Abstract
Experientially opening oneself to pain rather than avoiding it is said to reduce the mind’s tendency toward avoidance or anxiety which can further exacerbate the experience of pain. This is a central feature of mindfulness-based therapies. Little is known about the neural mechanisms of mindfulness on pain. During a meditation practice similar to mindfulness, functional magnetic resonance imaging was used in expert meditators (> 10,000 h of practice) to dissociate neural activation patterns associated with pain, its anticipation, and habituation. Compared to novices, expert meditators reported equal pain intensity, but less unpleasantness. This difference was associated with enhanced activity in the dorsal anterior insula (aI), and the anterior mid-cingulate (aMCC) the so-called ‘salience network’, for experts during pain. This enhanced activity during pain was associated with reduced baseline activity before pain in these regions and the amygdala for experts only. The reduced baseline activation in left aI correlated with lifetime meditation experience. This pattern of low baseline activity coupled with high response in aIns and aMCC was associated with enhanced neural habituation in amygdala and pain-related regions before painful stimulation and in the pain-related regions during painful stimulation. These findings suggest that cultivating experiential openness down-regulates anticipatory representation of aversive events, and increases the recruitment of attentional resources during pain, which is associated with faster neural habituation.
Keywords: Pain, emotion regulation, neuroimaging, insula cortex, salience network, Open Presence meditation, meditation expertise
Introduction
Many cognitive strategies regulate pain and distress by actively controlling the sensory, cognitive, or affective components of pain. These states include strategies which involve orienting attention away from the pain, such as listening to music to relieve distress, and those which involve altering the context of the experience, such as expressive suppression, cognitive reappraisal, hypnosis, or placebo (Rainville et al., 1997; Wager et al., 2004; Wiech et al., 2008b; Tracey, 2010). Recently developing bodies of clinical theory on acceptance and mindfulness suggest that a state or disposition that instead cultivates a quality of openness and experiential acceptance, that does not strive to ignore, reject or avoid pain through cognitive control should be more adaptive. This is especially true in circumstances where pain is unavoidable but known to be safe, because of these states’ capacity to regulate the mind’s conditioned tendency toward avoidance or anxiety, which overall could exacerbate the experience (Kabat-Zinn, 1982; Cioffi and Holloway, 1993; Gross and Levenson, 1997; Grossman et al., 2007; Wetherell et al., 2011; Gross and Levenson, 1997; Hayes, 2004; McCracken, 1998). A growing body of evidence is beginning to provide support for this framework (Kabat-Zinn, 1982; Cioffi and Holloway, 1993; Gross and Levenson, 1997; Grossman et al., 2007; Wetherell et al., 2011; Gross and Levenson, 1997; Hayes, 2004; McCracken, 1998). Actively suppressing the experience of pain produces a slower recovery from pain than merely monitoring the sensation of pain and strengthens the interpretation of a subsequent noxious sensation as being aversive (Cioffi and Holloway, 1993). Actively suppressing negative emotion also increases the intensity and frequency of sympathetic and cardiovascular activities (Gross and Levenson, 1997), which can have detrimental health consequences (Gross and Levenson, 1997; Chambers et al., 2009). Clinical interventions that cultivate experiential openness and acceptance, such as mindfulness-based stress reduction (MBSR) (Kabat-Zinn, 1982) or acceptance and commitment therapy (ACT) (Hayes, 2004) can reduce pain unpleasantness (Brown and Jones, 2010; Perlman et al., 2010; Grant et al., 2011; Zeidan et al., 2011) and lead to a reduction of symptoms in chronic pain patients (Kabat-Zinn, 1982; Grossman et al., 2007; Morone et al., 2008; Wetherell et al., 2011). In this study we used neuroimaging to explore the effects of a state of acceptance and openness on pain anticipation and processing and also the effects of this state on avoidance and anxiety-related processes across time.
States of acceptance and openness central to MBSR and ACT interventions are at the core of meditation practices labeled here Open Monitoring (Hayes, 2004; Bishop et al., 2006; Lutz et al., 2006, 2008; Chambers et al., 2009; Dunne, 2011). Open Monitoring practices aim to cultivate an effortless, open, and accepting awareness of whatever is occurring in the present moment, without reacting or being absorbed in the contents of the experience. Open Monitoring is said to increase pain acceptance and decrease unpleasantness by training one to recognize experientially that all components of the experience of pain are merely mental events, and thus do not necessarily need to be acted upon. Thus, the aim of this training is not to explicitly change the content of experience, but rather to change one’s relationship to it. In that sense, the sensation produced by the painful stimulus can be experienced during this state with equal or increased vividness in the moment it occurs, without fear. This is said to reduce emotional reactivity, enhance behavioral flexibility, and lessen automatic action patterns (Bishop et al., 2006; Lutz et al., 2008; Chambers et al., 2009). Within a cognitive framework, this could be understood as faster habituation and weaker conditioning to harmless but aversive events, and these processes are established as playing a role in pain perception and disability (McCracken et al., 1992). The neural mechanisms of this role are explored in the present study.
Several neuroimaging studies of the impact of meditation on pain processing have recently appeared (Brown et al., 2008; Gard et al., 2011; Grant et al., 2011; Zeidan et al., 2011). Grant et al. found reduced pain ratings and reduced activity in areas including the amygdala, but increased activation in anterior cingulate cortex and insula during pain for experienced meditators compared to controls (Grant et al., 2011). Similarly, Zeidan et al. found a reduction in pain ratings and increased activity in the anterior cingulate cortex and anterior insula (aI) during pain after a brief mindfulness meditation training (Zeidan et al., 2011). Gard et al. also found a mindfulness-related reduction in unpleasantness, which was associated with decreased activation in the lateral prefrontal cortex (PFC) and increased activation in the right pIns during stimulation (Gard et al., 2011). Meditation also affects neural processes of pain anticipation. Brown et al. reported reduced pain unpleasantness ratings and less anticipatory neural activity prior to the pain stimulus as measured by EEG event-related potentials for experienced meditators compared to controls (Brown et al., 2008). Gard et al. reported increased rostral anterior cingulate cortex (rACC) activation during the anticipation of pain for mindfulness practitioners compared to controls (Gard et al., 2011). The effects of meditation on anticipation and habituation to pain have not yet been investigated with neuroimaging.
