Abstract
Rationale
The synthetic progestin medroxyprogesterone acetate (MPA), widely used in hormone therapy (HT) and as the contraceptive Depo Provera, is implicated in detrimental cognitive effects in women. Recent evidence in aged ovariectomized (Ovx) rodents shows that short-term MPA treatment impairs cognition and alters the GABAergic system.
Objectives
Using rats, we evaluated the long-lasting cognitive and GABAergic effects of MPA administered in young adulthood (Early-MPA), modeling contraception, and how this early exposure interacts with later MPA treatment (Late-MPA), modeling HT.
Methods
Early-MPA treatment involved weekly anti-ovulatory MPA injections (3.5 mg) from 4 to 8 months of age in ovary-intact rats. At 10 months old, rats were Ovx and weekly MPA injections were re-initiated and continued throughout testing for Late-MPA treatment.
Results
On the water radial-arm maze, all MPA-treated groups showed working memory impairment compared to Controls (p<0.05); Early+Late-MPA rats were impaired on multiple dimensions of working memory (p<0.05). On the Morris maze, Late-MPA rats showed greater overnight forgetting compared to Controls (p<0.05). At study conclusion, MPA was detected in serum in all MPA-treated groups except Early-MPA, confirming treatment and clearance from serum in Early-MPA rats. In animals with detectable serum MPA, higher MPA levels were associated with less dorsal-hippocampal glutamic acid decarboxylase, the synthesizing enzyme for GABA (p=0.0059).
Conclusions
Findings suggest that MPA treatment leads to long-lasting cognitive impairments in the rodent, even in the absence of circulating MPA in animals given prior MPA treatment, which may relate to the GABAergic system. Further research defining the parameters of the negative impact of this widely used progestin on brain and cognition is warranted.
Keywords: Hormone therapy, Contraceptive, Cognition, Progestins, Menopause, Aging, Learning and memory
Introduction
Nearly every woman will face the decision of whether to take exogenous hormones, either for contraception or for hormone therapy (HT). Medroxyprogesterone acetate (MPA), a synthetic progestin, is the sole hormone component of the contraceptive Depo Provera and the most commonly prescribed progestin component of HT (Prempro and Premphase—MPA+conjugated equine estrogen). Depo Provera was approved by the Food and Drug Administration in 1992 (Bakry et al. 2008) and has been prescribed to over 10 million women in the USA since this time (Mosher et al. 2004). This widely prescribed contraceptive is often the choice for women who cannot take estrogen-containing contraceptives because of medical conditions (Spencer et al. 2009), for nursing mothers (Rodriguez and Kaunitz 2009), and for those who prefer the convenience of an intermittent injection once every 3 months over the daily pill. Prempro has been prescribed since 1995 (Stefanick 2005) with as many as 19.7 million prescriptions written per year (Hersh et al. 2004). At menopause, many women choose to take HT to attenuate menopause-induced symptoms, including memory decline (Freedman 2002). Women with a uterus must include a progestin in their HT regimen to offset the increased risk of endometrial hyperplasia related to unopposed estrogen treatment (Smith et al. 1975; Ziel and Finkle 1975). While there have been no methodical clinical evaluations of the cognitive effects of MPA alone in any population of women, there is evidence implicating that MPA is detrimental to cognition. For Depo Provera, there is a documented case study of amnestic effects corresponding with its use (Gabriel and Fahim 2005). Moreover, the large, placebo-controlled, randomized Women’s Health Initiative Memory Study (WHIMS) reported that twice as many women receiving Prempro were diagnosed with dementia as compared to the placebo group, a significant finding (Shumaker et al. 2003), as opposed to those women taking Premarin (conjugated equine estrogen only), who showed a non-significant increase in risk of dementia compared to those taking placebo (p=0.18) (Shumaker et al. 2004).
Recently, we showed that MPA impaired learning and memory in aged ovariectomized (Ovx) rodents (Braden et al. 2010). In that study, we also replicated previous findings (Bimonte-Nelson et al. 2004) that natural progesterone impairs cognition in aged Ovx rodents. Others have shown that infusions of the progesterone metabolite, allopregnanolone, into the lateral ventricles of young Ovx rats can lead to impaired spatial reference and working memory performance (Frye and Sturgis 1995). Progesterone and allopregnanolone have also been shown to impair working and reference memory, respectively, in young intact male and female rats (Johansson et al. 2002; Sun et al. 2010). However, the literature on progesterone and allopregnanolone cognitive effects in young rodents reports mixed results, with task-specific benefits noted sometimes, but not always (Frye et al. 2007, 2010; Frye and Walf 2008; Harburger et al. 2008; Lewis et al. 2008; Orr et al. 2009). The animal study findings of progestin-induced cognitive impairments are corroborated by clinical reports of the detrimental effects of progesterone and allopregnanolone on memory in women (Freeman et al. 1992; Kask et al. 2008). Yet, progesterone can enhance other cognitive processes such as attention, response/processing speed, cognitive flexibility, and visuomotor coordination (Sofuoglu et al. 2011). These clinical findings highlight the need for more scientific investigations, both clinical and preclinical, of the impact progestins could have on cognition. A progestin is included in every contraceptive, and in HT when given to women with a uterus; thus, safe progestin use is a critical medical issue affecting every woman who chooses to take exogenous hormones.
MPA’s cognitive-impairing effects may be mediated via the GABAergic system. Indeed, we have shown that both progesterone and MPA alter the GABAergic system in cognitive brain regions of behaviorally tested aged Ovx animals that showed cognitive impairments; in this study, MPA decreased glutamic acid decarboxylase (GAD), the synthesizing enzyme for GABA, in the dorsal hippocampus and increased GAD in the entorhinal cortex (Braden et al. 2010). MPA has also been shown to enhance synaptic and extrasynaptic GABAA receptor-mediated inhibition (Belelli and Herd 2003). Further, in vitro evidence implicates that MPA has a negative effect on neuronal function by exacerbating glutamate-induced excitotoxicity (Nilsen et al. 2006). Taken together, in vitro, preclinical, and clinical evidence of MPA’s negative effects on the brain and its function are particularly salient. Moreover, there have been no evaluations of the possible long-term cognitive effects of MPA administration or interactions with subsequent MPA use as HT, nor have there been assessments regarding whether there are long-term or interactive impacts on the GABAergic system in vivo. These issues are especially relevant as we embark on a new generation of menopausal women who were much more likely to have been prescribed contraceptives, as compared to generations before (Diczfalusy 1991). We hypothesize that MPA treatment during young adulthood and/or middle age impairs cognition in the rat model by enhancing GABAA receptor-mediated inhibition in the hippocampus, as evident by a compensatory decrease in GAD. Thus, the current study used the rodent model to investigate the long-lasting cognitive and GABAergic effects of MPA treatment given in young adulthood (to model contraception) and tested in middle age, as well as whether MPA treatment in young adulthood interacts with MPA treatment in middle age (to model HT).
Materials and methods
Subjects
Subjects were 44 Fischer-344 female rats born and reared at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN, USA). Rats were 4 months old at the beginning of the study, had unrestricted access to food and water, and were maintained on a 12-h light/dark cycle at the Arizona State University animal facility. All procedures were in accordance with the local IACUC committee and follow NIH standards.
Hormone treatment and ovariectomy
Rats were randomly assigned to one of four treatment groups: Control, Early-MPA, Late-MPA, and Early+Late-MPA. The study was divided into two phases of injections (Fig. 1). Phase 1 of weekly injections of either vehicle [0.4 ml sesame oil+0.02 ml dimethyl sulfoxide (DMSO), both from Sigma-Aldrich, St. Louis, MO, USA] or MPA (3.5 mg, Sigma-Aldrich) dissolved in 0.4 ml sesame oil+ 0.02 ml DMSO began at four months of age and continued until eight months of age. This regimen of MPA treatment was selected based on previously established anti-ovulatory effects (Bhowmik and Mukherjea 1988) in order to mimic the biological action of Depo Provera taken by women (Depo Provera Prescribing Information 2006). We chose to keep animals ovary-intact to most closely model the hormone milieu of fertile women prescribed Depo Provera.
