in 20% of patients (OR 2.41, 95% CI 1.04–4.74).7 Furthermore, 5%–10% of patients have a hereditary pancreatic cancer syndrome (Box 27, 10–13), in which cancer is caused by one of several germline mutations.7,10
Box 2: Hereditary pancreatic cancer syndromes7,10–13.
-
Familial pancreatic cancer: Two or more first-degree relatives with pancreatic cancer failing to satisfy the criteria of another hereditary pancreatic cancer syndrome (below). Familial pancreatic cancer is genetically heterogenous, with some cases caused by germline mutations in BRCA2 (2.8%–17.2%) and PALB2 (1%–3%), despite a paucity of breast, ovary or other cancers in these families.
Association with pancreatic cancer: standardized incidence ratio 6.79, 95% confidence interval (CI) 4.54–9.75.
Prevalence: Not well-defined, but possibly the most common inherited cause of pancreatic cancer.
-
Lynch syndrome (hereditary nonpolyposis colon cancer): Colon cancer predisposition syndrome associated with tumour microsatellite instability and mutations involving DNA mismatch repair (hMSH2, hMLH1, hPMS2, hMSH6).
Association with pancreatic cancer: odds ratio (OR) 3.68, 95% CI 1.45–5.88.
Prevalence: 1/500 to 1/1000.
-
Hereditary breast/ovarian cancer: Families with early-onset breast cancer (age < 50 yr) or multiple breast or ovarian cancers with underlying BRCA1/2 mutations.
Prevalence: 1/400 to 1/500.
-
Familial atypical mole melanoma: Two or more blood relatives with melanoma and an underlying CDKN2A/p16 mutation.
Assocation with pancreatic cancer: OR 47.8, 95% CI 28.4–74.7.
Prevalence: >1/1000.
-
Hereditary pancreatitis: Two or more first-degree relatives across 2 generations with recurrent pancreatitis and an underlying PRSS1 mutation.
Association with pancreatic cancer: OR 53 (95% CI 50–60).
Prevalence: 0.3/100 000.
-
Peutz–Jeghers syndrome: A hamartomatous polyposis syndrome of the gastrointestinal tract associated with a STK11 mutation.
Prevalence: 1/25 000–1/300 000.
Screening
A latency period of about 10 years between the start of pancreatic carcinogenesis and symptomatic disease has been shown.14 Thus, there is a theoretical benefit to screening; however, there is no consensus as to its optimal modality, interval or duration. Prospective observational studies of screening have included patients at high risk (Box 2) and have used a combination of endoscopic ultrasound, computed tomography (CT) imaging, magnetic resonance imaging (MRI) or endoscopic retrograde cholangiopancreatography.1,15 Relatives with a hereditary pancreatic cancer syndrome or unaffected adults in a familial pancreatic cancer kindred should be referred to a genetic counselor for further assessment and possible screening in a research setting.16,17 Most investigational protocols begin screening 10 years earlier than the age at which the youngest relative with pancreatic cancer received the diagnosis or at the age of 40–45 years, whichever occurs first.