In the current study we focus on the amygdala and on the so-called ‘salience network’ (Seeley et al., 2007; Menon and Uddin, 2010; Legrain et al., 2011) that encompasses the anterior insula (aI) and anterior midcingulate cortex (aMCC) as well as subcortical areas important for emotion (e.g. amygdala), homeostatic regulation, and reward (e.g. VTA) (Ongür and Price, 2000; Seeley et al., 2007). As discussed above, Open Monitoring results in reduced pain ratings and increased activity in the salience network during pain. There is also increasing evidence from neuroimaging studies that activity in the salience network is associated with anticipatory processes about incoming pain and that this activity strongly influences pain experience (Atlas et al., 2010; Wiech et al., 2010). For instance, believing that upcoming pain stimuli are entirely safe reduces anticipatory activity in aI and its connectivity to MCC (Wiech et al., 2008a), and this anticipatory activity is linked to trial-to-trial variations in pain ratings (Atlas et al., 2010). More generally, aI and MCC are activated to varied forms of pain, including the emotional dimensions of pain (Peyron et al., 2000), subjective magnitude of pain (Baliki et al., 2009; Moayedi and Weissman-Fogel, 2009), empathy for pain (Singer et al., 2006), lack of perceived controllability (Salomons et al., 2004), uncertainty (Preuschoff et al., 2008), and social rejection (Eisenberger et al., 2003) (for review (Friebel et al., 2011)). Lesion studies also indicate that a subjectively available experience of pain can be instantiated by brain mechanisms that do not require the insular cortex (Starr et al., 2009). Abnormal processing in these regions also contributes to clinical conditions like anxiety and depression where pain perception and anticipation are subjectively amplified (Paulus and Stein, 2010). Importantly for understanding the value of acceptance, the magnitude of pain perception and anticipation processes influence the degree of conditioning and habituation toward future pain. Compared to controls, anxious patients showed less extinction-related activity in the amygdala during extinction in a fear-conditioning paradigm (Sehlmeyer et al., 2011). The expectation of increased pain intensity decreases the rate of pain habituation in right operculum, amygdala, and insula (Rodriguez-Raecke et al., 2010). Overall this suggests that experiential acceptance and openness will decrease neural activity underlying avoidance and pain-related anxiety during anticipation and increase the rate of neural habituation in these regions to painful stimuli.
To investigate the impact of experiential acceptance and openness on pain we compared a group of long-term Buddhist practitioners with more than 10,000 hours of formal meditation, who performed an advanced style of Open Monitoring meditation called Open Presence (OP; see Material and methods) during a neuroimaging pain paradigm to a control group with no meditation experience who were given matching meditation instructions. OP meditation consists, at least theoretically, of a state where the qualities of effortless openness and acceptance are vividly experienced with minimal control-oriented elaborative processes (Lutz et al., 2006; Dunne, 2011).
According to the framework described above, we examined the hypothesis that OP meditation will affect the subjective representations of pain throughout the task, including its immediate appraisal and its temporal representations of the future (anticipation) and past (habituation) painful trials and that these changes will be correlated to changes in neural activity in pain-related regions during the baseline preceding pain, during pain and across the experimental blocks. More specifically, our first hypothesis was that expert practitioners would show lower unpleasantness ratings and stronger BOLD activity in the salience network (aI, aMCC) during painful stimulation compared to novices, as a consequence of the enhanced acceptance and openness to pain during OP (Perlman et al., 2010; Grant et al., 2011; Zeidan et al., 2011). Our second hypothesis was that experts would display less anxiety-related anticipatory activity in the amygdala and salience network prior to pain compared to novices, as a result of the present-centered nature of this state (Bishop et al., 2006; Farb et al., 2007). Our third hypothesis was that experts compared to novices would show faster neural habituation to pain and its anticipation, here defined as a more negative temporal slope across experimental blocks of activity in amygdala and pain-related regions before pain and in pain-related regions during pain; and that this measure would be correlated with unpleasantness ratings.
Material and methods
Participants
Fourteen long-term meditation practitioners (45.2±9.9 years old, 11 Caucasian, 3 Tibetan, 9 males and 5 females, stimulus temperature 48.1±0.8°C) and fourteen age-, sex-, and stimulus-temperature matched controls (45.6±11.5 years old, 13 Caucasian, 1 Hispanic, 9 males and 5 females, stimulus temperature 48.2±0.8°C) participated in the experimental procedure. Long-term meditation practitioners were selected based on a criterion of at least 10,000 hours of formal meditation practice in the Nyingma and Kagyu traditions of Tibetan Buddhism, which have closely similar styles of practice (mean 27,000 hours, SD 12,500). Based on this criterion, these practitioners are referred to as “experts” here for brevity. Fourteen control participants were recruited from the local community and had no previous experience with any type of meditation, but expressed interest in learning meditation. Participants were screened for pain-related disorders and use of analgesic or psychiatric medication. One long-term practitioner reported a diagnosis of fibromyalgia nearly 20 years ago, but considered him/herself mostly improved and would no longer meet the diagnostic criteria for FM. Analysis of data excluding this practitioner did not change significance of any tests, so he/she was included in the analysis due to the difficulty recruiting participants in this population. One long-term practitioner had a lesion on the left frontal cortex following the removal of an abscess during childhood. Analysis of data excluding this practitioner did not change significance of any tests in the main ROIs.
Meditation practices and training
Open Presence (OP) practice (Tibetan, “ma bcos rang babs”, pronounced “ma chö rang bap”, literally “resting as it is, without fabrication”, in the Dzogchen tradition (Third Dzogchen Rinpoche, 2008) and “tha mal gyi shes pa yengs med” pronounced “tamel gyi shepa yeng me”, literally “undistracted, ordinary consciousness” in the Mahamudra tradition (Namgyal et al., 2004)) aims at cultivating the state of Open Presence (Tibetan, “rig pa cog gzhag”, pronounced “rigpa chok shak”, literally “freely resting in what consciousness manifests”) are exemplified by instructions to “relax (..…) the mind into mere non-distraction. Within a state free of hopes and fears, devoid of evaluation or judgment, be carefree and open. And within that state, do not linger on the past; do not invite the future; place [awareness] within the present, without alteration, without hopes or fears.” (as quoted in (Dunne, 2011)). Based on its traditional presentation (Namgyal et al., 2004; Third Dzogchen Rinpoche, 2008), OP practice is viewed here as an advanced form of Open Monitoring practice, in which practitioners might be found in various levels of achievement. OP meditation consists theoretically of a state where the qualities of effortless openness and acceptance are vividly experienced with minimal control-oriented elaborative processes.
In addition to the OP meditation described above, for a supplementary analysis S1 we also used a concentrative meditation labeled Focused Attention (FA) practice (Namgyal et al., 2004) (translated from rtse gcig ting nge’dzin, pronounced Ts’e-cig Ting-ng’e-dzin, literally one-pointed concentration). FA practice refers to maintaining selective attention on a chosen object. In this particular case, FA was directed at a fixation cross away from the stimulation. This attentional strategy might be expected to regulate pain through a sensory gating mechanism akin to distraction (see Supplementary Materials). From this view, FA could be understood here as control condition for OP.