Fig. 1.
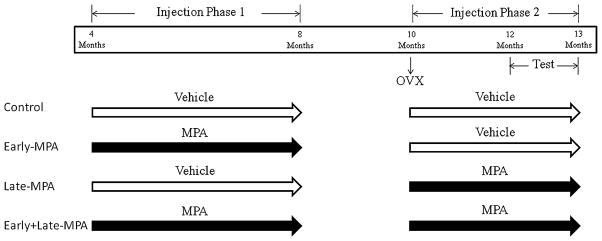
A time line summarizing injections, ovariectomy, and behavioral testing for each group
At 10 months of age, all rats received Ovx surgery. Rats were anesthetized via isofluorane inhalation, bilateral dorso-lateral incisions were made in the skin and peritoneum, and the ovaries and tips of uterine horns were ligatured and removed. Muscle and skin were sutured. At time of surgery, rats received a single injection of Rimadyl (carprofen; 5 mg/ml/kg) for pain and saline (2 ml) to prevent dehydration. Phase 2 of weekly injections (either vehicle or MPA) began two-three days after Ovx surgery and continued throughout behavior testing until sacrifice. Testing began at 12 months of age, approximately two months after the beginning of phase 2 injections.
Animals in the Control (n=11) group received vehicle injections during both injection phases. Early-MPA (n=12) animals received MPA injections during phase 1 and vehicle injections during phase 2, Late-MPA (n=9) animals received vehicle injections during phase 1 and MPA injections during phase 2, and Early+Late-MPA (n=12) animals received MPA injections during both phases (Fig. 1). During both injection phases, body weights were recorded weekly.
Vaginal smears and uterine weights
To assess the long-term effects of four months of MPA injections on ovulation, vaginal smears were taken at eight months of age for three days in the behaviorally tested animals, starting nine days after the last injection (Fig. 1). Smears were classified as proestrus, estrous, metestrus, or diestrous per prior protocol (Goldman et al. 2007; Acosta et al. 2009). At sacrifice, the uteri of all subjects were removed, trimmed of visible fat, and immediately weighed (wet weight) in order to examine drug effects on uterine tissues (Braden et al. 2010).
Water radial-arm maze
Two months after initiation of phase 2 of injections, subjects began 11 days of testing on the eight-arm win-shift water radial-arm maze (WRAM) to evaluate spatial working and reference memory, including performance as working memory load increased, as described previously (e.g., Bimonte and Denenberg 1999). There were escape platforms hidden under the water surface in the ends of four of the eight arms. The temperature of the water was 18°C. Platform locations varied across subjects, but remained fixed for each individual subject, throughout the experiment. Once released from the start arm, a subject had 3 min to locate a platform. After a platform was found, the animal remained on it for 15 s and was then placed in its heated cage for a 30 s inter-trial interval (ITI) until its next trial. During the interval, the just-chosen platform was removed from the maze. This process was repeated until each platform was found. This totaled to four trials in each animal’s daily session, with the number of platformed arms reduced by one on each subsequent trial. The working memory system was increasingly challenged as trials progressed, allowing assessment of working memory load. Each subject was given one session a day for 11 consecutive days.
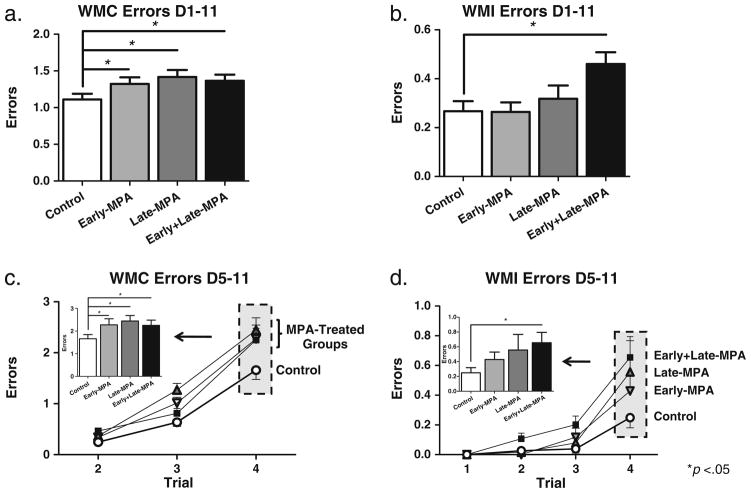
Quantification and blocking into acquisition and asymptotic phases were based on prior studies (e.g., Bimonte and Denenberg 1999). An arm entry was counted when the tip of a rat’s snout reached a mark 11 cm into the arm. Orthogonal measures of working and reference memory errors were quantified as done previously in WRAM studies (Bimonte et al. 2000; Braden et al. 2010). Working memory correct (WMC) errors were the number of first and repeat entries into an arm that previously contained a platform during that session. Reference memory (RM) errors were the number of first entries into an arm that never contained a platform. Working memory incorrect (WMI) errors were repeat entries into a reference memory arm. Errors were analyzed across all days (1–11) of testing and on the latter portion of regular testing at the highest working memory load, as this has revealed progesterone-and MPA-induced impairments (Bimonte-Nelson et al. 2003; Bimonte-Nelson et al. 2004; Braden et al. 2010).
Morris maze
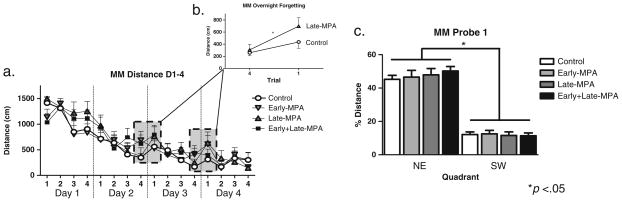
Two days after WRAM completion, subjects were tested on the Morris maze (MM) for four trials per day for four days to test spatial reference memory. For this task (Morris et al. 1982; Bimonte-Nelson et al. 2006), a tub (188-cm diameter) filled with black water made opaque using non-toxic paint was used, with a hidden platform (10-cm wide) remaining in a fixed location. The temperature of the water was 18°C. During initial testing (days 1–4), the rat was started at the north, south, east, or west location in the maze, and had 60 s to locate the hidden platform in the northeast (NE) quadrant. The trial was terminated once the rat found the platform. After 15 s on the platform, the rat was placed into its heated cage until its next trial. The ITI was approximately 10 min. Swim path distance (cm) and latency (sec) to the platform across all days of initial testing (days 1–4) were used to asses MM performance. Distance scores from the last trial of each day (Trial 6) were compared to the first trial on the following day (Trial 1) in order to test overnight forgetting of the platform location (Markham et al. 2002; Bimonte-Nelson et al. 2006), as this measure has revealed MPA-induced impairments (Braden et al. 2010). The first overnight interval was omitted from this analysis, as we noted that all animals were extending their learning curve without forgetting during this time interval (Fig. 3a). In order to determine which treatment groups increased their swim distance across the overnight interval, thus showing overnight forgetting of the platform location, we examined within-group trial comparisons of the overnight interval (Trial 6 to Trial 1).
Fig. 3.