Clinical presentation
The pancreas is located in the retroperitoneum, where initial growth of the cancer is silent; therefore, symptoms are usually a sign of advanced disease. Clinical presentation depends on the stage of disease and the location of the primary tumour: the pancreatic head, neck or uncinate process (70%); the body or tail (20%); or multifocal disease (10%).18 Because most tumours arise in the pancreatic head, signs and symptoms may include right–upper quadrant or epigastric pain (79%), jaundice (56%), nausea or vomiting secondary to obstruction of the gastric outlet (51%), diarrhea (43%) and steatorrhea due to pancreatic insufficiency (25%).19 New onset or worsening of previously stable diabetes, although not usually due to the cancer, should alert the physician to the possibility of pancreatic cancer (OR 7.9, 95% CI 4.7–12.5).20 Furthermore, new or worsening back pain (49%) could signal cancer in the pancreatic body or tail. Finally, systemic manifestations may include profound and rapid weight loss (85%), anorexia (83%) or thromboembolic disease (3%).19
Diagnostic tests
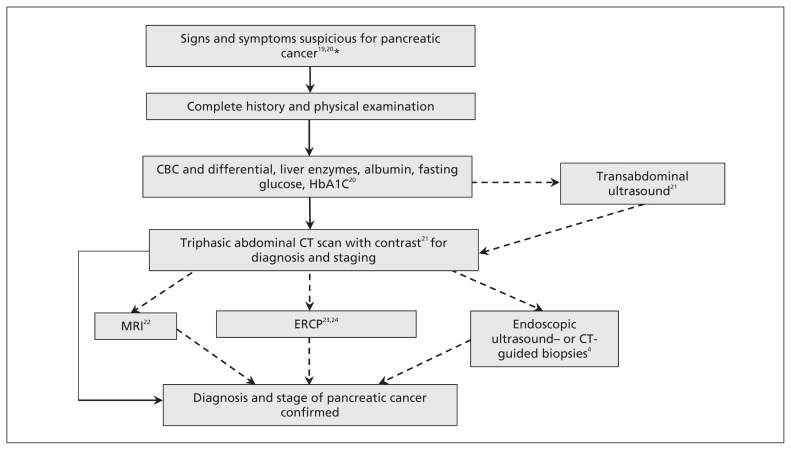
Tumour markers have minimal diagnostic utility in pancreatic cancer (Figure 1).19–24 Biomarkers that have been evaluated include CA 19-9 (sensitivity 70%–90%, specificity 68%–91%), which has poor positive predictive value in both asymptomatic (0.9%) and symptomatic patients (72%), and carcinoembryonic antigen, which also has a low diagnostic yield (sensitivity 25%–54%, specificity 75%–91%).25
Figure 1:
Diagnostic algorithm for pancreatic cancer. Laboratory investigations include a complete blood count (CBC), liver enzyme tests for biliary obstruction, and fasting glucose and glycated hemoglobin (HbA1C) tests to monitor for new onset or worsening diabetes. A transabdominal ultrasound can be performed for patients presenting with jaundice or nonspecific abdominal pain, followed by computed tomography (CT) if suspicious for pancreatic cancer. Patients with contraindications to CT, intolerance to contrast or in whom resectability is questioned can undergo magnetic resonance imagining (MRI). Endoscopic retrograde cholangiopancreatography (ERCP) is not routinely used, but cytologic brushings for diagnosis can be taken in those with cholangitis and an unknown pancreatic mass, or with jaundice who are unfit for immediate surgery. Endoscopic ultrasound- or CT-guided biopsies are used when diagnosis is unclear after imaging, in unresectable cases before palliative treatment or before neoadjuvant treatment. *Painless jaundice, pain in the right–upper quadrant or epigastric pain, indigestion, early satiety, steatorrhea, weight loss, abdominal mass. Dashed lines indicate imaging modalities that can be contained but are not routinely necessary.
Abdominal ultrasound is often used as an initial diagnostic test for patients with nonspecific abdominal pain. The sensitivity and specificity of ultrasound in diagnosing pancreatic tumours is 90% and 95%, respectively, but worsens for masses smaller than 3 cm.21 Ultrasound is limited by its operator dependency and inability to distinguish cancer from chronic or autoimmune pancreatitis; thus, ultrasound serves as a bridge to CT imaging. The putative diagnosis and stage of pancreatic cancer is usually made with triphasic contrast-enhanced abdominal CT (sensitivity 89%–97%, specificity 95%), which provides orientation of the tumour with surrounding vessels and organs.21 Although MRI is considered equivalent to CT (sensitivity 81%–99%, specificity 70%–93%), its more limited availability has restricted its use to patients with contraindications to CT (e.g., pregnancy, nephropathy) or where resectability is unclear after CT.22
The diagnostic accuracy of these imaging modalities obviates the need for preoperative tissue diagnosis in surgically resectable tumours. Endoscopic ultrasound (sensitivity 92%, specificity 96%) or CT-guided biopsy (sensitivity 80%–90%, specificity 98%–100%) is warranted in cases where malignancy is uncertain (autoimmune or chronic pancreatitis) and in unresectable disease before chemoradiation therapy.4 Routine preoperative biliary drainage with endoscopic retrograde cholangiopancreatography is unnecessary; however, brushings (sensitivity 40%–60%, specificity 91%–100%) may be helpful during therapeutic stenting for sepsis management in patients with cholangitis, an unknown pancreatic head mass or patients unable to undergo immediate surgery.23,24
When and how can pancreatic cancer be surgically resected?