Controls were given instructions in the practices written by a scholar who is familiar with the practices, (see Supplementary Materials) and then told to practice at home 30 minutes a day for 7 days prior to the experiment. To reduce effects of likely motivational differences between novices and experts, control participants were told that the four novices who demonstrated the largest reduction in pain-induced brain activity (i.e. BOLD signal) during meditation would receive a $50 bonus payment. We hoped that a bonus based on neural activity would motivate them to exert themselves in the meditation practices, while not incentivizing them to misrepresent their ratings.
Protocol
Before the MRI scanning session, participants had a simulation session during which they acclimated to the fMRI environment by lying in a mock MRI scanner (including head coil and digitized scanner sounds) and underwent a calibration procedure for stimulus temperature. Painful stimuli were provided by a TSA-2001 thermal stimulator (Medoc Advanced Medical Systems, Haifa, Israel) with a 30 mm × 30 mm flat thermode, which was applied to the inside of the left forearm, just below the wrist. Temperature was increased from 32°C to 49°C at 0.7°C/sec and then held for 5 seconds before returning to baseline at the maximum slew rate. Participants were instructed to hit a key to indicate when the pain level had reached 8 on a scale of 0–10, where 0 indicates no pain at all, and 10 indicates unbearable pain. At the indicated time the temperature returned to the 32°C baseline at the maximum rate. The temperature remained at the 32 C baseline for 30 seconds before beginning again. There were ten trials; the average temperature reached over the last five trials was used for that participant in the protocol. If a participant did not indicate the pain level had reached 8, 49°C was used for that participant. Temperatures used ranged from 46°C–49°C.
The experiment consisted of 32 trials, broken up into 8 blocks of 4 trials each, with a resting period and comfort check in between. We also checked after each block that controls and experts comply with the instructions. We also controlled for participants’ compliance to the task by monitoring the picture of their right eye throughout the task, which was recorded online using eye-tracking googles. In each trial, participants were presented with a cue for either FA or OP meditation, and then given 45 seconds to settle into the meditation state. Then there was a 12-second warm period at 38°C, followed by 10 seconds at that participant’s painful temperature, or a non-painful temperature six degrees cooler. All temperature changes occurred at the device’s maximum setting of 10°C/sec (the actual slew rate was approximately 5°C/sec as measured externally). Order of FA/OM was counterbalanced across runs. Each run alternated once between meditation states so as to reduce the number of switch between and spill-over across meditation states. During each meditation state, one hot and one warm stimuli were presented. Order of hot/warm within each state was counterbalanced across runs, and participants were not informed in advance of the coming temperature. At the end of each stimulus, a blank screen was presented for ten seconds, then participants were asked to rate the stimulus for “intensity—how hot was it”, and then “unpleasantness—how much did it bother you”, each on a scale of 0 to 10. Each rating screen appeared for 5 seconds, with 1 second blank in between. The overall time between the beginning of one stimulus and the next was 93.3 seconds.
Data collection
MR images were collected with a GE Signa 3.0 Tesla scanner equipped with a high-speed, whole-body gradient and a whole-head transmit-receive quadrature birdcage headcoil. Whole-brain anatomical images were acquired at the end of each session using an axial 3D T1-weighted inversion-recovery fast gradient echo (or IR-prepped fast gradient echo) sequence. The field of view (FOV) was 240 × 240 mm with a 256 × 256 matrix. The slice thickness was 1–1.2 mm, with 0.9 by 0.9mm in-plane dimensions. Functional data were collected using whole-brain EPI (TR=2000, TE=30ms). For functional images, sagittal acquisition was used to obtain 30 interleaved 4 mm slices with a gap of 1 mm between slices. The resulting voxel size was 3.75 by 3.75 by 5 mm (FOV=240mm, matrix=64×64).
Data analysis
Behavioral data
Ratings of hot stimuli were analyzed in a 2 × 2 mixed ANOVA, with between-subject factor Group (Novice or Expert) and within-subject factor Rating Type (Intensity or Unpleasantness).
BOLD data analysis
Analysis techniques were similar to those described previously in our lab. (Lutz et al., 2009). Briefly, data processing was implemented via AFNI (Analysis of Functional Neural Images). Data processing steps included image reconstruction in conjunction with smoothing in Fourier space via a Fermi filter, correction for differences in slice timing, 6-parameter rigid-body motion correction, and conversion to percent signal change prior to deconvolution. Motion parameters were not used as nuisance regressors for deconvolution, rather times of movement greater than 2mm were censored for 3TRs. The BOLD time series was modeled with a least-squares general linear model (GLM) fit that included a 3rd degree polynomial baseline function and blocks of 82 seconds modeled with 42 parameter cubic spline functions for each of the 4 combinations of states (OP and FA) and conditions (hot and warm). The time interval between the trial beginning cue was at t=−45 sec and the onset of the thermal stimuli at t=0 sec. Fixing instead of randomizing the time of warning cue is known to increase the expectancy about the onset of pain stimuli (Lakatos et al., 2008). A BOLD correlate of this expectation was measured by comparing the beginning of the meditation interval a1=[−40 to −25 sec] and the end of the meditation interval a2=[−15 to 0 sec] before the thermal stimulus. We also computed a coefficient during pain processing a3=[12–22sec], as the difference between response to a painful hot stimulus and a non-painful warm stimulus. These coefficients were further spatially smoothed using a 8mm RMS Gaussian filter. The resultant parametric maps were transformed into the standardized Talairach space via the warp from the spatially normalized anatomicals T1 high-resolution anatomical scans spatially normalized to the TT_icbm452 template at 1 mm3 resolution.