Mean distance scores in centimeters (±SE) on Morris maze Initial Testing days 1–4 by treatment group [Control (white circle); Early-MPA (down-pointing gray triangle); Late-MPA (gray triangle); Early+Late-MPA (black square)]. a Distance scores across all days and trials of initial testing (days 1–4). b Distance scores across the overnight interval. c Percent distance in the target NE quadrant as compared to the opposite SW quadrant during the probe trial day 4. *p<0.05
After all test trials on day 4, a 60-s probe trial was initiated whereby the platform was removed. This was used to evaluate whether rats localized the platform to the spatial location. Percent of total distance in the previously platformed (target) quadrant was compared to the quadrant diagonally opposite the platform. Rats that spent the greatest percent distance in the target quadrant were interpreted as localizing the platform to the spatial location. To attenuate the possibility of extinction due to the probe trial, rats were placed back into the maze immediately following the probe trial with the platform replaced in the NE quadrant. Next, to test relearning of a novel platform location, on day 5 the platform was switched from the NE quadrant to the southwest (SW) quadrant, and rats were given eight trials with the new platform location. Distance and latency were analyzed across all trials for the platform switch (day 5). To evaluate whether rats localized the new platform to the spatial location, after the eighth trial, a 60-s probe trial was given whereby the platform was again removed. For each trial, a rat’s path was recorded from a camera suspended above the maze, and a tracking system (Ethovision 3.1; Noldus Instruments) analyzed each rat’s tracing.
Visible platform maze
To confirm that all subjects had intact vision and could perform the procedural task components of MM and WRAM spatial navigation without difficulty, subjects were tested on a visible platform water-escape task, as previously described (Braden et al. 2010). Briefly, animals were placed into a rectangular tub (39×23 in.) where they had to locate a flagged platform protruding from the water. The temperature of the water was 18°C. Extramaze cues were covered by opaque curtains. Animals were given six trials, with the drop-off location the same across trials, and the platform location for each trial varied in space semi-randomly. Performance was assessed by latency (sec) to the platform across all trials.
Brain dissections
One day after the conclusion of behavior testing, animals were anesthetized with isofluorane and brains were rapidly dissected and frozen. Dissected tissues were stored at −70°C in pre-weighed microcentrifuge tubes until analysis. Plate designations (Paxinos and Watson 1998) for dissections were as follows: frontal cortex (plates 5–14), anterior cingulate cortex (plates 5–14), posterior cingulate cortex (plates 15–35), entorhinal cortex (plates 39–42), and the CA1/CA2 region of the dorsal (plates 33–35) and ventral (plates 39–42) hippocampus. For each brain, the frontal cortex was taken first from the dorsal aspect of the intact brain. The medial border of the frontal cortex cut served as the lateral border for the cingulate cortex dissection, with the longitudinal fissure as the medial border. Designation between anterior and posterior cingulate cortex was made according to Paxinos and Watson (1998) plate 14. Next, the brain was cut in the coronal plane at plate 33 to obtain access to the dorsal CA1/CA2 region of the hippocampus. Finally, the brain was cut again in the coronal plane at plate 39 to obtain access to the last two brain regions (the hippocampus and entorhinal cortex). It was here that the ventral CA1/CA2 region of the hippocampus was taken. For the hippocampus, the dentate gyrus and the alveus were excluded. For the entorhinal cortex, the tissue was dissected from the same slice as the ventral hippocampus sample, taking a 2- to 3- mm sample ventral to the hippocampus.
Western blot analyses of GAD 65+67
GAD 65+67 protein expression levels were analyzed in frontal, anterior cingulate, posterior cingulate and entorhinal cortices, and the dorsal and ventral hippocampus, from the right hemisphere. Each sample was sonicated in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Na deoxycholate, 50 mM Tris) equivalent to 10 times its weight and centrifuged at 10,000 rpm for 10 m at 4°C. BCA protein assays determined protein concentration (ThermoFisher Scientific, Pittsburgh, PA, USA) and 10 μg of protein from each sample were run on a NuPAGE Bis–Tris gel, using the SureLock mini-cell (Invitrogen, Carlsbad, CA, USA). PVDF membranes were used for transfer (Millipore, Bedford, MA, USA) and immunoblotting was performed with working dilutions of a rabbit anti-GAD 65+67 (ab11070) and rabbit anti-beta Actin (ab25894 or ab8227) primary antibodies (Abcam Inc., Cambridge, MA, USA). Antibody dilutions were 1:10,000 for GAD 65+67 and a range of 1:400–1:20,000 for beta Actin primary antibodies. Membranes were then incubated with a peroxidase-conjugated goat anti-rabbit secondary antibody, 1:10,000 dilution (111-035-003; Jackson Immuno Research, West Grove, PA, USA) and visualized using Pierce ECL Western Blotting Substrate (ThermoScientific, Rockford, IL, USA) in a film developer. Precision Plus Protein WesternC Standards (Bio Rad, Hercules, CA, USA) were used to identify bands as the protein of interest, based on molecular weight. ImageJ software (Rasband 1997–2004) was used to quantify the density of GAD 65+67 and beta Actin (loading control protein). The dependent measure was a ratio of each subject’s GAD 65+ 67 density to their beta Actin density, brought to percent control of Control subjects run on the same gel, per prior protocol (Pandey et al. 1999; Braden et al. 2010).
Blood serum analyses for MPA, progesterone, and allopregnanolone
Serum MPA determinations were performed according to the method reported earlier (Braden et al. 2010). In addition, the assay was extended to measure progesterone and allopregnanolone, similar to an earlier procedure (Prokai et al. 2008). Briefly, levels were measured by the isotope dilution method through the addition of deuterium-labeled internal standards d9-progesterone and d4-allopregnanolone (C/D/N Isotopes, Pointe-Claire, Quebec, Canada); 10 μl each from a 1 μg/ml solution in ethanol to 1 ml of serum. Progesterone, d9-progesterone, allopregnanolone, and d4- allopregnanolone were measured through selected-reaction monitoring (SRM) signals of m/z 315→297, 324→306, 301→287, and 305→287, respectively, obtained by liquid chromatography–atmospheric-pressure chemical ionization tandem mass spectrometry (LC–APCI-MS/MS). Progester-one and allopregnanolone concentrations were calculated by dividing the areas under their corresponding SRM peaks in the LC–APCI-MS/MS chromatograms with those of d9-progesterone and d4-allopregnanolone, respectively, multiplied with the known concentrations (10 ng/ml each) of these added internal standards. Calibrations from the analysis of serum samples spiked with 1, 2, 5, and 10 ng/ml of analyte, respectively, were used to determine MPA concentrations. Additionally, d9-progesterone was used as an internal reference to compensate for slight changes in extraction efficiency and LC–APCI-MS/MS conditions among samples. The dependent measures of the assay were ng/ml of MPA, progesterone, and allopregnanolone in serum, respectively.
Statistical analyses
For behavior assessments, data were analyzed separately for each maze. Since our interest was to evaluate Treatment effects regarding timing of MPA administration, two-group planned comparisons were run on specific measures. To evaluate potentially complex higher order Treatment interactions with Days or Trials, we utilized an omnibus repeated measures ANOVA including all groups, with Treatment as the between variable and Days and/or Trials as the within variable/s. For GAD protein levels, serum levels of MPA, progesterone, and allopregnanolone, uterine weights, and body weights, data were assessed via a priori planned comparisons using t tests. For all of these analyses, alpha was two-tailed and set at 0.05, with two exceptions where analyses were one-tailed: for the Trial 4 WMC effects for the latter portion of testing, as we have previously established that MPA impairs performance on this measure (Braden et al. 2010), and uterine weights comparisons (Control vs. Late-MPA and Control vs. Early+ Late-MPA), as we have previously shown that MPA administration after Ovx increases uterine weight (Braden et al. 2010). For all planned comparisons, it was noted that type I error correction is not necessary (Keppel and Wickens 2004).
Correlations
Pearson r correlations were run between GAD levels in each brain region and (1) serum MPA levels, (2) serum progesterone levels, (3) serum allopregnanolone levels, and (4) memory scores from each of the mazes tested. Additionally, correlations were run between each serum hormone and memory scores. The memory scores used for correlations were, for WRAM: WMC, WMI, and RM errors across all days of regular testing; for MM: distance across all days of testing, and overnight forgetting on the last two overnight intervals. Alpha was adjusted to 0.01 to account for multiple correlations.