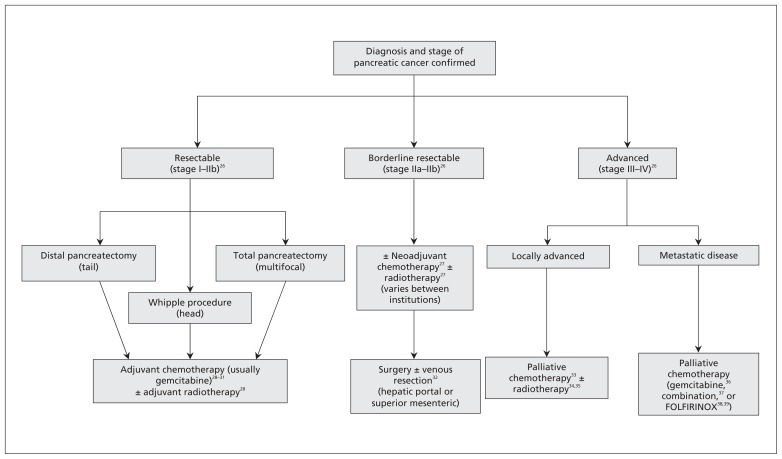
Pancreatic cancer is broadly classified into resectable, borderline resectable and advanced disease (Figure 2).26–39 Twenty percent of cases are candidates for surgery and have CT evidence of no distant metastases, with the primary tumour free from the hepatic portal and superior mesenteric veins and from the celiac, hepatic and superior mesenteric arteries.26 The surgical procedure depends on the location of the tumour: pancreaticoduodenectomy (Whipple procedure) is used for lesions of the head, neck and uncinate process; distal pancreatectomy is used for lesions of the body or tail; and total pancreatectomy is used in multifocal disease.
Figure 2:
Treatment algorithm for pancreatic cancer.
Owing to the technical complexity of these surgeries, minimally invasive approaches are not widely used, although laparoscopic distal pancreatectomy may provide similar oncologic results with the usual benefits of minimally invasive surgery.40
Common postsurgical complications include delayed gastric emptying (5%–45%) and pancreatic anastomotic leaks (0%–13%).41,42 Delayed gastric emptying manifests as failure of dietary progression after 7 days, prolonged use of nasogastric decompression or emesis upon removal requiring reinsertion.43 Management includes continued nasogastric decompression, use of promotility agents (metaclopromide, erythromycin) and ongoing nutritional support (jejunal or parenteral),41 with resolution usually within 2–6 weeks.
Pancreatic leaks (70%–90%) containing amylase-rich fluid occur within the first 1–2 weeks after surgery and present with abdominal pain and fever (temperature > 38.5°C). These symptoms are managed with antibiotics and percutaneous drainage, usually by interventional radiologists.41,44 Leaks with severe sepsis, peritonitis or hemorrhage require surgical irrigation and drainage, with rare cases requiring complete pancreatectomy.44 In-hospital mortality from pancreatic leaks is as high as 5% with nonsignificantly shortened survival compared with patients without leaks (16.5 mo v. 27.5 mo, p = 0.4).45 Diabetes and pancreatic insufficiency requiring lifelong treatment develop in patients undergoing total pancreatectomy.
Overall 5-year survival after pancreatic resection is 14.6%, but higher in well-differentiated disease (30%–40%) and disease that has not metastasized to the lymph nodes (25%–30%).46 Postoperatively, blood glucose monitoring every 3–6 months should be considered because diabetes develops in more than 50% of patients with partial pancreatectomy.47 Liberal use of enzyme replacements is recommended because of exocrine pancreatic insufficiency, occurring in 80% of patients and presenting as bloating, diarrhea and steatorrhea. Rising CA 19-9 levels may signal early recurrence; however, the appropriate surveillance interval and duration is unknown, and intensive follow-up may not be necessary outside of a clinical trial.48
Is there a role for adjuvant or neodjuvant therapy?