We assessed the effect of expertise on baseline activity and pain processing using two voxel-wise repeated ANOVAs with Group (Experts vs. Novices) as between factors, and with Temperature (Heat vs. Warm) or Time (a1 vs. a2) as within subject-factors for, respectively, the first and second ANOVAs. Monte Carlo simulations were run to correct for multiple testing to achieve an overall corrected mapwise alpha of 0.05. We found that the minimum cluster size was 154 contiguous voxels with the data thresholded at an uncorrected voxelwise p-value of p = 0.005. The data were then overlaid onto the average of the high-resolution anatomical images across participants. An identical approach was used to assess the degree of habituation and sensitization of the BOLD signal across the blocks. We explicitly modeled block order in the general linear model-based whole-brain analysis of BOLD activity during the meditation period (a1 and a2 interval combined) and of pain processing (interval a3). We defined the regressor for block order so that a negative coefficient corresponded to a reduction of activity across blocks, which was taken as a measure of neural habituation. We tested whether the two groups differed in their habituation to pain anticipation and pain processing in the right pI/S2, right mid-insula (mI) and MCC, subregions of the pain-related regions. These ROIs were defined by the intersection of the functionally-derived pain-related regions from Figure 2a and AFNI’s anatomical templates of these named regions. The ROI for the amygdala was provided by the Talairach Daemon database in AFNI (Cox, 1996). To refine the spatial specificity of these analyses, we complemented the ROI analyses with the corresponding voxel-wise t-tests. To test whether neural habituation was associated with behavioral habituation, we first measured and normalized (Fisher z transform) the correlation between block order (mean centered and normalized) and the difference between intensity and unpleasantness ratings. One participant without rating variability across blocks was not used in this analysis.
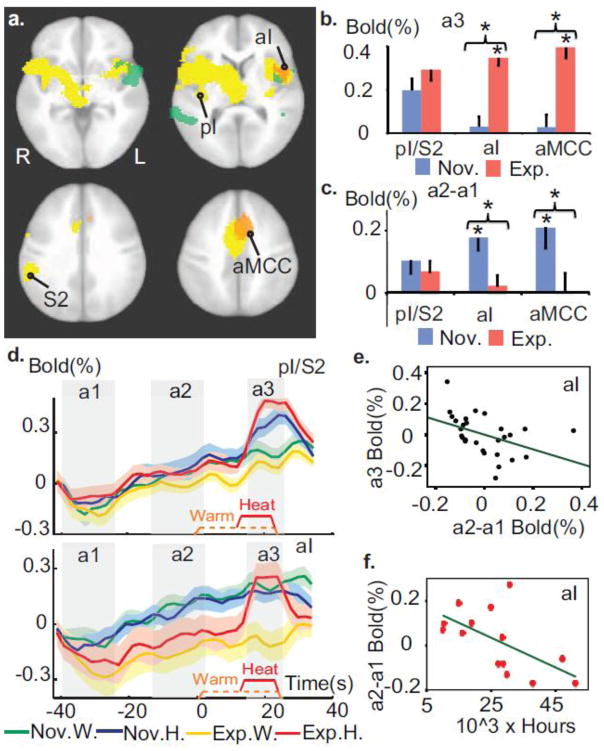
Figure 2. Expertise in OP modulates the temporal processing of painful stimuli.
a. Meditation experts had greater activity in primary pain regions during pain and decreased activity during the anticipatory period prior to pain. This is revealed by a voxel-wise group comparison of the response during (orange clusters) and prior to (green clusters) the pain stimulus (corrected, p<0.005). These maps are overlaid on the pain-related regions defined by the contrast heat vs. warm across groups (yellow clusters, t-test, corrected, p < 2×10^ −5). Talairach coordinates of axial views z= −2, 8, 31 and 42 mm. b. Experts differed more from novices in the anterior part of the pain-related regions than its sensory part during pain processing. The graph displays the response in sensory part of the pain-related regions, including posterior insula and secondary sensory cortex (labeled pI/S2), and in left aI (peak at (−38, 13, 7)) and aMCC (peak at (−8, 20, 39)) regions (in orange in 2a). Error bars are SEM. c. Experts had less anticipatory activity than novices in aI, aMCC but not in pI/S2. d. Average BOLD activity across time in pI/S2 and in left aI (baseline set to 0 at the onset of a1 for display purpose). e. Anticipatory activity in left aI predicted the pain-evoked response therein during pain controlling for Group factor and sensory activity in pI/S2 (Partial correlation r=−0.43, p<0.05). f. The amount of meditation practice in life for experts was negatively correlated to anticipatory activity in left aI (r= −0.63, p<0.05). * indicates p<0.05.
RESULTS
Behavioral results
In line with our preliminary behavioral report (Perlman et al., 2010) and our first hypothesis, the Group (Novice or Expert) × Rating type (Intensity or Unpleasantness) interaction was significant, F (1,25) =9.5, p = 0.005 (Figure 1b). This was driven by the experts’ lower unpleasantness (t (25)=3.6, p=0.001) but similar intensity ratings (t (25)=0.6, p=0.52) compared to novices (see Material and methods, and Supplementary Analysis S1, for a comparison to another control condition). This shows that OP-related expertise decreased pain unpleasantness.
Figure 1. Experimental design and behavioral results.
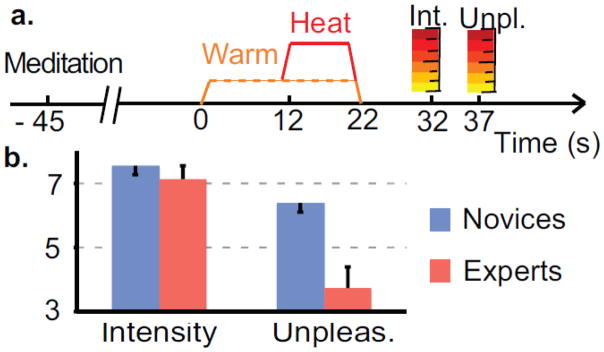
a. Trial structure. Each trial consisted of a visual cue followed by a 45 s meditation period, a 12 s warm thermal stimulation followed by either a warm or painful thermal stimulation. After each trial, subjects rated pain intensity and unpleasantness on a visual analog scale. b. Pain intensity and unpleasantness ratings for novices and experts performing OP. Error bars are SEM. Experts rated less unpleasantness than novices but rated intensity as comparable.
OP meditation enhances the salience network during pain processing
To test our first hypothesis of increased BOLD activity for experts in the salience network during painful stimulation, we ran a voxel-wise group comparison of the response during the pain stimulus, measured as the difference between response to a painful hot stimulus and a warm, non-painful control stimulus. As predicted, this pain response was greater for experts only in two clusters comprising the salience network, left aI and aMCC (see Table 1). This effect was present at a trend level in the right aI. These clusters overlapped with the pain-related regions, defined by the contrast heat vs. warm (Fig. 2a, orange clusters, see Supplementary Table S1 for details). This interaction was driven by a stronger response during pain compared to warm for experts (paired t-tests, p<0.001, t (13)>7.8, in aI and aMCC clusters) but not for novices (t (13)<0.6, p>0.5) (Fig. 2b). It is important to note that this effect was not merely produced by a group difference in pain sensitivity as indicated by the similarity between groups in intensity rating (Fig. 1b). Also, the effect appears specific to higher-order pain representation in the salience network as indicated by no difference in pain-evoked activity in sensory areas pI/S2 of the pain-related regions (p>0.2, Figs. 2a–b) and significant Group by Region (pI/S2 vs. aI or aMCC) interactions (repeated ANOVAs, F (1,26)=9.5, p<0.005 for aI, and F (1,26)=18.7, p<0.001 for aMCC). (Figs. 2a–b).