Results
Table 1 summarizes the significant behavior effects.
Table 1.
Summary of significant behavior effects
| Treatment | WRAM
|
MM
|
|||
|---|---|---|---|---|---|
| WMC D1-11 | WMC D5-11 Trial 4 | WMI D1-11 | WMI D5-11 Trial 4 | Overnight forgetting | |
| Early-MPA | Impaired | Impaired | No significant effect | No significant effect | No significant effect |
| Late-MPA | Impaired | Impaired | No significant effect | No significant effect | Impaired |
| Early+Late-MPA | Impaired | Impaired | Impaired | Impaired | No significant effect |
Water radial-arm maze
Two-group planned comparisons revealed that animals treated with MPA at any time point made more WMC errors than Control animals, across all days of testing [Control vs. Early-MPA—t(20)=2.17; p<0.05; Control vs. Late-MPA—t(18)=2.817; p<0.05; Control vs. Early+Late-MPA—t(21)=2.285; p<0.05] (Fig. 2a). For WMI errors, only animals that received MPA at both time points (Early+ Late-MPA) made more errors than Control animals [t(21)= 2.431; p<0.05] (Fig. 2b). There were no differences between Control and Early-MPA, or Control and Late-MPA, groups for WMI errors, and no Treatment group differences for RM. Across all testing days (days 1–11), there was a Day main effect for each error type for the omnibus ANOVA [WMC—F(10, 39)=5.627; p<0.0001; WMI—F(10, 39)= 13.009; p<0.0001; RM—F(10, 39)=10.288; p<0.0001], with errors decreasing across days demonstrating learning. There was no Day×Treatment interaction for any analysis.
Fig. 2.
Mean error scores (±SE) on the water radial-arm maze by treatment group [Control (white circle); Early-MPA (gray down-pointing triangle); Late-MPA (gray triangle); Early+Late-MPA (black square)]. a WMC errors across all days of testing. b WMI errors across all days of testing. c WMC errors across all trials, and at the highest working memory load (inset graph) during the latter portion of testing (days 5–11). d WMI errors across all trials, and at the highest working memory load (inset graph) during the latter portion of testing (days 5–11). *p<0.05
During the asymptotic phase of testing (days 5–11), we investigated group differences at the highest working memory load, Trial 4, as we have previously shown memory load impairments at the end of testing after progesterone treatment (Bimonte-Nelson et al. 2004; Braden et al. 2010), and after MPA treatment given to Ovx animals in old age (Braden et al. 2010). For WMC errors on Trial 4, animals treated with MPA at any time point made more errors than Control animals [Control vs. Early-MPA—t(20)=1.973; p<0.05; Control vs. Late-MPA—t(18)=2.681; p<0.05; Control vs. Early+Late-MPA—t(21)=1.990; p<0.05] (Fig. 2c). For WMI errors, only animals that received MPA at both time points (Early+Late-MPA) made more errors on Trial 4 than Control animals [t(21)=2.270; p<0.05] (Fig. 2d). There were no differences between Control and Early-MPA, or Control and Late-MPA for Trial 4 WMI errors, and no Treatment group differences for Trial 4 RM errors.
Morris maze
Initial testing (days 1–4, platform in the NE quadrant)
Planned comparisons revealed no effect of MPA treatment on initial MM performance, as measured by Distance and Latency. There was a Day main effect for Distance and Latency for the omnibus ANOVA [Distance—F(3, 40)= 114.966; p<0.0001 (Fig. 3a); Latency—F(3, 40)=107.947; p<0.0001 (data not shown)], with scores decreasing across days, demonstrating learning of the task. There was no Day× Treatment interaction for initial MM testing (NE platform location) performance as measured by Distance or Latency.
As we have shown previously (Braden et al. 2010), animals who received MPA after Ovx (Late-MPA) showed overnight forgetting. Distance scores increased across the overnight interval in the Late-MPA group [t(8)=2.538; p< 0.05], while they remained stable across the overnight interval in the Control group (Fig. 3b). There were no significant increases across the overnight interval for Early-MPA or Early+Late-MPA animals.
The first probe trial, assessing learning of the initial platform location (NE quadrant), revealed a main effect of Quadrant [F(1, 40)=221.798; p<0.0001]. We confirmed that each group spent more percent distance in the target NE quadrant as compared to the opposite SW quadrant, showing localization of the platform location [Control—t(10)=9.931; p<0.0001; Early-MPA—t(11)=6.134; p<0.0001; Late-MPA —t(8)=6.202; p<0.0005; Early+Late-MPA—t(11)=9.628; p<0.0001 (Fig. 3c)].
Platform switch (day 5, platform in the SW quadrant)
For the MM testing where the platform was moved from the NE to the SW quadrant (platform switch), planned comparisons revealed no effect of MPA treatment on Distance or Latency. There was a Trial main effect for both Distance and Latency for the omnibus ANOVA [Distance—F(7, 40)= 20.789; p<0.0001 (Fig. 4a); Latency—F(7, 40)=30.257; p<0.0001 (data not shown)], with both decreasing across trials. It is noteworthy that, when comparing distance for the first eight trials of the original location to the distance for the eight trials of the platform switch, there was an Original vs. Platform Switch main effect [F(1, 40)=44.171; p<0.0001], and an Original vs. Platform Switch×Trial interaction [F(7, 280)=4.325; p<0.0001], for the omnibus ANOVA. Collapsed across all groups, animals learned the platform switch location at a much faster rate than the original platform location [Trial 2—F(1, 40)=55.572; p<0.0001; Trial 3—F(1, 40)=11.133; p<0.005; Trial 4—F(1, 40)= 28.501; p<0.0001; Trial 5—F(1, 40)=6.783; p<0.05; Trial 6—F(1, 40)=6.857; p<0.05; Trial 7—F(1, 40)= 4.607; p<0.05 (Fig. 4a)], demonstrating understanding of the rules of the task. There were no interactions with Treatment.
Fig. 4.
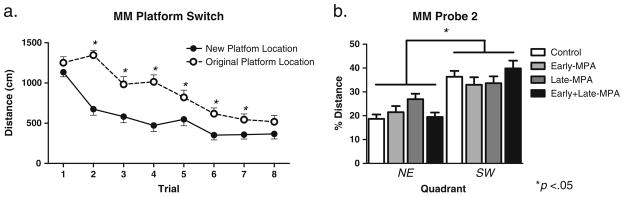
Mean distance scores in centimeters (±SE) on Morris maze Platform Switch testing day 5. a Distance across all eight trials by new (white circle) vs. original (black circle) platform location. b Percent distance in the target SW quadrant as compared to the opposite NE quadrant during the probe trial day 5 by treatment group [Control (white square); Early-MPA (gray square); Late-MPA (dark gray square); Early+Late-MPA (black square)]. *p<0.05
The second probe trial, assessing learning of the new platform location (SW quadrant), revealed a main effect of Quadrant [F(1, 40)=33.950; p<0.0001 (Fig. 4b)] with a preference for the SW quadrant, and no interactions with Treatment or Treatment main effect.