Adjuvant chemotherapy is recommended for all patients based on results from multiple randomized trials (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.121368/-/DC1) and meta-analyses. Either gemcitabine or 5-fluorouracil (5-FU) prolongs median survival by 3 months (95% CI 0.3–5.7),28–31,49 but gemcitabine has been recommended as the first-line adjuvant agent owing to its lower toxicity profile versus 5-FU in the European Study Group for Pancreatic Cancer (ESPAC) 3 trial.50 Common adverse effects with gemcitabine include myelosuppression (32%–73%, grade 3/4 2%–22%), hepatotoxicity (50%–80%, grade 4 2%–16%), nausea and vomiting (26%–55%, grade 3/4 2%–3%) and diarrhea (38%, grade 3/4 2%). Toxicities with 5-FU include stomatitis (65%, grade 3/4 10%), nausea or vomiting (30%–60%, grade 3/4 3%–4%) and myelosuppresion (10%–55%, grade 3/4 0%–22%).31
The benefit of adjuvant radiation therapy is unclear based on the results of the ESPAC-1 trial.28 Median survival with chemoradiation (13.9 mo, 95% CI 12.2–17.3) was similar to that seen with observation alone (16.9 mo, 95% CI 12.3–24.8), but was longer when chemoradiation was followed by chemotherapy (19.9 mo, 95% CI 14.2–22.5) and longest with adjuvant chemotherapy alone (21.6 mo, 95% CI 13.5–27.3). Criticism of the ESPAC-1 study design has prevented total abandonment of adjuvant radiation therapy, and it is still administered at some institutions. Common adverse effects of radiotherapy include dermatitis, nausea and vomiting, diarrhea and myelosuppression.
The evidence for benefit of neoadjuvant therapy (i.e., administered before surgery) at the cost of delaying surgery is controversial. Theoretical benefits include preoperative eradication of micrometastatic disease, reduction in tumour volume facilitating resection, ensuring that patients receive this type of treatment (some never receive adjuvant therapy owing to prolonged recovery from surgery) and improved perfusion of peritumoral tissues before surgical disruption of the vasculature and lymphatics. A meta-analysis mainly consisting of heterogenous phase I and II studies found a median overall survival of 23.3 (range 12–54) months, with perioperative mortality of 5.3% (95% CI 4.1%–6.8%).51 These figures are comparable with those for upfront resection with adjuvant therapy. Because no phase III trials comparing outcomes between neoadjuvant and adjuvant therapy exist, most centres currently refrain from using neoadjuvant therapy outside of research protocols.
What is the management of borderline resectable disease?
Pancreatic cancer with partial abutment or encasement of the hepatic portal or superior mesenteric veins is considered borderline resectable, and surgery is attempted only if complete resection is possible.26 A 6-year prospective study involving 110 patients with resection of the hepatic portal vein, superior mesenteric vein or both for suspected tumour infiltration showed median overall survival of 14.5 (range 7.3–24) months, with perioperative mortality of 3.7%.32 These results suggest that major venous resection and reconstruction is safe in experienced hands and results in oncologic results equivalent to those of complete resection.
A role and optimal regimen for neoadjuvant therapy in borderline resectable disease is unclear, based on inconclusive findings regarding median survival in a retrospective study of neoadjuvant treatment versus immediate surgery (35 mo v. 27 mo; p = 0.7).27
What treatment options exist for advanced pancreatic cancer?
Patients with advanced pancreatic cancer include those with locally advanced or metastatic disease and have a median overall survival of 2–3 months without treatment.52 Locally advanced disease, representing 30% of cases, appears radiographically as extensive involvement of the hepatic portal vein, superior mesenteric vein and retroperitoneum, in addition to encasement of major arteries (celiac, hepatic, superior mesenteric) or infiltration of the aorta.4,26 A meta-analysis of palliative chemotherapy versus supportive care showed improved survival in locally advanced disease (hazard ratio [HR] 0.64, 95% CI 0.42–0.98), with equivalent results between gemcitabine and 5-FU (HR 0.75, 95% CI 0.42–1.31).33 A survival benefit with chemotherapy followed by chemoradiation (Appendix 1) was seen in the Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR) phase II/III study34 and furthered by the Eastern Cooperative Oncology Group-E4201 trial35 comparing gemcitabine plus radiotherapy with gemcitabine alone. Combination chemoradiation therapy is now central to the management of locally advanced disease.
