Abstract
Tocotrienol-rich fraction of palm oil, which contains the isomers of vitamin E, was shown to possess potent anticancer activity against mammary adenocarcinoma cell lines. Its clinical use, however, is limited by poor oral bioavailability and short half-life. Previously, we developed tocotrienol-rich lipid nanoemulsions for intravenous administration. The objective of this study was to investigate the effect of surface grafted polyethylene glycol (PEG) on the properties of the nanoemulsions. PEGylation was achieved by the addition of equimolar PEG groups using poloxamer or 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (PEG2000-DSPE). The effect of PEG surface topography on the antiproliferative activity of nanoemulsions against mammary adenocarcinoma cells, their susceptibility to protein adsorption, and its effect on blood hemolysis and circulation time was investigated. Nanoemulsions PEGylated with poloxamer or PEG2000-DSPE were stable under physical stress. Poloxamer nanoemulsion, however, displayed higher uptake and potency against MCF-7 tumor cells in 2D and 3D culture and increased hemolytic effect and susceptibility to IgG adsorption, which was reflected in its rapid clearance and short circulation half-life (1.7 h). Conversely, PEGylation with PEG2000-DSPE led to a 7-fold increase in mean residence time (12.3 h) after IV injection in rats. Reduced activity in vitro and improved circulation time suggested strong shielding of plasma proteins from the droplets. Differences between the nanoemulsions were attributed to polymer imbibitions and the differences in PEG conformation and density on the surface of the droplets.
Key words: nanoemulsion, PEG2000-DSPE, PEGylation, poloxamer, tocotrienol
INTRODUCTION
Vitamin E refers to a family of eight isomers divided into two subfamilies known as tocopherols and tocotrienols (1). The tocotrienol-rich fraction (TRF) of vitamin E was found to display potent anticancer activity (2). It targets multiple cell signaling pathways linked with tumorigenesis such as NF-κB, STAT3, death receptors, apoptosis, Nrf2, HIF1, and growth factor receptor kinases (3). TRF, however, is poorly bioavailable when taken orally and is negligibly absorbed when administered intraperitoneally and intramuscularly (4). Intravenous (IV) delivery was therefore considered a viable alternative route for TRF administration. Tocotrienols, however, were also reported to have a short duration and distribution time in circulating blood (5) with a half-life of approximately 2.5–3 h (6). Therefore, a main challenge associated with IV administration of TRF is to extend its blood circulation time and to evade its clearance by the reticuloendothelial system (RES) (7).
Extending the residence time of drugs in the blood was found to significantly correlate with their uptake by solid tumors (8). A longer circulation time is associated with repeated passage of drugs through the leaky endothelium of tumor microvasculature, which improves tumor uptake through the enhanced permeability and retention effect (8,9). Several approaches for prolonging the circulation time of nanoparticles have been reported in the literature (10). One of the most successful approaches is grafting the surface of nanoparticles with polyethylene glycol (PEG), also known as PEGylation, which was shown to help nanoparticles evade clearance by the RES and increase their residence time in the blood (8,11,12).
Previously our laboratory carried out various optimization studies to develop stable phospholipid-based parenteral lipid nanoemulsions enriched with vitamin E (13). Several factors, such as the viscosity of the oil phase, type of emulsifiers used, homogenizing pressure, and number of homogenization cycles, were found to have a significant effect on both physical stability and size of the nanoemulsion droplets (14). To stabilize vitamin E nanoemulsions against coalescence in biological media, a phospholipid blend with poloxamer 188 was used. Poloxamers are FDA-approved difunctional block copolymer surfactants that are sold under the trade name Pluronic® (15). Over the years, poloxamers drew interest due to their high PEG density. Presence of these surface grafted PEGs in nanoparticles creates a steric barrier and increases surface hydration, which reduces serum protein absorption and cell binding (16), decreases uptake by liver macrophages, and prolongs circulation time (7).
As with poloxamers, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000] (PEG2000-DSPE), a block copolymer of PEG2000 covalently linked to a distearoyl lipid tail, has been used in many nanoplatforms (e.g., liposomes, polymeric nanoparticles, solid lipid nanoparticles, and microemulsions) as a PEGylating agent to increase their in vivo circulation time. For example, the use of PEG2000-DSPEin the liposomal Doxil® formulation was shown to prolong its circulation time, reduce its volume of distribution, and consequently improve its tumor uptake (17,18). PEGylating Doxil® liposomes with PEG2000-DSPE extended the half-life of doxorubicin from 0.5 to 20–30 h in tumor xenograft-bearing mice and rats and 50–60 h in humans (18,19).
Other studies, nonetheless, have reported that PEGylation may sometimes lead to unexpected results (20). A number of PEG-coated formulations, for example, were shown to vary in circulation time and resistance to protein adsorption (21). The overall goal of the present work was therefore to compare and contrast between two TRF lipid nanoemulsions that were PEGylated with either poloxamer 188 or PEG2000-DSPE. The specific objectives of this study were to compare between the two nanoemulsions with respect to their (a) physical stability under stress, (b) hemolytic effect, (c) susceptibility to protein adsorption, (d) cellular uptake and in vitro antiproliferative activity against mammary adenocarcinoma cells in 2D and 3D culture, and (e) blood circulation time and clearance after IV injection in tumor-free rats.
MATERIALS AND METHODS
Materials
TRF, which contains approximately 30% α-tocopherol and 70% α, γ, and δ-tocotrienols, was a gift from Beta Pharmaceutical Ltd (West Perth, Australia). α-Tocopherol was purchased from Sigma (St. Louis, MO, USA). Polyoxyethylene sorbitan monooleate (Tween® 80) was provided by Uniqema (New Castle, DE, USA). Phospholipids (Lipoid® E80S) isolated from soybean oil (64–79% phosphatidylcholine and 12–18% phosphatidyl ethanolamine) were a generous gift from Lipoid® GmbH (Ludwigshafen, Germany). Medium-chain triglyceride (MCT; Miglyol® 812) was provided by Sasol (Witten/Ruhr, Germany). Lutrol® F 68 NF (poloxamer 188) was provided by BASF (Florham Park, NJ, USA). PEG2000-DSPE ammonium salt was purchased from Avanti Polar Lipids (Pelham, AL, USA). 2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl) triethylamine salt (NBD-DPPE) was purchased from NOF America (White Plains, NY, USA). Human IgG was purchased from Equitech-Bio, Inc (Kerrville, TX, USA). C8/C10 polyglycolyzed glycerides from coconut oil (Labrasol®) was provided by Gattefossé (Saint-Priest, Cedex, France). Hemoglobin reagent kit was purchased from Teco Diagnostic (Anaheim, CA, USA). Whole rabbit blood was obtained from Hemostat Laboratories (Dixon, CA, USA). Insulin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Penicillin–streptomycin was obtained from Cellgro® (Manassas, VA, USA). Detergent reagent sodium dodecyl sulfate (SDS) was obtained from Trevigen Inc. (Gaithersburg, MD, USA). Fetal bovine serum (HyClone Inc.) was obtained from VWR (Westchester, PA, USA). Deionized water (DI) was used for all preparations. All chemicals and solvents were of reagent grade or higher and were used as supplied without further modification.
Preparation of TRF Nanoemulsions
TRF nanoemulsions were prepared by the high-pressure homogenization techniques as described previously (14). Briefly, a blend of 2% TRF/MCT at a 1/1 w/w ratio was mixed to form the homogeneous oil phase. In a separate vial, primary and secondary emulsifiers (0.12% Lipoid® E80S and 0.05% Tween® 80) were dispersed in DI to which 0.25% poloxamer 188 or PEG2000-DSPE was added to form the aqueous phase of the nanoemulsion. Glycerol 2.25% was added to adjust tonicity. The two phases were then combined and passed through a high-pressure homogenizer (EmulsiFlex®C3, Avestin Inc., Ottawa, ON, Canada) for 25 cycles under homogenization pressure of 170 MPa. The pH was adjusted to 8 ± 0.05 using 0.1 N sodium hydroxide solutions. This was essential as lipid emulsions are most stable at pH values higher than 7.5 (22). For the uptake study, 0.02% NBD-DPPE fluorescent probe was incorporated into the nanoemulsion by mixing the lipid with TRF in the oil phase.