Table 1. Brain regions showing expertise-related effect during pain.
Brain regions showing a group difference in the contrast hot stimulus versus warm stimulus (voxel-wise T-test, corrected, p<0.005)
| Brain Regions | CM (X, Y, Z) | Peak (X, Y, Z) | t-value | Voxels # | |
|---|---|---|---|---|---|
| 1 | aMCC | (−3 15 44) | (−4 18 44) | 3.9 | 706 |
| 2 | L. aIns | (−47 7 9) | (−45 13 4) | 3.4 | 276 |
Because of the group difference in unpleasantness ratings, we tested for correlations between unpleasantness ratings (difference heat-warm) and BOLD activity (difference heat-warm) in the two clusters of the salience network, controlled for the rating of intensity. In line with previous studies (Rainville et al., 1997; Craig, 2010), we found a positive association between the two measures for the novices (r=0.7, p=0.01 in aI, r=0.65, p=0.02 in aMCC). Importantly, this association was not present for the experts (p>0.4, with r=−0.26, in aI, and r=−0.15 in aMCC) and the magnitude of these associations differed between groups (t-tests following Fisher r-to-z transformation, p= 0.01 in aI and p= 0.03 in aMCC) indicating a possible dissociation between salience and unpleasantness during pain for experts (See Supplementary Analysis S2 for additional control analysis). As predicted, this indicates that the state of OP induces a stronger response during heat in the salience network. In addition, the activity in these regions was associated with unpleasantness ratings for novices but not for experts.
Meditation expertise modulates baseline activity prior to pain
To test our second hypothesis of reduced anxiety-related anticipatory processes in the amygdala and salience network prior to pain, we ran region of interest (ROI)-based group comparisons of the baseline activity prior to the stimulus, measured as the difference between BOLD activity at the end of the meditation (interval a2=[−15 to 0 sec], Fig. 2d) and the beginning of the meditation (interval a1=[−40 to −25sec], Fig. 2d) for both hot and warm stimulus types (see Material and methods). As predicted, baseline activity was greater for novices than experts in the two clusters found in the salience network, left aI and aMCC (Fig. 2a, c). We then conducted several post-hoc exploratory correlation analyses: the baseline activity in aI was negatively correlated with the pain response in that region for experts (r=−0.4, p=0.15), novices (r=−0.61 p=0.02) and across groups with group effect factored out to ensure that any observed effect was not driven by group differences (r=−0.52, p<0.005). This relationship remained when also controlling for baseline activity and pain response of the sensory region pI/S2 (r=−0.43, p<0.05, Fig. 2e) indicating that this modulation did not occur at an early stage of pain sensory processing.
In addition, the negative correlation between baseline activity and pain response was not found in pI/S2 (p>0.8). Also as predicted, activity in an anatomically defined amygdala ROI exhibited a similar pattern of anticipatory activity greater for novices than experts (t (26)=2.3, p<0.05, Fig. 3a, no laterality effect p>0.4). We also performed a voxel-wise analysis of anticipatory activity as defined above, which found activity stronger for the novices than the experts in the left insula (Fig. 2a, green clusters, see Table 2). Together, these findings indicate that baseline activity in the salience network before pain negatively predicts its reactivity to pain, and this activity was greater for novices than experts. Despite these group differences, additional voxel-wise analysis showed that experts did have anticipatory neural activity in several overlapping brain regions (data not presented here) suggesting that OP modulated as opposed to suppressed neural processes that increased prior to pain delivery.
Figure 3. Expertise in OP impacts amygdala activity.
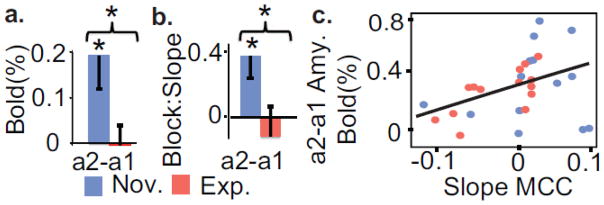
a. Experts showed less anticipatory activity before pain than novices in an anatomically-defined amygdala cluster (t (13)=2.6, p<0.05). b. The linear temporal slope across blocks was lower for experts than novices in amygdala during anticipation. This indicates a gradual increase over time of anticipatory activity for novices only (t (13)=2.2, p<0.05). c. The anticipatory activity in the amygdala was positively correlated to the temporal slope across blocks in MCC (r=0.45, p< 0.05, for experts only r= 0.68, p<0.01 and for novices only r=0.14, p=0.6). * indicates p<0.05.
Table 2. Brain regions showing expertise-related effect prior to pain.
Brain regions showing a group difference in anticipatory neural activity, voxel-wise T-test, corrected. A t-value of 3.1 corresponds to p<0.005 and a t-value of 4.0 corresponds to p<0.0005. Spatial coordinates are in Talairach space.
| Brain Regions | CM (X, Y, Z) | Peak (X, Y, Z) | t-value | Voxels # | |
|---|---|---|---|---|---|
| 1 | L. STG/pIns | (−50 0 −4) | −53 −1 −6) | −4.5 | 1102 |
| L. IFG/aIns | −41 13 −8) | −3.8 | |||
| 2 | R. ACC (BA25) | (1 16 −10) | (5 17 − 10) | −4.0 | 431 |
| L. ACC (BA25) | −9 15− 10) | −3.8 | |||
| 3 | R. MTG | (56− 50 8) | (47− 55 8) | −3.7 | 292 |
| R. STG | (65− 45 10) | −3.6 | |||
| 4 | L. STG | −48 −47 16) | −45 −47 16) | −4.1 | 214 |
Abbreviations: L: left, R: Right, IFG: inferior frontal gyrus, MTG: middle temporal gyrus, STG: superior temporal gyrus, Ins: Insula. The negative t-values below were produced by a stronger anticipatory neural activity for the novices compared to experts.
