Abstract
The administration of therapeutic proteins via the subcutaneous route (sc) is desired for compliance and convenience, but could be challenging due to perceived immunogenic potential or unwanted immune responses. There are clinical and preclinical data supporting as well as refuting the generalized notion that sc is more immunogenic. We provide a mechanistic perspective of immunogenicity of therapeutic proteins administered via the sc route and discuss strategies and opportunities for novel therapeutic approaches to mitigate immunogenicity.
KEY WORDS: biotechnology, immunogenicity, mitigation of immunogenicity, protein therapeutics, subcutaneous administration
IMMUNOGENICITY OF PROTEINS GIVEN VIA sc ROUTE
The subcutaneous (sc) route of administration of therapeutic proteins is desired over the intravenous (iv) route due to patient comfort and compliance. In addition, it could also prolong half-life of the therapeutic in circulation (1,2). However, this route of administration could be problematic due to a perceived potential for unwanted immunogenicity (3). As most of the vaccines are given via the sc route, it is expected that the sc route is more immunogenic than the iv route. However, a recent comparative clinical study of sc vs iv administration of abatacept, a fusion protein of Fc of human IgG and extracellular domain of CTLA-4, showed that the efficacy and immunogenicity are comparable between the two routes of administration (4,5). However, the immunogenic potential of chronic administration and long-term effects are not often adequately addressed during clinical trials (6). Few preclinical studies have shown that the sc route of administration does not increase immunogenicity (7–9). For example, the relative immunogenicity of Betaseron, interferon beta, is less for sc administration compared to iv administration (9). Based on clinical experience of head-to-head comparison and a few preclinical studies, one could argue that the generalization that the sc route is more immunogenic than the iv route is not universally valid.
A comparative immunogenicity study of three brands of insulin in type 1 diabetics showed an increase in incidence of anti-insulin titer development, across brands, in patients self-administering via sc route as compared to iv administration in hospital in the same cohort (10). In the therapeutic use of erythropoietin-α, incidence of immunogenicity increased with the change from iv to sc. It is appropriate to mention here that elimination of human serum albumin from the formulation as well as stopper material change (11) coincided with changes in route of administration, and thus, the effect of the sc route of administration on immunogenicity is not unambiguous for erythropoietin. There are several examples from preclinical relative immunogenicity studies that show the sc route is more immunogenic than the iv route. The sc administration of FVIII showed significantly higher total antibody titers compared to hemophilia A mice that were given FVIII via the iv route (12). A similar observation has been made for other therapeutic proteins such as interferon alpha and human growth hormone (3,13,14). In our recent relative immunogenicity studies in preclinical models, most of the mice that were given rituximab via the iv route did not produce antibodies to rituximab, whereas all sc administered animals responded with significant antibody titers (unpublished results). However, we could not make similar generalizations to other antibody therapeutics we tested in mice. Thus, the available immunogenicity data of therapeutic proteins supports as well as refutes the general notion that the sc route is more immunogenic.
MECHANISTIC PERSPECTIVE
The immunogenic potential of sc space is a conundrum. This is partly due to lack of mechanistic understanding of factors that drives the immunogenicity of subcutaneously administered protein. Based on antigen trafficking studies in the field of vaccines and on pharmacokinetics and distribution of protein, we propose a mechanistic model to understand the immunogenic potential of the sc route for therapeutic proteins. Our aim here is not to suggest any preferred route of administration of therapeutic proteins, but rather to propose a possible mechanistic basis of steps involved in presentation and processing of proteins following sc administration. The mechanistic studies for antigen trafficking discussed here are primarily carried out in mice, and one must be cautious of its relevance to the human immune system. However, both species share similar subtypes of antigen-presenting cells such as dendritic cells in sc space (15,16).
Primary Antigen-Presenting Cells Involved in Processing of Proteins Given via sc
In order to understand the mechanistic basis of immune response following sc administration, it is important to determine the primary antigen-processing cells involved in presentation and processing. The detection of peptide–MHC II complex using monoclonal antibodies provides an effective approach to track the fate of antigens and the cells that produce these complexes following different routes of administration. Germain and colleagues, followed by Reis D Sousa and colleagues, have shown that in the absence of endotoxin or adjuvant (situation similar to the administration of therapeutic proteins) B cells (that are not specific for the antigen in question) in the spleen and cutaneous dendritic cells (DCs) are the primary cell types that engage in antigen processing and presentation of the peptide–MHC II complexes following the iv and sc routes, respectively (17–19). Following iv administration of the hen egg lysozyme in mice, it has been shown that B cells not specific for the antigen in question rapidly take up the protein and present it in the spleen within 4 h of administration, and this is followed by presentation by dendritic cells after 24 h. Because antigen-unspecific B cells outnumber DCs in the spleen, the possibility of antigen-specific T cells encountering antigen presented by B cells is much higher than that which is presented by dendritic cells (19). In contrast, following sc administration, dendritic cells are the primary antigen-presenting cells that process and present the antigen (17). Dendritic cells are the primary initiators of T cell responses (20), and the cross talk between C-type lectin/Fc receptors and Toll-like receptors can mediate effective immune presentation (21).
