A retinal implant system recently approved for sale in the United States could give limited sight to the blind, but a high price tag, challenges with the device and a narrow target group of patients could be stumbling blocks.
The Argus II Retinal Prosthesis System, created by California-based Second Sight, is the only device approved for sale in the US and Europe, for the restoration of functional vision to people who have lost sight because of advanced retinal degenerative diseases such as retinitis pigmentosa. They hope to gain regulatory approval and market the system in Canada as well.
The device consists of a pair of glasses with a video camera embedded in the front and a retinal implant. The camera records images and sends them wirelessly through a cellphone-sized video-processing unit to the retinal implant, which uses a sheet of 60 electrodes in the posterior of the eye to stimulate the patient’s few remaining healthy retinal cells and transmit information to the optic nerve. The device allows patients to discern varying levels of light and the outline of objects.
“If you look at the patient you can’t tell that they have an implant,” says Second Sight Chief Executive Officer, Dr. Robert Greenberg. “Similarly the patient can’t feel the implant. And the glasses look like a pair of sport glasses.”
Dr. Phil Hooper, a retina specialist with the University of Western Ontario in London, says the device is a “significant improvement” for a very small group of people “who have an intact optic nerve and a reasonably intact inner retinal architecture.”
“The vision it produces is basically light in somebody who is living in a world of darkness … but beyond that the vision is very crude,” adds Hooper who is editor-in-chief of the peer-reviewed scientific journal, the Canadian Journal of Ophthalmology.
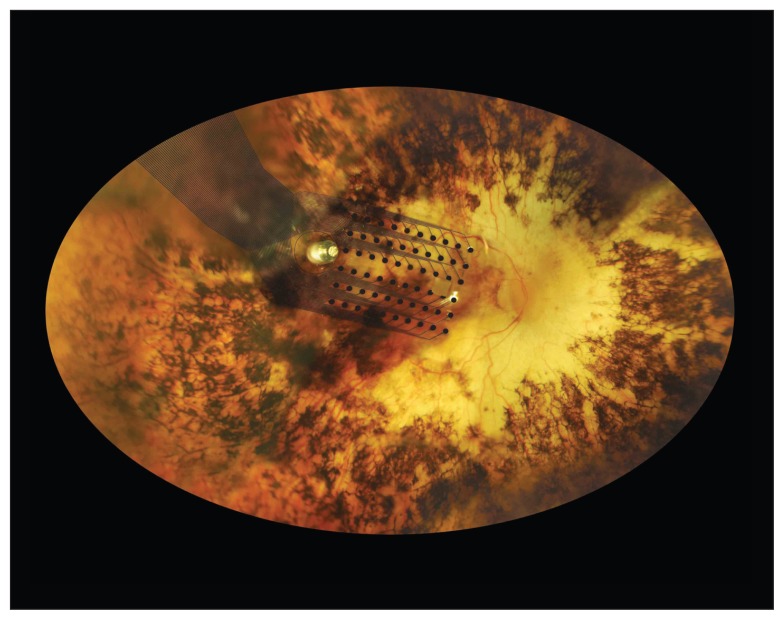
This a fundus image of the implant on the retina. The implant system costs $100 000 and is only suitable for patients with advanced retinal degenerative diseases.
Image courtesy of Second Sight
The device has several additional limitations.
For starters, it is primarily indicated for patients suffering from a specific degenerative eye disease called retinitis pigmentosa, which affects about 1 in 4000 people or more than 1 million people worldwide.
Most people are blind because of the effects of glaucoma, macular degeneration or diabetes, says Greenberg. “And all three of those diseases really don’t lend themselves well to this technology.”
Second, with a price tag close to US$100 000, few patients will be able to afford the device. The price may drop in a few years as manufacturing picks up, Greenberg says.
And third, the device’s durability is at present unknown. Hooper says, “I think the issues that remain to be determined is the long term … the longevity of the array on the retina, how long is it going to stay there without the damaging the retina.”
On the plus side, the surgery for the Argus II can be performed in two hours under general anesthesia by a single surgeon, Greenberg says. Patients go home the same day or the day after. Previous systems, such as the Argus I, often required eight-hour surgeries with multiple surgeons.
There has been no rejection of either Argus device; about 50 patients have been living with the Argus II implant for more than six years.
“There were adverse events during the [Argus II] clinical trial,” says Greenberg, which involved 30 patients and led up to the approval of the device in February.
“All of them were manageable with standard treatments. One of the 30 patients in the trial did have the device removed and the eye was fine after that.”
Reported adverse effects included infections such as conjunctivitis, which were treated with antibiotics, in addition to low eye pressure and the opening of the conjunctiva membrane, which had to be resutured where the cable enters the eye.
“That was part of the learning during the early part of the trial,” says Greenberg. “We haven’t seen that since.”
Another challenge with the device is patient satisfaction; so far reports have been mixed. “My original experience with it? I wasn’t impressed,” says Dean Lloyd, a US lawyer who suffers from retinitis pigmentosa. “What’s important here to note is that this device does not give you image vision, it gives you boundaries and borders and that’s very hard for normal sighted people to understand.”
The Argus II provides a grey-scale blurry outline of an object to patients, who then create the image in their mind by using memory and imagination, says Lloyd.
Greenberg says he hopes to launch the device commercially in the US later this year and in Canada within two years.
If Second Sight does attempt to venture north into Canada, Hooper says the company might decide to seek public health insurance coverage, but he’s not sure the evidence is there yet that it’s a cost effective treatment.
“I mean for $100 000 look at how many cataracts you can do and restore vision, and how many other things you could do with that money.”