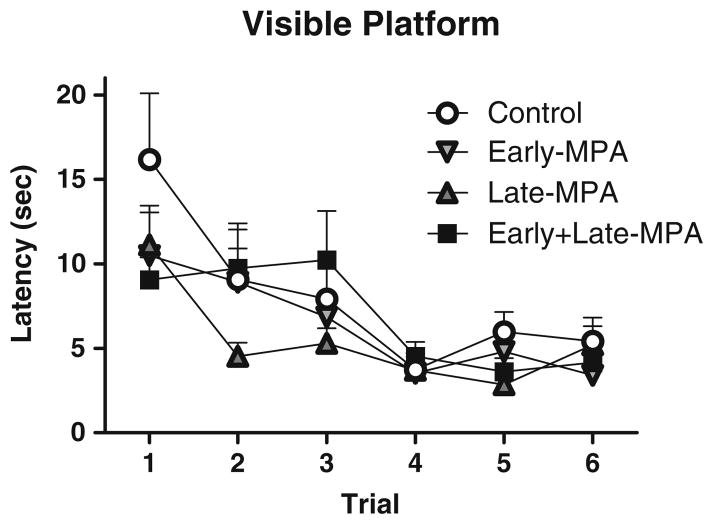
Visible platform
Planned comparisons revealed no Treatment effects for latency to the visible platform. There was a Trial main effect [F(5, 40)=13.259; p<0.0001(Fig. 5)], with latency decreasing across all trials, demonstrating learning of the task. By the fourth trial, all subjects found the visible platform within 6 s, confirming visual and motor competence to perform swim maze tasks for all groups.
Fig. 5.
Mean latency scores in seconds (±SE) on the visible platform maze by treatment group [Control (white circle); Early-MPA (down-pointing gray triangle); Late-MPA (gray triangle); Early+Late-MPA (black square)]
Blood serum levels of MPA, progesterone and allopregnanolone, vaginal smears, uterine weights, and body weights
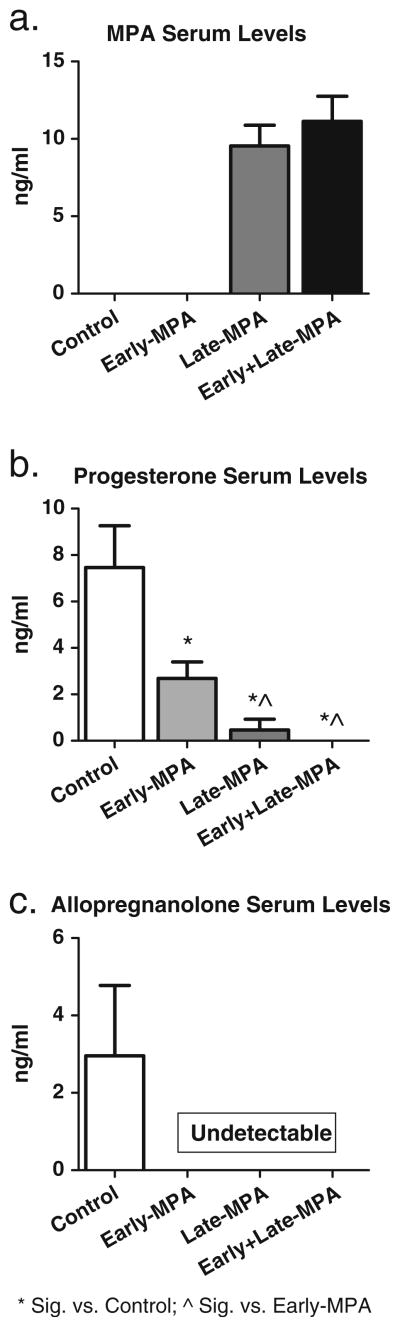
Groups with MPA administered in middle age were the only groups to show MPA concentrations in serum at sacrifice. Indeed, Late-MPA (mean=9.5 ng/ml) and Early+Late-MPA (mean=11.1 ng/ml)-treated groups demonstrated detectable MPA concentrations, and these groups did not significantly differ from each other. Control and Early-MPA animals had no detectable levels of MPA. This confirms presence of MPA in animals receiving the drug at sacrifice and successful clearing of the drug in animals that were not receiving MPA at time of sacrifice but had received it as earlier treatment (Fig. 6a). For progesterone, Control animals had higher levels than all MPA-treated groups [Control vs. Early-MPA—t(21)=2.553; p<0.05; Control vs. Late-MPA—t(18)=3.734; p<0.005; Control vs. Early+Late-MPA—t(21)=3.92; p<0.001] and animals that received MPA only during young adulthood (Early-MPA) had greater progesterone levels than animals treated with MPA during middle age [Early-MPA vs. Late-MPA—t(19)=3.288; p < 0.005; Early-MPA vs. Early + Late-MPA—t(22)= 2.642; p<0.05] (Fig. 6b). Only Control animals had detectable allopregnanolone levels (Fig. 6c). There were no significant correlations between serum hormone concentrations and memory scores.
Fig. 6.
Mean serum concentrations (±SE) by treatment group [Control (white square); Early-MPA (gray square); Late-MPA (dark gray square); Early+Late-MPA (black square)]. a MPA concentrations. b Progesterone concentrations. c Allopregnanolone concentrations. *p<0.05 vs. Control; ^p<0.05 vs. Early-MPA
Although it has previously been shown by Bhowmik and Mukherjea (1988) that weekly injections of 3.5 mg of MPA are sufficient to halt ovulation for at least 7 days, we confirmed this in a pilot study with animals, not included in behavioral testing, at 3 months of age and of the same strain as the behaviorally tested animals. Animals were given one injection of 3.5 mg MPA, and smears were then performed, classified as proestrus, estrous, metestrus, or diestrous per prior protocol (Goldman et al. 2007; Acosta et al. 2009). This one injection proved to be the minimum dose sufficient to inhibited ovulation for 9±2 days in these animals, as evidenced by continually diestrous smears, and no cornified estrous smears, which would indicate ovulation (Goldman et al. 2007). Smears were also taken at eight months of age for three days in the behaviorally tested animals, starting nine days after the last injection (Fig. 1). Eighty-eight percent of the MPA-treated animals (Early-MPA and Early+Late-MPA groups) showed the diestrous phase while 12% had begun cycling again; all animals given vehicle injections showed indication of cycling as evident by at least 1 day of cornified estrous smears.
Similar to our prior findings (Braden et al. 2010), animals treated with MPA during middle age had heavier uteri than Control animals [Control vs. Late-MPA—t(18)= 1.914; p<0.05; Control vs. Early+Late-MPA—t(21)= 1.787; p<0.05]. Control and Early-MPA uteri weights did not differ (Table 2).
Table 2.
Mean uterine and body weights in grams (±SE)
| Control | Early-MPA | Late-MPA | Early+Late-MPA | |
|---|---|---|---|---|
| Uterine weight (g) | 0.37±0.10 | 0.20±0.06 | 0.66±0.12* | 0.62±0.10* |
| Body weight (g) phase 1 | 189.8±0.9 | 215.4±1.2* | 191.6±0.1 | 209.3±1.4* |
| Body weight (g) phase 2 | 232.0±1.3 | 256.2±1.2* | 233.2±1.6 | 243.9±1.3* |
p<0.05 vs. Control
During phase 1 of injections, both groups receiving MPA had greater body weights than the Control group [Control vs. Early-MPA—t(21)=7.724; p<0.0001; Control vs. Early +Late-MPA—t(21)=4.147; p<0.001] (Table 2), and the group not receiving MPA (Late-MPA) did not differ from the Control group. During phase 2, animals that had received MPA during phase 1 (Early-MPA and Early+ Late-MPA) still had greater body weights than the Control group [Control vs. Early-MPA—t(21)=5.227; p<0.0001; Control vs. Early+Late-MPA—t(21)=2.353; p<0.05] (Table 2), but the Late-MPA animals did not weigh more than the Control group even though they were receiving MPA at the time [Control vs. Late-MPA—ns], which is in accordance with other reports of MPA treatment to middle-aged rodents (Isserow et al. 1995).