Physical Characterization of the Nanoemulsions
The intensity-weighed mean droplet size, population distribution (polydispersity index, PI), and zeta potential (ξ) of the nanoemulsions were measured by photon correlation spectroscopy at 23°C and a fixed angle of 90° using NicompTM 380 ZLS submicron particle size analyzer (PSS Inc., Santa Barbara, CA, USA). The size was recorded for 3 min with viscosity and dielectric constant of the medium set to 1.33 and 78.5, respectively. Analyses were performed in triplicates unless otherwise specified.
Stress Testing
Nanoemulsions were subjected to a mechanical shaking and thermal stability tests. Samples were placed randomly on a shaker (Barnstead International, Dubuque, IA, USA) and agitated at the maximum amplitude of 250 strokes/min for 24 h at room temperature. Separate samples were incubated at 37°C while rotating in a gravity convection oven (model 1350GM, Sheldon Manufacturing Inc., Cornelius, OR, USA) for 24 h. At the end of each test, samples were analyzed for droplet size and were visually inspected for signs of phase separation. For stability in culture medium and isotonic dextrose solution, an aliquot (0.05 mL) of each nanoemulsion was mixed with 0.5 mL of serum-free RPMI + GlutaMaxTM medium or dextrose 5% solution. Samples were then incubated at 37°C in the gravity convection oven for 24 h. At the end of the experiments, samples were visually inspected and then analyzed for droplet size.
Hemolysis Testing
Hemolysis studies were performed as described previously (23,24). Briefly, red blood cells were obtained by first centrifuging rabbit blood with normal blood chemistry at 800×g for 5 min to remove debris and serum proteins. The supernatant was discarded and the erythrocytes were re-suspended in isotonic phosphate buffer (pH 7.4). The washing step was repeated until a clear supernatant was obtained. Collected erythrocytes were then used to prepare a stock dispersion in Dulbecco’s phosphate-buffered saline (DPBS)/modified buffer with a fixed hemoglobin concentration of 8 g/dL. For hemolysis testing, 100 μL aliquot of each nanoemulsion was first diluted with 0.8 mL of the DPBS/modified buffer to which 100 μL of the stock erythrocyte dispersion was added. Mixtures were incubated at 37°C for up to 8 h. After 0.5, 3, and 8 h, debris and intact erythrocytes were removed by centrifuging the mixtures at 750×g for 3 min. From the supernatant, 100 μL was removed and dissolved in 2 mL of an ethanol/HCL mixture [39 parts of 99% ethanol (v/v) and one part of 37% hydrochloric acid (w/v)]. The absorbance of the resulting solution at 398 nm against a blank sample was used to estimate % hemolysis.
Stealth Properties of Lipid Nanoemulsions
To evaluate the ability of poloxamer 188 and PEG2000-DSPE to shield the nanoemulsion droplets from protein adsorption and consequently uptake by the mononuclear phagocyte system (MPS), droplet size of the nanoemulsions was monitored while incubated with increasing concentration of immunoglobulin IgG. Of each nanoemulsion, 100 μL was diluted with 900 μL of IgG stock solution to achieve a final IgG concentration of 0.5, 1, 2, and 4 mg/mL. Mixtures were incubated while rotating at 37°C in a gravity convection oven for 24 h. At the conclusion of the test, samples were collected and analyzed for droplet size and were visually inspected for signs of phase separation.
Antiproliferative Activity and Cellular Uptake Studies
The growth inhibitory activity of the nanoemulsions was evaluated against MCF-7 human mammary adenocarcinoma cells. MCF-7 cell line was obtained from ATCCTM (Manassas, VA, USA) and was maintained in RPMI + GlutaMaxTM medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, 1% insulin, and 1% penicillin–streptomycin at 37°C in an environment of 95% air and 5% CO2 in humidified incubator. Once confluent, cells were seeded at a density of 1 × 104 cells/well (six replicates per group) in 2D 96-well plates. After overnight incubation, cells were treated with different concentrations of TRF loaded in either poloxamer 188 or PEG2000-DSPE stabilized nanoemulsions. Cells in medium and cells treated with 0.1% SDS were examined in parallel as negative and positive controls, respectively. After 96 h, the number of viable cells was determined using the Cell Titer-Glo® Luminescent Cell Viability Assay by following the manufacturer’s protocol (Promega, Madison, WI, USA). The average luminescence reading obtained at each concentration was expressed as a percentage of the average luminescence readings obtained from the control wells. Cell viabilities were calculated as percentage living cells. Fluorescent NBD-DPPE-loaded nanoemulsions were used to measure the uptake of the nanoemulsions by the MCF-7 cells. For uptake studies, cells at a density of 2 × 105 cell/well were seeded in a 24-well plate and treated with various concentrations of TRF in either poloxamer 188 or PEG2000-DSPE stabilized fluorescent nanoemulsions. Cells were incubated at 37°C for 4 h in a 5% CO2 environment. After 2 and 4 h, cells were washed three times with cold PBS, and the fluorescence intensity was measured using a BioTek Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA).
3D Antiproliferative Assay
The antiproliferative activity of the TRF nanoemulsions was evaluated against MCF-7 cells grown in a 3D cell culture. MCF-7 cell were initially maintained in RPMI + GlutaMaxTM medium as described in the antiproliferative studies above. For 3D culturing, cells were seeded at a density of 1 × 105 cells/well (three replicates per group) in a 24-well Alvetex® scaffold plate. After overnight incubation at 37°C in a 5% CO2 environment, cells were treated with different concentrations of TRF in either poloxamer 188 or PEG2000-DSPE stabilized nanoemulsions. At the end of the 96-h treatment exposure period, the viable cell number was determined by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide colorimetric assay. For imaging, cells were fixed with methanol and then washed with PBS buffer. For staining, cells were treated with KaryoMAX® Giemsa Stain (Invitrogen, San Diego, CA, USA) for 40 s after which the cells were washed overnight with PBS. Nikon Eclipse TS-1000 inverted microscope was used to capture images and view the cells at ×40 magnification.
Pharmacokinetic Studies
Male Sprague-Dawley rats weighing 250–350 g were acquired from Harlan Laboratories (Houston, TX, USA). All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Louisiana at Monroe, and all the surgical and treatment procedures were consistent with the Institutional Animal Care and Use Committee policies and procedures. The rats were maintained on a 12-h light/dark cycle before the study and had free access to food and water ad libitum before each experiment.
To obtain the plasma profile of the TRF nanoemulsions after IV injection, animals were divided into three groups, three rats per each. In the control group, 10 mg/kg of TRF in non-PEGylated micelles prepared from a Tween® 80 (14%, v/v) and Labrasol® (2%, v/v) blend in sterilized water was injected into the tail vein of the rats. For the second and third group, the rats were administered 10 mg/kg of TRF in either poloxamer 188 or PEG2000-DSPE stabilized nanoemulsions, respectively. Blood samples were withdrawn at 0, 0.084, 0.25, 0.5, 1, 2, 4, 6, 8, and 10 h and collected in heparinized Eppendorf tubes. Samples were then centrifuged at 13,000 rpm for 10 min. The separated plasma was stored at −20°C until analyzed for γ-tocotrienol (γ-T3) by high-performance liquid chromatography (HPLC) using a fluorescent detector set at 298 nm excitation and 325 nm emission wavelengths.