Anatomy of the Skin and sc Space
The anatomy of the sc site contributes to effective presentation of the protein to lymph node-resident and cutaneous dendritic cells (Fig. 1). The most superficial layer of the skin is the epidermis, and Langerhans dendritic cells (LCs) reside in the epidermis. The next layer is the dermis and is separated from the epidermis by a membrane that supports the vascular network for nutrient supply to the epidermis. This layer contains dermis dendritic cells that are functionally distinct from LCs (22). The third layer of the tissue is called the hypodermis and is also called sc connective tissue. The proteins deposited in this area trigger the uptake, processing, and maturation of cutaneous dendritic cells and LCs in the epidermis and dermis that subsequently migrate into draining lymph nodes and secondary lymphoid tissues (23). The intensity of adaptive response depends upon the transport of proteins by dendritic cells to lymphoid organs for effective presentation to T cells. Further, proteins distribute in lymph nodes (determined by their pharmacokinetics and lymphatic transport), where these proteins are processed by lymph node-resident dendritic cells (24). This is consistent with two waves of antigen presentation following sc administration of a protein antigen.
Fig. 1.
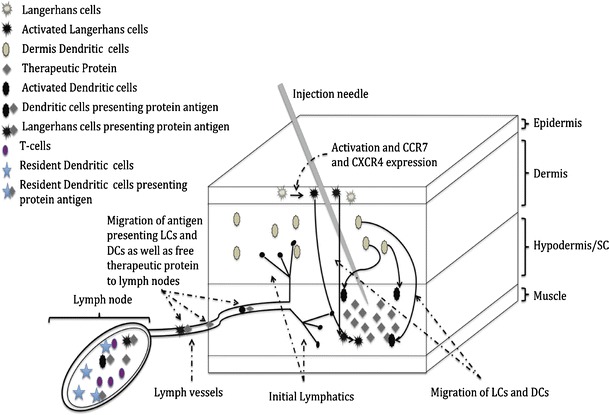
Schematic representation of the sc space and mechanism involved in immunogenic response against sc administered therapeutic proteins
Two Waves of Antigen Presentation
Using the peptide–MHC II complex of a fluorescent antigen and CD11c/CD40 markers, evidence of two waves of antigen processing and presentation was observed. This approach has been used to identify cells that present the antigen to CD4+ T cells following sc administration of antigen (25,26). The protein deposited in the sc space is presented by resident DCs in lymph nodes first and later by DCs that migrate from the epidermis and dermis.
First Wave and Lymph Node Distribution
The first wave of antigen presentation is influenced by pharmacokinetics and lymph node distribution of the protein. It has been demonstrated that following sc administration, lymphatic transport of the protein leads to its distribution in lymph nodes (27). Our PK and lymph node distribution studies showed that proteins deposited in the sc space arrive within hours to the lymph node (data not shown). These proteins and their fragments are processed by lymph node-resident DCs and subsequently present the antigen to T cells in peripheral lymph nodes. CD11chigh/CD40high, increased E-cadherin expression, and Birbeck granules characterize this antigen presentation. This set of DCs arrives within hours of administration of the antigen and is accompanied by IL-2 production and effective proliferation of T cells.
Second Wave and Migration of Cutaneous Dendritic Cells
The sc injection of proteins also triggers the migration of cutaneous dendritic cells. The use of CD11c/CD40 markers showed that both DCs from the epidermis and dermis migrate to peripheral lymph nodes via the G-protein-dependent mechanism following the subcutaneous deposition of antigen in about 1–3 days (25). These migratory cells display CD11cintCD40high and much lower expression of E-cadherin. This presentation produces a distinct function and is associated with a sustained expression of the IL-2 receptor and delayed hypersensitivity responses. The migration of the cutaneous DCs and molecular process that drives this migration are well characterized. Following sc administration of a protein antigen, the migration of LCs from epidermis and dermis DCs is triggered by inflammatory cytokines such as IL-1 beta and TNF alpha, and these cytokines up-regulate VEG-F C that in turn increases the number of lymphatic vessels at the inflammatory site (23). The migration of dendritic cells is also triggered by up-regulation of two receptors CCR7 and CXCR4 (23,28). The ligands for these receptors are expressed in lymphatic vessels, and the receptor–ligand interaction drives the migration of cutaneous DCs to draining lymph nodes. These migratory dendritic cells can also transfer the antigen to lymph node dendritic cells (29). Overall, the immunogenicity of subcutaneously administered protein antigen appears to be driven by migratory cutaneous dendritic cells and by lymph node-resident dendritic cells. Certainly, investigations are necessary to test several hypotheses based on this mechanistic model to completely understand the immunogenicity of subcutaneously administered proteins.