GAD 65 and 67
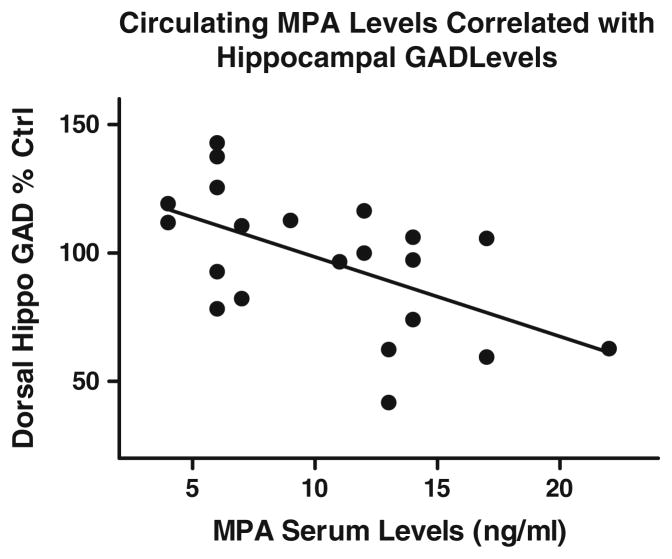
There were no Treatment effects for any brain region analyzed (Table 3). Pearson r correlations revealed that in animals that had detectable levels of serum MPA (Late-MPA and Early+Late-MPA), there was a negative correlation between MPA levels and GAD protein in the dorsal hippocampus [r(19)=−0.571, p=0.0059] (Fig. 7) indicating that higher MPA serum levels were associated with decreased GAD protein levels in the dorsal hippocampus. There were no correlations between GAD protein and the memory scores evaluated.
Table 3.
Mean GAD protein levels (% control)
| Frontal cortex | Anterior cingulate cortex | Posterior cingulate cortex | Entorhinal cortex | Dorsal hippocampus | Ventral hippocampus | |
|---|---|---|---|---|---|---|
| Control | 100±6 | 100±20 | 100±14 | 100±7 | 100±7 | 100±5 |
| Early-MPA | 100±6 | 84±18 | 79±8 | 104±11 | 96±6 | 99±12 |
| Late-MPA | 89±3 | 71±15 | 103±18 | 97±5 | 98±8 | 107±10 |
| Early+late-MPA | 98±4 | 92±20 | 87±11 | 93±5 | 96±9 | 96±9 |
Fig. 7.
Relation between MPA serum concentrations and GAD protein in the dorsal hippocampus in animals that had detectable levels of MPA at sacrifice (Late-MPA and Early+Late-MPA)
Discussion
The synthetic progestin MPA is widely prescribed as the contraceptive Depo Provera, and is in the HTs Prempro and Premphase. The current study supports the hypothesis that MPA has detrimental effects on cognition in the female rodent. We previously found that MPA impaired learning and memory in aged Ovx rats when given in old age (Braden et al. 2010). Here, we show that MPA administered during young adulthood, middle age, or both impaired working memory in middle-aged Ovx rats. Specifically, MPA given at any time point tested herein increased WMC errors on the WRAM across all days of testing and at the highest working memory load on the latter portion of testing, relative to vehicle treatment. MPA given at both time points was particularly detrimental to WRAM performance, as evidenced by a concomitant increase in errors on two orthogonal working memory measures. Notably, the detrimental effects of MPA were especially prominent when the task was most taxing and the load on working memory was highest. MPA seems to have less of an impact on reference memory, as compared to working memory. In fact, the only finding on a reference memory measure tested here was the replication of our prior work showing that MPA induced overnight forgetting, as evidenced when administered after Ovx only (Braden et al. 2010). Indeed, the current results show that the Late-MPA group, who only received MPA post-Ovx, had poorer retention of the platform location overnight. Table 1 summarizes the significant behavior effects.
The current findings are in agreement with clinical evidence implicating MPA as detrimental to cognition in young women when given as a contraceptive, as reported in one case study (Gabriel and Fahim 2005), and may be related to the increased risk of dementia seen in the WHIMS MPA+conjugated equine estrogen trial (Shumaker et al. 2003). Further, administration of progesterone or its metabolite, allopregnanolone, is detrimental to memory in healthy pre-menopausal women (Freeman et al. 1992; Kask et al. 2008). In rodents, we and others have shown progesterone-related decrements in working memory (Frye and Sturgis 1995; Bimonte-Nelson et al. 2004; Braden et al. 2010; Sun et al. 2010) and reference memory (Johansson et al. 2002; Bimonte-Nelson et al. 2004; Braden et al. 2010). However, the literature on progesterone and allopregnanolone’s effects on cognition in young rodents is mixed, with some studies showing benefits and others not showing benefits (Frye et al. 2007, 2010; Frye and Walf 2008; Harburger et al. 2008; Orr et al. 2009). Likewise, in ovary-intact middle-aged rodents, for some memory tasks higher levels of progesterone or allopregnanolone in cognitive brain regions were associated with better performance, while for others, lower levels were associated with better performance (Paris et al. 2010). Post-training injections of progesterone had no effect on MM performance in middle-age rodents, but attenuated overnight forgetting in aged Ovx rodents, as well as differentially facilitated object recognition across different age groups (Lewis et al. 2008).
It must be considered that the water maze tasks used in the present study could involve a stress response, exacerbated by colder water temperatures which can facilitate memory formation (Sandi et al. 1997). Although MPA does bind to the glucocorticoid receptor at one tenth of the potency as the synthetic glucocorticoid, dexamethasone (Pridjian et al. 1987), and at high doses has been shown to curb the stress response seen in cancer patients (Lang et al. 1990), in the current report the working memory load effect lends support to the hypothesis that MPA modulates memory, specifically. The nature of the working memory load in the radial-arm maze is such that the only aspect that changes across trials is the memory demand and not the motivator involved. Indeed, in the earlier trials when memory load was lowest but the non-cognitive stressors of the task were the same, MPA had no effect on performance.
MPA, progesterone, and allopregnanolone have each been shown to affect the GABAergic system (Wallis and Luttge 1980; Paul and Purdy 1992; Belelli and Herd 2003; Pazol et al. 2009; Braden et al. 2010). In the current report, there was a negative correlation between serum MPA levels and GAD in the dorsal hippocampus. In animals receiving MPA treatment at test (Late-MPA and Early+Late-MPA), higher MPA levels correlated with less GAD in the dorsal hippocampus, which is in agreement with our previous findings of MPA-induced decreases in GAD in the dorsal hippocampus in aged Ovx animals (Braden et al. 2010). There is an increasing focus on research, including both in vitro and in vivo studies, evaluating how MPA could impact the GABAergic system. Findings have indicated that although MPA’s ring-A reduced metabolites do not directly bind to the GABAA receptor (McAuley et al. 1993) like natural progesterone’s ring-A reduced metabolites (Paul and Purdy 1992), MPA alters progesterone’s metabolic conversions (Penning et al. 1985) which may be responsible for MPA-induced enhanced GABAA receptor-mediated inhibition (Belelli and Herd 2003) as well as changes in GAD (Braden et al. 2010). Similar to the relation between reduced GAD levels in dorsal hippocampus and higher serum MPA levels as we show here, Raol et al. (2005) demonstrated a decrease in GAD levels in the dorsal hippocampus after administration of the GABAA agonist diazepam in rodents, in turn suggesting that MPA-induced GAD decreases may be a consequence of increased GABAA receptor activation. In fact, increased GABAA receptor activation impaired learning as well as immediate and delayed recall in humans (Curran 1986). There is also evidence that MPA has a negative effect on neuronal function via exacerbation of glutamate-induced excitotoxicity when tested in vitro (Nilsen et al. 2006) and lacks neuroprotective effects seen with natural progesterone (Nilsen and Brinton 2002). However, further research is needed to understand the complete mechanism behind MPA’s detrimental effects on neuronal function and cognition in order to determine safe progestin use.