The extraction of γ-T3 from the plasma was performed as described previously (16). In brief, 50 μL plasma and 50 μL ethanol containing 1% ascorbic acid and 1 μg/mL δ-tocopherol (internal standard) were mixed in a glass tube. After the addition of 1.0 mL hexane, the mixture was vortexed for 90 s before centrifugation for 10 min at 5,000 rpm. Eight hundred microliters of the supernatant was then transferred to another tube and evaporated to dryness by a centrifugal evaporator (Centrivap concentrator, Kansas City, MO, USA). Precipitate was reconstituted with 100 μL methanol of which 20 μL was injected into a XDB-C18 HPLC Column (5 μm, 150 × 4.6 mm i.d.; Agilent, Santa Clara, CA, USA) at a flow rate of 1.0 mL/min using methanol, ethanol, and acetonitrile (85:7.5:7.5, v/v/v) as the mobile phase.
The HPLC method for γ-T3 analysis was fully validated (16,25). The inter-day precision of this method ranged from 5.8% to 12.8%, and the accuracy ranged from 92.4% to 108.5%, while the intra-day precision ranged from 0.7% to 7.9% in rat plasma. The standard curve range used was from 10 to 10,000 ng/mL. HPLC analysis was performed using an isocratic Prominence Shimadzu HPLC system (Columbia, MD, USA). The system consisted of a SIL 20-AHTautosampler, fluorescence detector (Shimadzu, RF10A XL) and a LC-20AB pump connected to a Dgu-20A3 degasser.
Data were acquired by LC Solution software version 1.22 SP1 (Shimadzu). Plasma levels versus time profiles of each nanoemulsion were plotted and fitted using a PK-Plus module in Gastroplus™ (Simulation Plus Inc., Lancaster, CA, USA). Non-compartmental model was used to calculate the mean residence time and the systemic clearance. The area under the curve (AUC) was calculated by the trapezoidal rule method. The IV data were found to best fit a two-compartmental model from which the central compartment (Vc) and peripheral compartment volume of distribution (V2) were calculated.
Statistical Analysis
Analysis of variance followed by Bonferroni’s or unpaired t test was used to test for significant differences between the treatment groups. A difference of P < 0.05 was considered to be statistically significant. IC50 values (dose resulting in 50% cell growth inhibition) for the nanoemulsions were determined by non-linear regression curve fit analysis using GraphPad Prism 4 (GraphPad Software, La Jolla, CA, USA).
RESULTS AND DISCUSSION
Two TRF lipid nanoemulsions were prepared using either poloxamer 188or PEG2000-DSPE as stabilizing and PEGylating agents. The size, zeta potential (ξ), and PI of the two nanoemulsions are given in Table I. After preparation, these nanoemulsions were subjected to several tests, which are discussed in the following sections. General characteristics of the nanoemulsions, such as TRF entrapment efficiency, drug release, chemical and storage stability, and surface morphology, were discussed in our published work (14). The emphasis of the present study was to elucidate the impact of PEGylating agents on the properties and behavior of parenteral lipid nanoemulsions and their impact on TRF delivery.
Table I.
Physical Characteristics of the Poloxamer 188 and PEG2000-DSPE Nanoemulsions
| PEGylating agent | Particle size (nm) | Polydispersity index | Zeta potential (ξ) |
|---|---|---|---|
| Poloxamer® 188 | 202 ± 1 | 0.12 ± 0.01 | −31 ± 3 |
| PEG2000-DSPE | 214 ± 4 | 0.19 ± 0.02 | −46 ± 1 |
Each value represents the mean ± SD
PEG 2000 -DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000]
Stress Testing
The average particle size of poloxamer 188 and PEG2000-DSPE nanoemulsions was monitored during shaking and exposure to heat. These are reliable tests that are often used to evaluate the consistency of emulsions under exposure to vigorous handling and transportation (17). No significant change in particle size was observed after shaking for 24 h (Fig. 1a). Upon visual inspection, both formulations appeared homogenous, and no visible oil or breakage of the emulsions was observed. Since lipid emulsions are thermodynamically unstable, TRF nanoemulsions were stored at 37°C for 24 h to accelerate their coalescence. As with shaking, the two nanoemulsions were stable with no significant change in droplet size (Fig. 1a). Since injectable emulsions are known to be readily admixed with saline solutions, the stability of the TRF nanoemulsions upon dilution with an isotonic 5% dextrose solution and culture media at a 1:10 ratio was also investigated (26,27). Dilution test is critical for subsequent intravenous applications as flocculation and coalescence of emulsion droplets upon dilution before or during administration into the blood may cause adverse effects, including the blockage of lung capillaries (28). No significant change in particle size was found when the nanoemulsions were diluted with either 5% dextrose or media (Fig. 1b). The consistency in droplet size of the nanoemulsions in the media was crucial to validate subsequent in vitro cell uptake and viability studies. It has been shown that cellular uptake and molecular response to nanoparticles is significantly influenced by particle size (19–21). The observed stability of the nanoemulsions after shaking and during storage at high temperatures could be attributed to the steric stabilization effect of the PEG moiety provided by poloxamer 188 or PEG2000-DSPE.
Fig. 1.
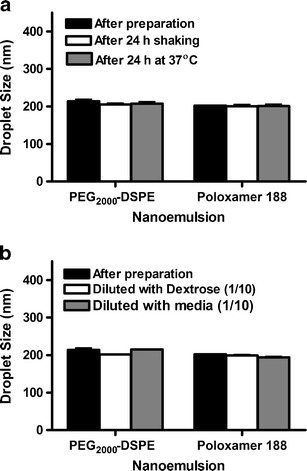
Droplet size of poloxamer 188 and PEG2000-DSPE stabilized TRF nanoemulsions immediately after preparation and after a exposure to physical stress by mechanical shaking for 24 h or incubation at 37°C for 24 h and b dilution in isotonic 5% dextrose solution and cell culture media. Vertical bars are expressed as the mean ± SD (n = 3)
Hemolysis Testing
Measuring the hemolytic effect of IV administered lipid emulsions is a viable in vitro toxicity screen and a reliable gauge for estimating erythrocytes membrane damage caused by emulsions (24). Since phospholipids and emulsifiers, such as Lipoid® E80S and Tween® 80, which were used in this study, are known to cause hemolysis, harmful effects are expected upon direct contact between emulsion droplets and erythrocytes (29). Hemolysis has been found to highly correlate with the severity of adverse effects and lesions (30). This effect was also found to be proportional to incubation time (31). Therefore, the hemolytic effect of the two TRF nanoemulsions was measured in human blood at a 1:10 nanoemulsion to blood ratio after 0.5, 3, and 8 h. After 3 h of incubation, both formulations did not induce significant hemolytic effect (Fig. 2a). Presence of the bulky PEG head groups on the surface of the nanoemulsion droplets hindered their ability to approach the erythrocytes and thereby reduced direct contact between phospholipids and cell membrane (24,32,33). These results are in agreement with previous studies in which erythrocyte morphology was found to remain unchanged after treatment with poloxamer 188 (34). Likewise, FDA approval of PEG2000-DSPEfor use in Doxil® formulation is an evidence of its safety and blood compatibility (35). The hemolytic effect of the poloxamer nanoemulsion after 8 h could be attributed to the differences between the two formulations in the conformation of the PEG chains on the surface of the droplets. Differences in PEG conformation were found to have a profound impact on protein adsorption, tumor cell viability, and in vivo circulation time, which are discussed in greater detail in later sections of this study. Briefly, it was found that PEG chains on the surface of the poloxamer nanoemulsion droplets assume a brush conformation that is believed to be ineffective in preventing binding with other hydrophobic surfaces (e.g., red blood cells) in close proximity, while PEG2000-DSPE nanoemulsion droplets were found to assume a dense brush confirmation that has the ability to shield the surface of the droplets from contact with nearby surfaces.