Strategies to Mitigate Immunogenicity
This understanding could be useful in developing strategies to mitigate immunogenicity of therapeutic proteins given via the sc route. A few possible strategies are discussed. An important step in the mediation of immunogenicity of therapeutic proteins given via the sc route arises from migration of cutaneous DCs (23,28). The migration of DCs is initiated by the inflammatory stimuli at the injection site. Thus, reduction in impurities such as LPS and aggregates in the injected proteins would be expected to ameliorate the immunogenic effect of the sc injection. The presence of LPS has been shown to increase the migration and presentation of antigen by dendritic cells to T cells (17,19). Further, our studies showed that presence of native-like aggregates in sc administered FVIII produced a much higher antibody response and possible secondary memory response compared to native FVIII and nonnative aggregates (30). Thus, avoiding inflammatory impurities and aggregates in the formulation is critical for decreasing the immunogenic effects of the sc administration. Further, the DC migration is accompanied by expression of surface receptors such as CCR7 and CXCR4, and thus, an effective approach could be use of molecules such as function-blocking antibodies specific for these receptors or formulating therapeutic proteins with molecules capable of inhibiting these receptors. These inhibitors or antibodies against CCR7 or CXCR4 can also interfere with receptor binding to ligands that are expressed in lymphatics following antigen administration, and this inhibition could reduce migration of DCs into the secondary lymphoid tissues. These approaches may limit the processing and presentation of antigens by migratory DCs, but contribute very little to control effective presentation to lymph node-resident dendritic cells.
The challenge of immunological exposure to dendritic cells for immunogenicity also provides an opportunity for mitigation approaches. Induction of immunological tolerance using dendritic cells is one of the effective ways to mitigate immunogenicity (31). It has been established that the presentation of an antigen to dendritic cells in a tolerogenic manner can promote peripheral tolerance via induction of regulatory T cells (32). We have shown that administration of therapeutic proteins such as FVIII in the presence of an immune-modulatory lipid phosphatidyl serine (PS) reduces development of inhibitory antibody responses against FVIII and promotes a hyporesponsiveness by tolerogenic presentation to dendritic cells (33–36). As the preexposure of FVIII in the presence of PS induced tolerance/hyporesponsiveness, we proposed a novel strategy called reverse vaccination to induce tolerance and thereby reduce immunogenicity of therapeutic proteins given sc. Unlike conventional vaccination, this approach induces antigen-specific hyporesponsiveness. The success of this vaccination strategy depends on effective exposure of the antigen and PS to dendritic cells, and the sc route of administration is very attractive from the “immunological exposure” perspective.
Conclusions
Overall, the immunogenicity of subcutaneously administered protein is dependent upon antigen presentation and processing by lymph node and migratory cutaneous dendritic cells in the sc space. A better understanding of this process provides opportunities to design strategies to reduce antibody responses to sc delivered therapeutic proteins and for the development and use of novel approaches to modify proteins that make them less immunogenic or tolerogenic.
ACKNOWLEDGMENTS
The authors are grateful for the financial support from National Heart, Lung and Blood Institute, National Institute of Health, HL-70227, to svb.
Conflict of Interest
The authors declare that there is no financial conflict of interest.
REFERENCES
- 1.Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93:2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 2.Tiede A, et al. Safety and pharmacokinetics of subcutaneously administered recombinant activated factor VII (rFVIIa) J Thromb Haemostasis: JTH. 2011;9:1191–1199. doi: 10.1111/j.1538-7836.2011.04293.x. [DOI] [PubMed] [Google Scholar]
- 3.Braun A, Kwee L, Labow MA, Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-alpha) in normal and transgenic mice. Pharm Res. 1997;14:1472–1478. doi: 10.1023/A:1012193326789. [DOI] [PubMed] [Google Scholar]
- 4.Genovese MC, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63:2854–2864. doi: 10.1002/art.30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiff M. Subcutaneous abatacept for the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2013;52:986–97. doi: 10.1093/rheumatology/ket018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartelds GM, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 7.Peng A, Kosloski MP, Nakamura G, Ding H, Balu-Iyer SV. PEGylation of a factor VIII-phosphatidylinositol complex: pharmacokinetics and immunogenicity in hemophilia A mice. AAPS J. 2012;14:35–42. doi: 10.1208/s12248-011-9309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA. Drug approval package. Adalimumab. 2002. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/125057s110TOC.cfm. Accessed March 2013.