We then examined whether these baseline patterns before pain were correlated with pain ratings. Baseline activity in these ROIs (aI, aMCC, pI/S2, and amygdala) were not correlated with unpleasantness ratings (all p>0.17, with or without controlling for intensity), but single trial variability in unpleasantness rating across these practices, when modeled in the GLM as a regressor, was positively related with single trial variability in amygdala activity for both groups (t (27)=2.7, p<05). The amount of meditation practice in life was also negatively correlated with baseline activity in left aI (r=−0.63, p<0.05, Fig. 2f) when the contribution of age was regressed out (age showed some association with hours of meditation in life, r=0.41, p=0.15; without controlling for age, r=−0.60, p<0.05 in aI). Together this indicates that several brain regions previously linked to pain anticipation were activated before the pain stimulation and this baseline activity was negatively correlated with the amount of meditation experience in life in the left aI.
Effect of meditation expertise on neural habituation
To test our third hypothesis of faster neural habituation to pain and its anticipation in the amygdala and pain-related regions, we explicitly modeled block order in the general linear model-based whole-brain analysis. We defined the regressor for block order so that a negative coefficient corresponded to a negative linear temporal slope across experimental blocks, which was used here as an operational definition of neural habituation. In line with our prediction, we found that before pain, the slope was more negative for experts than novices in amygdala (Fig. 3b) and in the right pI/S2, right mid-insula (mI) and MCC subregions of the pain-related regions (Fig. 4, see Material and methods). This effect was driven by a positive slope for all these clusters for the novices indicating a gradual increase over time of activity during anticipation (t(26)=−2.3, p< 0.05 in Amyg., Fig. 3b and t(26)<−4, p<0.001 in all clusters in Fig. 4b). Also in line with our prediction, we found that during pain processing, the slope was lower for experts than novices in pI/S2 and mI, and this effect was driven by a negative slope for experts only indicating a gradual decrease over time of activity during pain (p<0.05) (Fig. 4c). Figure 4a displays the spatial distribution of these temporal slopes before pain and during pain, as obtained by a similar voxel-wise complementary analysis (Fig. 4, and Supplementary Tables S2–3). Behavioral habituation (see Material and methods) was significantly positive for both groups, suggesting increased reduction in unpleasantness normalized for intensity across blocks for both groups (t (25)=2.5, p<0.05).
Figure 4. Expertise in OP, and anticipatory activity, modulate neural habituation.
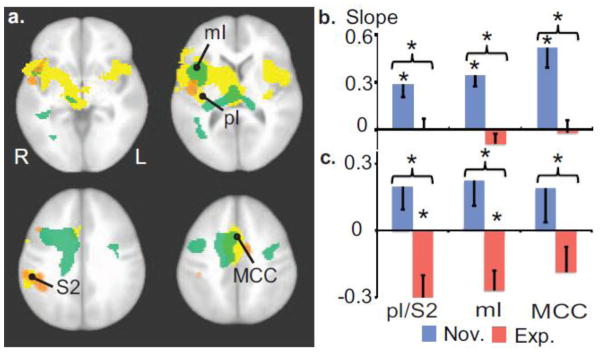
a. Voxel-wise t-tests between groups on the linear temporal slope across blocks of the anticipatory activity (green clusters) and activity during pain (orange clusters; both corrected, p<0.005). These maps are overlaid on the pain-related regions as in Figure 2. b. During anticipation, the slope was lower for experts than novices in the right pI/S2, right mid-insula (mI) and MCC, ROIs defined by the intersection of the functionally-derived pain-related regions and anatomical templates of these named regions. This effect was driven by a positive slope for all these clusters for the novices indicating a gradual increase over time of activity during anticipation (t (26)<-4, p<0.001 in all clusters). c. During pain processing, the slope was lower for experts than novices in pI/S2 and mI. This effect was driven by a negative slope for the experts indicating a gradual decrease over time of activity during pain. Error bars are SEM. * indicates p<0.05.
In an exploratory analysis, we found that behavioral habituation was positively correlated with the magnitude of neural habituation in MCC during anticipation across groups (partial correlation controlling for Group factor, r=0.40, p<0.05, r=0.6, p<0.05 for experts only, and r=0.29, p=0.3 for novices only). These effects were not driven by an accidental change in our experimental manipulation across blocks, because we did not detect any linear effect across blocks on pain intensity rating (p=0.26), nor a group difference in this linear effect (p=0.56). This finding indicates an association between behavioral and neural habituation in the anterior part of the pain-related regions.
As a post-hoc analysis, we explored the relationship between anxiety-related anticipatory processes in the amygdala and neural habituation during pain processing in the above four ROIs. In support of this possibility, we found that the baseline activity in the amygdala was positively correlated to the temporal slope across blocks in MCC (see Figure 3c for details). This indicates that the less baseline neural activity a participant had in the amygdala, a region previously linked to anxiety (Nitschke et al., 2009; Paulus and Stein, 2010; Sehlmeyer et al., 2011), the more rapidly the activity decreased over time in the MCC, a region important in the appraisal of pain.
DISCUSSION
Summary and interpretation of the findings
This study examined whether an Open Presence (OP) meditation practice, which cultivates a state of experiential openness, present-centeredness, and meta-awareness, modulates neural brain processes supporting acute pain, its anticipation, and the degree of habituation during the course of a pain experiment. The overall hypothesis was that OP meditation would affect the temporal representations of pain throughout the task, including increased phasic pain response (openness to the present), reduced baseline activity (representation of the future) and faster habituation (impact of the past) (Varela, 1999; Farb et al., 2007). There were three main findings in the study. Firstly, we found that expert practitioners gave lower unpleasantness ratings, and had stronger BOLD activity in two clusters of the salience network, left aI and aMCC, during pain compared to novices. Secondly, we found that experts had less anxiety-related baseline activity in these clusters and in the amygdala prior to pain, and that measure was predicted by hours of meditation in life in left aI. Finally, we found that experts had faster neural habituation to pain and its anticipation. The smaller the anticipatory activity in the amygdala, the faster the neural habituation in response to pain in MCC.
Our first finding replicated two recent studies that reported lower unpleasantness ratings and increased activity in aIns and aMCC during pain as a result of mindfulness experience or training (see Introduction). However, there remain inconsistencies in this growing literature. Whereas Grant et al. (Grant et al., 2011) and Gard et al. (Gard et al., 2011) reported enhanced activity during meditation in insula (consistent with our findings) and thalamus, Zeidan et al. (Zeidan et al., 2011) reported instead reduced activity in primary sensory regions. Here the mean activity was not different for experts than novices in pIns/S1–2. Our findings also did not replicate the role of OFC or dorsal PFC (Gard et al., 2011; Grant et al., 2011) in pain regulation during meditation (Zeidan et al., 2011) nor the lower activity in Amyg during pain for experts vs novices in (Grant et al., 2011). Some of these differences could merely reflect errors, as this field is young and still somewhat exploratory. Or, they may result from differences in the styles of meditation: for instance, some of these practices explicitly focus on an object whereas others are intended to sustain receptive, open awareness without any explicit object. In addition, the relative prominences of the various cognitive processes involved will also vary with the degrees of expertise. Finally, in contrast to Grant et al., our participants were explicitly instructed to engage in a formal meditation practice during the experiment. Future research will be needed to investigate the impact of these various factors on pain perception during meditation.