Fig. 2.
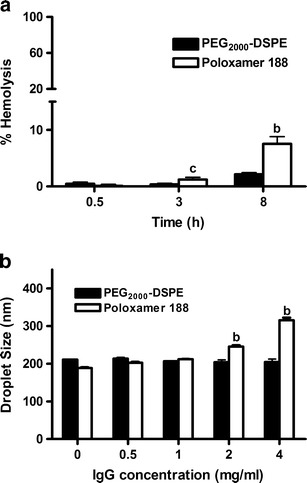
a % hemolysis of poloxamer 188 and PEG2000-DSPE stabilized TRF nanoemulsions as a function of time and b droplet size of poloxamer 188 and PEG2000-DSPE stabilized TRF nanoemulsions as a function of IgG concentration. Vertical bars are expressed as the mean ± SD (n = 3). b P < 0.001; c P < 0.05 versus PEG2000-DSPE of the same concentration or time point
Stealth Properties of Lipid Nanoemulsions
Plasma protein adsorption could be estimated by a 2D polyacrylamide gel electrophoresis (36–38) or by UV analysis of unbound proteins (7,39). Immunoglobulins, which are known as opsonic proteins that lead to fast recognition and uptake of nanoparticles by the MPS (7), have also been used in protein adsorption studies (40). In the present work, change in nanoemulsions droplet size when treated with free immunoglobulin IgG was used as an indirect measure of plasma protein adsorption (41,42). Poor PEG coverage is expected to result in higher protein adsorption and consequently emulsion aggregation and an increase in droplet size.
Poloxamer 188 and PEG2000-DSPE stabilized nanoemulsions were incubated with different concentrations of IgG over a period of 24 h to monitor changes in their droplet size. In the present experimental conditions, significant difference between the two nanoemulsions was observed when incubated with IgG (Fig. 2b). The average droplet size of PEG2000-DSPE nanoemulsion remained constant at around 210 nm, even at the highest IgG concentration of 4 mg/mL. In contrast, there was a significant increase in the droplet size of the poloxamer 188 nanoemulsions as a function of IgG concentration. The droplet size of the nanoemulsion increased from 188 to 310 nm when IgG concentration increased from 0.5 to 4 mg/mL. A significant increase in droplet size was also observed when the poloxamer 188-stabilized nanoemulsions were dispersed in human plasma and stored at 37°C for 24 h (unpublished data). These results indicate that poloxamer, as opposed to PEG2000-DSPE, was incapable of shielding the nanoemulsion droplets form IgG adsorption. This could be explained by the differences in the chemical structure and spatial arrangement of the two molecules within the lipid droplets. While both molecules project a PEG corona on the surface of the droplets, the density and conformation of PEG chains are different (Fig. 3). With PEG2000-DSPE, PEG groups are anchored to a phospholipid tail, which is aligned parallel within the phospholipid bilayer allowing the hydrophilic PEG chain to extend outward. This configuration was shown to decreases surface hydrophobicity, an effect known to reduce protein adsorption (41). On the other hand, the large hydrophobic polypropylene oxide block of poloxamer was probably positioned deeper and close to the oil core of the nanoemulsions droplet, thereby limiting the degree to which PEG chains are projected and extended on the surface of the droplets.
Fig. 3.
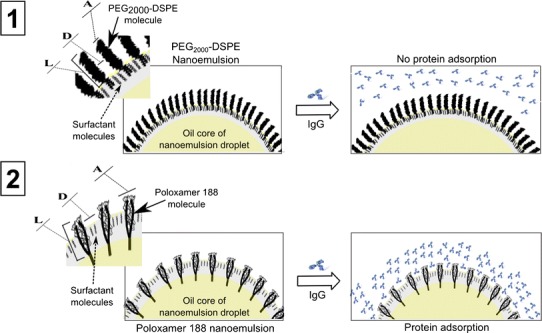
Schematic presentation of PEG conformations and their affinity for protein (IgG) adsorption in 1 poloxamer 188 stabilized nanoemulsion droplet and 2 PEG2000-DSPE stabilized nanoemulsion droplet. Showing in the figure are the PEG conformation parameters A, D, and L where A is the area occupied by one PEG chain (in square nanometers), D is the distance between PEG grafts, and L is the length/thickness of the grafted PEG layer
Antiproliferative Activity and Cellular Uptake Studies
TRF nanoemulsions were evaluated for their cytotoxicity against MCF-7 cells as a model human breast adenocarcinoma cell line. Cell viability was measured by the Cell Titer-Glo assay, which determines the number of viable cells based on quantifying the amount of ATP present. Both nanoemulsions exhibited a dose-dependent inhibition in cell growth and were found to significantly reduce cell viability at concentrations as low as 3 μg/mL (Fig. 4a). Higher activity, however, was observed with nanoemulsions stabilized with poloxamer 188. The IC50s of the TRF lipid nanoemulsions stabilized with either poloxamer or PEG2000-DSPE were 4 and 6 μg/mL, respectively (Fig. 4a). The biological activity of formulation constituents other than TRF may contribute to the observed differences in the activity of the nanoemulsions (43). Poloxamer, for example, was shown to have clinical and therapeutic applications for the treatment of various physiological conditions (44). To determine whether the biological activity of surfactants may have contributed to the activity of the nanoemulsions against MCF-7 cells, the antiproliferative activity of nanoemulsions loaded with α-tocopherol was tested as control. α-Tocopherol was used as a negative control as it was shown in several studies to lack anticancer activity at low concentrations. As shown in Fig. 4a, α-tocopherol nanoemulsions did not exhibit cytotoxic activity against the MCF-7 cells. For instance, approximately 70% and 100% of MCF-7 cells remained viable when treated with α-tocopherol emulsions stabilized by poloxamer 188 and PEG2000-DSPE at concentrations as high as 15 μg/mL, respectively (Fig. 4a). These data confirmed previous reports on the antiproliferative activity of tocotrienols against mammary adenocarcinoma cell lines (14,45) and demonstrated the potential benefits of nanoemulsions in cancer therapy.
Fig. 4.
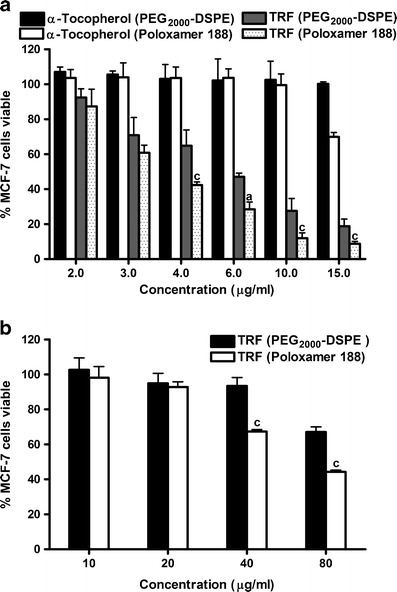
Dose–response relationship showing the antiproliferative activity of a TRF and α-tocopherol-loaded poloxamer 188 and PEG2000-DSPE nanoemulsions in 2D cell culture using 96-well plates and b TRF-loaded poloxamer 188 and PEG2000-DSPE nanoemulsions in 3D cell culture using the Alvetex® scaffolds against the estrogen-receptor positive MCF-7 cells. Vertical bars indicate the mean cell count ± SD (n = 6). a P < 0.0001; b P < 0.001; c P < 0.05 versus PEG2000-DSPE of the same concentration
The results from the cell viability assay were also supported by the results from the cell uptake study. Lower uptake of PEG2000-DSPE nanoemulsions was observed in MCF-7 cells when compared to the uptake of poloxamer nanoemulsions (Fig. 5). A gradual increase in uptake as a function of incubation time and the concentration of TRF in the media was, nonetheless, observed with both formulations. The difference in droplet size between the two nanoemulsions (Table I) could partially explain the observed differences in their cellular uptake and anticancer activity. Several studies demonstrated that the cellular uptake of nanoparticles was significantly affected by their characteristics including particle size (18,46). For instance, a size-dependent uptake was reported for PLGA nanoparticles by Caco-2 and HT-29 cells (18). The subtle differences in droplet size, however, may not be significant to warrant the observed differences in cell viability. Differences in PEG conformation on the surface of the nanoemulsions may have contributed to the observed differences in cytotoxicity and uptake of the two formulations. Furthermore, the ability of poloxamer 188 to inhibit P-gp and CYP3A4 may also contribute to its higher activity and uptake when compared with PEG2000-DSPE nanoemulsions (15,47,48).