Distant organ involvement, typically that of the liver, peritoneum or lung, occurs in 50% of cases. For patients with such involvement, gemcitabine provides a slight improvement in overall survival over 5-FU (5.65 mo v. 4.41 mo, p = 0.003).36 Individual clinical trials of combination gemcitabine with various cytotoxic agents53–56 have failed to show a survival benefit over gemcitabine alone (Appendix 1), but significantly improved overall survival was seen when these studies were pooled (HR 0.91, 95% CI 0.85–0.97).37 Furthermore, patients with a good performance status (Karnof-sky performance score > 90%) survived longer with combination gemcitabine (HR 0.76, 95% CI 0.67–0.87), whereas patients with a poor performance status did not (HR 1.08, 95% CI 0.90–1.29). Combination therapy also showed higher grade 3/4 toxicity: neutropenia (risk difference [RD] 5%, 95% CI 1%–10%), thrombocytopenia (RD 5%, 95% CI 2%–8%) and nausea or vomiting (RD 3%, 95% CI 0%–5%).57 Combination chemotherapy is reserved for patients with a good performance status.
Targeted therapy in pancreatic cancer has centred on the epidermal growth factor pathway. A phase III study of the epidermal growth factor receptor inhibitor erlotinib with gemcitabine versus gemcitabine alone showed marginally increased overall survival (6.24 v. 5.91 mo, p = 0.04)58 and 1-year survival (23% [95% CI 18%–28%] v. 17% [95% CI 12%–21%], p = 0.02). However, toxicity was increased at all grades, including fatigue (32%; percent difference gemcitabine–erlotinib v. gemcitabine 0.85%), rash (25%; percent difference 15.2%), diarrhea (20%; percent difference 5.2%) and, rarely, interstitial lung disease (0.7%; percent difference 0.6%). Currently, combination gemcitabine/erlotinib is considered for patients with good performance status in the metastatic setting.
More recently, FOLFIRINOX (5-FU, leucovorin, irinotecan and oxaliplatin) has shown a substantial increase in median overall survival over gemcitabine alone in metastatic pancreatic cancer (11.1 mo [95% CI 9.0–13.1] v. 6.8 mo [95% CI 5.5–7.6 mo]; HR 0.57, 95% CI 0.45–0.73, p < 0.001).38 However, a higher grade 3/4 toxicity was also seen: neutropenia (45.7% v. 21.0%, p < 0.001), thrombocytopenia (9.1% v. 3.6%, p = 0.04), diarrhea (12.7% v. 1.8%, p < 0.001) and sensory neuropathy (9.0% v. 0%, p < 0.001). A longitudinal follow-up study (median 26.6 mo, 95% CI 20.5–44.9) showed a longer time to symptomatic deterioration for patients in the FOLFIRINOX group.39
Future directions
Accelerated progress in understanding pancreatic cancer relies on robust partnerships between clinicians and basic scientists, such as the current global effort to develop more integrated translational pancreatic cancer programs.59 Next-generation sequencing has revolutionized the field of pancreatic cancer genetics, and notable findings include the recent discovery of germline mutations in the ataxia telangiectasia mutated gene (ATM) in a small proportion (2.4%–4.6%) of familial pancreatic cancer, and the involvement of axon guidance pathways as an unexpected molecular change in some tumours.60,61 Further investigations are ongoing to determine what role these mutations and pathways play in pancreatic carcinogenesis. Other initiatives, such as the International Cancer Genome Consortium efforts to sequence the genomes of 750 pancreatic cancer specimens, will generate new data and catalyze genotype-specific “personalized” treatment strategies.62
Key points
Smoking cessation remains the only recommended measure for the prevention of pancreatic cancer.
Triphasic abdominal contrast computed tomography scan is the imaging modality of choice for diagnosis.
Surgery (pancreaticoduodenectomy, or distal or total pancreatectomy, depending on tumour location) remains the only therapy with curative potential.
Adjuvant chemotherapy, with or without radiation therapy, should be administered to all patients following curative resection for pancreatic cancer.