In aged Ovx rats, we previously found a main effect whereby MPA treatment decreased GAD levels in the dorsal hippocampus (Braden et al. 2010). Here, we show a similar relationship via correlation, in the absence of a main effect, in middle-aged Ovx rats. We hypothesize it is a difference in age of the animals in the current study (12 months old) versus our previous report (20 months old; Braden et al. 2010). This may represent that some animals receiving MPA when middle-aged still showed resilience to MPA-induced GABAergic changes while others did not, thereby enriching variability conducive to significant correlations among individual subjects rather than main effects via ANOVA. While the relation between progestins (including MPA, progesterone, and allopregnanolone) and the GABAergic system has been evaluated using in vitro techniques with tissues from young adult animals (Paul and Purdy 1992; McAuley et al. 1993; Belelli and Herd 2003; Pazol et al. 2009), whether these findings translate to similar effects in vivo, or in aged animals, is an exciting and clinically relevant question that remains to be explored. Specifically, how brain aging alters the trajectory of progestin-induced GABAergic changes has yet to be methodically detailed.
There were MPA-induced changes in serum levels of MPA, progesterone, and allopregnanolone. For serum MPA, animals receiving MPA at test (Late-MPA and Early+Late-MPA) were the only groups to show MPA concentrations in serum at sacrifice, confirming presence of the drug after administration, as well as successful clearing of the drug in blood for the group that received MPA treatment in young adulthood only. Further, serum MPA levels are within the range of MPA used clinically (Depo Provera Prescribing Information 2006; Prempro Prescribing Information 2009). The prescribing information for Depo Provera and Prempro states that MPA is absorbed, distributed, metabolized, and excreted in an identical manner for both formulations. Presence of progesterone after Ovx is assumed to be from the adrenal glands, which have been shown to significantly contribute to total circulating progesterone levels in rodents (Fajer et al. 1971). In the current study, MPA administration at any time inhibited serum levels of both progesterone and allopregnanolone, as compared to Controls. For progester-one, our data suggest that this inhibition was partially recovered over time as the Early-MPA animals had greater levels than animals treated with MPA during middle age. Within this context, the long-lasting effects of the MPA-induced decrease in endogenous progesterone and allopregnanolone levels represent another possible mechanism by which MPA may impair cognition. This outcome of MPA treatment is particularly striking because it is the only biological effect that is present in all groups that showed cognitive impairments. Indeed, ovarian hormone removal via Ovx (thereby removing the major source of endogenous progesterone as well as estrogen) impairs cognition in young rats (Bimonte and Denenberg 1999; Talboom et al. 2008), but not in middle-aged or aged rats (Savonenko and Markowska 2003; Talboom et al. 2008), under normal test conditions without pharmacological challenge. Removal of elevated progesterone levels may be one mechanism by which Ovx improves cognitive performance in aged rats (Bimonte-Nelson et al. 2003, 2004). Indeed, while in some cases progesterone treatment can improve cognitive performance in Ovx rats (Walf et al. 2006; Frye et al. 2007), and in stroke and cortical contusion rodent models (Roof et al. 1993; Roof and Hall 2000), these improvements were seen in young animals. The collected findings suggest that age may be a factor in these effects since progesterone treatment impairs cognition in aged Ovx rats (Bimonte-Nelson et al. 2004; Braden et al. 2010), and hormone status at middle-age impacts cognition (Markowska 1999) with the estropause state characterized with high progesterone levels related to poorer cognitive performance (Warren and Juraska 2000). Additionally, in one study, progesterone treatment improved memory in young Ovx mice, but progesterone plus MPA did not (Frye et al. 2010), further demonstrating the lack of efficacy of MPA on improving cognition in the rodent model, even when tested in young adulthood. Recently, there have been investigations of combined estrogens and MPA treatment in middle-aged rodents. In Ovx animals, long-term treatment of 17β-estradiol plus MPA led to impaired spatial memory performance as compared to chronic or cyclic 17β-estradiol alone or 17β-estradiol plus progesterone, suggesting that the addition of MPA was detrimental to cognition (Lowry et al. 2010).
Another possible mechanism of MPA-related memory impairment is halting ovulation. It is tempting to speculate that the negative effects on cognition in Early-MPA and Early+Late-MPA animals could be due to prolonged acyclicity in young adulthood, as opposed to direct effects of MPA on the brain. Indeed, animals were ovary-intact in Phase 1 of the experiment. Inherent to the complexities associated with administration of a contraceptive to evaluate cognition is that we are stopping ovulation. While we cannot rule out the possibility that the cascade of events related to a lack of ovulation, including alteration of estrogen levels, might have impacted our findings, a recent publication shows no effect of MPA treatment on plasma 17β-estradiol levels in middle-aged ovary intact rodents (Frye et al. 2010). Further, we observed memory impairment in the Late-MPA group that had no evidence of acyclicity in young adulthood because they were ovary-intact and not exposed to MPA at that young timepoint.
MPA impacted uterine and body weights. For uterine weight, we replicated our previous findings that animals treated with MPA after Ovx had heavier uterine weights than Control animals (Braden et al. 2010), while Early-MPA animals did not differ from Control animals. Finally, for body weight, MPA treatment in young adulthood but not middle age (Isserow et al. 1995) caused an increase in body weight as compared to Control treatment. The body weights of animals treated with MPA during young adulthood only (Early-MPA) did not return to Control-like weights after the cessation of treatment, suggesting that early MPA treatment causes long-lasting weight gain in the rodent model. There is some evidence of this in clinical research as well (Bigrigg et al. 1999; Hani et al. 2009).
In conclusion, the current report indicates that, in the rodent model: (1) MPA administration during young adulthood results in long-lasting working memory impairments evident later in life (middle age), even in the absence of circulating MPA levels at the time of test; (2) MPA administered during middle age, extending through time of test, impairs working memory and overnight retention; and (3) MPA administered during young adulthood and again at middle age impairs working memory, an effect that is robust as it is seen on two orthogonal working memory measures. Based on the latter result, our study suggests there is added risk to working memory when MPA is given both in young adulthood and middle age. These detrimental effects may, in part, be mediated by changes to the GABAergic system in the dorsal hippocampus. Overall, the current study builds on earlier in vivo and vitro research indicating that MPA is detrimental to brain health and function. Given the large number of prescriptions that are written every year containing MPA, as it is contained in both contraceptives and HTs, more investigation into this synthetic hormone’s effect on cognition and brain is warranted.
Acknowledgments
This research was funded by grants awarded to HAB-N from the National Institute on Aging (AG028084), state of Arizona, ADHS and the Arizona Alzheimer’s Disease Core Center. LP recognizes support by the NIH Grant AG027956 and an endowment (BK-0031) from the Welch Foundation. We are grateful to Dr. Xiaoqian Liu for performing the LC–APCI-MS/MS assay and to Dr. Craig Enders for his expert statistical consultation. The authors have nothing to disclose regarding financial disclosures. The experiments comply with the current laws of the country in which they were performed.
Contributor Information
B. Blair Braden, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA. Arizona Alzheimer’s Consortium, Tempe, AZ, USA.
Alexandra N. Garcia, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA
Sarah E. Mennenga, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA. Arizona Alzheimer’s Consortium, Tempe, AZ, USA
Laszlo Prokai, University of North Texas Health Science Center, Fort Worth, TX, USA.
Stephanie R. Villa, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA
Jazmin I. Acosta, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA. Arizona Alzheimer’s Consortium, Tempe, AZ, USA
Natalie Lefort, Center for Metabolic Biology, Arizona State University, Tempe, AZ 85287, USA.
Alain R. Simard, Barrow Neurological Institute, Phoenix, AZ, USA
Heather A. Bimonte-Nelson, Email: bimonte.nelson@asu.edu, Department of Psychology, Arizona State University, P.O. Box 871104, Tempe, AZ 85287, USA. Arizona Alzheimer’s Consortium, Tempe, AZ, USA
References
- Depo Provera Prescribing Information. [Accessed 28 Jan 2011];Pfizer for Professionals. 2006 Available at http://media.pfizer.com/files/products/uspi_depo_provera_contraceptive.pdf.