The second finding of reduced baseline neural activity prior pain is consistent with a previous study that showed lower anticipatory EEG activity in MCC during a laser-evoked pain paradigm for meditators compared to novices (Brown et al., 2008), and a negative correlation of anticipatory EEG activity with hours of practice in life. Another neuroimaging study reported decrease activity in bilateral aI, ACC and medial prefrontal cortex during a mindfulness practice, which was interpreted as a decreased in self-referential thoughts (Ives-Deliperi et al., 2011). Anticipatory activity in amygdala has been previously linked to anxiety (Nitschke et al., 2009; Sehlmeyer et al., 2011), so the reduction of this activity is also consistent with positive outcomes of mindfulness-based therapy on anxiety disorders (Hofmann et al., 2010). Our study extends this literature by showing that reduced baseline activity predicted the enhanced activity in the salience network during pain. This functional relationship between baseline and pain appraisal is important because it suggests a neural mechanism for the recent theoretical claims of a more adaptive role of openness rather than active control. The salience network plays a critical role in recruiting attentional resources at a given moment (Menon and Uddin, 2010). We propose that the phasic enhancement of this network during pain could relate to an enhanced capacity to flexibly modulate conditioned automatic reactions to an aversive event. This interpretation also potentially accounts for our novel finding of decoupling of unpleasantness ratings from salience network activity for experts. This view is compatible with novel neuroimaging finding on mindfulness (Hasenkamp et al., 2011; Hasenkamp and Barsalou, 2012) and with recent models implicating the salience network not only in affective processes but also in cognitive control (Shackman et al., 2011), subjective awareness (Craig, 2009, 2010), conscious presence (Seth et al., 2011), and the representation of current and predictive feeling states (Singer et al., 2009). The contrast with previous studies of cognitive modulation of pain perception that have reported reduced activity in these regions during pain under placebo analgesia (Wager et al., 2004), hypnosis (Rainville et al., 1997), or active distraction (see (Wiech et al., 2008b; Tracey, 2010) for reviews) could represent the aforementioned distinction between canonical strategies of active control, and strategies of openness and experiential acceptance (Kabat-Zinn, 1982; Teasdale et al., 2000; Hayes, 2004; Bishop et al., 2006; Lutz et al., 2008; Chambers et al., 2009).
Our third finding on neural habituation further extends the support for this framework: OP increases neural habituation to pain and its anticipation for experts, which would be predicted if, as proposed above, OP enhances the capacity to flexibly modulate conditioned automatic reactions to an aversive event. This flexible modulation might reasonably be expected to lead to a reduction over time in response to a harmless aversive stimulus. The relationship between anticipation and neural habituation was also explicitly confirmed by our finding that the reduced anticipatory activity in the amygdala predicted faster neural habituation in brain regions activated during pain. Overall this finding indicates that the magnitude of pain perception and anticipation processes influenced the degree of habituation toward future pain.
A possible objection to this framework is that ordinary anticipatory and conditioning processes are usually adaptive, so suppressing them would be maladaptive. However, the framework does not suggest completely suppressing adaptive anticipatory processes, but rather avoiding over-reacting to pain and other aversive stimuli in a way that overall would be maladaptive. In support of this, we found, using a conjunction analysis, overlapping brain regions showing anticipatory activity for both groups (See Fig. S2) indicating that expertise in OP does not lead to complete suppression of these anticipatory processes.
CONCLUSIONS
Implication of this study for mindfulness-based psychotherapeutic interventions
In this report we chose to make a conceptual distinction between OP meditation, a so called “nondual” meditation, and Open Monitoring meditation (for details see (Lutz et al., 2006; Dunne, 2011)). This choice is meant to acknowledge the complexity and length of the meditation training in traditional settings. The OP form of mental training featured in this study is likely to induce different effects than a short 8-week meditation-based therapy, not withstanding possible overlaps between the techniques and meditation instructions. Despite this distinction, we are predicting from the current study that Open Monitoring-based interventions akin to Mindfulness-Based Stress Reduction (Kabat-Zinn, 1982) should also lead to a modulation of some of the elaborative processes that account for pain anticipation, pain habituation, and emotional reactivity during pain (Brown and Jones, 2010; Grant et al., 2011; Zeidan et al., 2011). Yet, we also anticipate that the capacity to regulate emotional reactivity and habituation will be more enhanced during OP meditation, as a result of the capacity of this state to further suspend subtle implicit elaborative processes associated with subjectivity itself (Dor-Je, 1981; Namgyal et al., 2004; Lutz et al., 2006; Dunne, 2011). This distinction needs future empirical validation.
Limitations
We found a correlation between baseline activity in dorsal anterior insula and its response activity during pain. We controlled that this correlation did not trivially resulted from the modeling of the global baseline of the GLM by controlling for baseline activity and pain response of the sensory region pI/S2. Yet it is possible that a ceiling effect of regional hemodynamic activation at baseline could decrease the reactivity to the stimulus during pain, accounting for some of this correlation. Notable limitations in our design came from the lack of experimental manipulation of pain anticipatory processes (for a recent example see (Atlas et al., 2010)). Our interpretation of the baseline activity prior to pain thus relied on reverse inference, and correlation with pain ratings and the amount of meditation practice in life. Yet, these novel data on a rare sample of meditation experts could help generating new hypothesis about the neural regulatory mechanisms of meditation. Another limitations came from the possibility of differential demand characteristics between groups and our cross-sectional design. In particular, the experts likely have greater motivation to demonstrate effectiveness of the meditation practices due to their long-term personal investment in them. To provide some motivation for the novices, we offered a bonus of $50 to the four novices who demonstrated the largest reduction in pain induced brain activity during meditation, but we acknowledge that this is unlikely to match the motivation of lifelong practitioners. We also performed a supplementary analysis (Supplementary Analysis S1) that indicated that among the experts, pain perception was affected more by OP than by another familiar meditation practice, supporting the interpretation of the present findings as specific to OP, which further reduces the possibility that they reflect demand characteristics. Because novices and experts differ in many respects other than simply the extent of meditative training, longitudinal research that follows individuals over time in response to meditation training will be needed to further substantiate our findings.