In advanced pancreatic cancer, gemcitabine alone, gemcitibine plus erlotinib and FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin) have each shown a survival benefit over other chemotherapy options.
Supplementary Material
Footnotes
Competing interests: Zaheer Kanji has received grant funding from the University of British Columbia Clinician Investigator Program. Steven Gallinger has received grant funding from the W. Garfield Weston Foundation, National Cancer Institutes/National Institutes of Health and Pancreas Cancer Canada; he is a consultant for Therapure. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Zaheer Kanji and Steven Gallinger conceived the article. Zaheer Kanji drafted the article, which was revised by Steven Gallinger. Both authors approved the final version submitted for publication.
References
- 1.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high risk cohort: an eight year experience. J GastroIntestinal Surg 2012;16:771–83 [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Steering Committee on Cancer Statistics Canadian Cancer Statistics 2012. Toronto (ON): Canadian Cancer Society; 2012 [Google Scholar]
- 3.Woodmass J, Lipschitz J, McKay A. Physician attitudes and treatment of patterns for pancreatic cancer. World J Surg Oncol 2011;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo M. Pancreas cancer. NEJM 2010;362:1605–17 [DOI] [PubMed] [Google Scholar]
- 5.Pancreatic cancer survival by stage. Atlanta (GA): American Cancer Society; 2013. Available: www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-survival-rates (accessed 2013 Mar. 21). [Google Scholar]
- 6.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol 2012;9:77–87 [DOI] [PubMed] [Google Scholar]
- 7.Klein AP. Identifying people at high risk of developing pancreatic cancer. Nat Rev Cancer 2013;13:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolpin BM, Kraft P, Gross M, et al. Genotype derived ABO blood groups alleles and the risk of pancreatic cancer: data from the Pancreatic Cancer Consortium. Cancer Res 2010;70:1015–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary and medical history factors associated with pancreas cancer risk in Ontario, Canada. Cancer Causes Control 2009;20:825–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med 2009;133:365–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn SA, Greenhalf B, Ellis I, et al. BRCA 2 mutations in familial pancreatic carcinoma. J Natl Cancer Inst 2003;95:214–21 [DOI] [PubMed] [Google Scholar]
- 12.Jones S, Hruban RH, Kamiyama M, et al. Exome sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slater EP, Langer P, Niemczyk E, et al. Prevalence of BRCA2 and CDKN2A mutations in German familial pancreatic cancer families. Fam Cancer 2010;9:335–43 [DOI] [PubMed] [Google Scholar]
- 14.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4:766–81 [DOI] [PubMed] [Google Scholar]
- 16.Brentnall TA. Pancreatic cancer surveillance: learning as we go. Am J Gastroenterol 2011;106:955–6 [DOI] [PubMed] [Google Scholar]
- 17.Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology 2010;139:1076–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB 2008;10:371–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porta M, Fabregat X, Malats N, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumor site and stage. Clin Transl Oncol 2005;7:189–97 [DOI] [PubMed] [Google Scholar]
- 20.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura F, Takada T, Amano H, et al. Diagnosis of pancreatic cancer. HPB 2006;8:337–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birchard KR, Semelka RC, Hyslop WB, et al. Suspected pancreatic cancer: evaluation by dynamic gadolinium-enhanced 3D gradient-echo MRI. AJR Am J Roentgenol 2005;185:700–3 [DOI] [PubMed] [Google Scholar]
- 23.Wight CO, Zaitoun AM, Boulton-Jones JR, et al. Improving diagnostic yield of biliary brushings cytology for pancreatic cancer and cholangiocarcinoma. Cytopathology 2004;15:87–92 [DOI] [PubMed] [Google Scholar]
- 24.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. NEJM 2010;362:129–37 [DOI] [PubMed] [Google Scholar]
- 25.Ruckert F, Pilarsky C, Grutzmann R. Serum tumor markers in pancreatic cancer — recent discoveries. Cancers 2010;2:1107–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2012: Featured updates to the NCCN guidelines. J Natl Compr Can Netw 2012;10:703–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg 2012;203:132–9 [DOI] [PubMed] [Google Scholar]
- 28.Neoptolemos JP, Stocken DB, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200–10 [DOI] [PubMed] [Google Scholar]