- Prempro Prescribing Information. [Accessed 28 Jan 2011];Pfizer for Professionals. 2009 Available at http://www.pfizerpro.com/content/showlabeling.asp?id-133.
- Acosta JI, Mayer L, Talboom JS, Tsang CW, Smith CJ, Enders CK, Bimonte-Nelson HA. Transitional versus surgical menopause in a rodent model: etiology of ovarian hormone loss impacts memory and the acetylcholine system. Endocrinology. 2009;150:4248–4259. doi: 10.1210/en.2008-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakry S, Merhi ZO, Scalise TJ, Mahmoud MS, Fadiel A, Naftolin F. Depot-medroxyprogesterone acetate: an update. Arch Gynecol Obstet. 2008;278:1–12. doi: 10.1007/s00404-007-0497-z. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA (A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmik T, Mukherjea M. Histological changes in the ovary and uterus of rat after injectable contraceptive therapy. Contraception. 1988;37:529–538. doi: 10.1016/0010-7824(88)90022-4. [DOI] [PubMed] [Google Scholar]
- Bigrigg A, Evans M, Gbolade B, Newton J, Pollard L, Szarewski A, Thomas C, Walling M. Depo Provera. Position paper on clinical use, effectiveness and side effects. Br J Fam Plann. 1999;25:69–76. [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Braden BB, Talboom JS, Crain ID, Simard AR, Lukas RJ, Prokai L, Scheldrup MR, Bowman BL, Bimonte-Nelson HA. Medroxyprogesterone acetate impairs memory and alters the GABAergic system in aged surgically menopausal rats. Neurobiol Learn Mem. 2010;93:444–453. doi: 10.1016/j.nlm.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV. Tranquillising memories: a review of the effects of benzodiazepines on human memory. Biol Psychol. 1986;23:179–213. doi: 10.1016/0301-0511(86)90081-5. [DOI] [PubMed] [Google Scholar]
- Diczfalusy E. Contraceptive prevalence, reproductive health and our common future. Contraception. 1991;43:201–227. doi: 10.1016/0010-7824(91)90141-2. [DOI] [PubMed] [Google Scholar]
- Fajer AB, Holzbauer M, Newport HM. The contribution of the adrenal gland to the total amount of progesterone produced in the female rat. J Physiol. 1971;214:115–126. doi: 10.1113/jphysiol.1971.sp009422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman MA. Quality of life and menopause: the role of estrogen. J Womens Health (Larchmt) 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. Br J Clin Pharmacol. 1992;33:293–298. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3alpha, 5alpha-THP. Neuroreport. 2010;21:590–595. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel A, Fahim G. Do depot medroxyprogesterone acetate contraceptive injections cause mood changes and memory impairment? Prim Psychiatry. 2005;12:59–60. [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Hani D, Imthurn B, Merki-Feld GS. Weight gain due to hormonal contraception: myth or truth? Gynäkol Geburtshilfliche Rundsch. 2009;49:87–93. doi: 10.1159/000197907. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Pechenino AS, Saadi A, Frick KM. Post-training progesterone dose-dependently enhances object, but not spatial, memory consolidation. Behav Brain Res. 2008;194:174–180. doi: 10.1016/j.bbr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Isserow JA, Rucinski B, Romero DF, Mann GN, Liu CC, Epstein S. The effect of medroxyprogesterone acetate on bone metabolism in the oophorectomized, tamoxifen-treated rat. Endocrinology. 1995;136:713–719. doi: 10.1210/endo.136.2.7835304. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Kask K, Backstrom T, Nilsson LG, Sundstrom-Poromaa I. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacol Berl. 2008;199:161–168. doi: 10.1007/s00213-008-1150-7. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4. Pearson Prentice Hall; Upper Saddle River: 2004. [Google Scholar]
- Lang I, Zielinski CC, Templ H, Spona J, Geyer G. Medroxyprogesterone acetate lowers plasma corticotropin and cortisol but does not suppress anterior pituitary responsiveness to human corticotropin releasing factor. Cancer. 1990;66:1949–1953. doi: 10.1002/1097-0142(19901101)66:9<1949::aid-cncr2820660917>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Orr PT, Frick KM. Differential effects of acute progesterone administration on spatial and object memory in middle-aged and aged female C57BL/6 mice. Horm Behav. 2008;54:455–462. doi: 10.1016/j.yhbeh.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry NC, Pardon LP, Yates MA, Juraska JM. Effects of long-term treatment with 17 beta-estradiol and medroxyprogesterone acetate on water maze performance in middle aged female rats. Horm Behav. 2010;58:200–207. doi: 10.1016/j.yhbeh.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JW, Kroboth PD, Stiff DD, Reynolds IJ. Modulation of [3H]flunitrazepam binding by natural and synthetic progestational agents. Pharmacol Biochem Behav. 1993;45:77–83. doi: 10.1016/0091-3057(93)90089-c. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;(350):1–36. [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Morales A, Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006;22:355–361. doi: 10.1080/09513590600863337. [DOI] [PubMed] [Google Scholar]
- Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progester-one infusions enhance object recognition in young female mice. Pharmacol Biochem Behav. 2009;93:177–182. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–878. [PubMed] [Google Scholar]
- Paris JJ, Walf AA, Frye CA. II. Cognitive performance of middle-aged female rats is influenced by capacity to metabolize progesterone in the prefrontal cortex and hippocampus. Brain Res. 2010;1379:149–163. doi: 10.1016/j.brainres.2010.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME. Progesterone and medroxyprogesterone acetate differentially regulate alpha4 subunit expression of GABA (A) receptors in the CA1 hippocampus of female rats. Physiol Behav. 2009;97:58–61. doi: 10.1016/j.physbeh.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Sharp RB, Krieger NR. Purification and properties of 3 alpha-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985;260:15266–15272. [PubMed] [Google Scholar]
- Pridjian G, Schmit V, Schreiber J. Medroxyprogesterone acetate: receptor binding and correlated effects on steroidogenesis in rat granulosa cells. J Steroid Biochem. 1987;26:313–319. doi: 10.1016/0022-4731(87)90095-1. [DOI] [PubMed] [Google Scholar]
- Prokai L, Frycak P, Stevens SM, Nguyen V. Measurement of acetylcholine in rat brain microdialysates by LC-isotope dilution tandem MS. Chromatographia. 2008;68:s101–s105. doi: 10.1365/s10337-008-0697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Rasband W. Image J. National Institute of Health; Bethesda, Maryland, USA: 1997–2004. Available at http://rsb.info.nih.gov/ij/ [Google Scholar]
- Rodriguez MI, Kaunitz AM. An evidence-based approach to postpartum use of depot medroxyprogesterone acetate in breast-feeding women. Contraception. 2009;80:4–6. doi: 10.1016/j.contraception.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–388. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36:123–132. doi: 10.1016/j.psyneuen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer AL, Bonnema R, McNamara MC. Helping women choose appropriate hormonal contraception: update on risks, benefits, and indications. Am J Med. 2009;122:497–506. doi: 10.1016/j.amjmed.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Sun WL, Luine VN, Zhou L, Wu HB, Weierstall KM, Jenab S, Quiniones-Jenab V. Acute progesterone treatment impairs spatial working memory in intact male and female rats. Ethn Dis. 2010;20(S1):83–87. [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Influence of estrogen and progesterone on glutamic acid decarboxylase activity in discrete regions of rat brain. J Neurochem. 1980;34:609–613. doi: 10.1111/j.1471-4159.1980.tb11187.x. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Sex differences and estropausal phase effects on water maze performance in aged rats. Neurobiol Learn Mem. 2000;74:229–240. doi: 10.1006/nlme.1999.3948. [DOI] [PubMed] [Google Scholar]
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–1170. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]