Future research
In this report, we investigated the impact of OP meditation on pain brain processes related to anticipation, pain and habituation. It is important to note that a central goal of this practice is to be more open and accepting not only toward one’s pain, but also toward the suffering of others. For that reason, Open Monitoring-like meditations are traditionally viewed as a necessary component of compassion training. Further research is needed to characterize the impact of these practices on self-other interaction and pro-social behaviors.
Supplementary Material
Highlights.
Compared to novices, expert meditators reported less unpleasantness during pain.
This difference was associated with enhanced activity in the salience network.
Baseline activation in pain-related region and amygdala is decreased before pain for experts only.
Habituation in pain-related is enhanced during pain stimulation for experts only.
Acknowledgments
We would like to acknowledge Dr. Matthieu Ricard for constant assistance with participant recruitment, written meditation instructions, and, with Dr. John D. Dunne and Cort Dahl, for extensive help with understanding the different meditation practices; and Liz Aeschlimann and Claire Wahmanholm for data collection. This work was supported by grants from the National Center for Complementary and Alternative Medicine (U01-AT002114 to A.L. and P01-AT004952 to A.L. and R.J.D.) and the National Institute of Mental Health (NIMH P50-MH069315 to R.J.D.), and gifts from Bryant Wangard and Ralph Robinson, Keith and Arlene Bronstein, the Mental Insight Foundation, and the John W. Kluge Foundation (to R.J.D.). David Perlman was supported by NIH training Grant T32-MH018931, R.J.D. director. Tim Salomons was supported by a Clinician-Scientist award from the University of Toronto Centre for the Study of Pain.
Abbreviations
- aI
anterior insula
- aMCC
anterior mid-cingulate
- FA
Focused Attention
- GLM
general linear model
- OP
Open Presence
- MBSR
mindfulness-based stress reduction
- ACT
acceptance and commitment therapy
Footnotes
Contributions: A.L, T.V.S. and R.J.D. designed the experiment, A.L. and D.M.P. collected the data, A.L. and D.R.M. analyzed the data and A.L. prepared the manuscript. All of the authors revised the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, et al. Mindfulness: A Proposed Operational Definition. Clinical Psychology: Science and Practice. 2006;11:230–241. [Google Scholar]
- Brown CA, Jones AKP. Meditation experience predicts less negative appraisal of pain: electrophysiological evidence for the involvement of anticipatory neural responses. Pain. 2010;150:428–438. doi: 10.1016/j.pain.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Brown CA, Seymour B, Boyle Y, El-Deredy W, Jones AKP. Modulation of pain ratings by expectation and uncertainty: Behavioral characteristics and anticipatory neural correlates. Pain. 2008;135:240–250. doi: 10.1016/j.pain.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gullone E, Allen NB. Mindful emotion regulation: An integrative review. Clin Psychol Rev. 2009;29:560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Cioffi D, Holloway J. Delayed costs of suppressed pain. Journal of Personality and Social Psychology. 1993;64:274–282. doi: 10.1037//0022-3514.64.2.274. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig ADB. How do you feel now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig ADB. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Dor-Je W-C. The Mahamudra Eliminating the Darkness of Ignorance (Library of Tibetan Works and Archives) 1981. [Google Scholar]
- Dunne J. Toward an understanding of non-dual mindfulness. Contemporary Buddhism. 2011;12:71–88. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, Bean J, Mckeon D, Fatima Z, Anderson AK. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebel U, Eickhoff SB, Lotze M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. Neuroimage. 2011;58:1070–1080. doi: 10.1016/j.neuroimage.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T, Hölzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, Ott U. Pain Attenuation through Mindfulness is Associated with Decreased Cognitive Control and Increased Sensory Processing in the Brain. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–156. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Gross J, Levenson R. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of post intervention and 3-year follow-up benefits in well-being. Psychother Psychosom. 2007;76:226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of Meditation Experience on Functional Connectivity of Distributed Brain Networks. Front Hum Neurosci. 2012:6. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hayes S. Acceptance and commitment therapy, relational frame theory, and the third wave of behavioral and cognitive therapies*. Behavior Therapy. 2004 doi: 10.1016/j.beth.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78:169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, Meintjes EM. The neural substrates of mindfulness: an fMRI investigation. Soc Neurosci. 2011;6:231–242. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lutz A, Dunne J, Davidson R. Meditation and the neuroscience of consciousness: An introduction. The Cambridge Handbook of Consciousness. 2006:19. [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ. BOLD signal in insula is differentially related to cardiac function during compassion meditation in experts vs. novices. Neuroimage. 2009;47:1038–1046. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci (Regul Ed) 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM. Learning to live with the pain: acceptance of pain predicts adjustment in persons with chronic pain. Pain. 1998;74:21–27. doi: 10.1016/S0304-3959(97)00146-2. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayedi M, Weissman-Fogel I. Is the insula the “how much” intensity coder? J Neurophysiol. 2009;102:1345–1347. doi: 10.1152/jn.00356.2009. [DOI] [PubMed] [Google Scholar]
- Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134:310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgyal DT, Kunsang EP, Rinpoche KT. Clarifying the Natural State: A Principal Guidance Manual for Mahamudra. North Atlantic Books; 2004. [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, Kalin NH. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman DM, Salomons TV, Davidson RJ, Lutz A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion. 2010;10:65–71. doi: 10.1037/a0018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Raecke R, Doganci B, Breimhorst M, Stankewitz A, Büchel C, Birklein F, May A. Insular cortex activity is associated with effects of negative expectation on nociceptive long-term habituation. J Neurosci. 2010;30:11363–11368. doi: 10.1523/JNEUROSCI.2197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24:7199–7203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Dannlowski U, Schöning S, Kugel H, Pyka M, Pfleiderer B, Zwitserlood P, Schiffbauer H, Heindel W, Arolt V, et al. Neural correlates of trait anxiety in fear extinction. Psychol Med. 2011;41:789–798. doi: 10.1017/S0033291710001248. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol. 2011;2:395. doi: 10.3389/fpsyg.2011.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci (Regul Ed) 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Third Dzogchen Rinpoche. Great Perfection, Volume II: Separation and Breakthrough. Ithaca, New York: Snow Lion Publication; 2008. [Google Scholar]
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- Varela F. Present-time consciousness. Journal of Consciousness Studies 1999 [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Solomon BC, Lehman DH, Liu L, Lang AJ, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011 doi: 10.1016/j.pain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Wiech K, Farias M, Kahane G, Shackel N, Tiede W, Tracey I. An fMRI study measuring analgesia enhanced by religion as a belief system. Pain. 2008a;139:467–476. doi: 10.1016/j.pain.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin C, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci (Regul Ed) 2008b;12:306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31:5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.