Fig. 5.
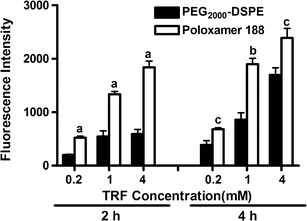
Cellular uptake of poloxamer 188 and PEG2000-DSPE stabilized nanoemulsions by MCF-7 cells after 2 and 4 h incubation as a function of TRF concentration. Vertical bars indicate the mean fluorescence intensity ± SD (n = 6). a P < 0.0001; b P < 0.001; c P < 0.05 versus PEG2000-DSPE of the same concentration
3D Antiproliferative Assay
The assessment of drug’s anticancer activity is usually carried out on monolayer cells seeded on 2D plates. While this routine provides useful preliminary observations, it does not imitate the environment and shape that cells experience in vivo (40). The limitations of the 2D culture due to cells’ monolayer morphology and the limited cell to cell contact could be addressed in vitro by using 3D culture models (49). 3D in vitro models are increasingly used to overcome the shortcomings of 2D culture and to minimize the gap between in vitro culture assays and animal models (50,51). 3D systems provide a more realistic environment for the cells to grow while retaining their native 3D morphology. Therefore, Alvetex® scaffolds were used in this study to test the activity of the TRF nanoemulsions in 3D culture. These scaffolds employ a highly porous inert polystyrene membrane in which cells can grow in three dimensions (52). A representative image of MCF-7 cells grown in 2D and 3D culture (Fig. 6) shows the monolayer morphology of cells in 2D plates and the xenograft-like structure of the matrix (53) when grown in Alvetex® scaffold plates. Data generated using the Alvetex® scaffolds (Fig. 4b) confirmed the lower activity of PEG2000-DSPE nanoemulsion when compared to the activity of the poloxamer 188 nanoemulsion. Results from 3D culture also revealed the lower overall activity of both formulations against MCF-7 cells in a 3D model as opposed to their activity in 2D plates (Fig. 4a). In standard 96-well plates (2D model), nearly 0% of the cells remained viable when treated with 20 μg/mL of TRF (Fig. 4a), whereas in 3D culture (Fig. 4b) >45% of the cells remained viable when treated with as high as 80 μg/mL TRF in lipid nanoemulsions. The observed lower activity of TRF in 3D culture is in agreement with previously reported studies in which lower apparent cytotoxicity of drugs, such as tamoxifen, doxorubicin, and docetaxel, was observed in 3D culture (54).
Fig. 6.
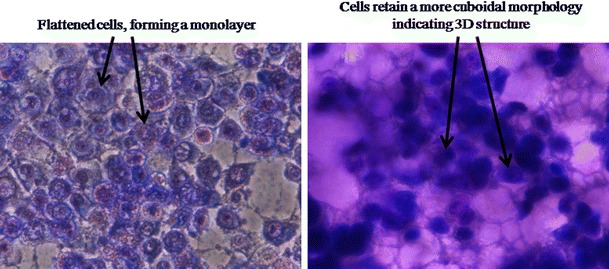
Image of MCF-7 cells stained with KaryoMAX® Giemsa and grown in 2D (left) and 3D (right) culture plates. The geometry and shape of cultured cells in the two environments were significantly different. Cells in 2D environment adopt flattened morphology in a mono-layer, while in 3D cells maintain their natural 3D ellipsoidal shape and structure. Images were taken with Nikon Eclipse TS-1000 inverted microscope at ×40 magnification
Based on the 3D culture data in this study, a low potency of TRF is expected in vivo. TRF, nonetheless, is an attractive adjuvant to cancer prevention and therapy when used in combination with other cytotoxic drugs. TRF at low doses was shown in several studies to significantly potentiate the activity of many classes of drugs, such as COX-2 inhibitors (55), tyrosine kinase inhibitors (56), and statins (57) against breast adenocarcinoma cells. TRF may not be useful as monotherapy in cancer; rather, its benefits may be attained when used in combination with other drugs. The fact that TRF activity was demonstrated in 3D culture and was not abolished by incorporation in PEGylated nanoemulsions confirmed its potential use in combination therapy. The activity of TRF/drug combinations and the physical properties and biological behavior of TRF/drug nanoemulsions are, however, beyond the scope of this study and are the subject of future work.
Pharmacokinetic Studies
After IV injection, nanoparticles are cleared quickly from the circulation, usually within minutes, by elements of the RES, particularly the hepatic Kupffer cells (10). Stealth nanoparticles that are shielded by hydrophilic PEG moieties, nonetheless, have a prolonged circulation time with half-lives ranging from 2 to 24 h in mice and rats and up to 45 h in humans depending on particle size, degree of surface hydrophobicity, and type of copolymer used (58,59). For example, PEGylated liposomes loaded with cisplatin were shown to have a better antitumor activity, lesser kidney toxicity, and an extended circulation time with a 60-fold larger AUC than the free unencapsulated drug (60).
In our study, the half-life of the TRF nanoemulsions after IV injection in tumor-free mice was indirectly estimated from the plasma concentration of the γ-T3 isomer of the TRF. Plasma half-life ranged from 1.7 h for the poloxamer nanoemulsion to 12.3 h for the PEG2000-DSPE nanoemulsion. The mean plasma concentration versus time profiles of γ-T3 following IV administration of nanoemulsions to rats are shown in Fig. 7, and the associated pharmacokinetic parameters are given in Table II.
Fig. 7.
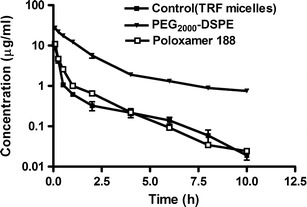
Plasma concentration (in nanograms per milliliter) versus time (in hours) profiles of γ-T3 after IV administration of 10 mg/kg of TRF loaded in non-PEGylated micelles or PEGylated poloxamer 188 or PEG2000-DSPE nanoemulsions to rats (n = 3). The plots represent profiles of the IV data. Each value represents the mean ± SD
Table II.
Pharmacokinetic Parameters of γ-T3 Following I.V. Administration of 10 mg/kg of TRF Poloxamer 188, PEG2000-DSPE Nanoemulsions, and Control
| Pharmacokinetic parameter | Control micelles | Poloxamer188 nanoemulsions | PEG2000-DSPE nanoemulsion |
|---|---|---|---|
| AUC (μg h/mL) | 3.9 ± 0.5 | 5.4 ± 0.4 | 53.5 ± 4.3a |
| MRT (h) | 1.4 ± 0.13 | 1.5 ± 0.1 | 8.7 ± 1b |
| Cl (L/h/kg) | 6 ± 0.85 | 3.5 ± 1.1 | 0.55 ± 0.03b |
| V c (L/kg) | 1.3 ± 0.45 | 1.5 ± 0.42 | 0.78 ± 0.1 |
| V 2 (L/kg) | 5.7 ± 2 | 3.1 ± 0.3 | 2 ± 0.9 |
| T 1/2 (h) | 2 ± 0.3 | 1.7 ± 0.03 | 12.3 ± 2b |
Each value represents the mean ± SD (n = 3)
AUC area under the curve, MRT mean residence time, Cl clearance, V c central compartment, V 2 peripheral compartment volume of distribution, T 1/2 half-time, PEG 2000 -DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000]
aIndicates significantly different between PEG2000-DSPE and poloxamer 188 or control (P < 0.001)
bIndicates significantly different between PEG2000-DSPE and poloxamer 188 or control (P < 0.01)
Plasma profiles were fitted into a two-compartmental model where γ-T3 exhibited bi-exponential pharmacokinetics in plasma with the apparent terminal elimination phase well-defined 3 h post-dose. These results were in agreement with previously reported data for tocotrienols (61). A biphasic plasma profile with initial rapid elimination by macrophages of the RES followed by a sustained effect has also been reported for PEGylated nanoparticles (42). This was attributed to the heterogeneity in their surface coating, which allows for opsonic binding to unshielded areas (10).