- 9.Torosantucci R, et al. Identification of oxidation sites and covalent cross-links in metal catalyzed oxidized interferon beta-1a: potential implications for protein aggregation and immunogenicity. Mol Pharmaceutics. 2013;10:2311–2322. doi: 10.1021/mp300665u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mianowska B, et al. Immunogenicity of different brands of human insulin and rapid-acting insulin analogs in insulin-naive children with type 1 diabetes. Pediatr Diabetes. 2011;12:78–84. doi: 10.1111/j.1399-5448.2010.00659.x. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt KU, Casadevall N. Pure red-cell aplasia due to anti-erythropoietin antibodies. Nephrol Dial Transplant. 2003;18:865–869. doi: 10.1093/ndt/gfg182. [DOI] [PubMed] [Google Scholar]
- 12.Peng A, et al. Effect of route of administration of human recombinant factor VIII on its immunogenicity in hemophilia A mice. J Pharm Sci. 2009;98:4480–4484. doi: 10.1002/jps.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schellekens H. Immunogenicity of therapeutic proteins. Nephrol Dial Transplant: Off Publ Eur Dial Transpl Assoc - Eur Renal Assoc. 2003;18:1257–1259. doi: 10.1093/ndt/gfg164. [DOI] [PubMed] [Google Scholar]
- 14.Schellekens H. The immunogenicity of therapeutic proteins. Discov Med. 2010;9:560–564. [PubMed] [Google Scholar]
- 15.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 16.Bogunovic M, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis e Sousa C, Germain RN. Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J Immunol. 1999;162:6552–6561. [PubMed] [Google Scholar]
- 18.Manickasingham S, Reis e Sousa C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J Immunol. 2000;165:5027–5034. doi: 10.4049/jimmunol.165.9.5027. [DOI] [PubMed] [Google Scholar]
- 19.Zhong G, Reis e Sousa C, Germain RN. Antigen-unspecific B cells and lymphoid dendritic cells both show extensive surface expression of processed antigen-major histocompatibility complex class II complexes after soluble protein exposure in vivo or in vitro. J Exp Med. 1997;186:673–682. doi: 10.1084/jem.186.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 21.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 22.Nagao K, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang ST. Homeward bound: how do skin dendritic cells find their way into the lymph system? J Investig Dermatol. 2012;132:1070–1073. doi: 10.1038/jid.2012.39. [DOI] [PubMed] [Google Scholar]
- 24.Iezzi G, et al. Lymph node resident rather than skin-derived dendritic cells initiate specific T cell responses after Leishmania major infection. J Immunol. 2006;177:1250–1256. doi: 10.4049/jimmunol.177.2.1250. [DOI] [PubMed] [Google Scholar]
- 25.Ruedl C, Koebel P, Bachmann M, Hess M, Karjalainen K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J Immunol. 2000;165:4910–4916. doi: 10.4049/jimmunol.165.9.4910. [DOI] [PubMed] [Google Scholar]
- 26.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/S1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 27.Porter CJ, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89:297–310. doi: 10.1002/(SICI)1520-6017(200003)89:3<297::AID-JPS2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Kabashima K, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–1257. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Pisal DS, Kosloski MP, Middaugh CR, Bankert RB, Balu-Iyer SV. Native-like aggregates of factor VIII are immunogenic in von Willebrand factor deficient and hemophilia a mice. J Pharm Sci. 2012;101:2055–2065. doi: 10.1002/jps.23091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idoyaga J, et al. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Investig. 2013;123:844–54. doi: 10.1172/JCI65260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Purohit VS, Ramani K, Sarkar R, Kazazian HH, Jr, Balasubramanian SV. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J Biol Chem. 2005;280:17593–17600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramani K, et al. Phosphatidylserine containing liposomes reduce immunogenicity of recombinant human factor VIII (rFVIII) in a murine model of hemophilia A. J Pharm Sci. 2008;97:1386–1398. doi: 10.1002/jps.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaitonde P, Peng A, Straubinger RM, Bankert RB, Balu-Iyer SV. Phosphatidylserine reduces immune response against human recombinant factor VIII in hemophilia A mice by regulation of dendritic cell function. Clin Immunol. 2011;138:135–145. doi: 10.1016/j.clim.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaitonde P, et al. Exposure to factor VIII protein in the presence of phosphatidylserine induces hypo-responsiveness toward factor VIII challenge in hemophilia A mice. J Biol Chem. 2013;288:17051–17056. doi: 10.1074/jbc.C112.396325. [DOI] [PMC free article] [PubMed] [Google Scholar]