- 29.Neuhaus P, Riess H, Post S, et al. CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resectable pancreatic cancer. J Clin Oncol 2008. (May 20 suppl) [abstract]. [Google Scholar]
- 30.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine base chemotherapy before and after fluorouracil based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019–26 [DOI] [PubMed] [Google Scholar]
- 31.Neoptolemos JP, Stocken DB, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer 2009;100:246–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller SA, Hartel M, Mehrab A, et al. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg 2009;13:784–92 [DOI] [PubMed] [Google Scholar]
- 33.Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607–15 [DOI] [PubMed] [Google Scholar]
- 34.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326–31 [DOI] [PubMed] [Google Scholar]
- 35.Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol 2008. (26 suppl) [abstract]. [Google Scholar]
- 36.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13 [DOI] [PubMed] [Google Scholar]
- 37.Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817–25 [DOI] [PubMed] [Google Scholar]
- 39.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 Randomized Trial. J Clin Oncol 2013;31:23–9 [DOI] [PubMed] [Google Scholar]
- 40.Venkat R, Edit BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: A systematic review and meta-analysis. Ann Surg 2012;255:1048–59 [DOI] [PubMed] [Google Scholar]
- 41.Ho CK, Kleeff J, Friess H, et al. Complications of pancreatic surgery. HPB (Oxford) 2005;7:99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long term survival. Ann Surg 2009;250:282–7 [DOI] [PubMed] [Google Scholar]
- 43.Malleo G, Crippa S, Buttirini G, et al. Delayed gastric emptying after pylorus — preserving pancreaticoduodenectomy: validation of international study group of pancreatic surgery classification and analysis of risk factors. HPB 2010;12:610–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Berge Henegouwen MI, De Wit LT, Van Gulik TM, et al. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 1997;185:18–24 [DOI] [PubMed] [Google Scholar]
- 45.Ausania F, Cook N, Jamieson N, et al. Impact of pancreatic leaks on survival following pancreaticoduodenectomy. JOP 2010;11:226–9 [PubMed] [Google Scholar]
- 46.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg 2004;198:722–31 [DOI] [PubMed] [Google Scholar]
- 47.Keim V, Klar E, Poll M, et al. Postoperative care following pancreatic surgery: surveillance and treatment. Dtsch Arztebl Int 2009; 106:789–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy MJ, Sturgeon C, Lamerz R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol 2010;21:441–7 [DOI] [PubMed] [Google Scholar]
- 49.Boeck S, Ankerst DP, Heinemann V. The role of adjuvant chemotherapy for patients with resected pancreatic cancer: systematic review of randomized controlled trials and meta-analysis. Oncology 2007;72:314–21 [DOI] [PubMed] [Google Scholar]
- 50.Neoptolemos JP, Stocken DB, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073–81 [DOI] [PubMed] [Google Scholar]
- 51.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol 2010;7:163–72 [DOI] [PubMed] [Google Scholar]
- 53.Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern cooperative oncology group trial E2297. J Clin Oncol 2002;20: 3270–5 [DOI] [PubMed] [Google Scholar]
- 54.Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 2006;24: 3946–52 [DOI] [PubMed] [Google Scholar]
- 55.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23: 3509–16 [DOI] [PubMed] [Google Scholar]
- 56.Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol 2007;25:2212–7 [DOI] [PubMed] [Google Scholar]
- 57.Xie DR, Liang HL, Wang Y, et al. Meta-analysis on inoperable pancreatic cancer: a comparison between gemcitabine-based chemotherapy therapy and gemcitabine alone. World J Gastroenterol 2006;12: 6973–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trial Group. J Clin Oncol 2007;25:1960–6 [DOI] [PubMed] [Google Scholar]
- 59.Petersen GM, de Andrade M, Goggins M, et al. Pancreatic Cancer Genetic Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2006;15:704–10 [DOI] [PubMed] [Google Scholar]
- 60.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal abberations in axon guidance pathways. Nature 2012;491:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hudson TJ, Anderson W, Artez A, et al. International network of cancer genome projects. Nature 2010;464:993–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.