The AUC of γ-T3 from the PEG2000-DSPE nanoemulsion was 10-fold higher than its AUC from the poloxamer nanoemulsion (Table II). The half-life and mean residence time of γ-T3 from the PEG2000-DSPE nanoemulsion were 7- and 6-fold higher than the poloxamer nanoemulsion, respectively. The volume of distribution and clearance of γ-T3 from PEG2000-DSPE nanoemulsion was also lower when compared to the other groups. The clinical significance of prolonging the blood circulation time of TRF stems from the fact that γ-T3 have a short half-life of 2.5–3.0 h in circulating blood (6). Prolonging the circulation time of TRF nanoemulsions in the blood would minimize their rapid clearance and/or accumulation in the skin and adipose tissue (61). It would also increase the probability of their uptake by the leaky tumor microvasculature.
The half-life of γ-T3 when incorporated into non-PEGylated micelles in the control group was found to be 2 h. A short half-life of the control non-PEGylated micelles was expected since no protective PEG groups were present. Encapsulating TRF into nanoemulsion droplets containing poloxamer 188, however, was expected to extend their half-life and blood circulation time. Unexpectedly, the half-life of the poloxamer nanoemulsion was only 1.7 h, which was significantly lower than the half-life of PEG2000-DSPE nanoemulsion (12.3 h) (Table II). Differences in half-life between the two nanoemulsions could be explained by the difference in the PEG coverage on the surface of the droplets, which was previously found to contribute to the hemolytic effect of poloxamer nanoemulsion and its higher uptake in tumor cells. Even though an equivalent amount of poloxamer and PEG2000-DSPE (25 mg) with equal number of PEG moles was used to prepare both nanoemulsions (Table III), PEG surface conformation and cover density were different. These factors play a key role in determining the efficacy of PEG chains in extending circulation time (42). The two main conformations that PEG chains can acquire on the surface of nanoparticles are the “mushroom” and “brush” conformations. The prevailing conformation type in a particular system depends on three main parameters: Flory radius (RF) of the PEG graft, the distance (D) between PEG grafts, and the length/thickness (L) of the grafted PEG layer (42). Mushroom conformation is seen when there is low PEG density coverage, where D > RF, resulting in poor projection of PEG chains from the surface of nanoparticles and consequently poor PEGylation effect. Increasing the concentration of PEG density causes the PEG chains to rearrange into a brush conformation where D approaches RF and results in thick layer of PEG grafts. Dense brush is obtained when L > 2RF (42). In several studies, it was reported that the beneficial effects of PEGylation occur when the PEG grafts are in the dense brush conformation (62). A schematic presentation of these parameters is shown in Fig. 3.
Table III.
Comparison of Parameters Used to Determine Surface PEG Density of the Poloxamer 188 and PEG2000-DSPE Stabilized Nanoemulsions
| Poloxamer 188 | PEG2000-DSPE | |
| MWt (g/mol) | ≈9,510 | ≈2,791 |
| PEG MWt (g/mol) | 7,700 | 2,000 |
| Total amount of PEG (mg)a | 20.25 | 17.75 |
| PEGs number in each molecule | 160 | 45 |
| Total PEGs (mol)b | 0.4 | 0.4 |
| Particles parameters | Poloxamer 188 nanoemulsion | PEG2000-DSPE nanoemulsion |
| Droplet radius (nm) | 101 | 107 |
| Droplet density (g/cm2) | 0.95 | 0.95 |
| N | 80 | 45 |
| a (nm)c | 0.35 | 0.35 |
| A (nm2)d | 4.8 | 1.3 |
| D (nm)d | 2.5 | 1.3 |
| R F (nm)d | 4.9 | 3.4 |
| L (nm)d | 7.5 | 6.61 |
MWt molecular weight, N number of monomers per polymer chain, a the length of one monomer, A the area that one PEG chain occupies, D the distance between PEG graft sites, R F Flory radius, L length/thickness of the grafted PEG layer, PEG 2000 -DSPE 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)2000]
aCalculated using [amount of surfactant × PEG fraction of MWt of surfactant]
bCalculated using [moles of PEGs × PEGs number in each molecule]
cLength of one ethylene glycol monomer was from (39)
The conformation of the PEG chains on the surface of the poloxamer and PEG2000-DSPE nanoemulsions could therefore be predicted by calculating the value of the D, RF, and L parameters (Table II) using the following equations (42):
| 1 |
| 2 |
| 3 |
| 4 |
N is the number of ethylene glycol monomers per polymer chain, a is the length of one monomer, A is the area occupied by one PEG chain (per square nanometers), MPEG is the molecular weight of the PEG graft, f is mass fraction of PEG in the particle, ρ is the density of the droplets, NA is Avogadro’s number, and d is the diameter of the particle.
The values of D, RF, and L for both nanoemulsions are given in Table III. From the results, it could be concluded that PEG chains on the surface of the poloxamer nanoemulsion droplets assume a brush conformation (D < RF), whereas they assume a dense brush confirmation on the surface of the PEG2000-DSPE nanoemulsion droplets (L ≈ 2RF). While these differences in PEG conformation may contribute to the observed differences in uptake and circulation time between the two nanoemulsions, differences in D, RF, and L parameters alone cannot account for the significant difference in their plasma profile. Other factors such as polymer chain architecture and surface PEG concentration may have also contributed to this effect. For example, it was shown that addition of PEG2000-DSPE to liposomes would significantly reduce protein adsorption by lateral repulsion of PEG chains (60) and increase their plasma circulation time when added at 0.5 mol% of the total formulation (63). In the same study, it was also shown that aggregation of liposomes was completely prevented when formulated with 2 mol% PEG2000-DSPE, which is the approximate amount used in formulating TRF nanoemulsions in this study (63).
CONCLUSION
In this study, we compared TRF nanoemulsions made with poloxamer 188 as the PEGylating agent versus one PEGylated with PEG2000-DSPE. While equimolar PEG concentration was used in both formulations, the poloxamer 188 nanoemulsion had higher uptake and potency against MCF-7 cell. Yet, it was inferior to the PEG2000-DSPE nanoemulsions in eliciting a PEGylation effect. On the other hand, the T1/2 of γ-T3 from the PEG2000-DSPE nanoemulsion was 7-fold higher than the poloxamer nanoemulsion and the control group which could be explained by different PEG surface density and conformation on the surface of the nanoemulsion droplets and the overall polymer chain architecture.
Acknowledgments
This work was supported in part by a grant from the First Tech International Ltd (Wanchai, Hong Kong).
References
- 1.Sylvester PW, Kaddoumi A, Nazzal S, El Sayed KA. The value of tocotrienols in the prevention and treatment of cancer. J Am Coll Nutr. 2010;29(3 Suppl):324S–333S. doi: 10.1080/07315724.2010.10719847. [DOI] [PubMed] [Google Scholar]
- 2.Nesaretnam K, Meganathan P, Veerasenan SD, Selvaduray KR. Tocotrienols and breast cancer: the evidence to date. Genes Nutr. 2012;7(1):3–9. doi: 10.1007/s12263-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannappan R, Gupta SC, Kim JH, Aggarwal BB. Tocotrienols fight cancer by targeting multiple cell signaling pathways. Genes Nutr. 2012;7(1):43–52. doi: 10.1007/s12263-011-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap SP, Yuen KH, Lim AB. Influence of route of administration on the absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats. J Pharm Pharmacol. 2003;55(1):53–58. doi: 10.1111/j.2042-7158.2003.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 5.Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alqahtani S, Alayoubi A, Nazzal S, Sylvester P, Kaddoumi A. Nonlinear absorption kinetics of self-emulsifying drug delivery systems (SEDDS) containing tocotrienols as lipophilic molecules: in vivo and in vitro studies. AAPS J. 2013;1-12. doi:10.1208/s12248-013-9481-7. [DOI] [PMC free article] [PubMed]
- 7.Goppert TM, Muller RH. Plasma protein adsorption of Tween 80- and poloxamer 188-stabilized solid lipid nanoparticles. J Drug Target. 2003;11(4):225–231. doi: 10.1080/10611860310001615956. [DOI] [PubMed] [Google Scholar]
- 8.Le UM, Cui Z. Long-circulating gadolinium-encapsulated liposomes for potential application in tumor neutron capture therapy. Int J Pharm. 2006;312(1–2):105–112. doi: 10.1016/j.ijpharm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Soundararajan A, Bao A, Phillips WT, Perez R, Goins BA. [186Re]Liposomal doxorubicin (Doxil): in vitro stability, pharmacokinetics, imaging and biodistribution in a head and neck squamous cell carcinoma xenograft model. Nucl Med Biol. 2009;36(5):515–524. doi: 10.1016/j.nucmedbio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–478. doi: 10.1016/S0163-7827(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama K, Yuda T, Okamoto A, Kojima S, Suginaka A, Iwatsuru M. Prolonged circulation time in vivo of large unilamellar liposomes composed of distearoyl phosphatidylcholine and cholesterol containing amphipathic poly(ethylene glycol) Biochim Biophys Acta. 1992;1128(1):44–49. doi: 10.1016/0005-2760(92)90255-T. [DOI] [PubMed] [Google Scholar]
- 12.Moghimi SM. Prolonging the circulation time and modifying the body distribution of intravenously injected polystyrene nanospheres by prior intravenous administration of poloxamine-908. A ‘hepatic-blockade’ event or manipulation of nanosphere surface in vivo? Biochim Biophys Acta Gen Subj. 1997;1336(1):1–6. doi: 10.1016/S0304-4165(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 13.Alayoubi A, Nazzal M, Sylvester PW, Nazzal S. “Vitamin E” fortified parenteral lipid emulsions: Plackett–Burman screening of primary process and composition parameters. Drug Dev Ind Pharm. 2012 doi: 10.3109/03639045.2012.682223. [DOI] [PubMed] [Google Scholar]
- 14.Alayoubi AY, Anderson JF, Satyanarayanajois SD, Sylvester PW, Nazzal S. Concurrent delivery of tocotrienols and simvastatin by lipid nanoemulsions potentiates their antitumor activity against human mammary adenocarcinoma cells. Eur J Pharm Sci. 2013;48(3):385–392. doi: 10.1016/j.ejps.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Yan F, Zhang C, Zheng Y, Mei L, Tang L, Song C, et al. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomedicine. 2010;6(1):170–178. doi: 10.1016/j.nano.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Harvie P, Wong FMP, Bally MB. Use of poly(ethylene glycol)–lipid conjugates to regulate the surface attributes and transfection activity of lipid–DNA particles. J Pharm Sci. 2000;89(5):652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Davis SS, Washington C. Physical properties and stability of two emulsion formulations of propofol. Int J Pharm. 2001;215(1–2):207–220. doi: 10.1016/S0378-5173(00)00692-X. [DOI] [PubMed] [Google Scholar]
- 18.Xu A, Yao M, Xu G, Ying J, Ma W, Li B, et al. A physical model for the size-dependent cellular uptake of nanoparticles modified with cationic surfactants. Int J Nanomedicine. 2012;7:3547–3554. doi: 10.2147/IJN.S32188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 20.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Bu L, Xie J, Chen K, Cheng Z, Li X, et al. Effects of nanoparticle size on cellular uptake and liver MRI with polyvinylpyrrolidone-coated iron oxide nanoparticles. ACS Nano. 2010;4(12):7151–7160. doi: 10.1021/nn101643u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jumaa M, Müller BW. Development of a novel parenteral formulation for tetrazepam using a lipid emulsion. Drug Dev Ind Pharm. 2001;27(10):1115–1121. doi: 10.1081/DDC-100108374. [DOI] [PubMed] [Google Scholar]
- 23.Bock TK, Müller BW. A novel assay to determine the hemolytic activity of drugs incorporated in colloidal carrier systems. Pharm Res. 1994;11(4):589–591. doi: 10.1023/A:1018987120738. [DOI] [PubMed] [Google Scholar]
- 24.Jumaa M, Kleinebudde P, Müller BW. Physicochemical properties and hemolytic effect of different lipid emulsion formulations using a mixture of emulsifiers. Pharm Acta Helv. 1999;73(6):293–301. doi: 10.1016/S0031-6865(99)00003-5. [DOI] [Google Scholar]
- 25.Abuasal B, Thomas S, Sylvester PW, Kaddoumi A. Development and validation of a reversed-phase HPLC method for the determination of gamma-tocotrienol in rat and human plasma. Biomed Chromatogr. 2011;25(5):621–627. doi: 10.1002/bmc.1493. [DOI] [PubMed] [Google Scholar]
- 26.Gao K, Sun J, Liu K, Liu X, He Z. Preparation and characterization of a submicron lipid emulsion of docetaxel: submicron lipid emulsion of docetaxel. Drug Dev Ind Pharm. 2008;34(11):1227–1237. doi: 10.1080/03639040802005057. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Davis SS, Papandreou C, Melia CD, Washington C. Design and evaluation of an emulsion vehicle for paclitaxel. I. Physicochemical properties and plasma stability. Pharm Res. 2004;21(9):1573–1580. doi: 10.1023/B:PHAM.0000041451.70367.21. [DOI] [PubMed] [Google Scholar]
- 28.Ilium L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Blood clearance and organ deposition of intravenously administered colloidal particles. The effects of particle size, nature and shape. Int J Pharm. 1982;12(2–3):135–146. doi: 10.1016/0378-5173(82)90113-2. [DOI] [Google Scholar]
- 29.Ishii F, Nagasaka Y. Interaction between erythrocytes and free phospholipids as an emulsifying agent in fat emulsions or drug carrier emulsions for intravenous injections. Colloids Surf B: Biointerfaces. 2004;37(1–2):43–47. doi: 10.1016/j.colsurfb.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Bjerregaard S, Wulf-Andersen L, Stephens RW, Røge Lund L, Vermehren C, Söderberg I, et al. Sustained elevated plasma aprotinin concentration in mice following intraperitoneal injections of w/o emulsions incorporating aprotinin. J Control Release. 2001;71(1):87–98. doi: 10.1016/S0168-3659(00)00370-9. [DOI] [PubMed] [Google Scholar]
- 31.El-Hariri LM, Marriott C, Martin GP. The mitigating effects of phosphatidylcholines on bile salt- and lysophosphatidylcholine-induced membrane damage. J Pharm Pharmacol. 1992;44(8):651–654. doi: 10.1111/j.2042-7158.1992.tb05487.x. [DOI] [PubMed] [Google Scholar]
- 32.Forster D, Washington C, Davis SS. Toxicity of solubilized and colloidal amphotericin B formulations to human erythrocytes. J Pharm Pharmacol. 1988;40(5):325–328. doi: 10.1111/j.2042-7158.1988.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 33.Weingarten C, Santos Magalhaes NS, Baszkin A, Benita S, Seiller M. Interactions of a non-ionic ABA copolymer surfactant with phospholipid monolayers: possible relevance to emulsion stabilization. Int J Pharm. 1991;75(2–3):171–179. doi: 10.1016/0378-5173(91)90191-P. [DOI] [Google Scholar]
- 34.Lechmann T, Reinhart WH. The non-ionic surfactant Poloxamer 188 (RheothRx®) increases plasma and whole blood viscosity. Clin Hemorheol Microcirc. 1998;18(1):31–36. [PubMed] [Google Scholar]
- 35.Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Goppert TM, Muller RH. Protein adsorption patterns on poloxamer- and poloxamine-stabilized solid lipid nanoparticles (SLN) Eur J Pharm Biopharm. 2005;60(3):361–372. doi: 10.1016/j.ejpb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18(3–4):301–313. doi: 10.1016/S0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt S, Muller RH. Plasma protein adsorption patterns on surfaces of amphotericin B-containing fat emulsions. Int J Pharm. 2003;254(1):3–5. doi: 10.1016/S0378-5173(02)00667-1. [DOI] [PubMed] [Google Scholar]
- 39.Patil S, Sandberg A, Heckert E, Self W, Seal S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials. 2007;28(31):4600–4607. doi: 10.1016/j.biomaterials.2007.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X, Howard MD, Mazik M, Eldridge J, Rinehart JJ, Jay M, et al. Nanoparticles containing anti-inflammatory agents as chemotherapy adjuvants: optimization and in vitro characterization. AAPS J. 2008;10(1):133–140. doi: 10.1208/s12248-008-9013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gessner A, Waicz R, Lieske A, Paulke B, Mader K, Muller RH. Nanoparticles with decreasing surface hydrophobicities: influence on plasma protein adsorption. Int J Pharm. 2000;196(2):245–249. doi: 10.1016/S0378-5173(99)00432-9. [DOI] [PubMed] [Google Scholar]
- 42.Perry JL, Reuter KG, Kai MP, Herlihy KP, Jones SW, Luft JC, et al. PEGylated PRINT nanoparticles: the impact of PEG density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012;12(10):5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G, Zeng X, Li P. Nanomaterials in cancer-therapy drug delivery system. J Biomed Nanotechnol. 2013;9(5):741–750. doi: 10.1166/jbn.2013.1583. [DOI] [PubMed] [Google Scholar]
- 44.Tsoneva I, Iordanov I, Berger AJ, Tomov T, Nikolova B, Mudrov N, et al. Electrodelivery of drugs into cancer cells in the presence of poloxamer 188. J Biomed Biotechnol. 2010;2010:11. doi: 10.1155/2010/314213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alayoubi A, Kanthala S, Satyanarayanajois SD, Anderson JF, Sylvester PW, Nazzal S. Stability and in vitro antiproliferative activity of bioactive “Vitamin E” fortified parenteral lipid emulsions. Colloids Surf B: Biointerfaces. 2013;103(0):23–30. doi: 10.1016/j.colsurfb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang SH, Lee CW, Chiou A, Wei PK. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J Nanobiotechnology. 2010;8:33. doi: 10.1186/1477-3155-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Tang L, Sun L, Bao J, Song C, Huang L, et al. A novel paclitaxel-loaded poly(ε-caprolactone)/poloxamer 188 blend nanoparticle overcoming multidrug resistance for cancer treatment. Acta Biomater. 2010;6(6):2045–2052. doi: 10.1016/j.actbio.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Mei L, Zhang Y, Zheng Y, Tian G, Song C, Yang D, et al. A novel docetaxel-loaded poly (epsilon-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Res Lett. 2009;4(12):1530–1539. doi: 10.1007/s11671-009-9431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 50.dit Faute MA, Laurent L, Ploton D, Poupon MF, Jardillier JC, Bobichon H. Distinctive alterations of invasiveness, drug resistance and cell-cell organization in 3D-cultures of MCF-7, a human breast cancer cell line, and its multidrug resistant variant. Clin Exp Metastasis. 2002;19(2):161–168. doi: 10.1023/A:1014594825502. [DOI] [PubMed] [Google Scholar]
- 51.Schutte M, Fox B, Baradez MO, Devonshire A, Minguez J, Bokhari M, et al. Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three-dimensional culture on a polystyrene scaffold designed for routine use. Assay Drug Dev Technol. 2011;9(5):475–486. doi: 10.1089/adt.2011.0371. [DOI] [PubMed] [Google Scholar]
- 52.Knight E, Murray B, Carnachan R, Przyborski S. Alvetex(R): polystyrene scaffold technology for routine three dimensional cell culture. Methods Mol Biol. 2011;695:323–340. doi: 10.1007/978-1-60761-984-0_20. [DOI] [PubMed] [Google Scholar]
- 53.Katza E, Hadlington-Boothb W, Fauvinc D, Rettenbergerb P. Incorporating the extracellular matrix: new opportunities in cancer research. Development. 2007;134(23):4177–4186. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 54.Reinnervate. Routine assessment of cancer cell cytotoxicity in a novel three dimensional culture assay. Application note 2 2013.
- 55.Shirode AB, Sylvester PW. Synergistic anticancer effects of combined gamma-tocotrienol and celecoxib treatment are associated with suppression in Akt and NFkappaB signaling. Biomed Pharmacother. 2010;64(5):327–332. doi: 10.1016/j.biopha.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachawal SV, Wali VB, Sylvester PW. Enhanced antiproliferative and apoptotic response to combined treatment of gamma-tocotrienol with erlotinib or gefitinib in mammary tumor cells. BMC Cancer. 2010;10:84. doi: 10.1186/1471-2407-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wali VB, Sylvester PW. Synergistic antiproliferative effects of gamma-tocotrienol and statin treatment on mammary tumor cells. Lipids. 2007;42(12):1113–1123. doi: 10.1007/s11745-007-3102-0. [DOI] [PubMed] [Google Scholar]
- 58.Moghimi SM, Hunter AC. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Trends Biotechnol. 2000;18(10):412–420. doi: 10.1016/S0167-7799(00)01485-2. [DOI] [PubMed] [Google Scholar]
- 59.Woodle MC, Storm G. Long circulating liposomes: old drugs, new therapeutics. Austin: Landes; 1998.
- 60.Wang R, Xiao R, Zeng Z, Xu L, Wang J. Application of poly(ethylene glycol)-distearoylphosphatidylethanolamine (PEG-DSPE) block copolymers and their derivatives as nanomaterials in drug delivery. Int J Nanomedicine. 2012;7:4185–4198. doi: 10.2147/IJN.S34489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan B, Watson RR, Preedy VR. Tocotrienols: vitamin E beyond tocopherols. Boca Raton: CRC; 2012.
- 62.Louguet S, Kumar AC, Guidolin N, Sigaud G, Duguet E, Lecommandoux S, et al. Control of the PEO chain conformation on nanoparticles by adsorption of PEO-block-poly(l-lysine) copolymers and its significance on colloidal stability and protein repellency. Langmuir. 2011;27(21):12891–12901. doi: 10.1021/la202990y. [DOI] [PubMed] [Google Scholar]
- 63.Dos Santos N, Allen C, Doppen AM, Anantha M, Cox KA, Gallagher RC, et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: relating plasma circulation lifetimes to protein binding. Biochim Biophys Acta. 2007;1768(6):1367–1377. doi: 10.1016/j.bbamem.2006.12.013. [DOI] [PubMed] [Google Scholar]


